Dimethyl Fumarate Alleviates Adult Neurogenesis Disruption in Hippocampus and Olfactory Bulb and Spatial Cognitive Deficits Induced by Intracerebroventricular Streptozotocin Injection in Young and Aged Rats
Abstract
1. Introduction
2. Results
2.1. DMF-Containing Chow or Standard Chow Intake, Daily DMF Dose and Body Weight
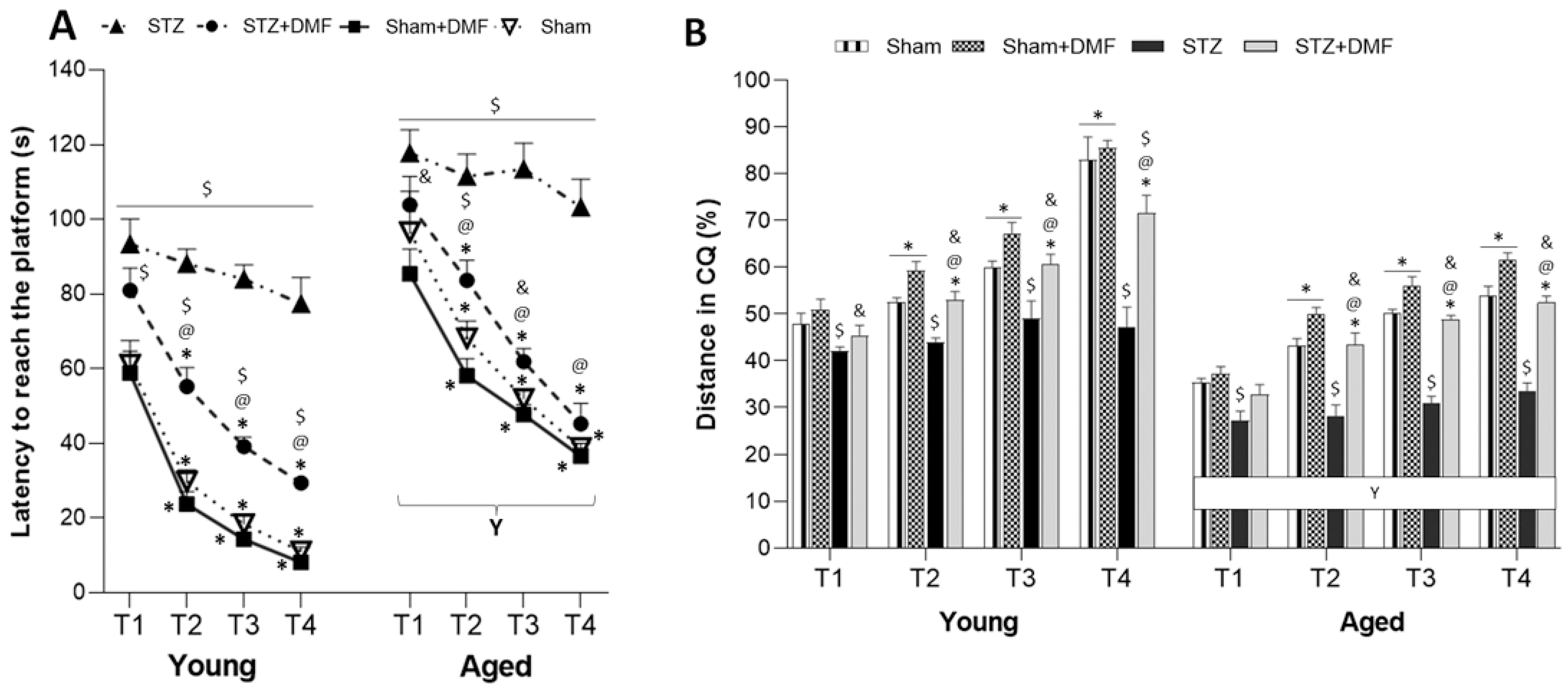
2.2. Spatial Learning and Memory—MWM Test
2.2.1. Spatial Learning—Acquisition of Long-Term Memory
2.2.2. Reference Memory Performance
2.2.3. Working Memory Performance
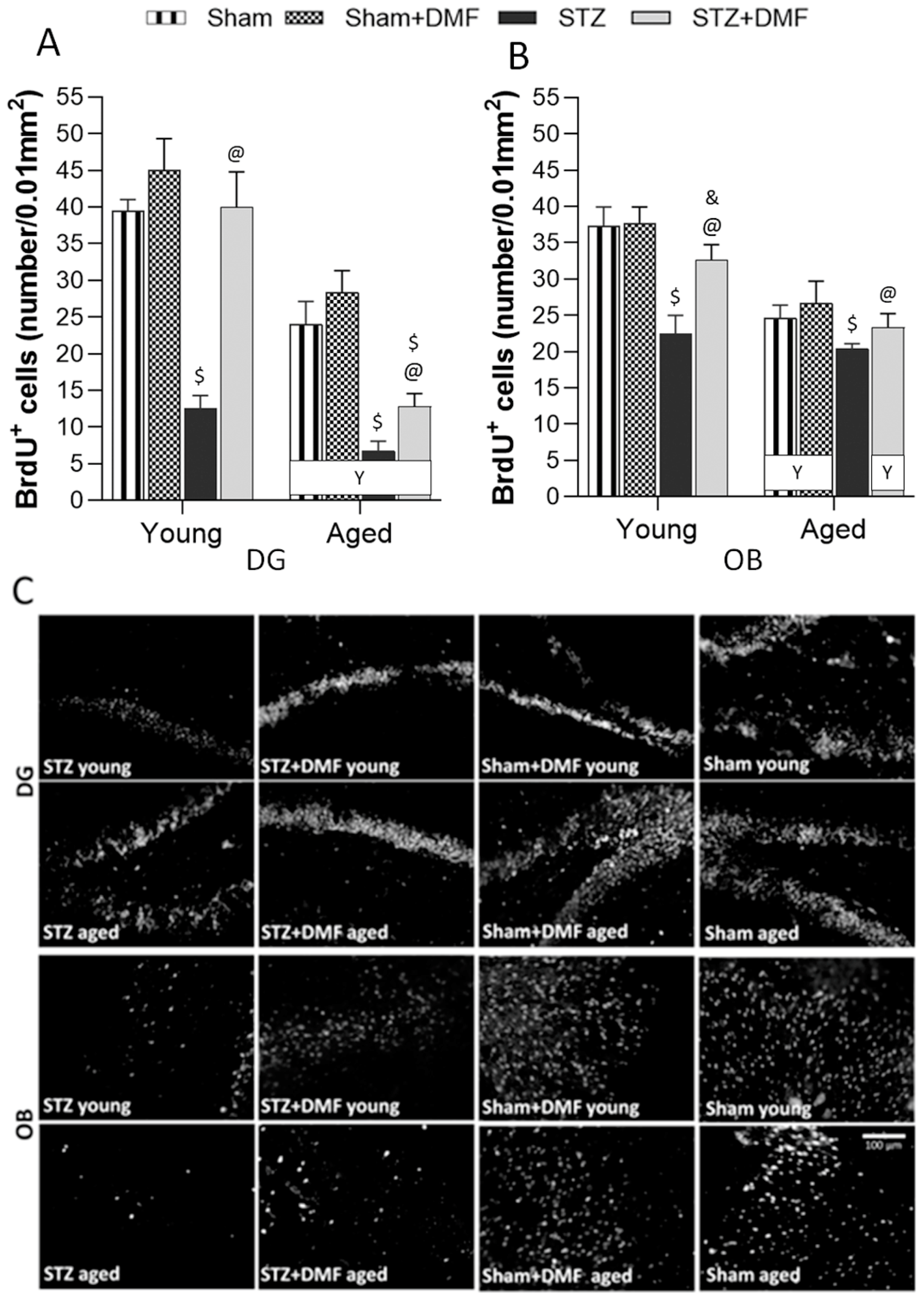
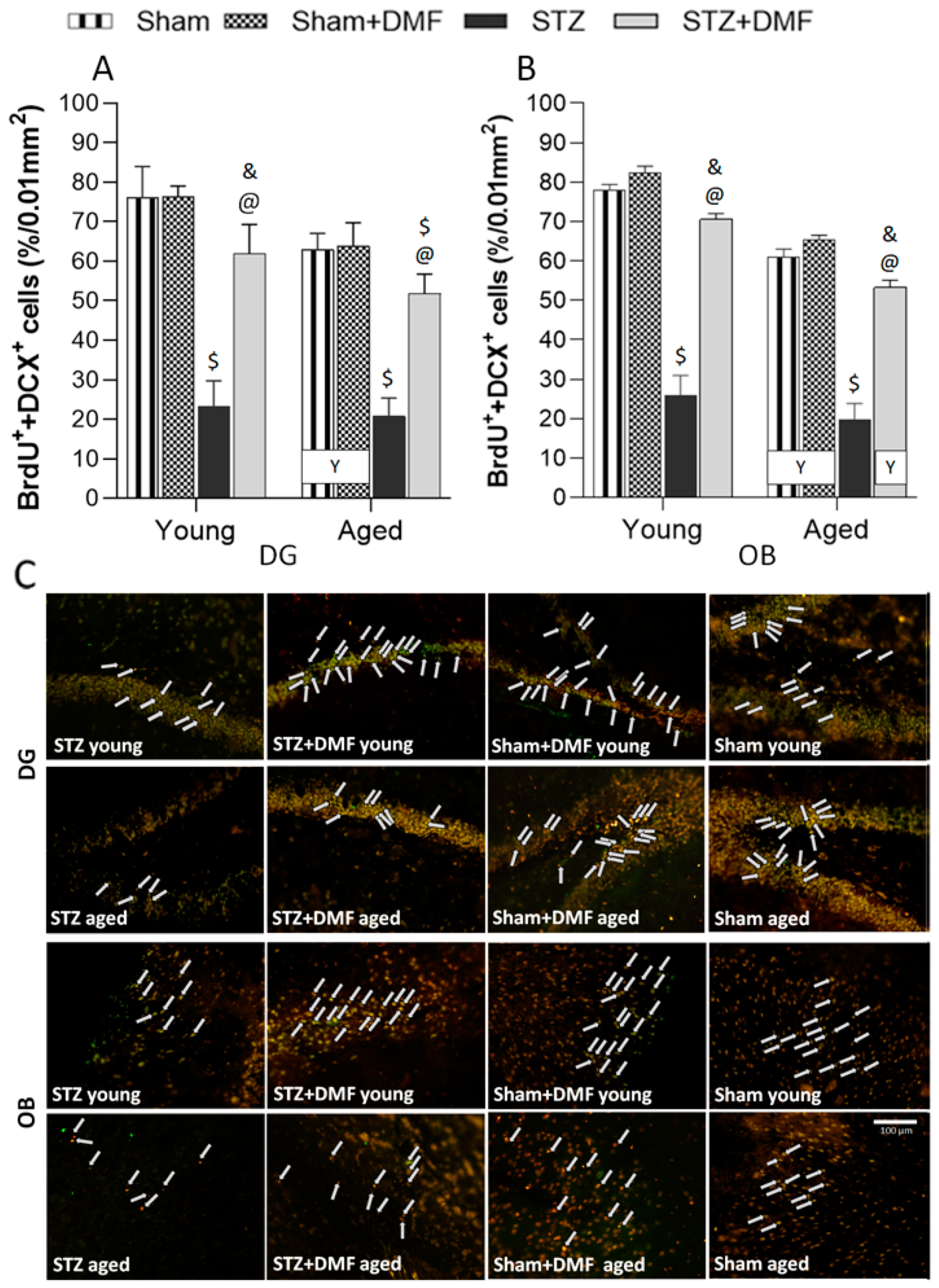
2.3. Adult Neurogenesis
2.3.1. Neural Cells Proliferation and Neuronal Differentiation
2.3.2. Level of the Newly Formed Immature Neurons
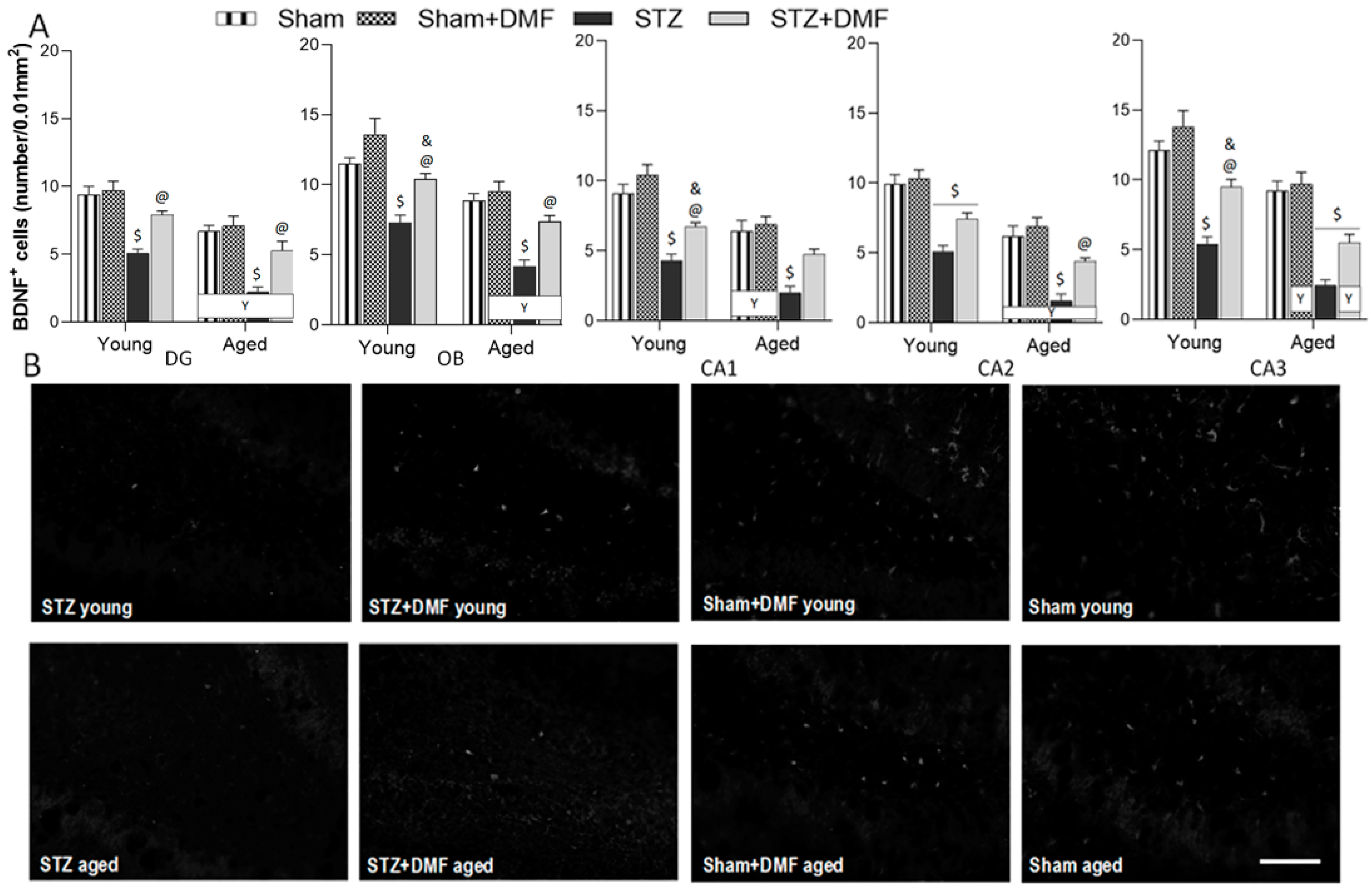
2.4. Neuroprotection—BDNF-Containing Cells
3. Discussion
4. Materials and Methods
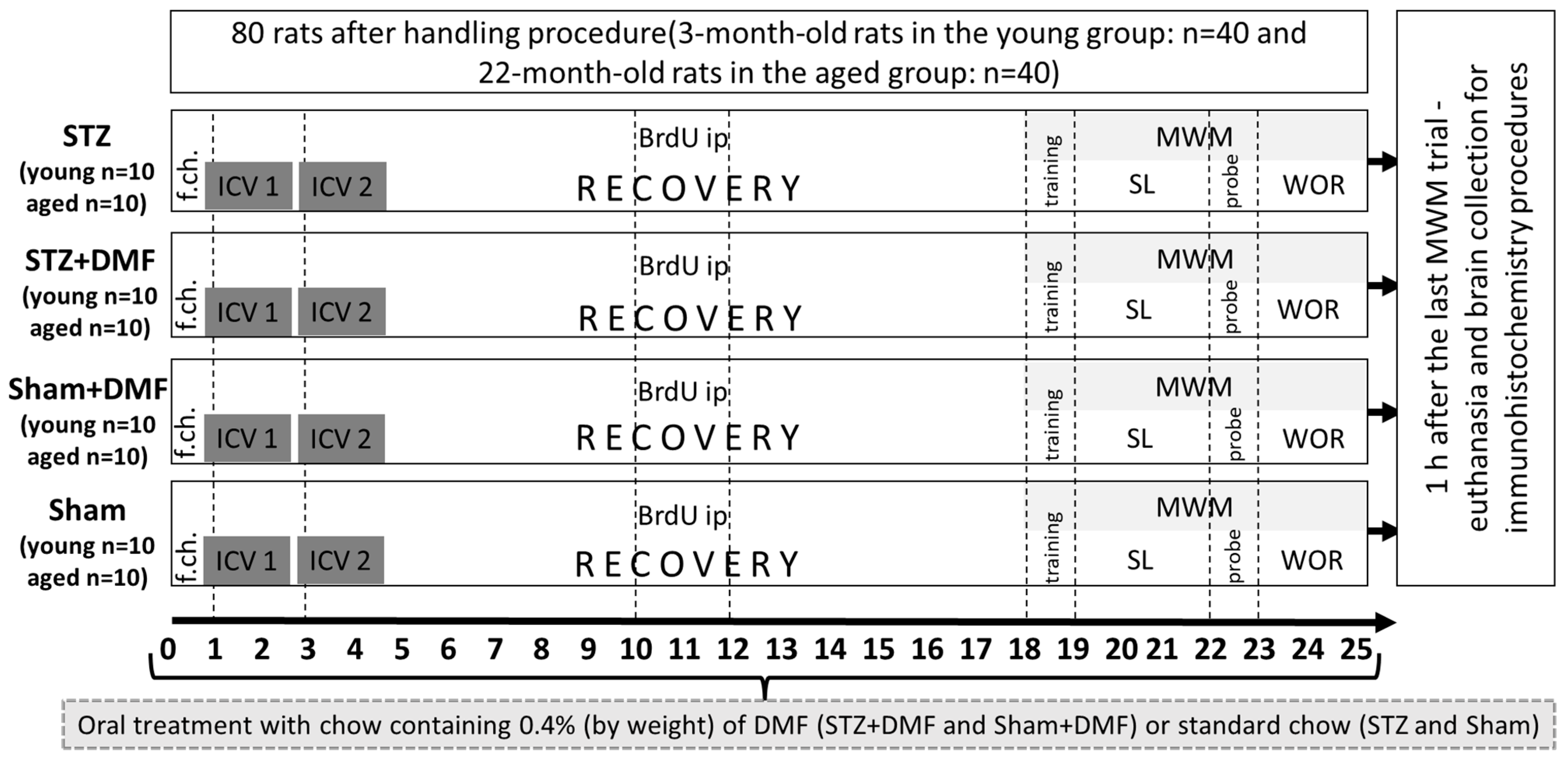
4.1. Animals and Experimental Approach
4.2. Dimethyl Fumarate Treatment
4.3. Intracerebroventricular Injection of STZ
4.4. 5-bromo-2′-Deoxyuridine (BrdU) Administration
4.5. Test of Spatial Memory in the Morris Water Maze (MWM)
4.6. Tissue Preparation
4.7. Immunohistochemistry
4.8. Quantification of Labelled Cells
4.9. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Aβ | amyloid beta protein |
| AD | Alzheimer’s disease |
| ANOVA | analysis of variance |
| BDNF | brain-derived neurotrophic factor |
| BrdU | 5-bromo-2′-deoxyuridine |
| CMI | mild cognitive impairment |
| CQ | critical quadrant (quadrant of the maze with the platform) in the Morris water maze |
| DCX | doublecortin |
| DG | dentate gyrus of the hippocampus |
| DMF | dimethyl fumarate |
| fAD | familial Alzheimer’s disease |
| GSK3β | Glycogen synthase kinase β |
| ICV | intracerebroventricular injection |
| ip | intraperitoneal injection |
| MWM | Morris water maze test |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| OB | olfactory bulb |
| PBS | phosphate-buffered saline |
| RRMS | relapsing–remitting multiple sclerosis |
| sAD | sporadic Alzheimer’s disease |
| SEM | standard error of the mean |
| SGZ | subgranular zone of the dentate gyrus |
| STZ | streptozotocin |
| SVZ | subventricular zone |
References
- Alves, S.S.; Silva-Junior, R.; Servilha-Menezes, G.; Homolak, J.; Salkovic-Petrisic, M.; Garcia-Cairasco, N. Insulin Resistance as a Common Link Between Current Alzheimer’s Disease Hypotheses. J. Alzheimers Dis. 2021, 82, 71–105. [Google Scholar] [CrossRef] [PubMed]
- Arvanitakis, Z.; Shah, R.C.; Bennett, D.A. Diagnosis and Management of Dementia: Review. JAMA 2019, 322, 1589–1599. [Google Scholar] [CrossRef] [PubMed]
- Karantzoulis, S.; Galvin, J.E. Distinguishing Alzheimer’s disease from other major forms of dementia. Expert Rev. Neurother. 2011, 11, 1579–1591. [Google Scholar] [CrossRef] [PubMed]
- Gazova, I.; Laczó, J.; Rubinova, E.; Mokrisova, I.; Hyncicova, E.; Andel, R.; Vyhnalek, M.; Sheardova, K.; Coulson, E.J.; Hort, J. Spatial navigation in young versus older adults. Front. Aging Neurosci. 2013, 5, 94. [Google Scholar] [CrossRef]
- Fu, H.; Rodriguez, G.A.; Herman, M.; Emrani, S.; Nahmani, E.; Barrett, G.; Figueroa, H.Y.; Goldberg, E.; Hussaini, S.A.; Duff, K.E. Tau Pathology Induces Excitatory Neuron Loss, Grid Cell Dysfunction, and Spatial Memory Deficits Reminiscent of Early Alzheimer’s Disease. Neuron 2017, 93, 533–541.e5. [Google Scholar] [CrossRef]
- Li Puma, D.D.; Piacentini, R.; Grassi, C. Does Impairment of Adult Neurogenesis Contribute to Pathophysiology of Alzheimer’s Disease? A Still Open Question. Front. Mol. Neurosci. 2021, 13, 578211. [Google Scholar] [CrossRef]
- Kuzumaki, N.; Ikegami, D.; Tamura, R.; Hareyama, N.; Imai, S.; Narita, M.; Torigoe, K.; Niikura, K.; Takeshima, H.; Ando, T.; et al. Hippocampal epigenetic modification at the brain-derived neurotrophic factor gene induced by an enriched environment. Hippocampus 2011, 21, 127–132. [Google Scholar] [CrossRef]
- Culig, L.; Chu, X.; Bohr, V.A. Neurogenesis in aging and age-related neurodegenerative diseases. Ageing Res. Rev. 2022, 78, 101636. [Google Scholar] [CrossRef]
- Takahashi, H.; Yoshihara, S.; Tsuboi, A. The Functional Role of Olfactory Bulb Granule Cell Subtypes Derived from Embryonic and Postnatal Neurogenesis. Front. Mol. Neurosci. 2018, 11, 229. [Google Scholar] [CrossRef]
- Devanand, D.P.; Lee, S.; Manly, J.; Andrews, H.; Schupf, N.; Doty, R.L.; Stern, Y.; Zahodne, L.B.; Louis, E.D.; Mayeux, R. Olfactory deficits predict cognitive decline and Alzheimer dementia in an urban community. Neurology 2015, 84, 182–189. [Google Scholar] [CrossRef]
- Numakawa, T.; Odaka, H. Brain-Derived Neurotrophic Factor Signaling in the Pathophysiology of Alzheimer’s Disease: Beneficial Effects of Flavonoids for Neuroprotection. Int. J. Mol. Sci. 2021, 22, 5719. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Jiménez, E.P.; Terreros-Roncal, J.; Flor-García, M.; Rábano, A.; Llorens-Martín, M. Evidences for Adult Hippocampal Neurogenesis in Humans. J. Neurosci. 2021, 41, 2541–2553. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Singh, S.; Shukla, S.; Shukla, R. Intracerebroventricular streptozotocin impairs adult neurogenesis and cognitive functions via regulating neuroinflammation and insulin signaling in adult rats. Neurochem. Int. 2018, 113, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.F.; Gu, S.; Shan, C.; Marchado, S.; Arias-Carrión, O. Oxidative Stress and Adult Neurogenesis. Stem Cell Rev. Rep. 2015, 11, 706–709. [Google Scholar] [CrossRef]
- Vaz, M.; Silva, V.; Monteiro, C.; Silvestre, S. Role of Aducanumab in the Treatment of Alzheimer’s Disease: Challenges and Opportunities. Clin. Interv. Aging 2022, 17, 797–810. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, C.; Mak, M.S.; Lu, J.; Wu, Z.; Chen, Q.; Han, Y.; Li, Y.; Pi, R. Advance of sporadic Alzheimer’s disease animal models. Med. Res. Rev. 2020, 40, 431–458. [Google Scholar] [CrossRef]
- Majkutewicz, I.; Kurowska, E.; Podlacha, M.; Myślińska, D.; Grembecka, B.; Ruciński, J.; Plucińska, K.; Jerzemowska, G.; Wrona, D. Dimethyl fumarate attenuates intracerebroventricular streptozotocin-induced spatial memory impairment and hippocampal neurodegeneration in rats. Behav. Brain Res. 2016, 308, 24–37. [Google Scholar] [CrossRef]
- Majkutewicz, I.; Kurowska, E.; Podlacha, M.; Myślińska, D.; Grembecka, B.; Ruciński, J.; Pierzynowska, K.; Wrona, D. Age-dependent effects of dimethyl fumarate on cognitive and neuropathological features in the streptozotocin-induced rat model of Alzheimer’s disease. Brain Res. 2018, 1686, 19–33. [Google Scholar] [CrossRef]
- Salkovic-Petrisic, M.; Knezovic, A.; Hoyer, S.; Riederer, P. What have we learned from the streptozotocin-induced animal model of sporadic Alzheimer’s disease, about the therapeuti.ic strategies in Alzheimer’s research. J. Neural Transm. (Vienna) 2013, 120, 233–252. [Google Scholar] [CrossRef]
- Montes Diaz, G.; Hupperts, R.; Fraussen, J.; Somers, V. Dimethyl fumarate treatment in multiple sclerosis: Recent advances in clinical and immunological studies. Autoimmun. Rev. 2018, 17, 1240–1250. [Google Scholar] [CrossRef]
- Piroli, G.G.; Manuel, A.M.; Patel, T.; Walla, M.D.; Shi, L.; Lanci, S.A.; Wang, J.; Galloway, A.; Ortinski, P.I.; Smith, D.S.; et al. Identification of Novel Protein Targets of Dimethyl Fumarate Modification in Neurons and Astrocytes Reveals Actions Independent of Nrf2 Stabilization. Mol. Cell Proteom. 2019, 18, 504–519. [Google Scholar] [CrossRef] [PubMed]
- Majkutewicz, I. Dimethyl fumarate: A review of preclinical efficacy in models of neurodegenerative diseases. Eur. J. Pharmacol. 2022, 926, 175025. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Casili, G.; Paterniti, I.; Cuzzocrea, S.; Esposito, E. Fumaric Acid Esters Attenuate Secondary Degeneration after Spinal Cord Injury. J. Neurotrauma 2017, 34, 3027–3040. [Google Scholar] [CrossRef]
- Pan, H.; Wang, Y.; Wang, X.; Yan, C. Dimethyl fumarate improves cognitive impairment by enhancing hippocampal brain-derived neurotrophic factor levels in hypothyroid rats. BMC Endocr. Disord. 2022, 22, 188. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Fatah, I.M.; Abdelrazek, H.; Ibrahim, S.M.; Abdallah, D.M.; El-Abhar, H.S. Dimethyl fumarate abridged tauo-/amyloidopathy in a D-Galactose/ovariectomy-induced Alzheimer’s-like disease: Modulation of AMPK/SIRT-1, AKT/CREB/BDNF, AKT/GSK-3β, adiponectin/Adipo1R, and NF-κB/IL-1β/ROS trajectories. Neurochem. Int. 2021, 148, 105082. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, Y.; Mao, Y.F.; Zheng, T.; Jiang, Y.; Yan, Y.; Yin, X.; Zhang, B. Long-term treatment with intranasal insulin ameliorates cognitive impairment, tau hyperphosphorylation, and microglial activation in a streptozotocin-induced Alzheimer’s rat model. Sci. Rep. 2017, 7, 45971. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Knezovic, A.; Parlak, M.; Cuber, J.; Karabeg, M.M.; Deckert, J.; Riederer, P.; Hua, Q.; Salkovic-Petrisic, M.; Schmitt, A.G. Long-Term Effects of Intracerebroventricular Streptozotocin Treatment on Adult Neurogenesis in the Rat Hippocampus. Curr. Alzheimer Res. 2015, 12, 772–784. [Google Scholar] [CrossRef] [PubMed]
- Knezovic, A.; Osmanovic-Barilar, J.; Curlin, M.; Hof, P.R.; Simic, G.; Riederer, P.; Salkovic-Petrisic, M. Staging of cognitive deficits and neuropathological and ultrastructural changes in streptozotocin-induced rat model of Alzheimer’s disease. J. Neural Transm. (Vienna) 2015, 122, 577–592. [Google Scholar] [CrossRef]
- Banik, A.; Brown, R.E.; Bamburg, J.; Lahiri, D.K.; Khurana, D.; Friedland, R.P.; Chen, W.; Ding, Y.; Mudher, A.; Padjen, A.L.; et al. Translation of Pre-Clinical Studies into Successful Clinical Trials for Alzheimer’s Disease: What are the Roadblocks and How Can They Be Overcome? J. Alzheimers Dis. 2015, 47, 815–843. [Google Scholar] [CrossRef]
- Bassani, T.B.; Turnes, J.M.; Moura, E.; Bonato, J.M.; Cóppola-Segovia, V.; Zanata, S.M.; Oliveira, R.; Vital, M. Effects of curcumin on short-term spatial and recognition memory, adult neurogenesis and neuroinflammation in a streptozotocin-induced rat model of dementia of Alzheimer’s type. Behav. Brain Res. 2017, 335, 41–54. [Google Scholar] [CrossRef]
- Bassani, T.B.; Bonato, J.M.; Machado, M.; Cóppola-Segovia, V.; Moura, E.; Zanata, S.M.; Oliveira, R.; Vital, M. Decrease in Adult Neurogenesis and Neuroinflammation Are Involved in Spatial Memory Impairment in the Streptozotocin-Induced Model of Sporadic Alzheimer’s Disease in Rats. Mol. Neurobiol. 2018, 55, 4280–4296. [Google Scholar] [CrossRef] [PubMed]
- Sasaki-Hamada, S.; Ikeda, M.; Oka, J.I. Glucagon-like peptide-2 rescues memory impairments and neuropathological changes in a mouse model of dementia induced by the intracerebroventricular administration of streptozotocin. Sci. Rep. 2019, 9, 13723. [Google Scholar] [CrossRef] [PubMed]
- Guardia de Souza E Silva, T.; do Val de Paulo, M.; da Silva, J.; da Silva Alves, A.; Britto, L.; Xavier, G.F.; Lopes Sandoval, M.R. Oral treatment with royal jelly improves memory and presents neuroprotective effects on icv-STZ rat model of sporadic Alzheimer’s disease. Heliyon 2020, 6, e03281. [Google Scholar] [CrossRef] [PubMed]
- Shoham, S.; Bejar, C.; Kovalev, E.; Schorer-Apelbaum, D.; Weinstock, M. Ladostigil prevents gliosis, oxidative-nitrative stress and memory deficits induced by intracerebroventricular injection of streptozotocin in rats. Neuropharmacology 2007, 52, 836–843. [Google Scholar] [CrossRef]
- Rostami, F.; Javan, M.; Moghimi, A.; Haddad-Mashadrizeh, A.; Fereidoni, M. Streptozotocin-induced hippocampal astrogliosis and insulin signaling malfunction as experimental scales for subclinical sporadic Alzheimer model. Life Sci. 2017, 188, 172–185. [Google Scholar] [CrossRef]
- Rojas-Gutierrez, E.; Muñoz-Arenas, G.; Treviño, S.; Espinosa, B.; Chavez, R.; Rojas, K.; Flores, G.; Díaz, A.; Guevara, J. Alzheimer’s disease and metabolic syndrome: A link from oxidative stress and inflammation to neurodegeneration. Synapse 2017, 71, e21990. [Google Scholar] [CrossRef]
- Curtis, M.A.; Faull, R.L.; Eriksson, P.S. The effect of neurodegenerative diseases on the subventricular zone. Nat. Rev. Neurosci. 2007, 8, 712–723. [Google Scholar] [CrossRef]
- Durante, M.A.; Kurtenbach, S.; Sargi, Z.B.; Harbour, J.W.; Choi, R.; Kurtenbach, S.; Goss, G.M.; Matsunami, H.; Goldstein, B.J. Single-cell analysis of olfactory neurogenesis and differentiation in adult humans. Nat. Neurosci. 2020, 23, 323–326. [Google Scholar] [CrossRef]
- Huang, J.S.; Kunkhyen, T.; Rangel, A.N.; Brechbill, T.R.; Gregory, J.D.; Winson-Bushby, E.D.; Liu, B.; Avon, J.T.; Muggleton, R.J.; Cheetham, C. Immature olfactory sensory neurons provide behaviourally relevant sensory input to the olfactory bulb. Nat. Commun. 2022, 13, 6194. [Google Scholar] [CrossRef]
- Mishra, S.K.; Hidau, M. Intranasal Insulin Enhances Intracerebroventricular Streptozotocin-Induced Decrease in Olfactory Discriminative Learning via Upregulation of Subventricular Zone-Olfactory Bulb Neurogenesis in the Rat Model. Mol. Neurobiol. 2021, 58, 1248–1259. [Google Scholar] [CrossRef]
- De Nuccio, C.; Bernardo, A.; Troiano, C.; Brignone, M.S.; Falchi, M.; Greco, A.; Rosini, M.; Basagni, F.; Lanni, C.; Serafini, M.M.; et al. NRF2 and PPAR-γ Pathways in Oligodendrocyte Progenitors: Focus on ROS Protection, Mitochondrial Biogenesis and Promotion of Cell Differentiation. Int. J. Mol. Sci. 2020, 21, 7216. [Google Scholar] [CrossRef] [PubMed]
- Rosito, M.; Testi, C.; Parisi, G.; Cortese, B.; Baiocco, P.; Di Angelantonio, S. Exploring the Use of Dimethyl Fumarate as Microglia Modulator for Neurodegenerative Diseases Treatment. Antioxidants 2020, 9, 700. [Google Scholar] [CrossRef] [PubMed]
- Scannevin, R.H.; Chollate, S.; Jung, M.Y.; Shackett, M.; Patel, H.; Bista, P.; Zeng, W.; Ryan, S.; Yamamoto, M.; Lukashev, M.; et al. Fumarates promote cytoprotection of central nervous system cells against oxidative stress via the nuclear factor (erythroid-derived 2)-like 2 pathway. J. Pharmacol. Exp. Ther. 2012, 341, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, A.; Kügler, S.; Lastres-Becker, I. Pharmacological targeting of GSK-3 and NRF2 provides neuroprotection in a preclinical model of tauopathy. Redox Biol. 2018, 14, 522–534. [Google Scholar] [CrossRef]
- Teixeira, C.M.; Pallas-Bazarra, N.; Bolós, M.; Terreros-Roncal, J.; Ávila, J.; Llorens-Martín, M. Untold New Beginnings: Adult Hippocampal Neurogenesis and Alzheimer’s Disease. J. Alzheimers Dis. 2018, 64, 497–505. [Google Scholar] [CrossRef]
- Pencea, V.; Bingaman, K.D.; Wiegand, S.J.; Luskin, M.B. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J. Neurosci. 2001, 21, 6706–6717. [Google Scholar] [CrossRef]
- Luo, H.; Xiang, Y.; Qu, X.; Liu, H.; Liu, C.; Li, G.; Han, L.; Qin, X. Apelin-13 Suppresses Neuroinflammation Against Cognitive Deficit in a Streptozotocin-Induced Rat Model of Alzheimer’s Disease Through Activation of BDNF-TrkB Signaling Pathway. Front. Pharmacol. 2019, 10, 395. [Google Scholar] [CrossRef]
- Santos, T.O.; Mazucanti, C.H.; Xavier, G.F.; Torrão, A.S. Early and late neurodegeneration and memory disruption after intracerebroventricular streptozotocin. Physiol. Behav. 2012, 107, 401–413. [Google Scholar] [CrossRef]
- Reeta, K.H.; Singh, D.; Gupta, Y.K. Chronic treatment with taurine after intracerebroventricular streptozotocin injection improves cognitive dysfunction in rats by modulating oxidative stress, cholinergic functions and neuroinflammation. Neurochem. Int. 2017, 108, 146–156. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Ismael, S.; Mirzahosseini, G.; Ishrat, T. Verapamil Prevents Development of Cognitive Impairment in an Aged Mouse Model of Sporadic Alzheimer’s Disease. Mol. Neurobiol. 2021, 58, 3374–3387. [Google Scholar] [CrossRef]
- Liu, X.; Song, W.; Yu, Y.; Su, J.; Shi, X.; Yang, X.; Wang, H.; Liu, P.; Zou, L. Inhibition of NLRP1-Dependent Pyroptosis Prevents Glycogen Synthase Kinase-3β Overactivation-Induced Hyperphosphorylated Tau in Rats. Neurotox Res. 2022, 40, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, G.; Laczó, J.; Hort, J.; Minihane, A.M.; Hornberger, M. Spatial navigation deficits—overlooked cognitive marker for preclinical Alzheimer disease? Nat. Rev. Neurol. 2018, 14, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Padurariu, M.; Ciobica, A.; Mavroudis, I.; Fotiou, D.; Baloyannis, S. Hippocampal neuronal loss in the CA1 and CA3 areas of Alzheimer’s disease patients. Psychiatr. Danub. 2012, 24, 152–158. [Google Scholar] [PubMed]
- Serino, S.; Morganti, F.; Di Stefano, F.; Riva, G. Detecting early egocentric and allocentric impairments deficits in Alzheimer’s disease: An experimental study with virtual reality. Front. Aging Neurosci. 2015, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.L.; Fagan, A.M.; Morris, J.C.; Head, D. Spatial Navigation in Preclinical Alzheimer’s Disease. J. Alzheimers Dis. 2016, 52, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Tangen, G.G.; Engedal, K.; Bergland, A.; Moger, T.A.; Hansson, O.; Mengshoel, A.M. Spatial navigation measured by the Floor Maze Test in patients with subjective cognitive impairment, mild cognitive impairment, and mild Alzheimer’s disease. Int. Psychogeriatr. 2015, 27, 1401–1409. [Google Scholar] [CrossRef]
- Laczó, J.; Markova, H.; Lobellova, V.; Gazova, I.; Parizkova, M.; Cerman, J.; Nekovarova, T.; Vales, K.; Klovrzova, S.; Harrison, J.; et al. Scopolamine disrupts place navigation in rats and humans: A translational validation of the Hidden Goal Task in the Morris water maze and a real maze for humans. Psychopharmacology 2017, 234, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Rojo, A.I.; Pajares, M.; García-Yagüe, A.J.; Buendia, I.; Van Leuven, F.; Yamamoto, M.; López, M.G.; Cuadrado, A. Deficiency in the transcription factor NRF2 worsens inflammatory parameters in a mouse model with combined tauopathy and amyloidopathy. Redox Biol. 2018, 18, 173–180. [Google Scholar] [CrossRef]
- Sun, X.; Suo, X.; Xia, X.; Yu, C.; Dou, Y. Dimethyl Fumarate is a Potential Therapeutic Option for Alzheimer’s Disease. J. Alzheimers Dis. 2022, 85, 443–456. [Google Scholar] [CrossRef]
- Möhle, L.; Brackhan, M.; Bascuñana, P.; Pahnke, J. Dimethyl fumarate does not mitigate cognitive decline and β-amyloidosis in female APPPS1 mice. Brain Res. 2021, 1768, 147579. [Google Scholar] [CrossRef]
- Huber, J.D.; VanGilder, R.L.; Houser, K.A. Streptozotocin-induced diabetes progressively increases blood-brain barrier permeability in specific brain regions in rats. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, 2660–2668. [Google Scholar] [CrossRef] [PubMed]
- Janota, C.S.; Brites, D.; Lemere, C.A.; Brito, M.A. Glio-vascular changes during ageing in wild-type and Alzheimer’s disease-like APP/PS1 mice. Brain Res. 2015, 1620, 153–168. [Google Scholar] [CrossRef][Green Version]
- Minogue, A.M.; Jones, R.S.; Kelly, R.J.; McDonald, C.L.; Connor, T.J.; Lynch, M.A. Age-associated dysregulation of microglial activation is coupled with enhanced blood-brain barrier permeability and pathology in APP/PS1 mice. Neurobiol. Aging 2014, 35, 1442–1452. [Google Scholar] [CrossRef] [PubMed]
- Bilkei-Gorzo, A. Genetic mouse models of brain a.ageing and Alzheimer’s disease. Pharmacol. Ther. 2014, 142, 244–257. [Google Scholar] [CrossRef] [PubMed]
- Villemagne, V.L.; Pike, K.E.; Chételat, G.; Ellis, K.A.; Mulligan, R.S.; Bourgeat, P.; Ackermann, U.; Jones, G.; Szoeke, C.; Salvado, O.; et al. Longitudinal assessment of Aβ and cognition in aging and Alzheimer disease. Ann. Neurol. 2011, 69, 181–192. [Google Scholar] [CrossRef]
- Jagust, W. Imaging the evolution and pathophysiology of Alzheimer disease. Nat. Rev. Neurosci. 2018, 19, 687–700. [Google Scholar] [CrossRef]
- Lester, A.W.; Moffat, S.D.; Wiener, J.M.; Barnes, C.A.; Wolbers, T. The Aging Navigational System. Neuron 2017, 95, 1019–1035. [Google Scholar] [CrossRef]
- Plácido, J.; de Almeida, C.; Ferreira, J.V.; de Oliveira Silva, F.; Monteiro-Junior, R.S.; Tangen, G.G.; Laks, J.; Deslandes, A.C. Spatial navigation in older adults with mild cognitive impairment and dementia: A systematic review and meta-analysis. Exp. Gerontol. 2022, 165, 111852. [Google Scholar] [CrossRef]
- Kuhn, H.G.; Dickinson-Anson, H.; Gage, F.H. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J. Neurosci. 1996, 16, 2027–2033. [Google Scholar] [CrossRef]
- Luo, J.; Daniels, S.B.; Lennington, J.B.; Notti, R.Q.; Conover, J.C. The aging neurogenic subventricular zone. Aging Cell 2006, 5, 139–152. [Google Scholar] [CrossRef]
- Lugert, S.; Basak, O.; Knuckles, P.; Haussler, U.; Fabel, K.; Götz, M.; Haas, C.A.; Kempermann, G.; Taylor, V.; Giachino, C. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell 2010, 6, 445–456. [Google Scholar] [CrossRef]
- Kempermann, G. The neurogenic reserve hypothesis: What is adult hippocampal neurogenesis good for? Trends Neurosci. 2008, 31, 163–169. [Google Scholar] [CrossRef]
- Hattiangady, B.; Shetty, A.K. Implications of decreased hippocampal neurogenesis in chronic temporal lobe epilepsy. Epilepsia 2008, 49, 26–41. [Google Scholar] [CrossRef]
- von Bohlen und Halbach, O. Involvement of BDNF in age-dependent alterations in the hippocampus. Front. Aging Neurosci. 2010, 2, 36. [Google Scholar] [CrossRef]
- Dong, B.E.; Chen, H.; Sakata, K. BDNF deficiency and enriched environment treatment affect neurotransmitter gene expression differently across ages. J. Neurochem. 2020, 154, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Solano Fonseca, R.; Mahesula, S.; Apple, D.M.; Raghunathan, R.; Dugan, A.; Cardona, A.; O’Connor, J.; Kokovay, E. Neurogenic Niche Microglia Undergo Positional Remodeling and Progressive Activation Contributing to Age-Associated Reductions in Neurogenesis. Stem Cells Dev. 2016, 25, 542–555. [Google Scholar] [CrossRef] [PubMed]
- Salkovic-Petrisic, M.; Osmanovic-Barilar, J.; Brückner, M.K.; Hoyer, S.; Arendt, T.; Riederer, P. Cerebral amyloid angiopathy in streptozotocin rat model of sporadic Alzheimer’s disease: A long-term follow up study. J. Neural Transm. 2011, 118, 765–772. [Google Scholar] [CrossRef]
- Spencer, S.R.; Wilczak, C.A.; Talalay, P. Induction of glutathione transferases and NAD(P)H:quinone reductase by fumaric acid derivatives in rodent cells and tissues. Cancer Res. 1990, 50, 7871–7875. [Google Scholar] [PubMed]
- Kuhn, H.G.; Cooper-Kuhn, C.M. Bromodeoxyuridine and the detection of neurogenesis. Curr. Pharm. Biotechnol. 2007, 8, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Aimone, J.B.; Li, Y.; Lee, S.W.; Clemenson, G.D.; Deng, W.; Gage, F.H. Regulation and function of adult neurogenesis: From genes to cognition. Physiol. Rev. 2014, 94, 991–1026. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, H.S. Adult Neurogenesis and the Promise of Adult Neural Stem Cells. J. Exp. Neurosci. 2019, 13, 1179069519856876. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: San Diego, CA, USA, 2007. [Google Scholar]



| Experimental and Age Group | STZ+DMF Young | Sham+DMF Young | STZ+DMF Aged | Sham+DMF Aged |
|---|---|---|---|---|
| Parameter | ||||
| Initial body weight (g) 1 | 337.30 ± 9.91 | 346.70 ± 8.93 | 505.80 ± 8.33 | 548.10 ± 15.83 |
| Final body weight (g) 2 | 319.90 ± 10.86 | 344.50 ± 10.15 | 465.00 ± 8.16 | 503.00 ± 15.68 |
| Daily chow intake (g) 3 | 3.21 ± 0.29 | 3.52 ± 0.25 | 5.74 ± 0.60 | 5.80 ± 0.40 |
| Daily DMF intake (mg) 4 | 12.83 ± 1.14 | 14.10 ± 1.00 | 22.98 ± 2.38 | 23.18 ± 1.59 |
| Daily DMF dose (mg/kg) 5 | 40.14 ± 3.51 | 41.02 ± 2.80 | 49.36 ± 5.06 | 46.63 ± 3.68 |
| Changes in body weight (%) 6 | 5.23 ± 0.88 | 0.65 ± 1.26 | 8.06 ± 0.73 | 8.28 ± 0.51 |
| Experimental and Age Group | STZ Young | Sham Young | STZ Aged | Sham Aged |
| Parameter | ||||
| Initial body weight (g) 1 | 356.20 ± 7.80 | 341.00 ± 6.93 | 521.60 ± 8.05 | 539.40 ± 13.15 |
| Final body weight (g) 2 | 329.60 ± 11.08 | 332.30 ± 11.26 | 509.10 ± 8.12 | 515.70 ± 7.50 |
| Daily chow intake (g) 3 | 5.50 ± 0.68 | 6.53 ± 1.00 | 5.82 ± 0.57 | 6.15 ± 0.54 |
| Changes in body weight (%) 6 | 5.28 ± 0.82 | 4.77 ± 0.61 | 4.53 ± 0.89 | 3.27 ± 0.83 |
| Factors | df | Latency to Reach the Platform (s) | % of Distance in CQ |
|---|---|---|---|
| Spatial Learning (sessions 1–3) | |||
| (1) sAD-like model (STZ_Sham) | 1, 216 | 194.83 # | 123.83 # |
| (2) Therapy (DMF_Stand) | 1, 216 | 64.44 & | 78.42 & |
| (3) Age (Young_Aged) | 1, 216 | 88.82 & | 54.59 & |
| (4) Day (1_2_3) | 2, 216 | 49.31 & | 15.34 & |
| Interactions: | |||
| sAD-like model × Therapy | 45.68 & | 82.40 & | |
| sAD-like model × Age | 8.96 * | 4.61 * | |
| Therapy × Age | 7.61 * | 0.06 | |
| sAD-like model × Day | 3.39 * | 1.22 | |
| Therapy × Day | 4.58 * | 0.33 | |
| Age × Day | 4.02 * | 5.90 * | |
| 1 × 2 × 3 | 6.18 * | 0.80 | |
| 1 × 2 × 4 | 3.98 * | 3.63 * | |
| 1 × 3 × 4 | 2.16 | 3.81 * | |
| 2 × 3 × 4 | 3.42 * | 0.09 | |
| 1 × 2 × 3 × 4 | 1.34 | 0.72 |
| Factors | df | Latency to Reach the CQ (s) | % of Distance in CQ | Number of Goal Crossings |
|---|---|---|---|---|
| Reference Memory (session 4—probe test) | ||||
| (1) sAD-like model (STZ_Sham) | 1, 72 | 86.84 # | 65.41 # | 18.42 & |
| (2) Therapy (DMF_Stand) | 1, 72 | 16.68 & | 34.23 & | 5.52 * |
| (3) Age (Young_Aged) | 1, 72 | 28.49 & | 51.99 # | 4.42 * |
| Interactions: | ||||
| sAD-like model × Therapy | 11.26 * | 18.86 & | 6.13 * | |
| sAD-like model × Age | 4.23 * | 9.04 * | 1.19 | |
| Therapy × Age | 4.89 * | 8.38 * | 0.48 | |
| 1 × 2 × 3 | 2.12 | 4.68 * | 0.41 |
| Factors | df | Latency to Reach the Platform (s) | % of Distance in CQ |
|---|---|---|---|
| Working Memory (sessions 5–7) | |||
| (1) sAD-like model (STZ_Sham) | 1, 288 | 154.56 # | 198.98 # |
| (2) Therapy (DMF_Stand) | 1, 288 | 159.27 # | 142.75 # |
| (3) Age (Young_Aged) | 1, 288 | 182.03 # | 162.89 # |
| (4) Trial (1_2_3_4) | 3, 288 | 81.46 & | 136.11 # |
| Interactions: | |||
| sAD-like model × Therapy | 48.39 & | 27.24 & | |
| sAD-like model × Age | 16.16 * | 0.74 | |
| Therapy × Age | 8.41 * | 9.10 * | |
| sAD-like model × Trial | 17.84 * | 14.71 * | |
| Therapy × Trial | 8.15 * | 7.39 * | |
| Age × Trial | 7.45 * | 8.93 * | |
| 1 × 2 × 3 | 0.01 | 2.68 | |
| 1 × 2 × 4 | 8.80 * | 4.16 * | |
| 1 × 3 × 4 | 0.59 | 4.58 * | |
| 2 × 3 × 4 | 1.32 | 2.06 | |
| 1 × 2 × 3 × 4 | 2.36 | 1.84 |
| Factors | df | Number of BrdU+ Cells | Number of DCX+ Cells | % of Newly Formed Immature Neurons (BrdU+DCX) |
|---|---|---|---|---|
| Neurogenesis in the DG | ||||
| (1) sAD-like model (STZ_Sham) | 1, 72 | 29.41 # | 33.11 # | 45.04 # |
| (2) Therapy (DMF_Stand) | 1, 72 | 13.15 & | 12.16 & | 15.30 & |
| (3) Age (Young_Aged) | 1, 72 | 29.58 # | 20.68 # | 24.49 # |
| Interactions: | ||||
| sAD-like model × Therapy | 9.09 & | 16.00 & | 14.29 & | |
| sAD-like model × Age | 0.05 | 0.79 | 5.23 * | |
| Therapy × Age | 5.85 * | 7.13 * | 1.41 | |
| 1 × 2 × 3 | 2.84 | 1.52 | 2.11 | |
| Neurogenesis in the OB | ||||
| (1) sAD-like model (STZ_Sham) | 1, 72 | 37.01 # | 79.55 # | 49.24 # |
| (2) Therapy (DMF_Stand) | 1, 72 | 4.96 * | 58.78 # | 21.16 # |
| (3) Age (Young_Aged) | 1, 72 | 22.16 # | 30.90 # | 11.52 & |
| Interactions: | ||||
| sAD-like model × Therapy | 9.35 * | 17.28 & | 21.79 & | |
| sAD-like model × Age | 11.95 & | 10.07 & | 3.93 | |
| Therapy × Age | 7.52 * | 7.37 * | 0.23 | |
| 1 × 2 × 3 | 2.53 | 3.36 | 3.79 |
| FACTORS | df | CA1 | CA2 | CA3 | DG | OB |
|---|---|---|---|---|---|---|
| Neuroprotection (BDNF-containing cells) | ||||||
| (1) sAD-like model (STZ_Sham) | 1, 69 | 45.47 # | 43.36 # | 57.60 # | 36.14 # | 34.41 # |
| (2) Therapy (DMF_Stand) | 1, 69 | 19.01 & | 11.07 * | 5.87 * | 11.77 * | 4.16 * |
| (3) Age (Young_Aged) | 1, 69 | 22.56 # | 35.44 # | 23.54 & | 26.48 & | 27.70 & |
| Interactions: | ||||||
| sAD-like model × Therapy | 4.55 * | 8.76 * | 10.92 * | 10.07 * | 14.36 & | |
| sAD-like model × Age | 1.31 | 0.11 | 0.01 | 0.02 | 0.09 | |
| Therapy × Age | 0.12 | 0.99 | 0.01 | 0.01 | 0.77 | |
| 1 × 2 × 3 | 0.42 | 0.13 | 1.20 | 0.05 | 0.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurowska-Rucińska, E.; Ruciński, J.; Myślińska, D.; Grembecka, B.; Wrona, D.; Majkutewicz, I. Dimethyl Fumarate Alleviates Adult Neurogenesis Disruption in Hippocampus and Olfactory Bulb and Spatial Cognitive Deficits Induced by Intracerebroventricular Streptozotocin Injection in Young and Aged Rats. Int. J. Mol. Sci. 2022, 23, 15449. https://doi.org/10.3390/ijms232415449
Kurowska-Rucińska E, Ruciński J, Myślińska D, Grembecka B, Wrona D, Majkutewicz I. Dimethyl Fumarate Alleviates Adult Neurogenesis Disruption in Hippocampus and Olfactory Bulb and Spatial Cognitive Deficits Induced by Intracerebroventricular Streptozotocin Injection in Young and Aged Rats. International Journal of Molecular Sciences. 2022; 23(24):15449. https://doi.org/10.3390/ijms232415449
Chicago/Turabian StyleKurowska-Rucińska, Ewelina, Jan Ruciński, Dorota Myślińska, Beata Grembecka, Danuta Wrona, and Irena Majkutewicz. 2022. "Dimethyl Fumarate Alleviates Adult Neurogenesis Disruption in Hippocampus and Olfactory Bulb and Spatial Cognitive Deficits Induced by Intracerebroventricular Streptozotocin Injection in Young and Aged Rats" International Journal of Molecular Sciences 23, no. 24: 15449. https://doi.org/10.3390/ijms232415449
APA StyleKurowska-Rucińska, E., Ruciński, J., Myślińska, D., Grembecka, B., Wrona, D., & Majkutewicz, I. (2022). Dimethyl Fumarate Alleviates Adult Neurogenesis Disruption in Hippocampus and Olfactory Bulb and Spatial Cognitive Deficits Induced by Intracerebroventricular Streptozotocin Injection in Young and Aged Rats. International Journal of Molecular Sciences, 23(24), 15449. https://doi.org/10.3390/ijms232415449






