MicroRNA Modulation during Orthodontic Tooth Movement: A Promising Strategy for Novel Diagnostic and Personalized Therapeutic Interventions
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Relationship between miRNA and OTM
3.1.1. Modulation of miRNAs in Canine Retraction
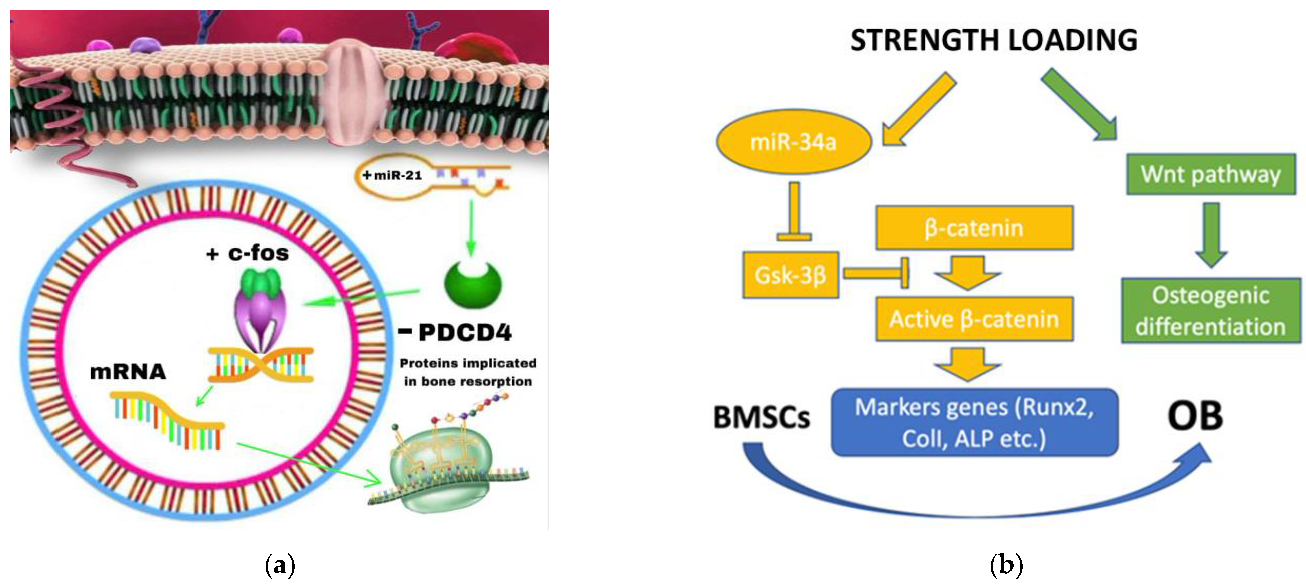
3.1.2. The Role of miRNA-21 during OTM
- Yuanyuan Zhang et al. investigated the role of miRNA-21 on four groups of rats: TM (Tooth Movement), PAOO (Periodontal Acceleration Orthodontic Osteogenesis), AgomiR-21 (MiR-21 Overexpression) and AntagomiR-21 (inhibition of miR-21). After 7 days of treatment, the tooth displacement obtained by the agomir-21 group was significantly greater than both TM and PAOO, demonstrating the role of miRNA-21 in promoting RANKL-mediated osteoblast differentiation via negative modulation of the PDCD4 gene and thus facilitating OTM [35].
- This study provides a demonstration of the miRna-21 increase in periodontal ligament cells (PDL) and an acceleration of orthodontic movement in mice treated with E. Coli LPS inoculation. This suggests a direct role of miRNA-21 during OTM even in an inflammatory microenvironment [36].
- It has also been shown that the role of miRna-21 on OC differentiation is not only due to the modulation of the PDCD4/C-fos pathway, but also due to the influence of RANKL secretion by T cells [37].
3.1.3. miRNA-34a
- An in vitro study of periodontal ligament stem cells (PDLSC) obtained from the periodontal ligament (PDL) of teeth extracted for orthodontic reasons demonstrated the inhibitory role of miRNA-34a and miRNA-146a against osteoblast by silencing the CELF3 gene [38].
- In contrast, Wenwen Yu et al. showed a positive role of miRNA 34a in osteogenic differentiation both in vitro and in vivo. The miRNA-34a, provided by the N-Ac-l-Leu-PEI vector, is capable of increasing dental anchorage in vivo and stimulating osteogenic differentiation in vitro. Its target gene is Gsk-3β which promotes the phosphorylation of the β-catenin protein, which is essential once accumulated in the cell nucleus to favor the Wnt/beta-catenin pathway and, therefore, the activation of OB differentiation gene [39].
3.1.4. Other miRNAs Implicated on OTM
- Other authors performed studies about the evaluation of miRNAs associated with frequent complications of orthodontic treatment, i.e., root resorption. miRNA-155-5p significantly decreases with increasing degree of root resorption (a sign of excessive osteoclast activation), so this miRNA could be implicated in the inhibition of osteoclast differentiation by suppressing transcription and expression of CXCR2 gene, involved in the synthesis of many OC enzymes linked to bone resorption [40].
- Wendan He et al. demonstrated that miRNA 125a-5p promotes M2 polarization of macrophages, which increases osteogenesis. Furthermore, this miRNA strongly increased under an orthodontic force and the OTM role is performed through the inhibition of the ETV6 gene [41].
- Considering that rBMSCs subjected to mechanical stress undergo osteogenic differentiation, the in vitro decrease in miRNA-503-5p levels (minimum level after 12 h) indicates its negative role in this type of differentiation. Furthermore, the confirmation occurred in vivo as the levels of this miRNA significantly decreased in the tension side (trend maintained for 3 days) and then increase again [42].
- A further study related the expression of miRNAs in the two sites identified during an orthodontic movement, namely compression and tension side. The authors demonstrated that miRNA-3198 is overexpressed on the compression side and at the same time there is a reduction in OPG levels in hPDL cells (as opposed to on the tension side). These results suggest that miRNA-3198 is capable to inhibit OPG (target) in response to a mechanical stimulus [43].
4. Discussion
5. Conclusions
- To study the miRNAs involved in other orthodontic movements (e.g.: extrusion, up-righting, torque, etc.) or between orthodontic and orthopedic movements (Rapid Maxillary Expansion (RME));
- To investigate the role of miRNA34a in the improvement of anchorage or other miRNAs involved in this orthodontic system. This could improve the force used during a rapid palate expansion (RME) treatment method, as it could provide guidance on the interdigitation degree of the median palatine suture and thus reduce possible side effects on the roots of the upper first molars or prevent their exit from the alveolar bone.
- To perform further studies to evaluate the modulation of miRNAs in the very early stages following the application of a predeterminated force to understand when the bone remodeling process begins, if there is a greater risk of undergoing root resorption or after how long it takes reduce or increase strength to optimize OTM.
- Conduct clinical investigations to measure the amount of miRNAs present in the crevicular fluid on either side of the tooth. This can give a more precise evaluation of how different the miRNA under investigation is from the standard.
- Use CGF sampling to evaluate miRNAs linked to OTM as salivary samples may not be accurate enough due to the possibility of other systemic factors.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, H.; Williams, R.C.; Kyrkanides, S. Accelerated orthodontic tooth movement: Molecular mechanisms. Am. J. Orthod. Dentofacial. Orthop. 2014, 146, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Van Schepdael, A.; Vander Sloten, J.; Geris, L. A mechanobiological model of orthodontic tooth movement. Biomech. Model Mechanobiol. 2013, 12, 249–265. [Google Scholar] [CrossRef] [PubMed]
- Ketting, R.F. microRNA Biogenesis and Function: An overview. Adv. Exp. Med. Biol. 2011, 700, 1–14. [Google Scholar] [CrossRef]
- Beertsen, W.; McCulloch, C.A.; Sodek, J. The periodontal ligament: A unique, multifunctional connective tissue. Periodontology 2000 1997, 13, 20–40. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Xiao, C.; Wan, X.; Cha, W.; Miao, Y.; Zhou, Y.; Qin, C.; Cui, T.; Su, F.; Shan, X. Small molecules with big roles in microRNA chemical biology and microRNA-targeted therapeutics. RNA Biol. 2019, 16, 707–718. [Google Scholar] [CrossRef]
- Cortez, M.A.; Bueso-Ramos, C.; Ferdin, J.; Lopez-Berestein, G.; Sood, A.K.; Calin, G.A. MicroRNAs in body fluids--the mix of hormones and biomarkers. Nat. Rev. Clin. Oncol. 2011, 8, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Tandon, M.; Alevizos, I.; Illei, G.G. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS ONE 2012, 7, e30679. [Google Scholar] [CrossRef]
- Mizoguchi, F.; Izu, Y.; Hayata, T.; Hemmi, H.; Nakashima, K.; Nakamura, T.; Kato, S.; Miyasaka, N.; Ezura, Y.; Noda, M. Osteoclast-specific Dicer gene deficiency suppresses osteoclastic bone resorption. J. Cell Biochem. 2010, 109, 866–875. [Google Scholar] [CrossRef]
- Sugatani, T.; Hruska, K.A. Impaired micro-RNA pathways diminish osteoclast differentiation and function. J. Biol. Chem. 2009, 284, 4667–4678. [Google Scholar] [CrossRef]
- Sugatani, T.; Vacher, J.; Hruska, K.A. A microRNA expression signature of osteoclastogenesis. Blood 2011, 117, 3648–3657. [Google Scholar] [CrossRef]
- Pi, C.; Li, Y.P.; Zhou, X.; Gao, B. The expression and function of microRNAs in bone homeostasis. Front. Biosci. (Landmark Ed.) 2015, 20, 119–138. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kapoor, P.; Kharbanda, O.P.; Monga, N.; Miglani, R.; Kapila, S. Effect of orthodontic forces on cytokine and receptor levels in gingival crevicular fluid: A systematic review. Prog. Orthod. 2014, 15, 65. [Google Scholar] [CrossRef]
- Kapoor, P.; Monga, N.; Kharbanda, O.P.; Kapila, S.; Miglani, R.; Moganty, R. Effect of orthodontic forces on levels of enzymes in gingival crevicular fluid (GCF): A systematic review. Dental Press J. Orthod. 2019, 24, 40.e1–40.e22. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [PubMed]
- Kular, J.; Tickner, J.; Chim, S.M.; Xu, J. An overview of the regulation of bone remodelling at the cellular level. Clin. Biochem. 2012, 45, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Udagawa, N.; Takahashi, N.; Jimi, E.; Matsuzaki, K.; Tsurukai, T.; Itoh, K.; Nakagawa, N.; Yasuda, H.; Goto, M.; Tsuda, E.; et al. Osteoblasts/stromal cells stimulate osteoclast activation through expression of osteoclast differentiation factor/RANKL but not macrophage colony-stimulating factor: Receptor activator of NF-kappa B ligand. Bone 1999, 25, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, L.C.; Khosla, S.; Dunstan, C.R.; Lacey, D.L.; Boyle, W.J.; Riggs, B.L. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J. Bone Miner. Res. 2000, 15, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Kenkre, J.S.; Bassett, J. The bone remodelling cycle. Ann. Clin. Biochem. 2018, 55, 308–327. [Google Scholar] [CrossRef]
- Nishijima, Y.; Yamaguchi, M.; Kojima, T.; Aihara, N.; Nakajima, R.; Kasai, K. Levels of RANKL and OPG in gingival crevicular fluid during orthodontic tooth movement and effect of compression force on releases from periodontal ligament cells in vitro. Orthod. Craniofac. Res. 2006, 9, 63–70. [Google Scholar] [CrossRef]
- Kanzaki, H.; Chiba, M.; Sato, A.; Miyagawa, A.; Arai, K.; Nukatsuka, S.; Mitani, H. Cyclical tensile force on periodontal ligament cells inhibits osteoclastogenesis through OPG induction. J. Dent. Res. 2006, 85, 457–462. [Google Scholar] [CrossRef]
- Hu, C.H.; Sui, B.D.; Du, F.Y.; Shuai, Y.; Zheng, C.X.; Zhao, P.; Yu, X.R.; Jin, Y. miR-21 deficiency inhibits osteoclast function and prevents bone loss in mice. Sci. Rep. 2017, 7, 43191. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Zhou, H.; Zeng, X.; Feng, S. Estrogen stimulates osteoprotegerin expression via the suppression of miR-145 expression in MG-63 cells. Mol. Med. Rep. 2017, 15, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Yete, S.; Saranath, D. MicroRNAs in oral cancer: Biomarkers with clinical potential. Oral Oncol. 2020, 110, 105002. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Zhou, X.; Naqvi, A.; Francis, M.; Foyle, D.; Nares, S.; Diekwisch, T.G.H. MicroRNAs and immunity in periodontal health and disease. Int. J. Oral Sci. 2018, 10, 24. [Google Scholar] [CrossRef]
- Luan, X.; Zhou, X.; Trombetta-eSilva, J.; Francis, M.; Gaharwar, A.K.; Atsawasuwan, P.; Diekwisch, T.G.H. MicroRNAs and Periodontal Homeostasis. J. Dent. Res. 2017, 96, 491–500. [Google Scholar] [CrossRef]
- Grassia, V.; Lombardi, A.; Kawasaki, H.; Ferri, C.; Perillo, L.; Mosca, L.; Delle Cave, D.; Nucci, L.; Porcelli, M.; Caraglia, M. Salivary microRNAs as new molecular markers in cleft lip and palate: A new frontier in molecular medicine. Oncotarget 2018, 9, 18929–18938. [Google Scholar] [CrossRef][Green Version]
- Wang, J.; Du, Y.; Deng, J.; Wang, X.; Long, F.; He, J. MicroRNA-506 Is Involved in Regulation of the Occurrence of Lipopolysaccharides (LPS)-Induced Pulpitis by Sirtuin 1 (SIRT1). Med. Sci. Monit. 2019, 25, 10008–10015. [Google Scholar] [CrossRef]
- Zhou, W.; Su, L.; Duan, X.; Chen, X.; Hays, A.; Upadhyayula, S.; Shivde, J.; Wang, H.; Li, Y.; Huang, D.; et al. MicroRNA-21 down-regulates inflammation and inhibits periodontitis. Mol. Immunol. 2018, 101, 608–614. [Google Scholar] [CrossRef]
- Scribante, A.; Gallo, S.; Pascadopoli, M.; Soleo, R.; Di Fonso, F.; Politi, L.; Venugopal, A.; Marya, A.; Butera, A. Management of Periodontal Disease with Adjunctive Therapy with Ozone and Photobiomodulation (PBM): A Randomized Clinical Trial. Photonics 2022, 9, 138. [Google Scholar] [CrossRef]
- Kapoor, P.; Chowdhry, A.; Bagga, D.K.; Bhargava, D.; Aishwarya, S. MicroRNAs in oral fluids (saliva and gingival crevicular fluid) as biomarkers in orthodontics: Systematic review and integrated bioinformatic analysis. Prog. Orthod. 2021, 22, 31. [Google Scholar] [CrossRef]
- Atsawasuwan, P.; Lazari, P.; Chen, Y.; Zhou, X.; Viana, G.; Evans, C.A. Secretory microRNA-29 expression in gingival crevicular fluid during orthodontic tooth movement. PLoS ONE 2018, 13, e0194238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yang, L.; Zheng, W.; Lin, T. MicroRNA-34 expression in gingival crevicular fluid correlated with orthodontic tooth movement. Angle Orthod. 2020, 90, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Lazari, P. Secretory micro-RNA 29 in Gingival Crevicular Fluid during Canine Retraction [Internet] [Thesis]. University of Illinois at Chicago. 2016. Available online: /articles/thesis/Secretory_Micro-RNA_29_in_Gingival_Crevicular_Fluid_During_Canine_Retraction/10812575/1 (accessed on 12 March 2021).
- Seagraves, A.L. Circulatory microRNA-27, -146, and -214 in Gingival Crevicular Fluid during Orthodontic Tooth Movement [Internet] [Thesis]. University of Illinois at Chicago. 2020. Available online: /articles/thesis/Circulatory_MicroRNA-27_-146_and_-214_in_Gingival_Crevicular_Fluid_During_Orthodontic_Tooth_Movement/13475250/1 (accessed on 24 December 2020).
- Zhang, Y.; Tian, Y.; Yang, X.; Zhao, Z.; Feng, C.; Zhang, Y. MicroRNA-21 serves an important role during PAOO-facilitated orthodontic tooth movement. Mol. Med. Rep. 2020, 22, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Sui, B.D.; Hu, C.H.; Cao, J.; Zheng, C.X.; Hou, R.; Yang, Z.K.; Zhao, P.; Chen, Q.; Yang, Q.J.; et al. microRNA-21 Contributes to Orthodontic Tooth Movement. J. Dent. Res. 2016, 95, 1425–1433. [Google Scholar] [CrossRef]
- Wu, L.; Su, Y.; Lin, F.; Zhu, S.; Wang, J.; Hou, Y.; Du, J.; Liu, Y.; Guo, L. MicroRNA-21 promotes orthodontic tooth movement by modulating the RANKL/OPG balance in T cells. Oral Dis. 2020, 26, 370–380. [Google Scholar] [CrossRef]
- Meng, X.; Wang, W.; Wang, X. MicroRNA-34a and microRNA-146a target CELF3 and suppress the osteogenic differentiation of periodontal ligament stem cells under cyclic mechanical stretch. J. Dent. Sci. 2022, 17, 1281–1291. [Google Scholar] [CrossRef]
- Yu, W.; Zheng, Y.; Yang, Z.; Fei, H.; Wang, Y.; Hou, X.; Sun, X.; Shen, Y. N-AC-l-Leu-PEI-mediated miR-34a delivery improves osteogenic differentiation under orthodontic force. Oncotarget. 2017, 8, 110460–110473. [Google Scholar] [CrossRef]
- Jiang, H.; Kitaura, H.; Liu, L.; Mizoguchi, I.; Liu, S. The miR-155-5p inhibits osteoclast differentiation through targeting CXCR2 in orthodontic root resorption. J. Periodontal. Res. 2021, 56, 761–773. [Google Scholar] [CrossRef]
- He, W.; Zhang, N.; Lin, Z. MicroRNA-125a-5p modulates macrophage polarization by targeting E26 transformation-specific variant 6 gene during orthodontic tooth movement. Arch. Oral Biol. 2021, 124, 105060. [Google Scholar] [CrossRef]
- Liu, L.; Liu, M.; Li, R.; Liu, H.; Du, L.; Chen, H.; Zhang, Y.; Zhang, S.; Liu, D. MicroRNA-503-5p inhibits stretch-induced osteogenic differentiation and bone formation. Cell Biol. Int. 2017, 41, 112–123. [Google Scholar] [CrossRef]
- Kanzaki, H.; Wada, S.; Yamaguchi, Y.; Katsumata, Y.; Itohiya, K.; Fukaya, S.; Miyamoto, Y.; Narimiya, T.; Noda, K.; Nakamura, Y. Compression and tension variably alter Osteoprotegerin expression via miR-3198 in periodontal ligament cells. BMC Mol. Cell Biol. 2019, 20, 6. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ou, Y.; Liao, C.; Liang, S.; Wang, Y. High-throughput sequencing analysis of the expression profile of microRNAs and target genes in mechanical force-induced osteoblastic/cementoblastic differentiation of human periodontal ligament cells. Am. J. Transl. Res. 2019, 11, 3398–3411. [Google Scholar] [PubMed]
- Jia, J.; Tian, Q.; Ling, S.; Liu, Y.; Yang, S.; Shao, Z. miR-145 suppresses osteogenic differentiation by targeting Sp7. FEBS Lett. 2013, 587, 3027–3031. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Morris, J.H.; Cook, H.; Kuhn, M.; Wyder, S.; Simonovic, M.; Santos, A.; Doncheva, N.T.; Roth, A.; Bork, P.; et al. The STRING database in 2017: Quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017, 45, D362–D368. [Google Scholar] [CrossRef] [PubMed]
- Kagiya, T.; Nakamura, S. Expression profiling of microRNAs in RAW264.7 cells treated with a combination of tumor necrosis factor alpha and RANKL during osteoclast differentiation. J. Periodontal. Res. 2013, 48, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Franceschetti, T.; Kessler, C.B.; Lee, S.K.; Delany, A.M. miR-29 promotes murine osteoclastogenesis by regulating osteoclast commitment and migration. J. Biol. Chem. 2013, 288, 33347–33360. [Google Scholar] [CrossRef] [PubMed]
- Kook, S.H.; Jang, Y.S.; Lee, J.C. Human periodontal ligament fibroblasts stimulate osteoclastogenesis in response to compression force through TNF-α-mediated activation of CD4+ T cells. J. Cell Biochem. 2011, 112, 2891–2901. [Google Scholar] [CrossRef]
- Toyono, T.; Usui, T.; Villarreal, G., Jr.; Kallay, L.; Matthaei, M.; Vianna, L.M.; Zhu, A.Y.; Kuroda, M.; Amano, S.; Jun, A.S. MicroRNA-29b Overexpression Decreases Extracellular Matrix mRNA and Protein Production in Human Corneal Endothelial Cells. Cornea. 2016, 35, 1466–1470. [Google Scholar] [CrossRef]
- Zhang, Y.; Ghazwani, M.; Li, J.; Sun, M.; Stolz, D.B.; He, F.; Fan, J.; Xie, W.; Li, S. MiR-29b inhibits collagen maturation in hepatic stellate cells through down-regulating the expression of HSP47 and lysyl oxidase. Biochem. Biophys. Res. Commun. 2014, 446, 940–944. [Google Scholar] [CrossRef]
- Villarreal, G., Jr.; Oh, D.J.; Kang, M.H.; Rhee, D.J. Coordinated regulation of extracellular matrix synthesis by the microRNA-29 family in the trabecular meshwork. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3391–3397. [Google Scholar] [CrossRef]
- Isola, G.; Matarese, G.; Cordasco, G.; Perillo, L.; Ramaglia, L. Mechanobiology of the tooth movement during the orthodontic treatment: A literature review. Minerva Stomatol. 2016, 65, 299–327. [Google Scholar] [PubMed]
- Kapinas, K.; Kessler, C.B.; Delany, A.M. miR-29 suppression of osteonectin in osteoblasts: Regulation during differentiation and by canonical Wnt signaling. J. Cell Biochem. 2009, 108, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hassan, M.Q.; Jafferji, M.; Aqeilan, R.I.; Garzon, R.; Croce, C.M.; van Wijnen, A.J.; Stein, J.L.; Stein, G.S.; Lian, J.B. Correction: Biological functions of miR-29b contribute to positive regulation of osteoblast differentiation. J. Biol. Chem. 2019, 294, 10018. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, C.; Yue, J.; Huang, X.; Chen, M.; Gao, J.; Wu, B. miR-21 and miR-101 regulate PLAP-1 expression in periodontal ligament cells. Mol. Med. Rep. 2012, 5, 1340–1346. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cheng, H.S.; Sivachandran, N.; Lau, A.; Boudreau, E.; Zhao, J.L.; Baltimore, D.; Delgado-Olguin, P.; Cybulsky, M.I.; Fish, J.E. MicroRNA-146 represses endothelial activation by inhibiting pro-inflammatory pathways. EMBO Mol. Med. 2013, 5, 1017–1034. [Google Scholar] [CrossRef] [PubMed]
- Sugatani, T.; Hruska, K.A. Down-regulation of miR-21 biogenesis by estrogen action contributes to osteoclastic apoptosis. J. Cell Biochem. 2013, 114, 1217–1222. [Google Scholar] [CrossRef]
- Yang, N.; Wang, G.; Hu, C.; Shi, Y.; Liao, L.; Shi, S.; Cai, Y.; Cheng, S.; Wang, X.; Liu, Y.; et al. Tumor necrosis factor α suppresses the mesenchymal stem cell osteogenesis promoter miR-21 in estrogen deficiency-induced osteoporosis. J. Bone Miner. Res. 2013, 28, 559–573. [Google Scholar] [CrossRef]
- Frankel, L.B.; Christoffersen, N.R.; Jacobsen, A.; Lindow, M.; Krogh, A.; Lund, A.H. Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. J. Biol. Chem. 2008, 283, 1026–1033. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Li, X.; Zhang, Z.; Zhao, X.; Wang, C.; Wei, F. MicroRNA-21 promotes bone reconstruction in maxillary bone defects. J. Oral Rehabil. 2020, 47 (Suppl. 1), 4–11. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, F.; Kou, X.; Liu, D.; Yang, R.; Wang, X.; Song, Y.; He, D.; Gan, Y.; Zhou, Y. T Cells Are Required for Orthodontic Tooth Movement. J. Dent. Res. 2015, 94, 1463–1470. [Google Scholar] [CrossRef]
- Lu, T.X.; Hartner, J.; Lim, E.J.; Fabry, V.; Mingler, M.K.; Cole, E.T.; Orkin, S.H.; Aronow, B.J.; Rothenberg, M.E. MicroRNA-21 limits in vivo immune response-mediated activation of the IL-12/IFN-gamma pathway, Th1 polarization, and the severity of delayed-type hypersensitivity. J. Immunol. 2011, 187, 3362–3373. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.X.; Munitz, A.; Rothenberg, M.E. MicroRNA-21 is up-regulated in allergic airway inflammation and regulates IL-12p35 expression. J. Immunol. 2009, 182, 4994–5002. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zheng, J.; Yang, C.; Xie, Q.; Liu, X.; Abdelrehem, A. A new modified bone grafting technique for periodontally accelerated osteogenic orthodontics. Medicine (Baltimore) 2018, 97, e12047. [Google Scholar] [CrossRef]
- Hou, H.Y.; Li, C.H.; Chen, M.C.; Lin, P.Y.; Liu, W.C.; Cathy Tsai, Y.W.; Huang, R.Y. A novel 3D-printed computer-assisted piezocision guide for surgically facilitated orthodontics. Am. J. Orthod. Dentofacial. Orthop. 2019, 155, 584–591. [Google Scholar] [CrossRef]
- Boisvert, W.A.; Curtiss, L.K.; Terkeltaub, R.A. Interleukin-8 and its receptor CXCR2 in atherosclerosis. Immunol. Res. 2000, 21, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Li, X.; Lv, W.; Xu, Z. Inhibition of CXCL1-CXCR2 axis ameliorates cisplatin-induced acute kidney injury by mediating inflammatory response. Biomed. Pharmacother. 2020, 122, 109693. [Google Scholar] [CrossRef] [PubMed]
- Hammond, M.E.; Lapointe, G.R.; Feucht, P.H.; Hilt, S.; Gallegos, C.A.; Gordon, C.A.; Giedlin, M.A.; Mullenbach, G.; Tekamp-Olson, P. IL-8 induces neutrophil chemotaxis predominantly via type I IL-8 receptors. J. Immunol. 1995, 155, 1428–1433. [Google Scholar] [PubMed]
- Bickel, M. The role of interleukin-8 in inflammation and mechanisms of regulation. J. Periodontol. 1993, 64 (Suppl. 5), 456–460. [Google Scholar]
- Curtale, G.; Rubino, M.; Locati, M. MicroRNAs as Molecular Switches in Macrophage Activation. Front. Immunol. 2019, 10, 799. [Google Scholar] [CrossRef]
- Roy, S. miRNA in Macrophage Development and Function. Antioxid. Redox Signal. 2016, 25, 795–804. [Google Scholar] [CrossRef]
- Loh, P.G.; Yang, H.S.; Walsh, M.A.; Wang, Q.; Wang, X.; Cheng, Z.; Liu, D.; Song, H. Structural basis for translational inhibition by the tumour suppressor Pdcd4. EMBO J. 2009, 28, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.D. Transcriptional control of granulocyte and monocyte development. Oncogene 2007, 26, 6816–6828. [Google Scholar] [CrossRef] [PubMed]
- Huhe, M.; Liu, S.; Zhang, Y.; Zhang, Z.; Chen, Z. Expression levels of transcription factors c-Fos and c-Jun and transmembrane protein HAb18G/CD147 in urothelial carcinoma of the bladder. Mol. Med. Rep. 2017, 15, 2991–3000. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, Y.B.; Zhang, X.H.; Yu, X.L.; Wang, Z.B.; Cheng, X.C. MicroRNA-21 gene and cancer. Med. Oncol. 2013, 30, 376. [Google Scholar] [CrossRef]
- Fan, B.; Jin, Y.; Zhang, H.; Zhao, R.; Sun, M.; Sun, M.; Yuan, X.; Wang, W.; Wang, X.; Chen, Z.; et al. MicroRNA-21 contributes to renal cell carcinoma cell invasiveness and angiogenesis via the PDCD4/c-Jun (AP-1) signalling pathway. Int. J. Oncol. 2020, 56, 178–192. [Google Scholar] [CrossRef]
- Sehic, A.; Tulek, A.; Khuu, C.; Nirvani, M.; Sand, L.P.; Utheim, T.P. Regulatory roles of microRNAs in human dental tissues. Gene 2017, 596, 9–18. [Google Scholar] [CrossRef]
- Krzeszinski, J.Y.; Wei, W.; Huynh, H.; Jin, Z.; Wang, X.; Chang, T.C.; Xie, X.J.; He, L.; Mangala, L.S.; Lopez-Berestein, G.; et al. miR-34a blocks osteoporosis and bone metastasis by inhibiting osteoclastogenesis and Tgif2. Nature 2014, 512, 431–435. [Google Scholar] [CrossRef]
- Paiva, K.B.S.; Granjeiro, J.M. Matrix Metalloproteinases in Bone Resorption, Remodeling, and Repair. Prog. Mol. Biol. Transl. Sci. 2017, 148, 203–303. [Google Scholar] [CrossRef]
- Zeng, X.Z.; He, L.G.; Wang, S.; Wang, K.; Zhang, Y.Y.; Tao, L.; Li, X.J.; Liu, S.W. Aconine inhibits RANKL-induced osteoclast differentiation in RAW264.7 cells by suppressing NF-κB and NFATc1 activation and DC-STAMP expression. Acta Pharmacol. Sin. 2016, 37, 255–263. [Google Scholar] [CrossRef]
- Linsuwanont, B.; Takagi, Y.; Ohya, K.; Shimokawa, H. Expression of matrix metalloproteinase-9 mRNA and protein during deciduous tooth resorption in bovine odontoclasts. Bone 2002, 31, 472–478. [Google Scholar] [CrossRef]
- Zuo, B.; Zhu, J.; Li, J.; Wang, C.; Zhao, X.; Cai, G.; Li, Z.; Peng, J.; Wang, P.; Shen, C.; et al. microRNA-103a functions as a mechanosensitive microRNA to inhibit bone formation through targeting Runx2. J. Bone Miner. Res. 2015, 30, 330–345. [Google Scholar] [CrossRef] [PubMed]
- Marin, T.; Gongol, B.; Chen, Z.; Woo, B.; Subramaniam, S.; Chien, S.; Shyy, J.Y. Mechanosensitive microRNAs-role in endothelial responses to shear stress and redox state. Free Radic. Biol. Med. 2013, 64, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.Q.; Maeda, Y.; Taipaleenmaki, H.; Zhang, W.; Jafferji, M.; Gordon, J.A.; Li, Z.; Croce, C.M.; van Wijnen, A.J.; Stein, J.L.; et al. miR-218 directs a Wnt signaling circuit to promote differentiation of osteoblasts and osteomimicry of metastatic cancer cells. J. Biol. Chem. 2012, 287, 42084–42092. [Google Scholar] [CrossRef] [PubMed]
- Storey, E. The nature of tooth movement. Am. J. Orthod. 1973, 63, 292–314. [Google Scholar] [CrossRef]
- Persson, H.; Kvist, A.; Rego, N.; Staaf, J.; Vallon-Christersson, J.; Luts, L.; Loman, N.; Jonsson, G.; Naya, H.; Hoglund, M.; et al. Identification of new microRNAs in paired normal and tumor breast tissue suggests a dual role for the ERBB2/Her2 gene. Cancer Res. 2011, 71, 78–86. [Google Scholar] [CrossRef]
- Huang, X.; Xiong, X.; Liu, J.; Zhao, Z.; Cen, X. MicroRNAs-containing extracellular vesicles in bone remodeling: An emerging frontier. Life Sci. 2020, 254, 117809. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Li, Q.; Wang, H.; Peng, H.; Huyan, T.; Cacalano, N.A. Exosomes: Versatile Nano Mediators of Immune Regulation. Cancers 2019, 11, 1557. [Google Scholar] [CrossRef]
- Nawaz, M.; Fatima, F.; Vallabhaneni, K.C.; Penfornis, P.; Valadi, H.; Ekström, K.; Kholia, S.; Whitt, J.D.; Fernandes, J.D.; Pochampally, R.; et al. Extracellular Vesicles: Evolving Factors in Stem Cell Biology. Stem Cells Int. 2016, 2016, 1073140. [Google Scholar] [CrossRef]
- Zhang, L.; Jiao, G.; Ren, S.; Zhang, X.; Li, C.; Wu, W.; Wang, H.; Liu, H.; Zhou, H.; Chen, Y. Exosomes from bone marrow mesenchymal stem cells enhance fracture healing through the promotion of osteogenesis and angiogenesis in a rat model of nonunion. Stem Cell Res. Ther. 2020, 11, 38. [Google Scholar] [CrossRef]
- Rahmati, S.; Shojaei, F.; Shojaeian, A.; Rezakhani, L.; Dehkordi, M.B. An overview of current knowledge in biological functions and potential theragnostic applications of exosomes. Chem. Phys. Lipids 2020, 226, 104836. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Z.; Duan, N.; Zhu, G.; Schwarz, E.M.; Xie, C. Osteoblast-osteoclast interactions. Connect. Tissue Res. 2018, 59, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.L.; Wu, Q.Y.; Miao, Z.N.; Xu, M.H.; Xu, R.S.; Jiang, D.L.; Ye, J.X.; Chen, F.H.; Zhao, M.D.; Wang, H.J.; et al. Osteoclast-Derived Extracellular Vesicles: Novel Regulators of Osteoclastogenesis and Osteoclast-Osteoblasts Communication in Bone Remodeling. Front. Physiol. 2018, 9, 628. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhai, C.; Hu, J.; Li, Z.; Fei, H.; Wang, Z.; Fan, W. MiR-23a inhibited IL-17-mediated proinflammatory mediators expression via targeting IKKα in articular chondrocytes. Int. Immunopharmacol. 2017, 43, 1–6. [Google Scholar] [CrossRef]
- Yang, J.X.; Xie, P.; Li, Y.S.; Wen, T.; Yang, X.C. Osteoclast-derived miR-23a-5p-containing exosomes inhibit osteogenic differentiation by regulating Runx2. Cell. Signal. 2020, 70, 109504. [Google Scholar] [CrossRef]
- Xu, Q.; Cui, Y.; Luan, J.; Zhou, X.; Li, H.; Han, J. Exosomes from C2C12 myoblasts enhance osteogenic differentiation of MC3T3-E1 pre-osteoblasts by delivering miR-27a-3p. Biochem. Biophys. Res. Commun. 2018, 498, 32–37. [Google Scholar] [CrossRef]
- Yin, X.; Wang, J.Q.; Yan, S.Y. Reduced miR-26a and miR-26b expression contributes to the pathogenesis of osteoarthritis via the promotion of p65 translocation. Mol. Med. Rep. 2017, 15, 551–558. [Google Scholar] [CrossRef]
- Holliday, L.S.; McHugh, K.P.; Zuo, J.; Aguirre, J.I.; Neubert, J.K.; Rody, W.J., Jr. Exosomes: Novel regulators of bone remodelling and potential therapeutic agents for orthodontics. Orthod. Craniofac. Res. 2017, 20 (Suppl 1), 95–99. [Google Scholar] [CrossRef]
- Pan, Y.; Li, D.; Lou, S.; Zhang, C.; Du, Y.; Jiang, H.; Zhang, W.; Ma, L.; Wang, L. A functional polymorphism in the pre-miR-146a gene is associated with the risk of nonsyndromic orofacial cleft. Hum. Mutat. 2018, 39, 742–750. [Google Scholar] [CrossRef]
- Schoen, C.; Aschrafi, A.; Thonissen, M.; Poelmans, G.; Von den Hoff, J.W.; Carels, C.E.L. MicroRNAs in Palatogenesis and Cleft Palate. Front. Physiol. 2017, 8, 165. [Google Scholar] [CrossRef][Green Version]
- Mendes, S.M.D.A.; Espinosa, D.D.S.G.; Moreira, P.E.O.; Marques, D.; Fagundes, N.C.F.; Ribeiro-Dos-Santos, Â. miRNAs as biomarkers of orofacial clefts: A systematic review. J. Oral Pathol. Med. 2020, 49, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Janjic, M.; Docheva, D.; Trickovic Janjic, O.; Wichelhaus, A.; Baumert, U. In Vitro Weight-Loaded Cell Models for Understanding Mechanodependent Molecular Pathways Involved in Orthodontic Tooth Movement: A Systematic Review. Stem Cells Int. 2018, 2018, 3208285. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ishihara, Y.; Ishikawa, T.; Hoshijima, M.; Odagaki, N.; Ei Hsu Hlaing, E.; Kamioka, H. Screening of key candidate genes and pathways for osteocytes involved in the differential response to different types of mechanical stimulation using a bioinformatics analysis. J. Bone Miner. Metab. 2019, 37, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Klein, Y.; Fleissig, O.; Polak, D.; Barenholz, Y.; Mandelboim, O.; Chaushu, S. Immunorthodontics: In vivo gene expression of orthodontic tooth movement. Sci. Rep. 2020, 10, 8172. [Google Scholar] [CrossRef]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Taccardi, D.; Scribante, A. Home Oral Care of Periodontal Patients Using Antimicrobial Gel with Postbiotics, Lactoferrin, and Aloe Barbadensis Leaf Juice Powder vs. Conventional Chlorhexidine Gel: A Split-Mouth Randomized Clinical Trial. Antibiotics 2022, 11, 118. [Google Scholar] [CrossRef]
- Butera, A.; Gallo, S.; Maiorani, C.; Molino, D.; Chiesa, A.; Preda, C.; Esposito, F.; Scribante, A. Probiotic Alternative to Chlorhexidine in Periodontal Therapy: Evaluation of Clinical and Microbiological Parameters. Microorganisms 2021, 9, 69. [Google Scholar] [CrossRef]
- Yang, X.; Wang, H. miRNAs Flowing Up and Down: The Concerto of Psoriasis. Front. Med. 2021, 8, 646796. [Google Scholar] [CrossRef]
- Neagu, M.; Constantin, C.; Cretoiu, S.M.; Zurac, S. miRNAs in the Diagnosis and Prognosis of Skin Cancer. Front. Cell Dev. Biol. 2020, 8, 71. [Google Scholar] [CrossRef]
- Ramanathan, K.; Padmanabhan, G. miRNAs as potential biomarker of kidney diseases: A review. Cell Biochem. Funct. 2020, 38, 990–1005. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, H.; Zhao, M.; Chang, C.; Lu, Q. Clinical significance of miRNAs in autoimmunity. J. Autoimmun. 2020, 109, 102438. [Google Scholar] [CrossRef]
- Brandao, B.B.; Lino, M.; Kahn, C.R. Extracellular miRNAs as mediators of obesity-associated disease. J. Physiol. 2022, 600, 1155–1169. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Fan, P.; Chen, M.; Xu, Y.; Zhao, D. miRNAs and Leukotrienes in Respiratory Syncytial Virus Infection. Front. Pediatr. 2021, 9, 602195. [Google Scholar] [CrossRef] [PubMed]
| miRNA | Role in OTM | Type of Study | Biological Sample Tested | References |
|---|---|---|---|---|
| miR-34a, miR-146a | inhibition of osteoblast differentiation by downregulation of CELF3 | In vitro | PDLSCs obtained from PDL tissue scraped off from the middle third of the premolar root | [38] |
| miR-21 | Stimulation of OC differentiation by downregulation of PDCD4 | In vivo | Rats divided in four groups: TM, PAOO, agomiR-21 and antagomir-21 | [35] |
| miR-34 | Promotion of OTM by targeting MMPs | Clinical trial, in vitro | CGF samples performed on the canines of patients subject to orthodontic treatment + hPDL | [32] |
| miR-155-5p | inhibition of OC differentiation by targeting CXCR2 | Clinical trial | Patients with different degree of root resorption | [40] |
| miR-21 | Directly promotion of OC differentiation during OTM | In vivo | WT C57BL/6 mice and miR-21−/− mice | [36] |
| miR-21 | Promotion of OC differentiation by promoting the RANK level secreted by T cells | In vivo | WT C57BL/6 mice and MiR-21−/− mice | [37] |
| miR-29 | Promotion of OC differentiation | Clinical trials | GCF sample performed during canine retraction | [31] |
| miR-125a-5p | Promotion of bone healing by targeting ETV6 | In vitro | Periodontal ligament cells were isolated from normal human impacted third molars | [41] |
| miR-503-5p | Inhibition of osteogenic differentiation and bone formation in OTM tension sides | In vitro, In vivo | BMSCs and Rats (the left maxillary first molars were mesially stretched | [42] |
| miR-3198 | Downregulation of OPG expression in response to mechanical stress. | In vitro | hPDL subjected to compression force (2 g/cm2) or tension force (15% elongation) | [43] |
| miR-34a | Promotion osteogenic differentiation by targeting GSK-3beta under orthodontic force | In vitro, in vivo | Rat bone mineral stem cells (rBMSCs) and local alveolar bone tissue. Rats (maxillary bone) | [39] |
| miR-221-3p, miR-138-5p, miR-132-3p, miR-218-5p, miR-133a-3p, miR-145-3p, miR-143-5p, miR-486-3p, miR-21-3p | Osteogenesis-related by targeting some genes (YAP1, WWTR1, TEAD2, CTGF, DVL2 and GDF5) | in vitro and in vivo | PDLCs obtained from the middle one-third (in vitro) and rats in which a coil spring connected the incisors to the first molar: after 3 days the PDL tissue from each rat’s distal stretched side and the control side was collected for RT-qPCR analysis (in vivo) | [44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cultrera, G.; Lo Giudice, A.; Santonocito, S.; Ronsivalle, V.; Conforte, C.; Reitano, G.; Leonardi, R.; Isola, G. MicroRNA Modulation during Orthodontic Tooth Movement: A Promising Strategy for Novel Diagnostic and Personalized Therapeutic Interventions. Int. J. Mol. Sci. 2022, 23, 15501. https://doi.org/10.3390/ijms232415501
Cultrera G, Lo Giudice A, Santonocito S, Ronsivalle V, Conforte C, Reitano G, Leonardi R, Isola G. MicroRNA Modulation during Orthodontic Tooth Movement: A Promising Strategy for Novel Diagnostic and Personalized Therapeutic Interventions. International Journal of Molecular Sciences. 2022; 23(24):15501. https://doi.org/10.3390/ijms232415501
Chicago/Turabian StyleCultrera, Giovanni, Antonino Lo Giudice, Simona Santonocito, Vincenzo Ronsivalle, Cristina Conforte, Giuseppe Reitano, Rosalia Leonardi, and Gaetano Isola. 2022. "MicroRNA Modulation during Orthodontic Tooth Movement: A Promising Strategy for Novel Diagnostic and Personalized Therapeutic Interventions" International Journal of Molecular Sciences 23, no. 24: 15501. https://doi.org/10.3390/ijms232415501
APA StyleCultrera, G., Lo Giudice, A., Santonocito, S., Ronsivalle, V., Conforte, C., Reitano, G., Leonardi, R., & Isola, G. (2022). MicroRNA Modulation during Orthodontic Tooth Movement: A Promising Strategy for Novel Diagnostic and Personalized Therapeutic Interventions. International Journal of Molecular Sciences, 23(24), 15501. https://doi.org/10.3390/ijms232415501









