Neural Regeneration in Regenerative Endodontic Treatment: An Overview and Current Trends
Abstract
1. Introduction
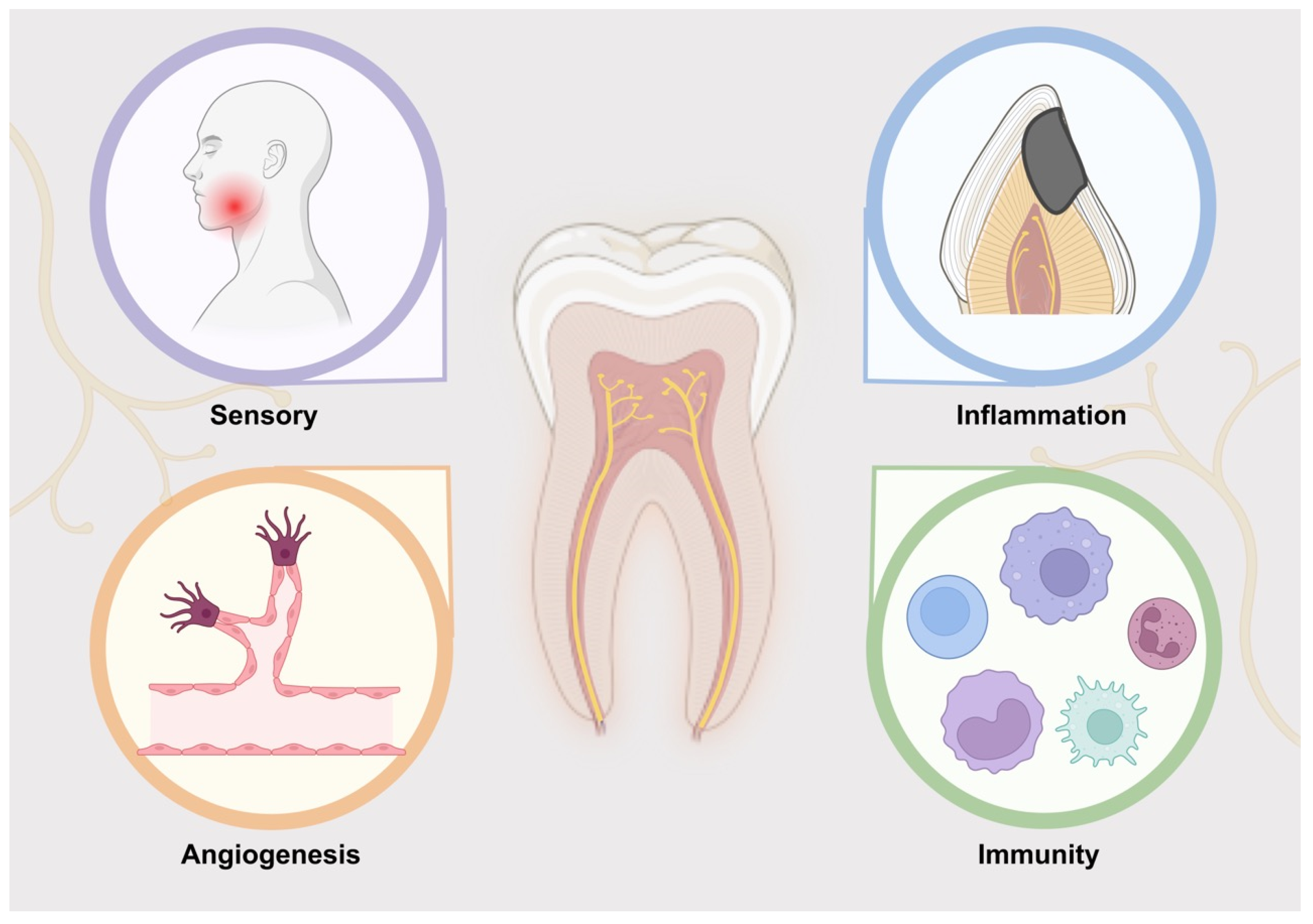
2. Function of Nerve in Dental Pulp
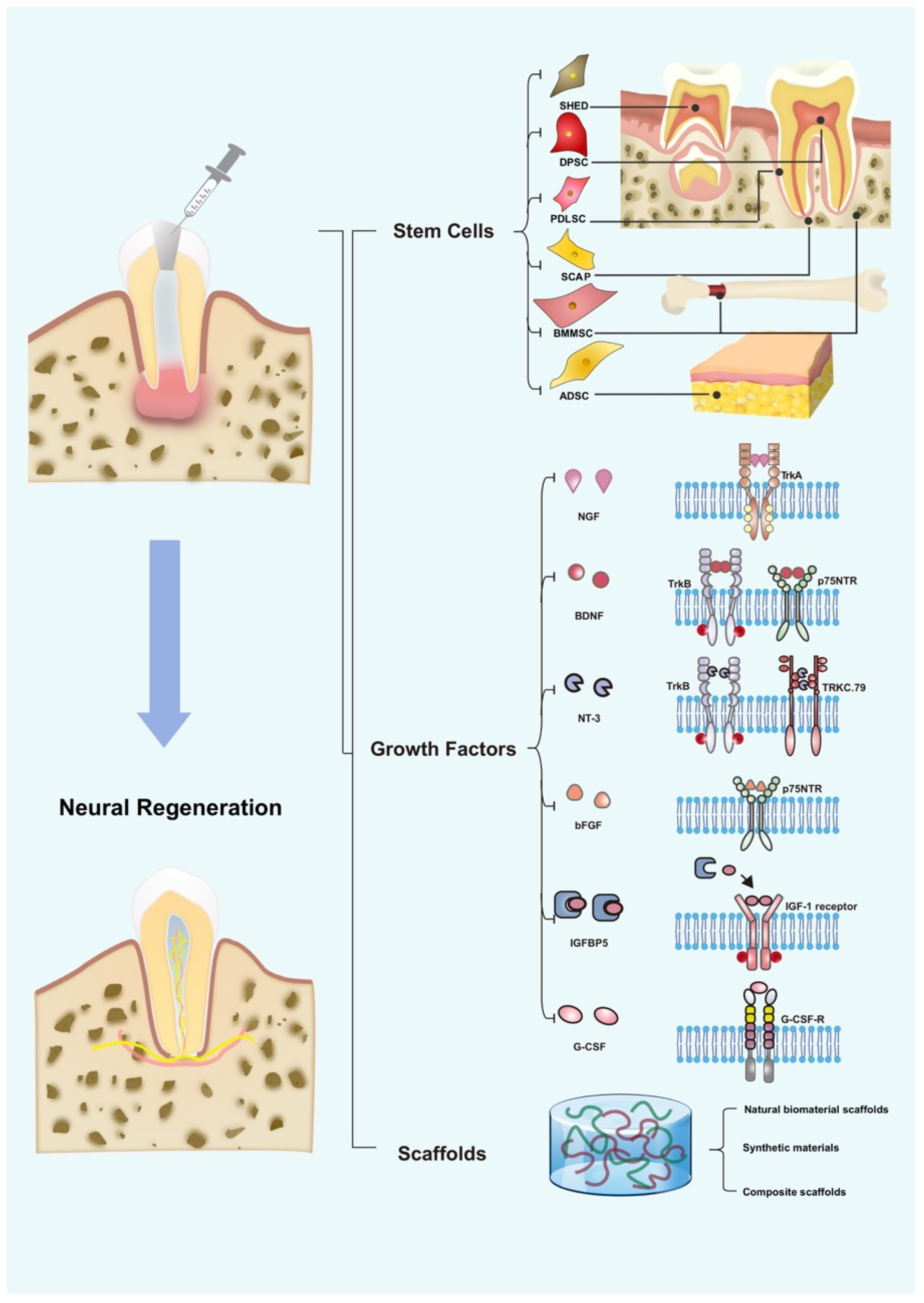
3. Development of Neural Regeneration in RET
4. Role of Stem Cells in Neural Regeneration
4.1. DPSCs
4.2. SHEDs
4.3. PDLSCs
4.4. SCAPs
4.5. BMMSCs
4.6. ADSCs
5. Role of GFs in Neural Regeneration
5.1. NGF
5.2. BDNF
5.3. NT-3
5.4. bFGF
5.5. IGFBP5
5.6. G-CSF
6. The Role of Scaffolds in Promoting Neural Regeneration
6.1. Natural Materials
6.1.1. APC
6.1.2. Collagen
6.1.3. Polysaccharides
6.1.4. Other Biomaterials
6.2. Synthetic Materials
6.3. Composite Scaffolds
7. Interaction between Nerve Regeneration and Maxillofacial Bone Regeneration
8. Limitations and Future Direction
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nör, J.E. Tooth regeneration in operative dentistry. Oper. Dent. 2006, 31, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhou, Y.; Zhou, X.; Sun, F.; Gao, B.; Wan, M.; Zhou, X.; Sun, J.; Xu, X.; Cheng, L.; et al. MicroRNA 224 Regulates Ion Transporter Expression in Ameloblasts To Coordinate Enamel Mineralization. Mol. Cell. Biol. 2015, 35, 2875–2890. [Google Scholar] [CrossRef]
- Hashemi-Beni, B.; Khoroushi, M.; Foroughi, M.R.; Karbasi, S.; Khademi, A.A. Tissue engineering: Dentin—Pulp complex regeneration approaches (A review). Tissue Cell 2017, 49, 552–564. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Wang, C.; Ye, L. Healing rate and post-obturation pain of single- versus multiple-visit endodontic treatment for infected root canals: A systematic review. J. Endod. 2011, 37, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.E.; Garcia-Godoy, F.; Hargreaves, K.M. Regenerative endodontics: A review of current status and a call for action. J. Endod. 2007, 33, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Eramo, S.; Natali, A.; Pinna, R.; Milia, E. Dental pulp regeneration via cell homing. Int. Endod. J. 2018, 51, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Shen, Z.; Zhan, P.; Yang, J.; Huang, Q.; Huang, S.; Chen, L.; Lin, Z. Functional Dental Pulp Regeneration: Basic Research and Clinical Translation. Int. J. Mol. Sci. 2021, 22, 8991. [Google Scholar] [CrossRef]
- Sui, B.; Chen, C.; Kou, X.; Li, B.; Xuan, K.; Shi, S.; Jin, Y. Pulp Stem Cell-Mediated Functional Pulp Regeneration. J. Dent. Res. 2019, 98, 27–35. [Google Scholar] [CrossRef]
- Hussain, A.; Tebyaniyan, H.; Khayatan, D. The Role of Epigenetic in Dental and Oral Regenerative Medicine by Different Types of Dental Stem Cells: A Comprehensive Overview. Stem Cells Int. 2022, 2022, 5304860. [Google Scholar] [CrossRef]
- Kim, J.Y.; Xin, X.; Moioli, E.K.; Chung, J.; Lee, C.H.; Chen, M.; Fu, S.Y.; Koch, P.D.; Mao, J.J. Regeneration of dental-pulp-like tissue by chemotaxis-induced cell homing. Tissue Eng. Part A 2010, 16, 3023–3031. [Google Scholar] [CrossRef]
- Hakim, L.K.; Yazdanian, M.; Alam, M.; Abbasi, K.; Tebyaniyan, H.; Tahmasebi, E.; Khayatan, D.; Seifalian, A.; Ranjbar, R.; Yazdanian, A. Biocompatible and Biomaterials Application in Drug Delivery System in Oral Cavity. Evid. Based Complement. Altern. Med. 2021, 2021, 9011226. [Google Scholar] [CrossRef] [PubMed]
- Fawzy El-Sayed, K.M.; Jakusz, K.; Jochens, A.; Dörfer, C.; Schwendicke, F. Stem Cell Transplantation for Pulpal Regeneration: A Systematic Review. Tissue Eng. Part B Rev. 2015, 21, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Ibuki, T.; Kido, M.A.; Kiyoshima, T.; Terada, Y.; Tanaka, T. An Ultrastructural Study of the Relationship between Sensory Trigeminal Nerves and Odontoblasts in Rat Dentin/Pulp as Demonstrated by the Anterograde Transport of Wheat Germ Agglutinin-Horseradish Peroxidase (WGA-HRP). J. Dent. Res. 1996, 75, 1963–1970. [Google Scholar] [CrossRef]
- Walton, R.E.; Ramachandran Nair, P.N. Neural elements in dental pulp and dentin. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1995, 80, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Närhi, M. Interaction between the autonomic and sensory nerves in the dental pulp. Proc. Finn. Dent. Soc. 1989, 85, 389–393. [Google Scholar] [PubMed]
- Abd-Elmeguid, A.; Yu, D.C. Dental pulp neurophysiology: Part 1. Clinical and diagnostic implications. J. Can. Dent. Assoc. 2009, 75, 55–59. [Google Scholar]
- Longhurst, J.C.; Dittman, L.E. Hypoxia, bradykinin, and prostaglandins stimulate ischemically sensitive visceral afferents. Am. J. Physiol. 1987, 253, H556–H567. [Google Scholar] [CrossRef] [PubMed]
- Chmilewsky, F.; About, I.; Cooper, L.F.; Chung, S.H. C5L2 Silencing in Human Pulp Fibroblasts Enhances Nerve Outgrowth Under Lipoteichoic Acid Stimulation. J. Endod. 2018, 44, 1396–1401. [Google Scholar] [CrossRef]
- Zhan, C.; Huang, M.; Yang, X.; Hou, J. Dental nerves: A neglected mediator of pulpitis. Int. Endod. J. 2021, 54, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P. Neurogenic vasodilatation and plasma leakage in the skin. Gen. Pharmacol. Vasc. Syst. 1998, 30, 5–11. [Google Scholar] [CrossRef]
- Fristad, I.; Heyeraas, K.J.; Jonsson, R.; Kvinnsland, I.H. Effect of inferior alveolar nerve axotomy on immune cells and nerve fibres in young rat molars. Arch. Oral Biol. 1995, 40, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.Z.; Lai, J.P.; Zhu, X.H.; Uvaydova, M.; Douglas, S.D. Human monocytes and macrophages express substance P and neurokinin-1 receptor. J. Immunol. 1997, 159, 5654–5660. [Google Scholar] [PubMed]
- Diogenes, A. Trigeminal Sensory Neurons and Pulp Regeneration. J. Endod. 2020, 46, S71–S80. [Google Scholar] [CrossRef] [PubMed]
- Meschi, N.; Hilkens, P.; Lambrichts, I.; Van den Eynde, K.; Mavridou, A.; Strijbos, O.; De Ketelaere, M.; Van Gorp, G.; Lambrechts, P. Regenerative endodontic procedure of an infected immature permanent human tooth: An immunohistological study. Clin. Oral Investig. 2016, 20, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Austah, O.; Joon, R.; Fath, W.M.; Chrepa, V.; Diogenes, A.; Ezeldeen, M.; Couve, E.; Ruparel, N.B. Comprehensive Characterization of 2 Immature Teeth Treated with Regenerative Endodontic Procedures. J. Endod. 2018, 44, 1802–1811. [Google Scholar] [CrossRef] [PubMed]
- Fried, K.; Nosrat, C.; Lillesaar, C.; Hildebrand, C. Molecular signaling and pulpal nerve development. Crit. Rev. Oral. Biol. Med. 2000, 11, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Fried, K.; Lillesaar, C.; Sime, W.; Kaukua, N.; Patarroyo, M. Target finding of pain nerve fibers: Neural growth mechanisms in the tooth pulp. Physiol. Behav. 2007, 92, 40–45. [Google Scholar] [CrossRef]
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef]
- Mittal, N.; Baranwal, H.C.; Kumar, P.; Gupta, S. Assessment of pulp sensibility in the mature necrotic teeth using regenerative endodontic therapy with various scaffolds—Randomised clinical trial. Indian J. Dent. Res. 2021, 32, 216–220. [Google Scholar] [CrossRef]
- Guo, H.; Zhao, W.; Liu, A.; Wu, M.; Shuai, Y.; Li, B.; Huang, X.; Liu, X.; Yang, X.; Guo, X.; et al. SHED promote angiogenesis in stem cell-mediated dental pulp regeneration. Biochem. Biophys. Res. Commun. 2020, 529, 1158–1164. [Google Scholar] [CrossRef]
- Iohara, K.; Zayed, M.; Takei, Y.; Watanabe, H.; Nakashima, M. Treatment of Pulpectomized Teeth With Trypsin Prior to Transplantation of Mobilized Dental Pulp Stem Cells Enhances Pulp Regeneration in Aged Dogs. Front. Bioeng. Biotechnol. 2020, 8, 983. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Hu, L.; Zhou, Y.; Ge, Z.; Wang, H.; Wu, C.T.; Jin, J. A Sandwich Structure of Human Dental Pulp Stem Cell Sheet, Treated Dentin Matrix, and Matrigel for Tooth Root Regeneration. Stem Cells Dev. 2020, 29, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Iohara, K.; Utsunomiya, S.; Kohara, S.; Nakashima, M. Allogeneic transplantation of mobilized dental pulp stem cells with the mismatched dog leukocyte antigen type is safe and efficacious for total pulp regeneration. Stem Cell Res. Ther. 2018, 9, 116. [Google Scholar] [CrossRef]
- Xuan, K.; Li, B.; Guo, H.; Sun, W.; Kou, X.; He, X.; Zhang, Y.; Sun, J.; Liu, A.; Liao, L.; et al. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci. Transl. Med. 2018, 10, eaaf3227. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, Z.L. PDGF-BB, NGF and BDNF enhance pulp-like tissue regeneration via cell homing. RSC Adv. 2016, 6, 109519–109527. [Google Scholar] [CrossRef]
- Murakami, M.; Hayashi, Y.; Iohara, K.; Osako, Y.; Hirose, Y.; Nakashima, M. Trophic Effects and Regenerative Potential of Mobilized Mesenchymal Stem Cells From Bone Marrow and Adipose Tissue as Alternative Cell Sources for Pulp/Dentin Regeneration. Cell Transpl. 2015, 24, 1753–1765. [Google Scholar] [CrossRef]
- Iohara, K.; Murakami, M.; Nakata, K.; Nakashima, M. Age-dependent decline in dental pulp regeneration after pulpectomy in dogs. Exp. Gerontol. 2014, 52, 39–45. [Google Scholar] [CrossRef]
- Souron, J.B.; Petiet, A.; Decup, F.; Tran, X.V.; Lesieur, J.; Poliard, A.; Le Guludec, D.; Letourneur, D.; Chaussain, C.; Rouzet, F.; et al. Pulp cell tracking by radionuclide imaging for dental tissue engineering. Tissue Eng. Part C Methods 2014, 20, 188–197. [Google Scholar] [CrossRef]
- Iohara, K.; Murakami, M.; Takeuchi, N.; Osako, Y.; Ito, M.; Ishizaka, R.; Utunomiya, S.; Nakamura, H.; Matsushita, K.; Nakashima, M. A novel combinatorial therapy with pulp stem cells and granulocyte colony-stimulating factor for total pulp regeneration. Stem Cells Transl. Med. 2013, 2, 521–533. [Google Scholar] [CrossRef]
- Ishizaka, R.; Iohara, K.; Murakami, M.; Fukuta, O.; Nakashima, M. Regeneration of dental pulp following pulpectomy by fractionated stem/progenitor cells from bone marrow and adipose tissue. Biomaterials 2012, 33, 2109–2118. [Google Scholar] [CrossRef]
- Iohara, K.; Imabayashi, K.; Ishizaka, R.; Watanabe, A.; Nabekura, J.; Ito, M.; Matsushita, K.; Nakamura, H.; Nakashima, M. Complete pulp regeneration after pulpectomy by transplantation of CD105+ stem cells with stromal cell-derived factor-1. Tissue Eng. Part A 2011, 17, 1911–1920. [Google Scholar] [CrossRef] [PubMed]
- Sajjad, I.; Sajid, M.; Munir, B.; Akhlaq, H.; Zehra, T.; Ahmed, J. Success of Revascularization of Pulp in Necrotic Maxillary Anterior Immature Permanent Teeth. Pakiatan J. Med. Health Sci. 2022, 16, 420–422. [Google Scholar] [CrossRef]
- Youssef, A.; Ali, M.; ElBolok, A.; Hassan, R. Regenerative endodontic procedures for the treatment of necrotic mature teeth: A preliminary randomized clinical trial. Int. Endod. J. 2022, 55, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Abu Zeid, S.T.; Alamoudi, R.A.; Alothmani, O.S.; Mokeem Saleh, A.A.; Siddiqui, A.Y. A Prospective Study of Long-Term Regenerative Endodontics Outcomes of Necrotic Immature Permanent Teeth: An 8-Year Follow-Up. Healthcare 2021, 9, 1670. [Google Scholar] [CrossRef] [PubMed]
- Brizuela, C.; Meza, G.; Urrejola, D.; Quezada, M.A.; Concha, G.; Ramírez, V.; Angelopoulos, I.; Cadiz, M.I.; Tapia-Limonchi, R.; Khoury, M. Cell-Based Regenerative Endodontics for Treatment of Periapical Lesions: A Randomized, Controlled Phase I/II Clinical Trial. J. Dent. Res. 2020, 99, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Chrepa, V.; Joon, R.; Austah, O.; Diogenes, A.; Hargreaves, K.M.; Ezeldeen, M.; Ruparel, N.B. Clinical Outcomes of Immature Teeth Treated with Regenerative Endodontic Procedures-A San Antonio Study. J. Endod. 2020, 46, 1074–1084. [Google Scholar] [CrossRef]
- Mittmann, C.W.; Kostka, E.; Ballout, H.; Preus, M.; Preissner, R.; Karaman, M.; Preissner, S. Outcome of revascularization therapy in traumatized immature incisors. BMC Oral Health 2020, 20, 207. [Google Scholar] [CrossRef]
- Nagaveni, N.B.; Poornima, P.; Mathew, M.G.; Soni, A.J.; Khan, M.M. A Comparative Evaluation of Revascularization Done in Traumatized Immature, Necrotic Anterior Teeth with and without Platelet-rich Fibrin: A Case Report. Int. J. Clin. Pediatr. Dent. 2020, 13, 98–102. [Google Scholar] [CrossRef]
- Nazzal, H.; Ainscough, S.; Kang, J.; Duggal, M.S. Revitalisation endodontic treatment of traumatised immature teeth: A prospective long-term clinical study. Eur. Arch. Paediatr. Dent. 2020, 21, 587–596. [Google Scholar] [CrossRef]
- Arslan, H.; Ahmed, H.M.A.; Şahin, Y.; Doğanay Yıldız, E.; Gündoğdu, E.C.; Güven, Y.; Khalilov, R. Regenerative Endodontic Procedures in Necrotic Mature Teeth with Periapical Radiolucencies: A Preliminary Randomized Clinical Study. J. Endod. 2019, 45, 863–872. [Google Scholar] [CrossRef]
- Meza, G.; Urrejola, D.; Saint Jean, N.; Inostroza, C.; López, V.; Khoury, M.; Brizuela, C. Personalized Cell Therapy for Pulpitis Using Autologous Dental Pulp Stem Cells and Leukocyte Platelet-rich Fibrin: A Case Report. J. Endod. 2019, 45, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Ulusoy, A.T.; Turedi, I.; Cimen, M.; Cehreli, Z.C. Evaluation of Blood Clot, Platelet-rich Plasma, Platelet-rich Fibrin, and Platelet Pellet as Scaffolds in Regenerative Endodontic Treatment: A Prospective Randomized Trial. J. Endod. 2019, 45, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Chen, Y.; Cai, Z.; Lei, L.; Zhang, M.; Zhou, R.; Huang, X. The efficacy of platelet-rich fibrin as a scaffold in regenerative endodontic treatment: A retrospective controlled cohort study. BMC Oral Health 2018, 18, 139. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, M. Maturogenesis and Osseous Healing of a Necrotic Immature Premolar using Revascularization Procedure—A Case Report and Review of Literature. Ann. Med. Health Sci. Res. 2018, 8, 39–44. [Google Scholar]
- Nageh, M.; Ahmed, G.M.; El-Baz, A.A. Assessment of Regaining Pulp Sensibility in Mature Necrotic Teeth Using a Modified Revascularization Technique with Platelet-rich Fibrin: A Clinical Study. J. Endod. 2018, 44, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Nazzal, H.; Kenny, K.; Altimimi, A.; Kang, J.; Duggal, M.S. A prospective clinical study of regenerative endodontic treatment of traumatized immature teeth with necrotic pulps using bi-antibiotic paste. Int. Endod. J. 2018, 51 (Suppl. S3), e204–e215. [Google Scholar] [CrossRef] [PubMed]
- Neelamurthy, P.S.; Kumar, R.A.; Balakrishnan, V.; Venkatesan, S.M.; Narayan, G.S.; Karthikeyan, I. Revascularization in Immature and Mature Teeth with Necrotic Pulp: A Clinical Study. J. Contemp. Dent. Pract. 2018, 19, 1393–1399. [Google Scholar] [PubMed]
- Alagl, A.; Bedi, S.; Hassan, K.; AlHumaid, J. Use of platelet-rich plasma for regeneration in non-vital immature permanent teeth: Clinical and cone-beam computed tomography evaluation. J. Int. Med. Res. 2017, 45, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Liu, H.; Peng, C. Clinical and Radiographic Assessment of the Efficacy of a Collagen Membrane in Regenerative Endodontics: A Randomized, Controlled Clinical Trial. J. Endod. 2017, 43, 1465–1471. [Google Scholar] [CrossRef]
- Li, L.; Pan, Y.; Mei, L.; Li, J. Clinical and Radiographic Outcomes in Immature Permanent Necrotic Evaginated Teeth Treated with Regenerative Endodontic Procedures. J. Endod. 2017, 43, 246–251. [Google Scholar] [CrossRef]
- Shivashankar, V.Y.; Johns, D.A.; Maroli, R.K.; Sekar, M.; Chandrasekaran, R.; Karthikeyan, S.; Renganathan, S.K. Comparison of the Effect of PRP, PRF and Induced Bleeding in the Revascularization of Teeth with Necrotic Pulp and Open Apex: A Triple Blind Randomized Clinical Trial. J. Clin. Diagn. Res. 2017, 11, Zc34–Zc39. [Google Scholar] [CrossRef] [PubMed]
- Ulusoy, A.T.; Cehreli, Z.C. Regenerative Endodontic Treatment of Necrotic Primary Molars with Missing Premolars: A Case Series. Pediatr. Dent. 2017, 39, 131–134. [Google Scholar] [PubMed]
- Farhad, A.R.; Shokraneh, A.; Shekarchizade, N. Regeneration or replacement? A case report and review of literature. Dent. Traumatol. 2016, 32, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Nagaveni, N.B.; Pathak, S.; Poornima, P.; Joshi, J.S. Revascularization Induced Maturogenesis of Non-Vital Immature Permanent Tooth Using Platelet-Rich-Fibrin: A Case Report. J. Clin. Pediatr. Dent. 2016, 40, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Priya, M.H.; Tambakad, P.B.; Naidu, J. Pulp and Periodontal Regeneration of an Avulsed Permanent Mature Incisor Using Platelet-rich Plasma after Delayed Replantation: A 12-month Clinical Case Study. J. Endod. 2016, 42, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Ray, H.L., Jr.; Marcelino, J.; Braga, R.; Horwat, R.; Lisien, M.; Khaliq, S. Long-term follow up of revascularization using platelet-rich fibrin. Dent. Traumatol. 2016, 32, 80–84. [Google Scholar] [CrossRef]
- Subash, D.; Shoba, K.; Aman, S.; Bharkavi, S.K. Revitalization of an Immature Permanent Mandibular Molar with a Necrotic Pulp Using Platelet-Rich Fibrin: A Case Report. J. Clin. Diagn. Res. 2016, 10, Zd21–Zd23. [Google Scholar] [CrossRef]
- Bezgin, T.; Yilmaz, A.D.; Celik, B.N.; Kolsuz, M.E.; Sonmez, H. Efficacy of platelet-rich plasma as a scaffold in regenerative endodontic treatment. J. Endod. 2015, 41, 36–44. [Google Scholar] [CrossRef]
- Dudeja, P.G.; Grover, S.; Srivastava, D.; Dudeja, K.K.; Sharma, V. Pulp Revascularization- It’s your Future Whether you Know it or Not? J. Clin. Diagn. Res. 2015, 9, Zr01–Zr04. [Google Scholar] [CrossRef]
- Lei, L.; Chen, Y.; Zhou, R.; Huang, X.; Cai, Z. Histologic and Immunohistochemical Findings of a Human Immature Permanent Tooth with Apical Periodontitis after Regenerative Endodontic Treatment. J. Endod. 2015, 41, 1172–1179. [Google Scholar] [CrossRef]
- Nagaveni, N.B.; Poornima, P.; Joshi, J.S.; Pathak, S.; Nandini, D.B. Revascularization of immature, nonvital permanent tooth using platelet-rich fibrin in children. Pediatr. Dent. 2015, 37, 1–6. [Google Scholar] [PubMed]
- Sachdeva, G.S.; Sachdeva, L.T.; Goel, M.; Bala, S. Regenerative endodontic treatment of an immature tooth with a necrotic pulp and apical periodontitis using platelet-rich plasma (PRP) and mineral trioxide aggregate (MTA): A case report. Int. Endod. J. 2015, 48, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Kahler, B.; Mistry, S.; Moule, A.; Ringsmuth, A.K.; Case, P.; Thomson, A.; Holcombe, T. Revascularization outcomes: A prospective analysis of 16 consecutive cases. J. Endod. 2014, 40, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Farsi, N.; Abuzeid, S.; El Ashiry, E. Revascularization of dental pulp in human necrotic permanent teeth with immature apex: Three case reports. Life Sci. J. 2013, 10, 1516–1521. [Google Scholar]
- Keswani, D.; Pandey, R.K. Revascularization of an immature tooth with a necrotic pulp using platelet-rich fibrin: A case report. Int. Endod. J. 2013, 46, 1096–1104. [Google Scholar] [CrossRef]
- Mishra, N.; Narang, I.; Mittal, N. Platelet-rich fibrin-mediated revitalization of immature necrotic tooth. Contemp. Clin. Dent. 2013, 4, 412–415. [Google Scholar] [CrossRef]
- Cehreli, Z.C.; Sara, S.; Aksoy, B. Revascularization of immature permanent incisors after severe extrusive luxation injury. Tex. Dent. J. 2012, 129, 675–681. [Google Scholar]
- Miller, E.K.; Lee, J.Y.; Tawil, P.Z.; Teixeira, F.B.; Vann, W.F., Jr. Emerging therapies for the management of traumatized immature permanent incisors. Pediatr. Dent. 2012, 34, 66–69. [Google Scholar]
- Shivashankar, V.Y.; Johns, D.A.; Vidyanath, S.; Kumar, M.R. Platelet Rich Fibrin in the revitalization of tooth with necrotic pulp and open apex. J. Conserv. Dent. 2012, 15, 395–398. [Google Scholar] [CrossRef]
- Cehreli, Z.C.; Isbitiren, B.; Sara, S.; Erbas, G. Regenerative endodontic treatment (revascularization) of immature necrotic molars medicated with calcium hydroxide: A case series. J. Endod. 2011, 37, 1327–1330. [Google Scholar] [CrossRef]
- Iwaya, S.; Ikawa, M.; Kubota, M. Revascularization of an immature permanent tooth with periradicular abscess after luxation. Dent. Traumatol. 2011, 27, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Turman, M. Revitalization of tooth with necrotic pulp and open apex by using platelet-rich plasma: A case report. J. Endod. 2011, 37, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Petrino, J.A.; Boda, K.K.; Shambarger, S.; Bowles, W.R.; McClanahan, S.B. Challenges in regenerative endodontics: A case series. J. Endod. 2010, 36, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.; Kahler, B. Regenerative endodontics--biologically-based treatment for immature permanent teeth: A case report and review of the literature. Aust. Dent. J. 2010, 55, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.Y.; Cheung, G.S.; Chen, J.; Yin, X.Z.; Wang, Q.Q.; Zhang, C.F. Pulp revascularization of immature teeth with apical periodontitis: A clinical study. J. Endod. 2009, 35, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, K.; Johnson, J.D.; Cohenca, N. Pulp revascularization of necrotic bilateral bicuspids using a modified novel technique to eliminate potential coronal discolouration: A case report. Int. Endod. J. 2009, 42, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Banchs, F.; Trope, M. Revascularization of immature permanent teeth with apical periodontitis: New treatment protocol? J. Endod. 2004, 30, 196–200. [Google Scholar] [CrossRef]
- Iwaya, S.I.; Ikawa, M.; Kubota, M. Revascularization of an immature permanent tooth with apical periodontitis and sinus tract. Dent. Traumatol. 2001, 17, 185–187. [Google Scholar] [CrossRef]
- Botelho, J.; Cavacas, M.A.; Machado, V.; Mendes, J.J. Dental stem cells: Recent progresses in tissue engineering and regenerative medicine. Ann. Med. 2017, 49, 644–651. [Google Scholar] [CrossRef]
- Lyu, P.; Li, B.; Li, P.; Bi, R.; Cui, C.; Zhao, Z.; Zhou, X.; Fan, Y. Parathyroid Hormone 1 Receptor Signaling in Dental Mesenchymal Stem Cells: Basic and Clinical Implications. Front. Cell Dev. Biol. 2021, 9, 654715. [Google Scholar] [CrossRef]
- Bi, R.; Lyu, P.; Song, Y.; Li, P.; Song, D.; Cui, C.; Fan, Y. Function of Dental Follicle Progenitor/Stem Cells and Their Potential in Regenerative Medicine: From Mechanisms to Applications. Biomolecules 2021, 11, 0997. [Google Scholar] [CrossRef] [PubMed]
- Soudi, A.; Yazdanian, M.; Ranjbar, R.; Tebyanian, H.; Yazdanian, A.; Tahmasebi, E.; Keshvad, A.; Seifalian, A. Role and application of stem cells in dental regeneration: A comprehensive overview. Excli. J. 2021, 20, 454–489. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Bi, R.; Liu, W.; Guan, S.; Li, P.; Song, D.; Xu, R.; Zheng, L.; Yuan, Q.; Zhou, X.; et al. Role of PTH1R Signaling in Prx1(+) Mesenchymal Progenitors during Eruption. J. Dent. Res. 2020, 99, 1296–1305. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Mattei, V.; Santacroce, C.; Tasciotti, V.; Martellucci, S.; Santilli, F.; Manganelli, V.; Piccoli, L.; Misasi, R.; Sorice, M.; Garofalo, T. Role of lipid rafts in neuronal differentiation of dental pulp-derived stem cells. Exp. Cell Res. 2015, 339, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, C.; Rai, K.S.; Pinnelli, V.B.; Kutty, B.M.; Dhanushkodi, A. Neuroprotection by Human Dental Pulp Mesenchymal Stem Cells: From Billions to Nano. Curr. Gene Ther. 2018, 18, 307–323. [Google Scholar] [CrossRef]
- Wang, L.H.; Gao, S.Z.; Bai, X.L.; Chen, Z.L.; Yang, F. An Up-To-Date Overview of Dental Tissue Regeneration Using Dental Origin Mesenchymal Stem Cells: Challenges and Road Ahead. Front. Bioeng. Biotechnol. 2022, 10, 855396. [Google Scholar] [CrossRef] [PubMed]
- Kaukua, N.; Shahidi, M.K.; Konstantinidou, C.; Dyachuk, V.; Kaucka, M.; Furlan, A.; An, Z.; Wang, L.; Hultman, I.; Ahrlund-Richter, L.; et al. Glial origin of mesenchymal stem cells in a tooth model system. Nature 2014, 513, 551–554. [Google Scholar] [CrossRef]
- Gronthos, S.; Brahim, J.; Li, W.; Fisher, L.W.; Cherman, N.; Boyde, A.; DenBesten, P.; Robey, P.G.; Shi, S. Stem cell properties of human dental pulp stem cells. J. Dent. Res. 2002, 81, 531–535. [Google Scholar] [CrossRef]
- Ahmed, M.N.; Shi, D.; Dailey, M.T.; Rothermund, K.; Drewry, M.D.; Calabrese, T.C.; Cui, X.T.; Syed-Picard, F.N. Dental Pulp Cell Sheets Enhance Facial Nerve Regeneration via Local Neurotrophic Factor Delivery. Tissue Eng. Part A 2021, 27, 1128–1139. [Google Scholar] [CrossRef]
- Sharma, Y.; Shobha, K.; Sundeep, M.; Pinnelli, V.B.; Parveen, S.; Dhanushkodi, A. Neural Basis of Dental Pulp Stem Cells and its Potential Application in Parkinson’s Disease. CNS Neurol. Disord. Drug Targets 2022, 21, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Hill, L.J.; Blanch, R.J.; Ward, K.; Logan, A.; Berry, M.; Leadbeater, W.; Scheven, B.A. Mesenchymal stromal cell-mediated neuroprotection and functional preservation of retinal ganglion cells in a rodent model of glaucoma. Cytotherapy 2016, 18, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Kolar, M.K.; Itte, V.N.; Kingham, P.J.; Novikov, L.N.; Wiberg, M.; Kelk, P. The neurotrophic effects of different human dental mesenchymal stem cells. Sci. Rep. 2017, 7, 12605. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.D.; Liu, H.H.; Li, Y.F.; Xiang, L.; Hu, M. Research progress of dental pulp stem cells for peripheral nerve injury repair. Zhonghua Kou Qiang Yi Xue Za Zhi 2022, 57, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Iohara, K.; Zheng, L.; Ito, M.; Ishizaka, R.; Nakamura, H.; Into, T.; Matsushita, K.; Nakashima, M. Regeneration of dental pulp after pulpotomy by transplantation of CD31(-)/CD146(-) side population cells from a canine tooth. Regen. Med. 2009, 4, 377–385. [Google Scholar] [CrossRef]
- Nakamura, S.; Yamada, Y.; Katagiri, W.; Sugito, T.; Ito, K.; Ueda, M. Stem Cell Proliferation Pathways Comparison between Human Exfoliated Deciduous Teeth and Dental Pulp Stem Cells by Gene Expression Profile from Promising Dental Pulp. J. Endod. 2009, 35, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Martinez Saez, D.; Sasaki, R.T.; Neves, A.D.; da Silva, M.C. Stem Cells from Human Exfoliated Deciduous Teeth: A Growing Literature. Cells Tissues Organs 2016, 202, 269–280. [Google Scholar] [CrossRef]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef]
- Bar, J.K.; Lis-Nawara, A.; Grelewski, P.G. Dental Pulp Stem Cell-Derived Secretome and Its Regenerative Potential. Int. J. Mol. Sci. 2021, 22, 12018. [Google Scholar] [CrossRef]
- Oubenyahya, H. Stem Cells from Dental Pulp of Human Exfoliated Teeth: Current Understanding and Future Challenges in Dental Tissue Engineering. Chin. J. Dent. Res. 2021, 24, 9–20. [Google Scholar] [CrossRef]
- Kerkis, I.; Caplan, A.I. Stem cells in dental pulp of deciduous teeth. Tissue Eng. Part B Rev. 2012, 18, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.V.; Bento, R.F.; Cruz, D.B.; Marchi, C.; Salomone, R.; Oiticicca, J.; Costa, M.P.; Haddad, L.A.; Mingroni-Netto, R.C.; Costa, H. Stem Cells from Human Exfoliated Deciduous Teeth (SHED) Differentiate in vivo and Promote Facial Nerve Regeneration. Cell Transpl. 2019, 28, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Xing, J.; Feng, G.; Sang, A.; Shen, B.; Xu, Y.; Jiang, J.; Liu, S.; Tan, W.; Gu, Z.; et al. Age-dependent impaired neurogenic differentiation capacity of dental stem cell is associated with Wnt/β-catenin signaling. Cell. Mol. Neurobiol. 2013, 33, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, L.; Cordeiro, M.M.; Nör, S.A.; Nör, J.E. Dental pulp stem cells in regenerative dentistry. Odontology 2011, 99, 1–7. [Google Scholar] [CrossRef]
- Rosa, V.; Zhang, Z.; Grande, R.H.; Nör, J.E. Dental pulp tissue engineering in full-length human root canals. J. Dent. Res. 2013, 92, 970–975. [Google Scholar] [CrossRef]
- Seo, B.M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Chen, F.M.; Gao, L.N.; Tian, B.M.; Zhang, X.Y.; Zhang, Y.J.; Dong, G.Y.; Lu, H.; Chu, Q.; Xu, J.; Yu, Y.; et al. Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: A randomized clinical trial. Stem. Cell Res. Ther. 2016, 7, 33. [Google Scholar] [CrossRef]
- Tomokiyo, A.; Wada, N.; Maeda, H. Periodontal Ligament Stem Cells: Regenerative Potency in Periodontium. Stem. Cells Dev. 2019, 28, 974–985. [Google Scholar] [CrossRef]
- Queiroz, A.; Albuquerque-Souza, E.; Gasparoni, L.M.; de França, B.N.; Pelissari, C.; Trierveiler, M.; Holzhausen, M. Therapeutic potential of periodontal ligament stem cells. World J. Stem. Cells 2021, 13, 605–618. [Google Scholar] [CrossRef]
- Bueno, C.; Ramirez, C.; Rodríguez-Lozano, F.J.; Tabarés-Seisdedos, R.; Rodenas, M.; Moraleda, J.M.; Jones, J.R.; Martinez, S. Human adult periodontal ligament-derived cells integrate and differentiate after implantation into the adult mammalian brain. Cell Transpl. 2013, 22, 2017–2028. [Google Scholar] [CrossRef]
- Fortino, V.R.; Chen, R.S.; Pelaez, D.; Cheung, H.S. Neurogenesis of neural crest-derived periodontal ligament stem cells by EGF and bFGF. J. Cell Physiol. 2014, 229, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Dong, Z.; Wang, W.; Li, B.; Jin, Y. Dental stem cell and dental tissue regeneration. Front. Med. 2019, 13, 152–159. [Google Scholar] [CrossRef]
- Janebodin, K.; Horst, O.V.; Ieronimakis, N.; Balasundaram, G.; Reesukumal, K.; Pratumvinit, B.; Reyes, M. Isolation and characterization of neural crest-derived stem cells from dental pulp of neonatal mice. PLoS ONE 2011, 6, e27526. [Google Scholar] [CrossRef] [PubMed]
- Smeda, M.; Galler, K.M.; Woelflick, M.; Rosendahl, A.; Moehle, C.; Lenhardt, B.; Buchalla, W.; Widbiller, M. Molecular Biological Comparison of Dental Pulp- and Apical Papilla-Derived Stem Cells. Int. J. Mol. Sci. 2022, 23, 2615. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Sonoyama, W.; Liu, Y.; Yamaza, T.; Tuan, R.S.; Wang, S.; Shi, S.; Huang, G.T. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: A pilot study. J. Endod. 2008, 34, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Bakopoulou, A.; About, I. Stem Cells of Dental Origin: Current Research Trends and Key Milestones towards Clinical Application. Stem. Cells Int. 2016, 2016, 4209891. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.T.; Sonoyama, W.; Liu, Y.; Liu, H.; Wang, S.; Shi, S. The hidden treasure in apical papilla: The potential role in pulp/dentin regeneration and bioroot engineering. J. Endod. 2008, 34, 645–651. [Google Scholar] [CrossRef]
- Aydin, S.; Şahin, F. Stem Cells Derived from Dental Tissues. Adv. Exp. Med. Biol. 2019, 1144, 123–132. [Google Scholar] [CrossRef]
- Kim, S.G. A Cell-Based Approach to Dental Pulp Regeneration Using Mesenchymal Stem Cells: A Scoping Review. Int. J. Mol. Sci. 2021, 22, 4357. [Google Scholar] [CrossRef]
- de Almeida, J.F.; Chen, P.; Henry, M.A.; Diogenes, A. Stem cells of the apical papilla regulate trigeminal neurite outgrowth and targeting through a BDNF-dependent mechanism. Tissue Eng. Part A 2014, 20, 3089–3100. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, D.B.; Oliveira, A.R.; Seabra, C.M.; Palma, P.J.; Ramos, C.; Figueiredo, M.H.; Santos, A.C.; Cardoso, A.L.; Peça, J.; Santos, J.M. Regeneration of pulp-dentin complex using human stem cells of the apical papilla: In vivo interaction with two bioactive materials. Clin. Oral Investig. 2021, 25, 5317–5329. [Google Scholar] [CrossRef] [PubMed]
- Araújo, P.R.S.; Silva, L.B.; Neto, A.; Almeida de Arruda, J.A.; Álvares, P.R.; Sobral, A.P.V.; Júnior, S.A.; Leão, J.C.; Braz da Silva, R.; Sampaio, G.C. Pulp Revascularization: A Literature Review. Open. Dent. J. 2017, 10, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, M.E.; Locatelli, F.; Fibbe, W.E. Mesenchymal stromal cells. Ann. N. Y. Acad. Sci. 2009, 1176, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ghazanfari, R.; Zacharaki, D.; Lim, H.C.; Scheding, S. Isolation and characterization of primary bone marrow mesenchymal stromal cells. Ann. N. Y. Acad. Sci. 2016, 1370, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Guo, Z.K.; Jiang, X.X.; Li, H.; Wang, X.Y.; Yao, H.Y.; Zhang, Y.; Mao, N. A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nat. Protoc. 2010, 5, 550–560. [Google Scholar] [CrossRef]
- Baksh, D.; Song, L.; Tuan, R.S. Adult mesenchymal stem cells: Characterization, differentiation, and application in cell and gene therapy. J. Cell. Mol. Med. 2004, 8, 301–316. [Google Scholar] [CrossRef]
- Kaneko, T.; Gu, B.; Sone, P.P.; Zaw, S.Y.M.; Murano, H.; Zaw, Z.C.T.; Okiji, T. Dental Pulp Tissue Engineering Using Mesenchymal Stem Cells: A Review with a Protocol. Stem Cell Rev. Rep. 2018, 14, 668–676. [Google Scholar] [CrossRef]
- Ohazama, A.; Modino, S.A.; Miletich, I.; Sharpe, P.T. Stem-cell-based tissue engineering of murine teeth. J. Dent. Res. 2004, 83, 518–522. [Google Scholar] [CrossRef]
- Hu, B.; Unda, F.; Bopp-Kuchler, S.; Jimenez, L.; Wang, X.J.; Haïkel, Y.; Wang, S.L.; Lesot, H. Bone Marrow Cells Can Give Rise to Ameloblast-like Cells. J. Dent. Res. 2006, 85, 416–421. [Google Scholar] [CrossRef]
- Yang, Y.; Rossi, F.M.; Putnins, E.E. Periodontal regeneration using engineered bone marrow mesenchymal stromal cells. Biomaterials 2010, 31, 8574–8582. [Google Scholar] [CrossRef]
- Hu, L.; Liu, Y.; Wang, S. Stem cell-based tooth and periodontal regeneration. Oral Dis. 2018, 24, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Zhang, Y.; Gu, X.; Huang, L.; Zhang, K.; Qian, T.; Gu, X. Application of stem cells in peripheral nerve regeneration. Burn. Trauma 2020, 8, tkaa002. [Google Scholar] [CrossRef]
- Azizi, S.A.; Stokes, D.; Augelli, B.J.; DiGirolamo, C.; Prockop, D.J. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats--similarities to astrocyte grafts. Proc. Natl. Acad. Sci. USA 1998, 95, 3908–3913. [Google Scholar] [CrossRef] [PubMed]
- Mathot, F.; Saffari, T.M.; Rbia, N.; Nijhuis, T.H.J.; Bishop, A.T.; Hovius, S.E.R.; Shin, A.Y. Functional Outcomes of Nerve Allografts Seeded with Undifferentiated and Differentiated Mesenchymal Stem Cells in a Rat Sciatic Nerve Defect Model. Plast. Reconstr. Surg. 2021, 148, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Wen, R.; Wang, X.; Lu, Y.; Du, Y.; Yu, X. The combined application of rat bone marrow mesenchymal stem cells and bioceramic materials in the regeneration of dental pulp-like tissues. Int. J. Clin. Exp. Pathol. 2020, 13, 1492–1499. [Google Scholar]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- Chu, D.T.; Nguyen Thi Phuong, T.; Tien, N.L.B.; Tran, D.K.; Minh, L.B.; Thanh, V.V.; Gia Anh, P.; Pham, V.H.; Thi Nga, V. Adipose Tissue Stem Cells for Therapy: An Update on the Progress of Isolation, Culture, Storage, and Clinical Application. J. Clin. Med. 2019, 8, 917. [Google Scholar] [CrossRef]
- Fan, Y.; Hanai, J.I.; Le, P.T.; Bi, R.; Maridas, D.; DeMambro, V.; Figueroa, C.A.; Kir, S.; Zhou, X.; Mannstadt, M.; et al. Parathyroid Hormone Directs Bone Marrow Mesenchymal Cell Fate. Cell Metab. 2017, 25, 661–672. [Google Scholar] [CrossRef]
- Gronthos, S.; Franklin, D.M.; Leddy, H.A.; Robey, P.G.; Storms, R.W.; Gimble, J.M. Surface protein characterization of human adipose tissue-derived stromal cells. J. Cell. Physiol. 2001, 189, 54–63. [Google Scholar] [CrossRef]
- Salgado, A.J.; Reis, R.L.; Sousa, N.J.; Gimble, J.M. Adipose tissue derived stem cells secretome: Soluble factors and their roles in regenerative medicine. Curr. Stem Cell Res. Ther. 2010, 5, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Sasaki, R.; Matsumine, H.; Yamato, M.; Okano, T. Undifferentiated and differentiated adipose-derived stem cells improve nerve regeneration in a rat model of facial nerve defect. J. Tissue Eng. Regen. Med. 2017, 11, 362–374. [Google Scholar] [CrossRef]
- Liang, C.; Liang, Q.; Xu, X.; Liu, X.; Gao, X.; Li, M.; Yang, J.; Xing, X.; Huang, H.; Tang, Q.; et al. Bone morphogenetic protein 7 mediates stem cells migration and angiogenesis: Therapeutic potential for endogenous pulp regeneration. Int. J. Oral. Sci. 2022, 14, 38. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yuan, G.; Chen, Z. Pulp Regeneration: Current Approaches and Future Challenges. Front. Physiol. 2016, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Levi-Montalcini, R.; Hamburger, V. Selective growth stimulating effects of mouse sarcoma on the sensory and sympathetic nervous system of the chick embryo. J. Exp. Zool. 1951, 116, 321–361. [Google Scholar] [CrossRef]
- Tomlinson, R.E.; Li, Z.; Li, Z.; Minichiello, L.; Riddle, R.C.; Venkatesan, A.; Clemens, T.L. NGF-TrkA signaling in sensory nerves is required for skeletal adaptation to mechanical loads in mice. Proc. Natl. Acad. Sci. USA 2017, 114, E3632–E3641. [Google Scholar] [CrossRef]
- Tsutsui, T.W. Dental Pulp Stem Cells: Advances to Applications. Stem Cells Cloning 2020, 13, 33–42. [Google Scholar] [CrossRef]
- Gage, F.H.; Batchelor, P.; Chen, K.S.; Chin, D.; Higgins, G.A.; Koh, S.; Deputy, S.; Rosenberg, M.B.; Fischer, W.; Bjorklund, A. NGF receptor reexpression and NGF-mediated cholinergic neuronal hypertrophy in the damaged adult neostriatum. Neuron 1989, 2, 1177–1184. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, H.; Huang, S. Role of NGF and its receptors in wound healing (Review). Exp. Ther. Med. 2021, 21, 599. [Google Scholar] [CrossRef]
- Vega, J.A.; García-Suárez, O.; Hannestad, J.; Pérez-Pérez, M.; Germanà, A. Neurotrophins and the immune system. J. Anat. 2003, 203, 1–19. [Google Scholar] [CrossRef]
- Moattari, M.; Kouchesfehani, H.M.; Kaka, G.; Sadraie, S.H.; Naghdi, M. Evaluation of nerve growth factor (NGF) treated mesenchymal stem cells for recovery in neurotmesis model of peripheral nerve injury. J. Craniomaxillofac. Surg. 2018, 46, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Cheng, Y.; Gao, S.; Chen, J. Effects of nerve growth factor and Noggin-modified bone marrow stromal cells on stroke in rats. J. Neurosci. Res. 2011, 89, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.; Kubota, H.; Ozaki, Y.; Uda, S.; Kuroda, S. Timing-dependent actions of NGF required for cell differentiation. PLoS ONE 2010, 5, e9011. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, X.; Feng, G.; Gu, Z.; Sun, Y.; Bao, G.; Xu, G.; Lu, Y.; Chen, J.; Xu, L.; et al. Chitosan scaffolds induce human dental pulp stem cells to neural differentiation: Potential roles for spinal cord injury therapy. Cell Tissue Res. 2016, 366, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, N.; Shiba, H.; Xu, W.P.; Inui, T.; Fujita, T.; Kajiya, M.; Takeda, K.; Hasegawa, N.; Kawaguchi, H.; Kurihara, H. Effect of neurotrophins on differentiation, calcification and proliferation in cultures of human pulp cells. Cell Biol. Int. 2007, 31, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
- Mitsiadis, T.A.; Pagella, P. Expression of Nerve Growth Factor (NGF), TrkA, and p75(NTR) in Developing Human Fetal Teeth. Front. Physiol. 2016, 7, 338. [Google Scholar] [CrossRef]
- Mitsiadis, T.A.; Magloire, H.; Pagella, P. Nerve growth factor signalling in pathology and regeneration of human teeth. Sci. Rep. 2017, 7, 1327. [Google Scholar] [CrossRef]
- Liu, Q.; Lei, L.; Yu, T.; Jiang, T.; Kang, Y. Effect of Brain-Derived Neurotrophic Factor on the Neurogenesis and Osteogenesis in Bone Engineering. Tissue Eng. Part A 2018, 24, 1283–1292. [Google Scholar] [CrossRef]
- Blais, M.; Lévesque, P.; Bellenfant, S.; Berthod, F. Nerve Growth Factor, Brain-Derived Neurotrophic Factor, Neurotrophin-3 and Glial-Derived Neurotrophic Factor Enhance Angiogenesis in a Tissue-Engineered In Vitro Model. Tissue Eng. Part A 2013, 19, 1655–1664. [Google Scholar] [CrossRef]
- Cen, L.P.; Ng, T.K.; Liang, J.J.; Zhuang, X.; Yao, X.; Yam, G.H.; Chen, H.; Cheung, H.S.; Zhang, M.; Pang, C.P. Human Periodontal Ligament-Derived Stem Cells Promote Retinal Ganglion Cell Survival and Axon Regeneration After Optic Nerve Injury. Stem Cells 2018, 36, 844–855. [Google Scholar] [CrossRef]
- Nosrat, C.A.; Fried, K.; Ebendal, T.; Olson, L. NGF, BDNF, NT3, NT4 and GDNF in tooth development. Eur. J. Oral Sci. 1998, 106, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Kim, J.H.; Druzinsky, R.E.; Ravindran, S.; Chung, S. Complement C5aR/LPS-induced BDNF and NGF modulation in human dental pulp stem cells. Sci. Rep. 2022, 12, 2042. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N.; Thor, D.; Yu, W.Y. Neurotrophins BDNF and NT4/5 accelerate dental pulp stem cell migration. Biomed. J. 2021, 44, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Luzuriaga, J.; Pineda, J.R.; Irastorza, I.; Uribe-Etxebarria, V.; García-Gallastegui, P.; Encinas, J.M.; Chamero, P.; Unda, F.; Ibarretxe, G. BDNF and NT3 Reprogram Human Ectomesenchymal Dental Pulp Stem Cells to Neurogenic and Gliogenic Neural Crest Progenitors Cultured in Serum-Free Medium. Cell. Physiol. Biochem. 2019, 52, 1361–1380. [Google Scholar] [CrossRef] [PubMed]
- Hohn, A.; Leibrock, J.; Bailey, K.; Barde, Y.A. Identification and characterization of a novel member of the nerve growth factor/brain-derived neurotrophic factor family. Nature 1990, 344, 339–341. [Google Scholar] [CrossRef]
- Tauszig-Delamasure, S.; Bouzas-Rodriguez, J. Targeting neurotrophin-3 and its dependence receptor tyrosine kinase receptor C: A new antitumoral strategy. Expert Opin. Targets 2011, 15, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.I.; Das, I.; Schwartz, G.M.; Tsoulfas, P.; Mikawa, T.; Hempstead, B.L. Trk C receptor signaling regulates cardiac myocyte proliferation during early heart development in vivo. Dev. Biol. 2000, 226, 180–191. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cristofaro, B.; Stone, O.A.; Caporali, A.; Dawbarn, D.; Ieronimakis, N.; Reyes, M.; Madeddu, P.; Bates, D.O.; Emanueli, C. Neurotrophin-3 is a novel angiogenic factor capable of therapeutic neovascularization in a mouse model of limb ischemia. Arter. Thromb. Vasc. Biol. 2010, 30, 1143–1150. [Google Scholar] [CrossRef]
- Zhang, S.; Jin, H.; Yao, L.; Deng, F.; Shen, L. Neurotrophin-3 enhances the osteogenesis ability of human bone marrow mesenchymal stem cells stimulated by lipopolysaccharide. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2018, 34, 47–52. [Google Scholar]
- Ji, W.C.; Li, M.; Jiang, W.T.; Ma, X.; Li, J. Protective effect of brain-derived neurotrophic factor and neurotrophin-3 overexpression by adipose-derived stem cells combined with silk fibroin/chitosan scaffold in spinal cord injury. Neurol. Res. 2020, 42, 361–371. [Google Scholar] [CrossRef]
- Yan, Z.; Shi, X.; Wang, H.; Si, C.; Liu, Q.; Du, Y. Neurotrophin-3 Promotes the Neuronal Differentiation of BMSCs and Improves Cognitive Function in a Rat Model of Alzheimer’s Disease. Front. Cell. Neurosci. 2021, 15, 629356. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Zhang, R.P.; Li, J.D. Transplantation of neurotrophin-3-expressing bone mesenchymal stem cells improves recovery in a rat model of spinal cord injury. Acta Neurochir. 2014, 156, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Zhang, X.; Ji, L.; Wang, K.; Qiu, Y. Effects of brain-derived neurotrophic factor and neurotrophin-3 on the neuronal differentiation of rat adipose-derived stem cells. Mol. Med. Rep. 2015, 12, 4981–4988. [Google Scholar] [CrossRef] [PubMed]
- Armelin, H.A. Pituitary extracts and steroid hormones in the control of 3T3 cell growth. Proc. Natl. Acad. Sci. USA 1973, 70, 2702–2706. [Google Scholar] [CrossRef] [PubMed]
- Ornitz, D.M.; Itoh, N. The Fibroblast Growth Factor signaling pathway. WIREs Dev. Biol. 2015, 4, 215–266. [Google Scholar] [CrossRef]
- Vaseenon, S.; Chattipakorn, N.; Chattipakorn, S.C. The possible role of basic fibroblast growth factor in dental pulp. Arch. Oral Biol. 2020, 109, 104574. [Google Scholar] [CrossRef]
- Xiao, L.; Sobue, T.; Esliger, A.; Kronenberg, M.S.; Coffin, J.D.; Doetschman, T.; Hurley, M.M. Disruption of the Fgf2 gene activates the adipogenic and suppresses the osteogenic program in mesenchymal marrow stromal stem cells. Bone 2010, 47, 360–370. [Google Scholar] [CrossRef]
- Qian, J.; Jiayuan, W.; Wenkai, J.; Peina, W.; Ansheng, Z.; Shukai, S.; Shafei, Z.; Jun, L.; Longxing, N. Basic fibroblastic growth factor affects the osteogenic differentiation of dental pulp stem cells in a treatment-dependent manner. Int. Endod. J. 2015, 48, 690–700. [Google Scholar] [CrossRef]
- Osathanon, T.; Nowwarote, N.; Pavasant, P. Basic fibroblast growth factor inhibits mineralization but induces neuronal differentiation by human dental pulp stem cells through a FGFR and PLCγ signaling pathway. J. Cell. Biochem. 2011, 112, 1807–1816. [Google Scholar] [CrossRef]
- Luo, L.; Albashari, A.A.; Wang, X.; Jin, L.; Zhang, Y.; Zheng, L.; Xia, J.; Xu, H.; Zhao, Y.; Xiao, J.; et al. Effects of Transplanted Heparin-Poloxamer Hydrogel Combining Dental Pulp Stem Cells and bFGF on Spinal Cord Injury Repair. Stem Cells Int. 2018, 2018, 2398521. [Google Scholar] [CrossRef]
- Liang, C.; Liao, L.; Tian, W. Stem Cell-based Dental Pulp Regeneration: Insights From Signaling Pathways. Stem Cell Rev. Rep. 2021, 17, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, Y.; Ueda, M.; Ozasa, M.; Anzai, J.; Takedachi, M.; Yanagita, M.; Ito, M.; Hashikawa, T.; Yamada, S.; Murakami, S. Fibroblast growth factor-2 regulates the cell function of human dental pulp cells. J. Endod. 2009, 35, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, C.; Nishihara, T.; Terashita, M.; Tabata, Y.; Washio, A. Local regeneration of dentin-pulp complex using controlled release of fgf-2 and naturally derived sponge-like scaffolds. Int. J. Dent. 2012, 2012, 190561. [Google Scholar] [CrossRef]
- Kim, J.; Park, J.C.; Kim, S.H.; Im, G.I.; Kim, B.S.; Lee, J.B.; Choi, E.Y.; Song, J.S.; Cho, K.S.; Kim, C.S. Treatment of FGF-2 on stem cells from inflamed dental pulp tissue from human deciduous teeth. Oral Dis. 2014, 20, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Sagomonyants, K.; Kalajzic, I.; Maye, P.; Mina, M. Enhanced Dentinogenesis of Pulp Progenitors by Early Exposure to FGF2. J. Dent. Res. 2015, 94, 1582–1590. [Google Scholar] [CrossRef]
- Forbes, B.E.; Blyth, A.J.; Wit, J.M. Disorders of IGFs and IGF-1R signaling pathways. Mol. Cell. Endocrinol. 2020, 518, 111035. [Google Scholar] [CrossRef]
- Liu, D.; Wang, Y.; Jia, Z.; Wang, L.; Wang, J.; Yang, D.; Song, J.; Wang, S.; Fan, Z. Demethylation of IGFBP5 by Histone Demethylase KDM6B Promotes Mesenchymal Stem Cell-Mediated Periodontal Tissue Regeneration by Enhancing Osteogenic Differentiation and Anti-Inflammation Potentials. Stem Cells 2015, 33, 2523–2536. [Google Scholar] [CrossRef]
- Hao, J.; Yang, H.; Cao, Y.; Zhang, C.; Fan, Z. IGFBP5 enhances the dentinogenesis potential of dental pulp stem cells via JNK and ErK signalling pathways. J. Oral Rehabil. 2020, 47, 1557–1565. [Google Scholar] [CrossRef]
- Saito, K.; Ohshima, H. The putative role of insulin-like growth factor (IGF)-binding protein 5 independent of IGF in the maintenance of pulpal homeostasis in mice. Regen. Ther. 2019, 11, 217–224. [Google Scholar] [CrossRef]
- Li, J.; Diao, S.; Yang, H.; Cao, Y.; Du, J.; Yang, D. IGFBP5 promotes angiogenic and neurogenic differentiation potential of dental pulp stem cells. Dev. Growth Differ. 2019, 61, 457–465. [Google Scholar] [CrossRef]
- Ahmadi, F.; Salmasi, Z.; Mojarad, M.; Eslahi, A.; Tayarani-Najaran, Z. G-CSF augments the neuroprotective effect of conditioned medium of dental pulp stem cells against hypoxic neural injury in SH-SY5Y cells. Iran. J. Basic Med. Sci. 2021, 24, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.T.; Chu, S.C.; Liu, S.H.; Pang, C.Y.; Hou, T.W.; Lin, S.Z.; Chen, S.Y. Neuroprotection of Granulocyte Colony-Stimulating Factor for Early Stage Parkinson’s Disease. Cell Transpl. 2017, 26, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.M.; Du, F.; Yang, D.; Wang, R.; Yu, C.J.; Huang, X.N.; Hu, H.Y.; Liu, W.; Fu, J. Granulocyte colony-stimulating factor increases the therapeutic efficacy of bone marrow mononuclear cell transplantation in cerebral ischemia in mice. BMC Neurosci. 2011, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.; Krüger, C.; Steigleder, T.; Weber, D.; Pitzer, C.; Laage, R.; Aronowski, J.; Maurer, M.H.; Gassler, N.; Mier, W.; et al. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J. Clin. Investig. 2005, 115, 2083–2098. [Google Scholar] [CrossRef]
- Iohara, K.; Fujita, M.; Ariji, Y.; Yoshikawa, M.; Watanabe, H.; Takashima, A.; Nakashima, M. Assessment of Pulp Regeneration Induced by Stem Cell Therapy by Magnetic Resonance Imaging. J. Endod. 2016, 42, 397–401. [Google Scholar] [CrossRef]
- Schmalz, G.; Widbiller, M.; Galler, K.M. Signaling Molecules and Pulp Regeneration. J. Endod. 2017, 43, S7–S11. [Google Scholar] [CrossRef]
- Takeuchi, N.; Hayashi, Y.; Murakami, M.; Alvarez, F.J.; Horibe, H.; Iohara, K.; Nakata, K.; Nakamura, H.; Nakashima, M. Similar in vitro effects and pulp regeneration in ectopic tooth transplantation by basic fibroblast growth factor and granulocyte-colony stimulating factor. Oral Dis. 2015, 21, 113–122. [Google Scholar] [CrossRef]
- Yu, J.; Liu, X.L.; Cheng, Q.G.; Lu, S.S.; Xu, X.Q.; Zu, Q.Q.; Liu, S. G-CSF and hypoxic conditioning improve the proliferation, neural differentiation and migration of canine bone marrow mesenchymal stem cells. Exp. Ther. Med. 2016, 12, 1822–1828. [Google Scholar] [CrossRef]
- Smojver, I.; Katalinić, I.; Bjelica, R.; Gabrić, D.; Matišić, V.; Molnar, V.; Primorac, D. Mesenchymal Stem Cells Based Treatment in Dental Medicine: A Narrative Review. Int. J. Mol. Sci. 2022, 23, 1662. [Google Scholar] [CrossRef]
- Moussa, D.G.; Aparicio, C. Present and future of tissue engineering scaffolds for dentin-pulp complex regeneration. J. Tissue Eng. Regen. Med. 2019, 13, 58–75. [Google Scholar] [CrossRef]
- Pinho, A.C.; Fonseca, A.C.; Serra, A.C.; Santos, J.D.; Coelho, J.F. Peripheral Nerve Regeneration: Current Status and New Strategies Using Polymeric Materials. Adv. Healthc Mater. 2016, 5, 2732–2744. [Google Scholar] [CrossRef]
- Ramachandran, N.; Singh, S.; Podar, R.; Kulkarni, G.; Shetty, R.; Chandrasekhar, P. A comparison of two pulp revascularization techniques using platelet-rich plasma and whole blood clot. J. Conserv. Dent. 2020, 23, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.E. Platelet-Rich Plasma and Platelet-Rich Fibrin Can Induce Apical Closure More Frequently Than Blood-Clot Revascularization for the Regeneration of Immature Permanent Teeth: A Meta-Analysis of Clinical Efficacy. Front. Bioeng. Biotechnol. 2018, 6, 139. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Gou, L.; Zhang, P.; Li, H.; Qiu, S. Platelet-rich plasma and regenerative dentistry. Aust. Dent. J. 2020, 65, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Raddall, G.; Mello, I.; Leung, B.M. Biomaterials and Scaffold Design Strategies for Regenerative Endodontic Therapy. Front. Bioeng. Biotechnol. 2019, 7, 317. [Google Scholar] [CrossRef] [PubMed]
- Cen, L.; Liu, W.; Cui, L.; Zhang, W.; Cao, Y. Collagen tissue engineering: Development of novel biomaterials and applications. Pediatr. Res. 2008, 63, 492–496. [Google Scholar] [CrossRef]
- Zein, N.; Harmouch, E.; Lutz, J.C.; Fernandez De Grado, G.; Kuchler-Bopp, S.; Clauss, F.; Offner, D.; Hua, G.; Benkirane-Jessel, N.; Fioretti, F. Polymer-Based Instructive Scaffolds for Endodontic Regeneration. Materials 2019, 12, 2347. [Google Scholar] [CrossRef]
- Abbass, M.M.S.; El-Rashidy, A.A.; Sadek, K.M.; Moshy, S.E.; Radwan, I.A.; Rady, D.; Dörfer, C.E.; Fawzy El-Sayed, K.M. Hydrogels and Dentin-Pulp Complex Regeneration: From the Benchtop to Clinical Translation. Polymers 2020, 12, 2935. [Google Scholar] [CrossRef]
- Dayi, B.; Bilecen, D.S.; Eröksüz, H.; Yalcin, M.; Hasirci, V. Evaluation of a collagen-bioaggregate composite scaffold in the repair of sheep pulp tissue. Eur. Oral Res. 2021, 55, 152–161. [Google Scholar] [CrossRef]
- Nakashima, M.; Iohara, K. Regeneration of dental pulp by stem cells. Adv. Dent. Res. 2011, 23, 313–319. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Lee, S.H.; Hwang, Y.C.; Rosa, V.; Lee, K.W.; Min, K.S. Behaviour of human dental pulp cells cultured in a collagen hydrogel scaffold cross-linked with cinnamaldehyde. Int. Endod. J. 2017, 50, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Han, Q.; Lu, P.; Zhang, L.; Zhang, Y.; Chen, S.; Zhang, P.; Zhang, L.; Cui, W.; Wang, H.; et al. Construction of Dual-Biofunctionalized Chitosan/Collagen Scaffolds for Simultaneous Neovascularization and Nerve Regeneration. Research 2020, 2020, 2603048. [Google Scholar] [CrossRef] [PubMed]
- Ouasti, S.; Donno, R.; Cellesi, F.; Sherratt, M.J.; Terenghi, G.; Tirelli, N. Network connectivity, mechanical properties and cell adhesion for hyaluronic acid/PEG hydrogels. Biomaterials 2011, 32, 6456–6470. [Google Scholar] [CrossRef]
- Ahmadian, E.; Eftekhari, A.; Dizaj, S.M.; Sharifi, S.; Mokhtarpour, M.; Nasibova, A.N.; Khalilov, R.; Samiei, M. The effect of hyaluronic acid hydrogels on dental pulp stem cells behavior. Int. J. Biol. Macromol. 2019, 140, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Turley, E.A.; Noble, P.W.; Bourguignon, L.Y. Signaling properties of hyaluronan receptors. J. Biol. Chem. 2002, 277, 4589–4592. [Google Scholar] [CrossRef]
- Almeida, L.D.F.; Babo, P.S.; Silva, C.R.; Rodrigues, M.T.; Hebling, J.; Reis, R.L.; Gomes, M.E. Hyaluronic acid hydrogels incorporating platelet lysate enhance human pulp cell proliferation and differentiation. J. Mater. Sci. Mater. Med. 2018, 29, 88. [Google Scholar] [CrossRef]
- Yang, J.; Hsu, C.C.; Cao, T.T.; Ye, H.; Chen, J.; Li, Y.Q. A hyaluronic acid granular hydrogel nerve guidance conduit promotes regeneration and functional recovery of injured sciatic nerves in rats. Neural. Regen. Res. 2023, 18, 657–663. [Google Scholar] [CrossRef]
- Issa, M.M.; Köping-Höggård, M.; Artursson, P. Chitosan and the mucosal delivery of biotechnology drugs. Drug Discov. Today Technol. 2005, 2, 1–6. [Google Scholar] [CrossRef]
- Chang, B.; Ahuja, N.; Ma, C.; Liu, X. Injectable scaffolds: Preparation and application in dental and craniofacial regeneration. Mater. Sci. Eng. R Rep. 2017, 111, 1–26. [Google Scholar] [CrossRef]
- El Ashiry, E.A.; Alamoudi, N.M.; El Ashiry, M.K.; Bastawy, H.A.; El Derwi, D.A.; Atta, H.M. Tissue Engineering of Necrotic Dental Pulp of Immature Teeth with Apical Periodontitis in Dogs: Radiographic and Histological Evaluation. J. Clin. Pediatr. Dent. 2018, 42, 373–382. [Google Scholar] [CrossRef]
- Feng, X.; Lu, X.; Huang, D.; Xing, J.; Feng, G.; Jin, G.; Yi, X.; Li, L.; Lu, Y.; Nie, D.; et al. 3D porous chitosan scaffolds suit survival and neural differentiation of dental pulp stem cells. Cell. Mol. Neurobiol. 2014, 34, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Delgado, M.E.; Gomez-Pinedo, U.; Feria-Velasco, A.; Huerta-Viera, M.; Castañeda, S.C.; Toral, F.A.; Parducz, A.; Anda, S.L.; Mora-Galindo, J.; García-Estrada, J. Ultrastructural analysis of guided nerve regeneration using progesterone- and pregnenolone-loaded chitosan prostheses. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 74, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, T.; Song, K.; Yao, L.; Ge, D.; Bao, C.; Ma, X.; Cui, Z. Culture of neural stem cells in calcium alginate beads. Biotechnol. Prog. 2006, 22, 1683–1689. [Google Scholar] [CrossRef]
- Dobie, K.; Smith, G.; Sloan, A.J.; Smith, A.J. Effects of alginate hydrogels and TGF-beta 1 on human dental pulp repair in vitro. Connect. Tissue Res. 2002, 43, 387–390. [Google Scholar] [CrossRef]
- Poongodi, R.; Chen, Y.L.; Yang, T.H.; Huang, Y.H.; Yang, K.D.; Lin, H.C.; Cheng, J.K. Bio-Scaffolds as Cell or Exosome Carriers for Nerve Injury Repair. Int. J. Mol. Sci. 2021, 22, 13347. [Google Scholar] [CrossRef]
- Benton, G.; Arnaoutova, I.; George, J.; Kleinman, H.K.; Koblinski, J. Matrigel: From discovery and ECM mimicry to assays and models for cancer research. Adv. Drug Deliv. Rev. 2014, 79–80, 3–18. [Google Scholar] [CrossRef]
- Wang, J.; Chu, R.; Ni, N.; Nan, G. The effect of Matrigel as scaffold material for neural stem cell transplantation for treating spinal cord injury. Sci. Rep. 2020, 10, 2576. [Google Scholar] [CrossRef]
- Luzuriaga, J.; Irurzun, J.; Irastorza, I.; Unda, F.; Ibarretxe, G.; Pineda, J.R. Vasculogenesis from Human Dental Pulp Stem Cells Grown in Matrigel with Fully Defined Serum-Free Culture Media. Biomedicines 2020, 8, 483. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Lee, S.; Choi, W.H.; Jee, J.H.; Kim, H.R.; Yoo, J. Fabrication of Dentin-Pulp-Like Organoids Using Dental-Pulp Stem Cells. Cells 2020, 9, 642. [Google Scholar] [CrossRef]
- Absalan, F.; Pasandi, M.S.; Ghasemi Hamidabadi, H.; Saeednia, S.; Bojnordi, M.N.; Zahiri, M.; Alizadeh, R.; Bagher, Z. Matrigel enhances differentiation of human adipose tissue-derived stem cells into dopaminergic neuron. Neurosci. Lett. 2021, 760, 136070. [Google Scholar] [CrossRef] [PubMed]
- Coulombe, P.A.; Bousquet, O.; Ma, L.; Yamada, S.; Wirtz, D. The ‘ins’ and ‘outs’ of intermediate filament organization. Trends Cell Biol. 2000, 10, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhang, L.; Wei, Y.; Chen, T.; Ji, X.; Ye, K.; Yu, J.; Tang, B.; Sun, X.; Hu, J. Human hair keratins promote the regeneration of peripheral nerves in a rat sciatic nerve crush model. J. Mater. Sci. Mater. Med. 2019, 30, 82. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Yang, C.; Jung, I.H.; Lim, H.C.; Lee, J.S.; Jung, U.W.; Seo, Y.K.; Park, J.K.; Choi, S.H. Regeneration of rabbit calvarial defects using cells-implanted nano-hydroxyapatite coated silk scaffolds. Biomater. Res. 2015, 19, 7. [Google Scholar] [CrossRef]
- Chen, S.; Liu, S.; Zhang, L.; Han, Q.; Liu, H.; Shen, J.; Li, G.; Zhang, L.; Yang, Y. Construction of injectable silk fibroin/polydopamine hydrogel for treatment of spinal cord injury. Chem. Eng. J. 2020, 399, 125795. [Google Scholar] [CrossRef]
- Vijayavenkataraman, S. Nerve guide conduits for peripheral nerve injury repair: A review on design, materials and fabrication methods. Acta Biomater. 2020, 106, 54–69. [Google Scholar] [CrossRef]
- Liu, X.; Ma, P.X. Polymeric scaffolds for bone tissue engineering. Ann. Biomed. Eng. 2004, 32, 477–486. [Google Scholar] [CrossRef]
- Fan, Y.; Bi, R.; Densmore, M.J.; Sato, T.; Kobayashi, T.; Yuan, Q.; Zhou, X.; Erben, R.G.; Lanske, B. Parathyroid hormone 1 receptor is essential to induce FGF23 production and maintain systemic mineral ion homeostasis. FASEB J. 2016, 30, 428–440. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, W.; Bi, R.; Densmore, M.J.; Sato, T.; Mannstadt, M.; Yuan, Q.; Zhou, X.; Olauson, H.; Larsson, T.E.; et al. Interrelated role of Klotho and calcium-sensing receptor in parathyroid hormone synthesis and parathyroid hyperplasia. Proc. Natl. Acad. Sci. USA 2018, 115, E3749–E3758. [Google Scholar] [CrossRef]
- Fan, Y.; Cui, C.; Rosen, C.J.; Sato, T.; Xu, R.; Li, P.; Wei, X.; Bi, R.; Yuan, Q.; Zhou, C. Klotho in Osx(+)-mesenchymal progenitors exerts pro-osteogenic and anti-inflammatory effects during mandibular alveolar bone formation and repair. Signal Transduct. Target. Ther. 2022, 7, 155. [Google Scholar] [CrossRef]
- Fan, Y.; Cui, C.; Li, P.; Bi, R.; Lyu, P.; Li, Y.; Zhu, S. Fibrocartilage Stem Cells in the Temporomandibular Joint: Insights From Animal and Human Studies. Front. Cell Dev. Biol. 2021, 9, 665995. [Google Scholar] [CrossRef] [PubMed]
- Brockes, J.P. Mitogenic growth factors and nerve dependence of limb regeneration. Science 1984, 225, 1280–1287. [Google Scholar] [CrossRef]
- Rinkevich, Y.; Montoro, D.T.; Muhonen, E.; Walmsley, G.G.; Lo, D.; Hasegawa, M.; Januszyk, M.; Connolly, A.J.; Weissman, I.L.; Longaker, M.T. Clonal analysis reveals nerve-dependent and independent roles on mammalian hind limb tissue maintenance and regeneration. Proc. Natl. Acad. Sci. USA 2014, 111, 9846–9851. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.E.; Salhotra, A.; Robertson, K.S.; Ransom, R.C.; Foster, D.S.; Shah, H.N.; Quarto, N.; Wan, D.C.; Longaker, M.T. Skeletal Stem Cell-Schwann Cell Circuitry in Mandibular Repair. Cell Rep. 2019, 28, 2757–2766.e2755. [Google Scholar] [CrossRef]
- Heine, W.; Conant, K.; Griffin, J.W.; Höke, A. Transplanted neural stem cells promote axonal regeneration through chronically denervated peripheral nerves. Exp. Neurol. 2004, 189, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Godinho, M.J.; Staal, J.L.; Krishnan, V.S.; Hodgetts, S.I.; Pollett, M.A.; Goodman, D.P.; Teh, L.; Verhaagen, J.; Plant, G.W.; Harvey, A.R. Regeneration of adult rat sensory and motor neuron axons through chimeric peroneal nerve grafts containing donor Schwann cells engineered to express different neurotrophic factors. Exp. Neurol. 2020, 330, 113355. [Google Scholar] [CrossRef]
- Frostick, S.P.; Yin, Q.; Kemp, G.J. Schwann cells, neurotrophic factors, and peripheral nerve regeneration. Microsurgery 1998, 18, 397–405. [Google Scholar] [CrossRef]
- Kajiya, M.; Shiba, H.; Fujita, T.; Ouhara, K.; Takeda, K.; Mizuno, N.; Kawaguchi, H.; Kitagawa, M.; Takata, T.; Tsuji, K.; et al. Brain-derived neurotrophic factor stimulates bone/cementum-related protein gene expression in cementoblasts. J. Biol. Chem. 2008, 283, 16259–16267. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, S.; Liu, B.; Lei, D.; Zhao, Y.; Lu, C.; Tan, A. Locally applied nerve growth factor enhances bone consolidation in a rabbit model of mandibular distraction osteogenesis. J. Orthop. Res. 2006, 24, 2238–2245. [Google Scholar] [CrossRef]
- Grills, B.L.; Schuijers, J.A.; Ward, A.R. Topical application of nerve growth factor improves fracture healing in rats. J. Orthop. Res. 1997, 15, 235–242. [Google Scholar] [CrossRef]
- Sun, S.; Diggins, N.H.; Gunderson, Z.J.; Fehrenbacher, J.C.; White, F.A.; Kacena, M.A. No pain, no gain? The effects of pain-promoting neuropeptides and neurotrophins on fracture healing. Bone 2020, 131, 115109. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Zeng, W.; Wu, Y.X.; Hou, C.L.; Chen, W.; Yang, M.C.; Li, L.; Zhang, Y.F.; Zhu, C.H. Neurotrophin-3 accelerates wound healing in diabetic mice by promoting a paracrine response in mesenchymal stem cells. Cell Transpl. 2013, 22, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.W.; Chim, S.M.; Zhou, L.; Hassanshahi, M.; Chung, R.; Fan, C.; Song, Y.; Foster, B.K.; Prestidge, C.A.; Peymanfar, Y.; et al. Osteoblast derived-neurotrophin-3 induces cartilage removal proteases and osteoclast-mediated function at injured growth plate in rats. Bone 2018, 116, 232–247. [Google Scholar] [CrossRef] [PubMed]
- Bucan, V.; Vaslaitis, D.; Peck, C.T.; Strauß, S.; Vogt, P.M.; Radtke, C. Effect of Exosomes from Rat Adipose-Derived Mesenchymal Stem Cells on Neurite Outgrowth and Sciatic Nerve Regeneration After Crush Injury. Mol. Neurobiol. 2019, 56, 1812–1824. [Google Scholar] [CrossRef] [PubMed]
| Scaffold | Stem Cells | Signal Molecules | Species | Outcome | Reference |
|---|---|---|---|---|---|
| (1) Periapical bleeding (2) PRF (3) Collagen (4) Hydroxyapatite | / | / | Human | Radiographically: all succeeded; success rate of cold test: (1) periapical bleeding: 11%; (2) PRF:66%; (3) collagen: 44.4%; (4) hydroxyapatite: 33.3% | Mittal et al., 2021 [29] |
| / | Human SHED aggregates | / | Pig | Dentin-pulp-like tissue with blood vessels and nerves | Guo et al., 2020 [30] |
| Atelocollagen | DPSC | G-CSF | Dog | Dentin-pulp-like tissue with blood vessels and nerves | Iohara et al., 2020 [31] |
| Human treated dentin matrix, and Matrigel | Human DPSC | / | Mice | Dentin-pulp-like tissue with blood vessels and nerves | Meng et al., 2020 [32] |
| Atelocollagen | DPSC | G-CSF | Dog | Dentin-pulp-like tissue with blood vessels and nerves | Iohara et al., 2018 [33] |
| / | SHED aggregate | / | Pig; Human | Pig: Dentin-pulp-like tissue with blood vessels and nerves; Human: Pulp-like tissue containing an odontoblast layer, connective tissue, blood vessels, and nerves | Xuan et al., 2018 [34] |
| Collagen gel | BMMSC | PDGF-BB NGF BDNF | Rat | Well-vascularized pulp-like tissue; positive signals for S-100 | Li and Wang, 2016 [35] |
| Atelocollagen | DPSC BMMSC ADSC | G-CSF | Dog | Dentin-pulp-like tissue with blood vessels and nerves | Murakami et al., 2015 [36] |
| Atelocollagen | DPSC | G-CSF | Dog | Dentin-pulp-like tissue with blood vessels and nerves | Iohara et al., 2014 [37] |
| Polymerizing type I collagen hydrogel | Indium-111-oxine -labeled rat pulp cells | / | Rat | Dentin-pulp-like tissue with blood vessels, nerves, and active fibroblastic cells | Souron et al., 2014 [38] |
| Atelocollagen | DPSC | G-CSF | Dog | Dentin-pulp-like tissue with blood vessels and nerves | Iohara et al., 2013 [39] |
| Collagen TE | CD31-side population cells from pulp, bone marrow, adipose | SDF-1 | Dog | Dentin-pulp-like tissue with blood vessels and nerves | Ishizaka et al., 2012 [40] |
| Collagen TE | CD105+cells form pulp or adipose | SDF-1 | Dog | Dentin-pulp-like tissue with blood vessels and nerves | Iohara et al., 2011 [41] |
| Collagen | / | bFGF VEGF/PDGF NGF BMP7 | Teeth from humans implanted in mice | Dentin-pulp-like tissue with blood vessels and nerves | Kim et al., 2010 [10] |
| Revascularization Treatment | Characteristics of Samples | Radiological or Pathological Results | Evidence of Successful Neural Regeneration | Reference |
|---|---|---|---|---|
| BC | Immature permanent teeth of 40 patients | Increased root length and thickness | The EPT on the sixth week demonstrated a positive response | Sajjad et al., 2022 [42] |
| BC PRF | Mature anterior teeth of 20 patients | Periradicular healing in both groups | Different readings of tooth sensitivity between preoperative, 6 months, and 12 months | Youssef et al., 2022 [43] |
| BC | 23 immature necrotic permanent teeth | Increased root length and thickness | 53.8% and 50% responded to EPT after 3 and 8 years, respectively | Abu Zeid et al., 2021 [44] |
| Plasma-derived biomaterial with human umbilical cord MSCs | Mature anterior teeth of 18 patients | After 12 months, the treatment showed 100% clinical efficacy | Increased positive pulp response at 12-month follow-up | Brizuela et al., 2020 [45] |
| BC | 51 immature permanent teeth with pulp necrosis | 91.4% showed root development. | 54% responded positively to cold or EPT, and 30% responded to both tests | Chrepa et al., 2020 [46] |
| BC | 16 traumatized permanent incisors with open apices | 56.3% root resorptions, 31.3% ankylosis, and 92.9% discolorations | 81.3% of teeth regained sensitivity | Mittmann et al., 2020 [47] |
| BC PRF | The upper front teeth of an 11-year-old child | Increased root length and thickness; closure of apex | After 12 months, both teeth were positive to the cold test and EPT | Nagaveni et al., 2020) [48] |
| BC | Traumatized upper immature anterior teeth of 15 children | Periapical healing, root walls thickened, and apical closure closed | One tooth was positive to the cold test; four teeth were positive to EPT | Nazzal et al., 2020 [49] |
| BC | 28 mature teeth | 92.3% favorable clinical and radiographic outcomes | Half of the teeth responded to the EPT | Arslan et al., 2019 [50] |
| MSCs PRF | Tooth #28 of a 50-year-old man | Normal under percussion and palpation | Delayed response to cold test; positive to EPT | Meza et al., 2019 [51] |
| BC PRP PRF PP | 88 immature incisors of 67 children | 86 teeth showed periapical healing and root development | 86 teeth were positive to sensitivity tests | Ulusoy et al., 2019 [52] |
| BC PRF | Five patients per group | Increased root length and thickness; closure of apex | Positive to sensitivity test between 6 and 9 months of follow-up | Lv et al., 2018 [53] |
| BC | An immature permanent tooth | Increased root length and thickness, and complete osseous healing | Positive to cold test | Mustafa, 2018 [54] |
| PRF | 15 patients with mature necrotic pulp | Complete healing of periapical lesion | Different readings of tooth sensitivity between preoperative and 12 months | Nageh et al., 2018 [55] |
| BC | Immature maxillary incisors of 15 children | Apical foramen narrowed | One-third of the teeth were responsive to EPT | Nazzal et al., 2018 [56] |
| BC | 15 patients with immature and mature permanent teeth | 10/13 root development and apical closure | 2/13 were positive to EPT | Neelamurthy et al., 2018 [57] |
| PRP BC | 30 non-vital immature permanent teeth | Lesion size decreased; bone density increased; continued root development | After 5 months, sensitivity tests elicited a delayed positive response in 23 sites | Alagl et al., 2017 [58] |
| Experimental: BC + Bio-Gide collagen membrane Control: BC | 46 non-vital immature teeth | Thickening of the dentin wall | EPT was achieved in 18% of the control group and 33% of the experimental group | Jiang et al., 2017 [59] |
| BC | 20 teeth with dens evaginatus treated | Apical diameter and root length increased | Five teeth responded to the pulp sensitivity test during the 1-year follow-up period | Li et al., 2017 [60] |
| PRF PRP BC | 60 patients with immature permanent teeth | Increased root length and thickness; healing of periapical wound | 15% in PRF, 13.30% in BC, and 15.8% in PRP were positive to the vitality test | Shivashankar et al., 2017 [61] |
| BC | 4 infected necrotic primary second molars | At six months, complete periradicular healing and remained symptomless | Positive to cold test | Ulusoy and Cehreli, 2017 [62] |
| BC | Maxillary left central incisor of an 8-year-old child | Increased root length and thickness; healing of periapical wound; closure of apex | Responded to cold and EPT as normal teeth | Farhad et al., 2016 [63] |
| BC | #45 of a 10-year-old girl | Pulp-like tissue with blood vessels | The neurovascular bundles in the pulp-like tissue were NF (+) | Meschi et al., 2016 [24] |
| PRF | Immature permanent tooth of an 11-year-old boy | Increased root length and thickness; healing of periapical wound; closure of apex | Positive to cold test and EPT similar to adjacent teeth after 3 months | Nagaveni et al., 2016 [64] |
| PRP | An avulsed mature incisor of an 11-year-old boy | Resolution of periapical radiolucency | Positive to thermal test and EPT | Priya et al., 2016 [65] |
| PRF | A traumatized, necrotic, immature tooth | Increase in root length and an intact lamina dura | Remained negative to cold test but positive to EPT at 24 and 36 months | Ray et al., 2016 [66] |
| PRF | Left posterior tooth of a 13-year-old male | Increased root length and thickness; resolution of periapical wound; closure of apex | Positive to cold test and EPT | Subash et al., 2016 [67] |
| PRP BC | 20 immature teeth | Complete apical closure and increased root length | 5 teeth in PRP and 2 teeth in BC were positive to vitality test | Bezgin et al., 2015 [68] |
| BC | 4 mature teeth | Root formation increased and dentin wall thickened | 3/4 teeth responded positively to sensitivity test. | Dudeja et al., 2015 [69] |
| BC | An immature permanent tooth | Increased root length and thickness; closure of apex | Neurons and nerve fibers were observed | Lei et al., 2015 [70] |
| PRF | An immature permanent tooth | Increased root length and thickness; closure of apex | Positive to cold test and EPT | Nagaveni et al., 2015 [71] |
| PRP | Maxillary left lateral incisor of a 16-year-old male | Increased root length and thickness; resolution of periapical wound; closure of apex | Not response to cold tests; delayed positive response to EPT | Sachdeva et al., 2015 [72] |
| BC | 3 premolars and 13 incisors | Incomplete apical closure: 47.2%; complete apical closure: 19.4%. Change in root length varying from −2.7% to 25.3%, and change in root dentin thickness ranging from −1.9% to 72.6% | 5/16 teeth were positive to EPT at 18-month recall | Kahler et al., 2014 [73] |
| BC | 3 immature permanent central incisors | Complete root development | Positive to pulp test | Farsi et al., 2013 [74] |
| PRF | Immature right maxillary central incisor of a child | Increased root length and thickness; closure of apex | Positive to cold test and EPT | Keswani and Pandey, 2013 [75] |
| PRF | Tooth #21 of an 11-year-old child | Resolution of periapical rarefaction, further root development, and apical closure of the tooth | Positive to EPT and cold test | Mishra et al., 2013 [76] |
| BC | Central incisors of an 8.5-year-old boy | Increased root length and thickness; closure of apex | Positive to cold tests at 12 months and EPT at 18 months | Cehreli et al., 2012 [77] |
| BC | An immature permanent incisor | Root development and apical closure | Positive to sensitivity tests at 1–3-months | Miller et al., 2012) [78] |
| PRF | Immature incisor of a 9-year-old child | Increased root length and thickness; resolution of periapical wound; closure of apex | Positive to cold test and EPT | Shivashankar et al., 2012 [79] |
| BC | 6 immature permanent molars of children | Increased root length and thickness; resolution of periapical wound; closure of apex | Two teeth were positive to cold test | Cehreli et al., 2011 [80] |
| BC | An immature permanent incisor of a child | Root development and apical closure | Positive to EPT | Iwaya et al., 2011 [81] |
| PRP | Maxillary premolar tooth of a child | Increased root length and thickness; resolution of periapical wound; closure of apex | Positive to cold test and EPT | Torabinejad and Turman, 2011 [82] |
| BC | 6 immature teeth with apical periodontitis | Resolution of periapical lesions; continued root development | Two teeth positive to vitality test | Petrino et al., 2010 [83] |
| BC | A mandibular premolar | Increased root length and thickness; resolution of periapical wound; closure of apex | Positive to sensibility test | Thomson and Kahler, 2010 [84] |
| BC | Immature permanent teeth of 12 patients | Increased root thickness; closure of apex | 3 teeth positive to pulp test | Ding et al., 2009 [85] |
| BC | 2 bilateral mandibular premolars with dens evaginatus of an 11-year-old girl | Increased root length and thickness; resolution of periapical wound; closure of apex | Positive to cold test | Reynolds et al., 2009 [86] |
| BC | Lower-right second premolar of a child | Increased root thickness; closure of apex | Positive to cold test | Banchs and Trope, 2004 [87] |
| BC | An immature premolar of a 13-year-old patient | Increased root thickness; closure of apex | Positive to EPT | Iwaya et al., 2001 [88] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Lyu, P.; Bi, R.; Chen, X.; Yu, Y.; Li, Z.; Fan, Y. Neural Regeneration in Regenerative Endodontic Treatment: An Overview and Current Trends. Int. J. Mol. Sci. 2022, 23, 15492. https://doi.org/10.3390/ijms232415492
Wei Y, Lyu P, Bi R, Chen X, Yu Y, Li Z, Fan Y. Neural Regeneration in Regenerative Endodontic Treatment: An Overview and Current Trends. International Journal of Molecular Sciences. 2022; 23(24):15492. https://doi.org/10.3390/ijms232415492
Chicago/Turabian StyleWei, Yali, Ping Lyu, Ruiye Bi, Xinyu Chen, Yanshen Yu, Zucen Li, and Yi Fan. 2022. "Neural Regeneration in Regenerative Endodontic Treatment: An Overview and Current Trends" International Journal of Molecular Sciences 23, no. 24: 15492. https://doi.org/10.3390/ijms232415492
APA StyleWei, Y., Lyu, P., Bi, R., Chen, X., Yu, Y., Li, Z., & Fan, Y. (2022). Neural Regeneration in Regenerative Endodontic Treatment: An Overview and Current Trends. International Journal of Molecular Sciences, 23(24), 15492. https://doi.org/10.3390/ijms232415492








