Abstract
The effect of single substitutions of protium for deuterium in hydrogen bonds between pairs of nitrogenous bases on the open states occurrence probability at high critical breaking energies of these bonds has been studied. The study was carried out using numerical methods based on the angular mathematical model of DNA. The IFNA17 gene was divided into three approximately equal parts. A comparison of the open states occurrence probability in these parts of the gene was done. To improve the accuracy of the results, a special data processing algorithm was developed. The developed methods have shown their suitability for taking into account the occurrence of open states in the entire range of high critical energies. It has been established that single 2H/1H substitutions in certain nitrogenous bases can be a mechanism for maintaining the vital activity of IFNA17 under critical conditions. In general, the developed method of the mathematical modeling provide unprecedented insight into the DNA behavior under the highest critical energy range, which greatly expands scientific understanding of nucleobases interaction.
1. Introduction
Experimental study of the DNA structure is difficult because of several problems; the most important of which is the limitation of the spatial resolution of available research tools [1,2]. Despite the development of methods for studying single molecules, they have a few limitations for studying the mechanics of DNA [3,4]. For this reason, mathematical modeling is one of the main modern methods for studying DNA molecular dynamics, its mechanical movements, open states, denaturation bubbles [5,6,7]. This method, despite several simplifications, allows us to consider various aspects of DNA functioning with great accuracy [8,9,10]. In the article [11], using a combination of atomic force microscopy (AFM) and modeling of atomic molecular dynamics (MD), an attempt was made to experimentally observe the dynamics of open states. The experiment was carried out on short ring DNA, while many microphotographs were taken, after which, using a custom Python script, the images were corrected for further morphological analysis. As a result, denaturation bubbles were recorded, which, in turn, leads to DNA compaction. This indicates the importance of combining the experimental and mathematical modeling methods, which allows us to obtain much more information about the dynamics and open states of the DNA molecule [12].
Earlier, we found that the ingress of a deuterium atom into hydrogen bonds between pairs of nitrogenous bases increases the probability of occurrence of open states by 0.22–0.60% [13]. In addition, it has been shown that the probability of the bubbles formation (length from 12 to 27 nucleotides) depends on the localization of the deuterium atom in the DNA molecule and may differ significantly from the probability of the occurrence of open states in general [14]. The participation of deuterium atoms in the formation of hydrogen bonds in the DNA molecule can cause a change in the time of transmission of genetic information, thus, even a slight change in the isotopic state of the medium can affect changes in metabolic processes in living systems [15,16,17]. It is known that non-radioactive isotopes of biogenic elements (2H/1H, 13C/12C, 15N/14N, 18O/17O/16O) have a significant effect on the rate of biochemical reactions, physiological processes, growth, and development of unicellular and multicellular living organisms with different levels of organization of energy metabolism and metabolic rate [18,19,20,21,22,23,24,25].
The aim of this work is to study the effect of a single deuterium substitution at the frequency of hydrogen bond dissociation in IFNA17 in the range of the highest critical energies, based on the mechanical model of DNA [26], without simplifications and averaging [27,28].
2. Results
On Figure 1 the graphs of the angular deviations of the 1-st chain of DNA molecule nitrogenous bases under the first , which equals N·m over period from 0 to s are presented.
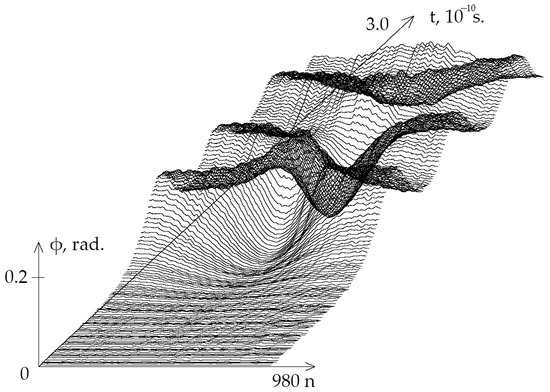
Figure 1.
Graphs of angular deviations of the 1st chain of DNA molecule nitrogenous bases in the gene encoding interferon alpha 17 under equals N·m over period [ s.].
The open state (OS) occurrence frequency was counted by using above-mentioned and modified Basov–Jimack algorithm, according to which, for the highest critical energy range (from N·m to N·m), on Figure 2 the dynamics of the OS occurrence in studied gene dependent on the H-bond dissociation energy under natural condition and after the single 2H/1H replacement are showed. This data, more than anything else, demonstrates that, in the highest critical energy range, the OS occurrence frequency under natural condition, when all hydrogen bonds in DNA nucleotides are 1H, is always lower than frequency of OS occurrence in the range “Maximum”, when is the single replacement of protium with deuterium at nitrogenous bases result in the increase of the OSs (). Moreover, after equals to N·m the natural OS occurrence frequency declines abruptly to 0.0 and, further, it has not positive value throughout diapason from N·m to N·m (Figure 2 and Figure 3). For instance, 22.7% 2H-substituted nucleobases in the whole gene increase OS occurrence frequency more than 0.0 under equals to N·m (Table 1).
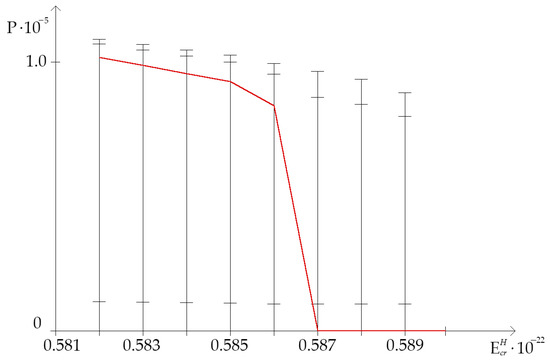
Figure 2.
Dynamics of the open state (OS) occurrence in gene encoding interferon alpha 17 dependent on the H-bond dissociation energy under natural condition and after the single 2H/1H replacement (with gradation of OS occurrence frequency by modified Basov–Jimack algorithm). Note: for each H-bond dissociation energy: the 1st cross dash is , the 2nd cross dash is the bottom of the range “Maximum”, the 3d cross dash is the top of the range “Minimum”, which was calculated by using non-modified Basov–Jimack algorithm [29]; the red line is OS occurrence frequency under condition when all hydrogen bonds in DNA are 1H ( ).
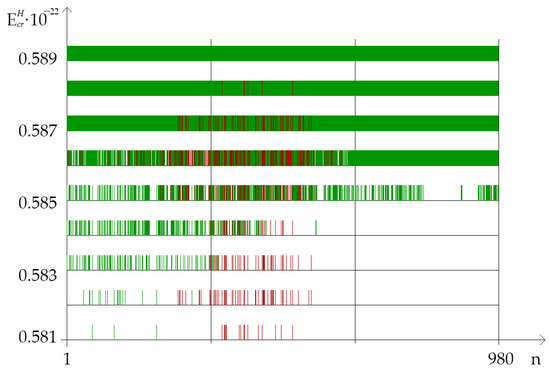
Figure 3.
Distribution of nucleobase pairs (which was counted by modified Basov–Jimack algorithm in the different parts of IFNA17), leading after the single 2H/1H replacement to the extreme frequencies of OS and CSNB occurrences. Note: red dot is the location of the deuterium atom in the DNA molecule, which leads to the maximum probability of OS occurrence (range); green dot is the location of the deuterium atom in the DNA molecule, which leads to the CSNB occurrence (range).

Table 1.
Quantity of closed states of nitrogenous bases for different nitrogenous bases in each of three parts of IFNA17 dependent on single 2H/1H replacement.
On Figure 3, in the different parts of IFNA17, showed the distribution of nucleobase pairs leading to the maximum probability of OS and CSNB occurrences after the single 2H/1H replacement under energy diapason from N·m to N·m (henceforth 0.581–0.589).
In our previous study for N·m energy range [29], the inequality in the frequency of the open states occurrence because of the single 2H/1H replacement in gene encoding interferon alpha 17 (IFNA17, which consists of 980 nucleotide pairs [30]) was proved through it conditionally divided into three equal parts: from the 1st to the 327th nucleobases (I part), from the 328th to the 653th nucleobases (II part) and from the 654th to the 980th nucleobases (III part). This approach allowed us to contemplate the influence on probabilities () of open state occurrences between different nitrogenous bases in double-stranded DNA dependent on the single 2H/1H replacement in basepair of each gene region. Although these studied parts of IFNA17 had approximately the same number of base pairs, the A-T/G-C ratios were significantly different in each part [31], which are presented in more detail in Table 2.

Table 2.
Distribution of adenine-thymine (A–T) and guanine-cytosine (G–C) base pairs in different parts of IFNA17.
According to this fact, for the range of the energies from N·m to N·m both quantity of closed states of nitrogenous bases (CSNB) and number of nucletides of the range “Maximum” () for different nitrogenous bases in each of three parts of IFNA17 under single 2H/1H replacement via BJ-algorithm were calculated, and obtained data are presented in Table 1 for CSNB and Table 3 for .

Table 3.
Quantity of A-T and G-C nucleobases, included in the range “Maximum”, in each of three parts of IFNA17 dependent on single 2H/1H replacement.
It is worth noting that the change in energy, which was taken into account when calculating CSNB (Table 1) and (Table 3) in the highest energy range, always equaled 0.001.
In our study was found out that quantity of CSNB and of OSs for the each gene part were different in the above-described energies ) and depend on A-T/G-C ratio (Table 1 and Table 3). In the range of from 0.581 to 0.589 the highest total number of counted by the BJ-algorithm was when the single 2H/1H replacement had taken place in the II part of IFNA17. The number of i, which were included in the range “Maximum” in this gene part was in 3.21 times higher than in the I part (: ); whereas counted was equal to 0.0 for any of studied energies when 2H/1H replacing had occurred in the III part (Figure 3). Albeit the A-T nitrogenous bases prevalence over G-C ones in IFNA17 (in 1.48 times), the distribution of the total in the whole gene due to the isotopic substitution at the A-T and G-C nucleotides showed the predominance of 2H/1H replacement at the cytosine and guanine bases, the number of which was in 3.67 times higher, than the number of 2H-substituted A-T (). Wherein, the difference between of 2H/1H-substituted A-T and G-C nucleotides was much more pronounced in the gene I part (in 8.5 times, Table 3) compared to II part (in 3.03 times, Table 3). In addition, the overwhelming majority of 2H/1H-replacement at the G-C pairs, leading to the , arose in the diapason of from 0.581 to 0.585, so that the number of them was higher in 7.56 times than the nmax via the single 2H-substituted adenine or thymine in the same range of energies (). For from 0.586 to 0.589, there is a less pronounced difference between deuterium substituted A-T and G-C , although the number of the last is more in 2.29 times (, Table 3).
The calculated quantity of CSNB firstly turned higher in the I part of IFNA17 (under from 0.581 to 0.582) compared to the II and III parts, which having nothing of these (Figure 3). Further, under from 0.583 to 0.584 the CSNBs arise in the gene II part; but even at energy equal to 0.584 their number keeps less in 2.75 times than in the I part. Only upon reaching the influence of 0.585 energy in the III part appears some CSNBs, the number of which was less compared to the I and II parts in 1.42 and 1.69 times, respectively (: , Figure 3). However, starting with equal to 0.586, the quantity of CSNBs in the III part was grown very abruptly, reaching 100% of the nucleotides of this gene part, and it exceeded the CSNB numbers, which were almost equal to each other, in the I and II parts of IFNA17 in 1.51 and 1.56 times, respectively (: , Figure 3). In addition, as in the coming into being of , the influence of A-T and the G-C ratio was explicit for generation of CSNBs under the single deuterium substitution in each part of the studied gene. In the 0.581–0.582 range of critical energies, the CSNBs were initiated and grew up specific via the 2H-substituted G-C pairs, and, moreover, in the 0.583–0.584 range the 2H-substituted G-C outnumbered the 2H-substituted A-T nitrogenous bases and were accounted at least 67% of the total nucleobases leading to the CSNB occurrence in the whole gene (, Table 1). However, at the equal to 0.585 this difference of CNBS numbers between 2H-substituted A-T and G-C was leveled (). It should be noted, that, under 0.589 , CSNB making was completed for the each nucleobase pairs of the whole IFNA17, and it observed when six G-C pairs had turned into close states in the gene II part specifically (). In addition, it was found that than the lower A-T/G-C ratio in the gene part, the CSNBs arose there earlier and under lower critical energies. So, for example, in the range of from 0.581 to 0.585 the Spearman correlation coefficient between A-T/G-C ratio and CSNB numbers was −0.547 (, Figure 3). The fractions of the 2H-substituted G-C bases in the initiative numbers of CSNB in the each gene parts were following: 100% in the I part under equal to 0.581 (), 100% in the II part under equal to 0.583 (), and only 30% in the III part under equal to 0.585 (, Table 1), that could be because of the last gene part had the least number of the G-C nucleobases (Table 2). For the highest acceleration rates of the CSNB numbers, with reaching them approximately half of the total nucleobases in each gene part (but less than all of the nitrogenous bases in the each part) were provided more often due to the 2H/1H replacement at the A-T pairs: 62% in the I part under equal to 0.586 (), 59% in the II part under equal to 0.586 () and 70% in the III part under equal to 0.585 (, Table 1).
3. Discussion
The influence of the isotopic 2H-effect on nucleic acids structure and their functions is being actively studied, and it is relevant for various fields of science [19,32,33,34,35,36,37,38,39,40]. So, our data allows to understand the mechanism of the possible changes in the OS occurrence frequency in IFNA17 by the single 2H/1H-replacement under specific energy range more precisely. It is well known about deuterium (D) ability to alter structure and activity of DNA and RNA via changing base-pairing interaction strength by the difference in the energy, stability and geometry between H– and D–bonds, and also through the inequality between 1H and 2H in the hydrogen bond zero-point vibration energies [32,35,36,41,42,43,44,45,46]. In the present study one of the most vital processes for DNA functioning, which can depend on isotopic 2H/1H-exchange, was considered—generation of OSs, surplus or deficiency of which can provoke various impairments of DNA stability. It is worth noting that occurrence of nucleotide open states in DNA is very critical conformational transition, which is necessarily needed for both hydrogen exchange and intermolecular interaction of proteins with specific DNA target, which, consequently, provide the realization of the vital function of nucleic acids [47]. In addition, expressing concisely, CSNB are logically inappropriate for the specific implementation of some essential processes in cell due to the formation in DNA many excessively unbreakable sections, which totally prevent the hydrogen exchange between proteins and DNA. Additionally, those energies, under which gene has one or more CSNB (which can equal in summary up to the entirety number of its nucleotides), form the highest critical energy range for the certain gene. Therefore, the study of the influence of 2H/1H exchange on the molecular dynamic of DNA for energy diapason (or the highest energy range), which initials the rising of the nucleotide closed states in IFNA17, and also taking into consideration A-T/G-C ratio in its different parts, is the great interest for a deeper understanding of the biological functioning of nucleic acids under critical environmental effects [41]. So, according to the data obtained for the studied IFNA17 [29], the highest energy range equals to from 0.581 to 0.589 for it (Table 1, Figure 3), and that was contemplated in this work.
According to the developed mathematical modeling it was found in the study, that the more increased stability of IFNA17 observed in its parts rich in G-C base pairs, which can be associated with the specific DNA folding of these regions providing more stabilizing energetic contributions and earlier transition to closed states of nucleobases under lower critical energies (starting from the equals to 0.581 in the gene I part). This statement is confirmed by the significant and negative Spearman correlation coefficient between A-T/G-C ratio and CSNB numbers in the each of the three studied parts of IFNA17. It is well known that even the change of closing nitrogenous bases from G-C to C-G can stabilize DNA hairpin structure approximately 2 kcal/mol and lead to the significant enhance of its melting temperature [48,49]. The similar phenomena are most possible associated with the formation in the alternative DNA structures additional H-bonds, for instance, due to the slightly twisted some nitrogenous base pairs. In addition, it is possible through the occurrence of electrostatically favorable contacts between the partially positively charged amino groups and partially negative oxygen-containing groups with the subsequent formation of different types of loops or other energetically auspicious DNA structures [50,51]. Another but less influenceable reason of the arising CSNB in the gene is increasing of van der Waals packing forces, which changed at the specific conformations of DNA. In opposing to CSNB, the increase OS occurrence frequency, which stems from the presence of the single 2H/1H-substituted nucleotide in the specific gene region, requires weakening of hydrogen bonds in DNA, reduction of electrostatic attraction between charged chemical groups and decreasing of van der Waals packing forces, that occurred firstly and more often when 2H/1H replacement takes place at the cytosine and guanine bases, especially in the II part of IFNA17. It can be induced by different reasons: some conformational change of the gene, specific molecular folding, and reducing rate of the rare tautomer formation within DNA because of the isotopic 2H/1H exchange in nitrogenous bases [52,53]. In our study was showed, that the single 2H/1H replacement at G-C pairs has the most influence on the initial arise both and . Moreover, the last OSs of IFNA17 under N·m were presented only G-C pairs (Table 1). The peculiarity of the gene III part with a significant higher content of A-T is the concise turning OSs into CSBNs within two critical energies, such as a collapse (Figure 3). It can point out the higher destabilizing of DNA region enriched A-T nucleobases and risk of its sharp dysfunction under critical conditions [54], nevertheless, the initial OS frequency changes occur foremost because of the 2H-substitution at G-C pairs.
In addition, the above modification of the BJ-algorithm was due to the fact that, in the highest critical energy range, there was a progressive accumulation of the number of CSNB (that was starting from eaqualed 0.581 when for the first time the minimum limit was reached: ). Further, this did not allow the differential consideration of the contribution of the de novo emerging CSNBs (under non-modified BJ-algorithm [29]), which were beginning continually rising from equaled 0.582 (Figure 3). So that, since the subsequent CSNBs additively accumulated in the total number of the previously formed CSNBs, the role of each emerging CSNB has been indistinguishable in the total number of CSNBs (Figure 3).
Bearing in mind following prerequisites: the contribution of earlier arisen CSNB is clearly higher in failure of the molecular dynamics of the gene, than the later ones, and for the newly formed CSNB it is also inversely proportional to the summary number of the ones in gene, as well as the number of CSNBs are limited by the total number of gene nucleobase pairs the modified BJ-algorithm can required additional below-presented changes for this study:
i ϵ range “Minimum” (CSNB): if :
; where:
CSNB is closed state of nitrogenous bases: PCSNB = 0;
is number of closed states of nitrogenous bases in the gene part x under certain ;
is total number of closed states of nitrogenous bases in the whole gene under certain ;
is number of nitrogenous base pairs in the whole gene;
If : i ϵ range from Q2-min to Q4-min (Q2–Q4-min) [29].
The new approach can not only take into account the bigger role earlier formed CSNBs for the certain gene functioning (due to accounting for depending on , but also avoid the cumulative effect distorting the total CSNB number (especially for the higher energies: , Figure 3 and Figure 4) by using square function for the component: . Foremost this approach allows us to reduce the number of false positive data when evaluating CSNBs (Figure 3 and Figure 5). Other benefits of the changes in the new approach of BJ-algorithm and some disadvantages of decile and quartile methods for selection of the nucleobases for the maximum and minimum ranges were presented in details in our earlier work [29].
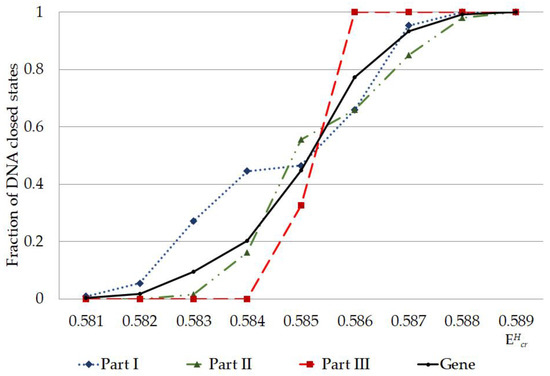
Figure 4.
Fraction of nucleobase pairs, which was counted by modified Basov-Jimack algorithm in the different parts of IFNA17, leading after the single 2H/1H replacement to the CSNB occurrences under the highest critical energy range. Note: fraction of CSNB was calculated as CSNB number under specific divided by the total nucleobase number in the each part of gene; fraction of CSNB in the gene was calculated as CSNB number in the whole gene under specific divided by the total nucleobase number in the gene (n = 980); part I is from 1st nucleotide to 327th nucleotide; part II is from 328th nucleotide to 653d nucleotide; part III is from 654th nucleotide to 980th nucleotide.
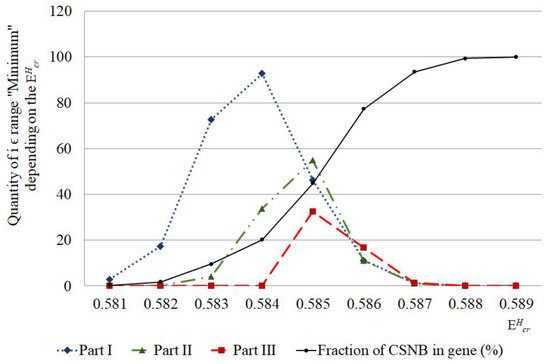
Figure 5.
Number of nucleobase pairs, which was counted by the new approach of modified Basov–Jimack algorithm in the different parts of IFNA17, leading after the single 2H/1H replacement to the CSNB occurrences under the highest critical energy range. Note: fraction of CSNB in the gene was calculated as CSNB number in the whole gene under specific divided by the total nucleobase number in the gene (n = 980) and then multiplying this ratio by 100%.
For instance, if we confront statistics of the data, processed by non-modified BJ-algorithm and the new approach, it can be found when comparing their results, there is no significant difference between the sums of , which were counted by the non-modified BJ-algorithm [29], in the second (II) and third (III) parts of the gene throughout 0.581–0.589 energy range (n(part-II) = 1371 bases, n(part-III) = 1415 bases, ), but there is significant difference on 205.9% between the sums of , higher in the II part compared to III part, are calculated by the new approach in the same energy diapason (n(part-II) = 105 bases, n(part-III) = 51 bases, ). It was reached due to the strong reduction of the false positive results because of the above-mentioned new approach of the modified BJ-algorithm (Figure 3 and Figure 5). Additionally, when CSNB numbers were counted by the new approach of the modified BJ-algorithm in the whole gene and for each throughout the energy diapason from 0.581 to 0.588, the strong correlation between the total was revealed (because of 2H-substitution at both A-T and G-C) and due to the 2H-substitution only at G-C nucleobases, with Spearman’s coefficient equaled to 0.881 (), which proved the far higher ability of 2H-substituted G-C produced more evenly and incessantly the closed states in IFNA17 compared to 2H-substituted A-T bases (Figure 6).
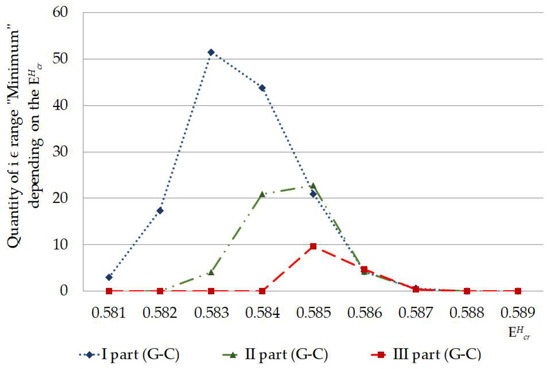
Figure 6.
Quantity of nucleobase pairs, which was counted by the new approach of modified Basov–Jimack algorithm in the different parts of IFNA17, leading after the single 2H/1H-substitution at G-C bases to the CSNB occurrences under the whole highest critical energy range.
In general, the developed method of the mathematical modeling provide unprecedented insight into the DNA behavior under the highest critical energy range, which greatly expands scientific understanding of nucleobases interaction [50]. Under the highest critical energy range, these described fluctuations of after single 2H/1H-substitutions in the different gene parts can provoke DNA dysfunction, for example, because of the sharp slowdown in the rate of the generation of vital non-superhelical denaturation bubbles, which have more than four nucleobase-pairs in the promoter regions and specific binding points for DNA repair proteins [55,56] that can be a predictor of DNA repair system lesion. In addition, obtained data demonstrate the possibility of higher risk, which increase abruptly in the whole energy range from 0.581 to 0.587 of altering and impairment of IFNA17 due to the single 2H/1H exchange in the A-T richest part compared to the both I or II parts, especially under more than 0.585 (Figure 3 and Figure 4). It should be noted, that in opposition to the natural frequency of OSs in the gene under the energy higher 0.586, after the single 2H-substitution at G-C in the II part observed lower , so this can provide more probability of DNA bubble occurrence, be one of nucleic acid adaptation mechanisms to critical conditions, and matter to the evolution process of living system [57]. According to the developed mathematical modeling and modified BJ-algorithm it was found, that, under the from 0.586 to 0.588 the occurrence rates, which matches the range “Maximum”, due to the 2H/1H exchange at G-C is always significant more than ones due to the same isotopic exchange at A-T, that can consider as the positive participating of the 2H-substituted guanine and cytosine in nucleic acid adaptation in opposing to the 2H-substituted adenine and thymine, which give no advantages to DNA denaturation process. For example, it can influence the rate of specific DNA bubble forming, which participating in generation of the pre-existing state of denaturation, that is required by specific DNA-binding enzymes [58,59]. The relationship study between total and produced due to the 2H/1H substitution at G-C in the I and II parts of IFNA17, which had more than one 2H-substituted bases from range “Maximum” in opposing to III part, for each throughout the energy diapason from 0.581 to 0.588 revealed Spearman’s coefficient equals to 0.994 (), that pointed out the obvious dominant role of 2H-substituted G-C bases in the generation in these gene parts compared to A-T ones (Figure 7). Moreover, the study demonstrated, that the single deuterium introduction in the gene III part, which is out of the coding region from 50th to 619th base pairs [60,61], had the most close data of the OS occurrence rate compared to the data of OS occurrence frequency under condition when all hydrogen bonds in IFNA17 are 1H, that was showed clearly the least possibility of 2H/1H replacement in this part to influence on gene adaptation process under critical conditions positively.
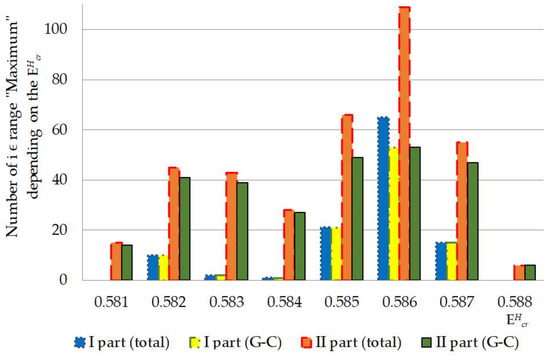
Figure 7.
Numbers of nucleobases from the range “Maximum”, which were counted by the modified Basov–Jimack algorithm, in the I and II parts of IFNA17 throughout 0.581–0.588 energy diapason. Note: Total in each gene part was calculated as number due to the single 2H/1H-exchange both at A-T and G-C pairs in total; G-C in each gene part was calculated as number due to the single 2H/1H-exchange only at G-C pairs.
Above-described cases of the double-stranded DNA dynamics obviously confirmed that under the highest critical energy diapason, the heterogeneous sensitivity of IFNA17 to isotopic 2H/1H exchange took place and frequency of OS occurrence strongly depended on not only the single 2H-modified nucleobase in the certain gene region (I, II, or III) but, additionally, on the A-T/G-C-ratio in each of its part; the last was, especially, correlated to the quantity of initial CSNB, that was proved due to significant and negative Spearman’s coefficient, reflecting the moderate strength of relationship between them under energy range from 0.581 to 0.585.
So, below are the main inferences of our work, confirming in detail the above-mentioned conclusion:
- In some cases, the single 2H/1H replacement result in positive value of OS occurrence frequency in the IFNA17 throughout diapason from N·m to N·m opposing to the OS occurrence frequency, when all hydrogen bonds in DNA nucleotides are 1H, which is always equaled to 0.0 after exceed N·m. This underlines that at least 22.7% of the total number 2H-substituted nucleobases can reduce molecular interaction in the studied gene and increase the hydrogen bond dissociation, foremost in its part from 328 to 653 nitrogenous bases;
- The counted occurrences of the OSs were much higher when the single 2H/1H replacement had taken place at nucleobases of the middle part IFNA17 (from 328 to 653 nucleotides) compared to its other parts, and this had a strong prevalence rate in the G-C pairs;
- The lowest rate of the OS occurrence was under the single deuterium substitution at the nitrogenous bases in the gene III part (from 654 to 980 nucleotides), which was also too rich in A-T pairs (72.2%) compared to the other parts of IFNA17, so that the calculated was equal to 0.0 for all of the studied critical energies (from N·m to N·m);
- Sum of was less significant when the single 2H/1H replacement occurred at the A-T nucleobase pairs compared to the G-C ones in the I and II parts of IFNA17, and the relationship between total and at G-C in these parts for each throughout the energy diapason from N·m to N·m was strong () that proves the obvious dominant role of 2H-substitutied G-C bases in the generation compared to the A-T;
- Earliest CSNBs (n = 3) arose under equal to N·m when IFNA17 had had the single 2H-substituted cytosine or guanine nitrogenous bases in its I part (from 1 to 327 nucleotides). Moreover, throughout the range energy from N·m to N·m, the single 2H/1H replacement, leading to the CSNBs, prevailed in the I part, especially for its G-C pairs making up at least 67% of the total CSNB quantity in the whole gene. So, for IFNA17 throughout the range of from N·m to N·m the Spearman correlation coefficient between A-T/G-C ratio in the each gene part and CSNB numbers was significant and negative ();
- The highest acceleration of CSNB occurrence was observed when the single 2H/1H replacement took place at nucleotides of the III part of IFNA17 under from N·m to N·m, and throughout it they very abruptly reached the value of 100% of the nucleobases in this gene part. It indicates the obvious and higher vulnerability of IFNA17 due to the single 2H-substitution at nucleobases from 654 to 980 compared with other gene parts exposed to studied critical energies, which increased the risk of permanent disorders of converting genetic information to mRNA messenger;
- All of the above-mentioned underline clearly the significant difference in the responsiveness of each IFNA17 parts under range of critical energies because of the single 2H/1H replacement in their nucleobases and with its strong dependence on A-T/G-C ratio with the prevalent contribution of the last pair, which leads to an increase due to the 2H-substitution into its nitrogenous bases both and CSNB, especially under diapason from N·m to N·m;
- The single 2H-substitution at G-C pairs not only had the most influence on the initial arise both and in the whole gene throughout the critical energy diapason from N·m to N·m but also made possible the existence of the last OS occurrence under equals to N·at least in six cases that proves the leading effect of the isotopic 2H/1H modifications at G-C compared to A-T on the molecular dynamics of IFNA17;
- In addition, in the study was presented a modified algorithm allowing for accounting for nucleobases with the single 2H/1H replacement, which leads to occurrence of both the highest rate of Oss and CSNBs. Also, it showed the developed approach, decreasing significantly the false positive results compared to non-modified BJ-algorithm [29] due to the differentiated counting of the total sum of CSNB occurrence in the gene with relevance to the critical energy in the highest diapason.
4. Materials and Methods
4.1. Mathematical Model
The open states of the DNA molecule and its dynamics are well described by a mechanical model, which is two chains of disks that are interconnected by transverse springs; this system is described by the following Newton equations:
here:
—is the angular deflection of the i-th nitrogenous base of the j-th chain counted counterclockwise at time t;
—is the rotational inertia of the i-th nitrogenous base of the j-th chain;
—is the distance between the center of inertia of the i-th nitrogenous base of the j-th chain to sugar phosphate chain;
—is the constant characterizing the torsion moment of the i-th segment of the j-th sugar phosphate chain;
—is the constant characterizing the bond elastic properties of the i-th nitrogenous base pairs;
—external influence on the i-th nitrogenous base of the j-th chain at a time t;
—is the number of nitrogenous base pairs in the system.
The magnitude of the external impact is assumed to be equal to , where the term is simulates the effects of dissipation caused by interaction with the water surrounding the DNA molecule, the term is an external periodic effect.
In Equations (1)–(6), the first term to the right of the equality sign describes the force acting on the i-th nitrogenous base from the sugar-phosphate filament, the second term—the force from the complementary nitrogenous base, the third term—external impact.
Thus, Equations (1)–(6) allow us to model the hydrogen bond in the i-th pair (, ), deuterium (, ) and the break of this connection (). We will assume that a break in base pairs occurs if the potential binding energy in these pairs exceeds a certain critical value for a hydrogen bond and for a deuterium bond, if the potential energy in a pair with a broken bond is less than the critical value, then the bond is restored.
To Equations (1)–(6) we add the initial conditions:
For the sake of certainty, we assume that at the system is in equilibrium, that is, in the initial conditions (7) and (8)
Problems (1)–(8) is a Cauchy problem for a system of ordinary differential equations; in this paper, all studies were carried out on the basis of a numerical solution of this system.
The study of the effect of 2H/1H exchange on the formation and dynamics of open states will be carried out using the example of the gene encoding interferon alpha 17. For this gene, , the values of the coefficients of Equations (1)–(6) are taken from [31]), the values of .
We assume that , if one of the hydrogen bonds in the i-th base pair replaced with deuterium, . The value of the coefficient will be chosen because the deuterium bond is 5% stronger than the hydrogen bond. The order of the critical energy is consistent with the experimental data from the work.
We designate by P0 the probability of an OS formation in a molecule in which all pairs of nitrogenous bases are connected by hydrogen bonds; by Pi, i =, the probability of an OS occurrence in a DNA molecule in which the i-th nitrogenous base pair one <any> hydrogen bond is replaced by deuterium.
The probabilities P0 и Pi, i = , will be sought on the basis of a numerical solution of the problem (1)–(8). To do this, we will create a set of points tj = jƮ, j =, Ʈ = T/m in the segment [0, T]. Calculate at t = tj the ratio qj of the number of base pairs with a broken bond to the total number of base pairs n, then the value of Pk is equal to the arithmetic mean value over the points tj of these ratio:
4.2. Modification of Basov–Jimack Algorithm
The affiliation of each nucleotide to I part, II part or III part of IFNA17 was determined due to its sequence (serial) number. Nucleobases with , and the higher were selected for the maximum range and their sum was , and nucleobases, which had CSNB (closed state of nitrogenous bases) (), were selected for the minimum range, and their number was designated as . According to the below-presented modified Basov–Jimack algorithm (BJ-algorithm [30]), which was changed for the highest critical energy range, every was arranged from CSNB to and their numbers were calculated in each gene part:
- (1)
- i ϵ range “Maximum” (BJ-max):
- if and :; or else:
- if and :;
- (2)
- i ϵ range “Minimum” (CSNB):
- if ;
where is number of i, which were included in the range “Maximum”; CSNB: ; is critical energy in the diapason, which has 1 or more CSNB: , in this case from N·m to N·m; is number of nitrogenous base pairs in x part of gene: x can be I, II or III part of IFNA17; is number of closed states of nitrogenous bases in the gene part x under specific ; is number of nitrogenous base pairs in the whole gene.
4.3. Statistics
In addition, some statistical methods were used to exact the significance of differences among and of the three gene parts. Yates corrected chi-squared test () was applied for a 2 2 contingency table (where degrees of freedom . As a short-cut, for a 2 2 table with the following entries (Table 4):

Table 4.
Chi-squared test with Yates correction.
Where A and B are the rows according to the parts of the gene: I, II, or III; S is the column of of the range “Minimum”, or of the range “Maximum”; F is the column of the rest number of nucleotide pairs; a and c are of the range “Minimum”, or of the range “Maximum” in the each compared part of the gene in the determined range of ; b and d equals the total number of nucleotide pairs of the gene part minus of the range “Minimum”, or of the range “Maximum” in the each compared part of the gene in the determined range of ;
Chi-square corrected by procedure for Bonferroni () was used for a 3 2 contingency table (where 3 is rows, 2 is columns, . The Kruskal–Wallis ANOVA by Ranks test (KWt) was applied for the comparison of the range “Minimum”, or of the range “Maximum” in the determined range of for two gene parts are mutually independent. The assess of the relationship between two variables was measured by Spearman’s rank correlation coefficient ().
5. Conclusions
Thus, in the study, it was found that under the highest critical energy range, the very significant inequality of the frequencies of OS occurrence in the full gene were due to the single 2H-substitution at the nucleobase of its different regions, which were almost equal to each other (gene I, II, III parts) by the number of nucleotides. In almost the entire highest critical energy range after the single 2H/1H replacement at the nitrogenous base of IFNA17, the values both in the range “Maximum” and “Minimum” were much different compared to the OS occurrence frequency under condition when all hydrogen bonds in DNA are 1H (, Figure 2). Foremost, the A-T/G-C ratio had a strong influence on the frequency of CSNB in the diapason from N·m to N·m that can be not only the reason of the function impairment of IFNA17 but can also demonstrate the nucleic acid adaptation mechanisms to critical conditions and matter to the evolution process of living system [57]. For instance, under the highest critical energy range, gene function impairment can occur because of the increase in , leading to the sharp slowdown of DNA bubble generation, which has more than four nucleobases, participating in forming of the preexisting denaturation state that is required by specific DNA-binding proteins [58,59]. In turn, via the single 2H/1H replacement at certain bases, the arising can be the mechanism of preserving the vital functioning of IFNA17 under critical conditions. The modified algorithm and new approach allow for calculating the and throughout the highest critical energy diapason, in opposition to the non-modified Basov–Jimack algorithm, which is appropriate for counting the base-pair number in the range “Maximum” and “Minimum” under only the critical energies less than the highest range.
In addition, it should be noted, that the developed mathematical modeling showed its appropriateness for counting of the OS occurrence under the whole highest critical energy range. However, our study had a limitation, which was following: all results had been obtained in the frameworks of the mathematical model based on Newton’s equations and are representative of the Cauchy problem for the system of 2n ordinary differential equations, which did not entirely take into account the all contribution of DNA–protein interactions [62,63]. Nevertheless, we do not contemplate this limitation as an insurmountable obstacle and consider the modified algorithm and new approach as appropriate methods that can be generalized and used for more other complex models of DNA dynamics.
Author Contributions
Conceptualization, A.B.; software, A.S.; formal analysis, A.D. (Andrey Drozdov); data curation, V.M.; writing—original draft preparation, M.D. and S.D.; visualization, M.B. (Maria Bezhenar); supervision, M.B. (Mikhail Baryshev); project administration, A.M. and A.D. (Anna Dorohova); funding acquisition, E.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Kuban Scientific Foundation project No. H-21.1/11 and the state assignment of the SSC RAS No. 122020100351-9.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The research was carried out using the equipment of the Research Center for Food and Chemical Technologies of KubSTU (CKP_3111), which is supported by the Ministry of Science and Higher Education of the Russian Federation (Agreement No. 075-15-2021-679).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ye, F.; Inman, J.T.; Hong, Y.; Hall, P.M.; Wang, M.D. Resonator nanophotonic standing-wave array trap for single-molecule manipulation and measurement. Nat. Commun. 2022, 13, 77. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Kayikcioglu, T.; Ngo, T.T.M.; Ranjan, A.; Eustermann, S.; Cieza, B.; Morgan, M.T.; Hejna, M.; Rube, H.T.; Hopfner, K.P.; et al. Measuring DNA mechanics on the genome scale. Nature 2021, 589, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Jie, M.; Wang, M.D. DNA supercoiling during transcription. Biophys. Rev. 2016, 8, 75–87. [Google Scholar] [CrossRef]
- Konyshev, I.; Byvalov, A. Model systems for optical trapping: The physical basis and biological applications. Biophys. Rev. 2021, 13, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Manghi, M.; Destainville, N. Physics of base-pairing dynamics in DNA. Phys. Rep. 2016, 631, 1–41. [Google Scholar] [CrossRef]
- Chevizovich, D.; Michieletto, D.; Mvogo, A.; Zakiryanov, F.; Zdravković, S. A review on nonlinear DNA physics. R. Soc. Open Sci. 2020, 7, 200774. [Google Scholar] [CrossRef]
- Dzhimak, S.S.; Drobotenko, M.I.; Basov, A.A.; Svidlov, A.A.; Fedulova, L.V.; Lyasota, O.M.; Baryshev, M.G. Mathematical Modeling of Open State in DNA Molecule Depending on the Deuterium Concentration in the Surrounding Liquid Media at Different Values of Hydrogen Bond Disruption Energy. Dokl. Biochem. Biophys. 2018, 483, 359–362. [Google Scholar] [CrossRef]
- De Santis, D.; Guarcello, C.; Spagnolo, B.; Carollo, A.; Valenti, D. Generation of travelling sine-Gordon breathers in noisy long Josephson junctions. Chaos Solitons Fractals 2022, 158, 112039. [Google Scholar] [CrossRef]
- Grinevich, A.A.; Masulis, I.S.; Yakushevich, L.V. Mathematical Modeling of Transcription Bubble Behavior in the pPF1 Plasmid and its Modified Versions: The Link between the Plasmid Energy Profile and the Direction of Transcription. Biophysics 2021, 66, 209–217. [Google Scholar] [CrossRef]
- Svidlov, A.A.; Drobotenko, M.I.; Basov, A.A.; Gerasimenko, E.O.; Malyshko, V.V.; Elkina, A.A.; Baryshev, M.G.; Dzhimak, S.S. DNA dynamics under periodic force effects. Int. J. Mol. Sci. 2021, 22, 7873. [Google Scholar] [CrossRef]
- Pyne, A.L.B.; Noy, A.; Main, K.H.S.; Velasco-Berrelleza, V.; Piperakis, M.M.; Mitchenall, L.A.; Cugliandolo, F.M.; Beton, J.G.; Stevenson, C.E.M.; Hoogenboom, B.W.; et al. Base-pair resolution analysis of the effect of supercoiling on DNA flexibility and major groove recognition by triplex-forming oligonucleotides. Nat. Commun. 2021, 12, 1053. [Google Scholar] [CrossRef] [PubMed]
- Drobotenko, M.I.; Dzhimak, S.S.; Svidlov, A.A.; Basov, A.A.; Lyasota, O.M.; Baryshev, M.G. A Mathematical Model for Basepair Opening in a DNA Double Helix. Biophysics 2018, 63, 177–182. [Google Scholar] [CrossRef]
- Dzhimak, S.S.; Svidlov, A.A.; Basov, A.A.; Baryshev, M.G.; Drobotenko, M.I. The effect of single deuterium substitutions for protium in a DNA molecule on the occurrence of open states. Biophysics 2018, 63, 497–500. [Google Scholar] [CrossRef]
- Svidlov, A.A.; Drobotenko, M.I.; Basov, A.A.; Elkina, A.A.; Gerasimenko, E.O.; Malyshko, V.V.; Baryshev, M.G.; Dzhimak, S.S. Influence of the 2H/1H isotope composition of the water environment on the probability of denaturation bubble formation in a DNA molecule. Phys. Wave Phenom. 2021, 29, 180–185. [Google Scholar] [CrossRef]
- Syroeshkin, A.V.; Antipova, N.V.; Zlatska, A.V.; Zlatskiy, I.A.; Skylska, M.D.; Grebennikova, T.V.; Goncharuk, V.V. The effect of the deuterium depleted water on the biological activity of the eukaryotic cells. J. Trace Elem. Med. Biol. 2018, 50, 629–633. [Google Scholar] [CrossRef]
- Basov, A.A.; Bykov, I.M.; Baryshev, M.G.; Dzhimak, S.S.; Bykov, M.I. Determination of deuterium concentration in foods and influence of water with modified isotopic composition on oxidation parameters and heavy hydrogen isotopes content in experimental animals. Vopr. Pitan. 2014, 83, 43–50. [Google Scholar]
- Yaglova, N.V.; Obernikhin, S.S.; Timokhina, E.P.; Yaglov, V.V. Response of Pituitary—Thyroid Axis to a Short-Term Shift in Deuterium Content in the Body. Bull. Exp. Biol. Med. 2021, 171, 262–264. [Google Scholar] [CrossRef]
- Dzhimak, S.S.; Basov, A.A.; Fedulova, L.V.; Naumov, G.N.; Baryshev, M.G. Correction of metabolic processes in rats during chronic endotoxicosis using isotope (D/H) exchange reactions. Biol. Bull. 2015, 42, 440–448. [Google Scholar] [CrossRef]
- Elkina, A.A.; Tumaev, E.N.; Basov, A.A.; Moiseev, A.V.; Malyshko, V.V.; Barisheva, E.V.; Churkina, A.V.; Dzhimak, S.S. The mechanisms of the interaction of stable isotopes with biological objects in the presence of an uncompensated neutron in chemical bonds. Biophysics 2020, 65, 883–888. [Google Scholar] [CrossRef]
- Schmidt, H.L.; Robins, R.J.; Werner, R.A. Multi-factorial in vivo stable isotope fractionation: Causes, correlations, consequences and applications. Isot. Environ. Health Stud. 2015, 51, 155–199. [Google Scholar] [CrossRef]
- Basov, A.A.; Kozin, S.V.; Bikov, I.M.; Popov, K.A.; Moiseev, A.V.; Elkina, A.A.; Dzhimak, S.S. Changes in prooxidant-antioxidant system indices in the blood and brain of rats with modelled acute hypoxia which consumed a deuterium-depleted drinking diet. Biol. Bull. 2019, 46, 531–535. [Google Scholar] [CrossRef]
- Xie, X.; Zubarev, R.A. Isotopic Resonance Hypothesis: Experimental Verification by Escherichia coli Growth Measurements. Sci. Rep. 2015, 5, 9215. [Google Scholar] [CrossRef] [PubMed]
- Kravtsov, A.; Kozin, S.; Basov, A.; Butina, E.; Baryshev, M.; Malyshko, V.; Moiseev, A.; Elkina, A.; Dzhimak, S. Reduction of deuterium level supports resistance of neurons to glucose deprivation and hypoxia: Study in cultures of neurons and on animals. Molecules 2022, 27, 243. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Liu, Y.; Wang, L.; Lu, F. Interaction between heavy water and single-strand DNA: A SERS study. Molecules 2022, 27, 6023. [Google Scholar] [CrossRef] [PubMed]
- Lisicin, A.B.; Barishev, M.G.; Basov, A.A.; Barisheva, E.V.; Bikov, I.M.; Didikin, A.S.; Tekutskaya, E.E.; Timakov, A.A.; Fedulova, L.V.; Chernuha, I.M.; et al. Influence of deuterium depleted water on the organism of laboratory animals in various functional conditions of nonspecific protective systems. Biophysics 2014, 59, 620–627. [Google Scholar] [CrossRef]
- Yakushevich, L.V. Nonlinear Physics of DNA; John Wiley & Sons: Hoboken, NJ, USA, 2007; p. 220. [Google Scholar] [CrossRef]
- Svidlov, A.A.; Drobotenko, M.I.; Basov, A.A.; Gerasimenko, E.O.; Elkina, A.A.; Baryshev, M.G.; Nechipurenko, Y.D.; Dzhimak, S.S. Influence of Environmental Parameters on the Stability of the DNA Molecule. Entropy 2021, 23, 1446. [Google Scholar] [CrossRef]
- Dzhimak, S.S.; Svidlov, A.A.; Elkina, A.A.; Gerasimenko, E.O.; Baryshev, M.G.; Drobotenko, M.I. Genesis of Open States Zones in a DNA Molecule Depends on the Localization and Value of the Torque. Int. J. Mol. Sci. 2022, 23, 4428. [Google Scholar] [CrossRef]
- Basov, A.A.; Drobotenko, M.I.; Svidlov, A.A.; Gerasimenko, E.O.; Malyshko, V.V.; Elkina, A.A.; Baryshev, M.G.; Dzhimak, S.S. Inequality in the Frequency of the Open States Occurrence Depends on Single 2H/1H Replacement in DNA. Molecules 2020, 25, 3753. [Google Scholar] [CrossRef]
- GeneCards: The Human Gene Database. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=IFNA17 (accessed on 19 October 2022).
- Yakushevich, L.V.; Krasnobaeva, L.A. Forced Oscillations of DNA Bases. Biophysics 2016, 61, 241–250. [Google Scholar] [CrossRef]
- Werner, R.M.; Stivers, J.T. Kinetic isotope effect studies of the reaction catalyzed by uracil DNA glycosylase: Evidence for an oxocarbenium ion-uracil anion intermediate. Biochemistry 2000, 39, 14054–14064. [Google Scholar] [CrossRef]
- Opitz, C.; Ahrné, E.; Goldie, K.N.; Schmidt, A.; Grzesiek, S. Deuterium induces a distinctive Escherichia coli proteome that correlates with the reduction in growth rate. J. Biol. Chem. 2019, 294, 2279–2292. [Google Scholar] [CrossRef] [PubMed]
- Galagedera, S.; Flechsig, G.U. Deuterium isotope effects upon the redox-switching of the viscosity of DNA layers observed by electrochemical quartz crystal micro-balance. Electroanalysis 2019, 31, 2074–2080. [Google Scholar] [CrossRef]
- Boros, L.G.; Somlyai, I.; Kovács, B.Z.; Puskás, L.G.; Nagy, L.I.; Dux, L.; Farkas, G.; Somlyai, G. Deuterium depletion inhibits cell proliferation, RNA and nuclear membrane turnover to enhance survival in pancreatic cancer. Cancer Control 2021, 28, 1073274821999655. [Google Scholar] [CrossRef] [PubMed]
- Basov, A.A.; Fedulova, L.V.; Baryshev, M.G.; Dzhimak, S.S. Deuterium-depleted water influence on the isotope 2H/1H regulation in body and individual adaptation. Nutrients 2019, 11, 1903. [Google Scholar] [CrossRef] [PubMed]
- Kalkur, R.S.; Ballast, A.C.; Triplett, A.R.; Spendier, K. Effects of deuterium oxide on cell growth and vesicle speed in RBL-2H3 cells. PeerJ 2014, 2, e553. [Google Scholar] [CrossRef]
- Englander, S.W.; Kallenbach, N.R. Hydrogen exchange and structural dynamics of proteins and nucleic acids. Q. Rev. Biophys. 1983, 16, 521–655. [Google Scholar] [CrossRef] [PubMed]
- Sviridova, D.A.; Smirnova, S.V.; Abilev, S.K. Deuterium Oxide Enhances Expression of the ada, alkA, and luxA Genes of Escherichia coli Induced by Methyl Methanesulfonate. Russ. J. Genet. 2022, 58, 481–484. [Google Scholar] [CrossRef]
- Ullah, S.; Ishimoto, T.; Williamson, M.P.; Hansen, P.E. Ab initio calculations of deuterium isotope effects on chemical shifts of salt-bridged lysines. J. Phys. Chem. B 2011, 115, 3208–3215. [Google Scholar] [CrossRef]
- Ignatov, I.; Mosin, O.; Velikov, B.; Bauer, E. Influence of isotopic composition of water with varrying deuterium content in composition with mountain water of bulgaria on human longevity. J. Med. Physiol. Biophys. 2014, 7, 46–78. [Google Scholar]
- Hohlefelder, L.S.; Stögbauer, T.; Opitz, M.; Bayerl, T.M.; Rädler, J.O. Heavy water reduces GFP expression in prokaryotic cell-free assays at the translation level while stimulating its transcription. BioMed Res. Int. 2013, 2013, 592745. [Google Scholar] [CrossRef]
- Ignatov, I.; Mosin, O.; Bauer, E. Mathematical model of melt water and mountain water from Bulgaria obtained by IR, NES and DNES-methods. J. Med. Physiol. Biophys. 2015, 17, 30–52. [Google Scholar]
- Basov, A.A.; Fedulova, L.V.; Vasilevskaya, E.R.; Dzhimak, S.S. Possible mechanisms of biological effects observed in living systems during 2H/1H isotope fractionation and deuterium interactions with other biogenic isotopes. Molecules 2019, 24, 4101. [Google Scholar] [CrossRef] [PubMed]
- Brini, E.; Fennell, C.J.; Fernandez-Serra, M.; Hribar-Lee, B.; Lukšič, M.; Dill, K.A. How water’s properties are encoded in its molecular structure and energies. Chem Rev. 2017, 117, 12385–12414. [Google Scholar] [CrossRef] [PubMed]
- Kleemann, J.; Reichenbach, G.; Zöller, N.; Jäger, M.; Kaufmann, R.; Meissner, M.; Kippenberger, S. Heavy water affects vital parameters of human melanoma cells in vitro. Cancer Manag. Res. 2020, 12, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.R.; Kim, N.H.; Jin, H.S.; Seo, Y.J.; Lee, J.; Lee, J.H. Base-pair opening dynamics of nucleic acids in relation to their biological function. Comput. Struct. Biotechnol. J. 2019, 17, 797–804. [Google Scholar] [CrossRef]
- Kannan, S.; Zacharias, M. Role of the closing base pair for d(GCA) hairpin stability: Free energy analysis and folding simulations. Nucleic Acids Res. 2011, 39, 8271–8280. [Google Scholar] [CrossRef]
- Bikard, D.; Loot, C.; Baharoglu, Z.; Mazel, D. Folded DNA in action: Hairpin formation and biological functions in prokaryotes. Microbiol. Mol. Biol. Rev. 2010, 74, 570–588. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, M.; Cranford, S. Ranking of molecular biomarker interaction with targeted DNA nucleobases via full atomistic molecular dynamics. Sci. Rep. 2016, 6, 18659. [Google Scholar] [CrossRef]
- Yamasaki, S.; Terada, T.; Shimizu, K.; Kono, H.; Sarai, A. A generalized conformational energy function of DNA derived from molecular dynamics simulations. Nucleic Acids Res. 2009, 37, e135. [Google Scholar] [CrossRef][Green Version]
- Brovarets, O.O.; Hovorun, D.M. Proton tunneling in the A·T Watson-Crick DNA base pair: Myth or reality? J. Biomol. Struct. Dyn. 2015, 33, 2716–2720. [Google Scholar] [CrossRef]
- Farthing, D.E.; Buxbaum, N.P.; Lucas, P.J.; Maglakelidze, N.; Oliver, B.; Wang, J.; Hu, K.; Castro, E.; Bare, C.V.; Gress, R.E. Comparing DNA enrichment of proliferating cells following administration of different stable isotopes of heavy water. Sci. Rep. 2017, 7, 4043. [Google Scholar] [CrossRef] [PubMed]
- Manalo, M.N.; Pérez, L.M.; LiWang, A. Hydrogen-bonding and pi-pi base-stacking interactions are coupled in DNA, as suggested by calculated and experimental trans-Hbond deuterium isotope shifts. J. Am. Chem. Soc. 2007, 129, 11298–11299. [Google Scholar] [CrossRef]
- Sicard, F.; Destainville, N.; Manghi, M. DNA denaturation bubbles: Free-energy landscape and nucleation/closure rates. J. Chem. Phys. 2015, 142, 034903. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, V.; Villa, A.; Hess, B. Sequence dependency of canonical base pair opening in the DNA double helix. PLoS Comput. Biol. 2017, 13, e1005463. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.G.; Bartolotti, L.; Li, L. Deuterium and its role in the machinery of evolution. J. Theor. Biol. 2006, 238, 914–918. [Google Scholar] [CrossRef] [PubMed]
- Priyakumar, U.D.; MacKerell, A.D., Jr. Computational approaches for investigating base flipping in oligonucleotides. Chem. Rev. 2006, 106, 489–505. [Google Scholar] [CrossRef]
- Grosjean, H. DNA and RNA Modification Enzymes: Structure, Mechanism, Function and Evolution, 1st ed.; CRC Press: Boca Raton, FL, USA, 2018; p. 682. [Google Scholar] [CrossRef]
- Dubois, A.; Francois, C.; Descamps, V.; Fournier, C.; Wychowski, C.; Dubuisson, J.; Castelain, S.; Duverlie, G. Enhanced anti-HCV activity of interferon alpha 17 subtype. Virology 2009, 6, 70. [Google Scholar] [CrossRef]
- GenBank: Homo Sapiens Interferon Alpha 17 (IFNA17), mRNA. Available online: http://www.ncbi.nlm.nih.gov/nuccore/NM_021268.2 (accessed on 20 October 2022).
- Ordu, O.; Lusser, A.; Dekker, N.H. Recent insights from in vitro single-molecule studies into nucleosome structure and dynamics. Biophys. Rev. 2016, 8, 33–49. [Google Scholar] [CrossRef]
- Noy, A.; Sutthibutpong, T.; Harris, S.A. Protein/DNA interactions in complex DNA topologies: Expect the unexpected. Biophys. Rev. 2016, 8, 233–243. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).