The CCN2 Polymorphism rs12526196 Is a Risk Factor for Ascending Thoracic Aortic Aneurysm
Abstract
:1. Introduction
2. Results
2.1. Genotype and Allele Frequencies from rs6918698, rs9402373 and rs12526196 Polymorphisms Do Not Differ in Thoracic Aortic Aneurism Patients
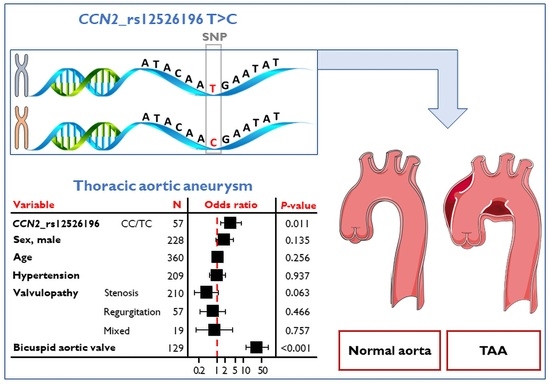
2.2. rs12526196 SNP Is Associated with Thoracic Aortic Aneurism Independently of Risk Factors
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Study Population
5.2. Genotyping
5.3. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leask, A.; Abraham, D.J. All in the CCN family: Essential matricellular signaling modulators emerge from the bunker. J. Cell Sci. 2006, 119, 4803–4810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perbal, B.; Tweedie, S.; Bruford, E. The official unified nomenclature adopted by the HGNC calls for the use of the acronyms, CCN1–6, and discontinuation in the use of CYR61, CTGF, NOV and WISP 1–3 respectively. J. Cell Commun. Signal. 2018, 12, 625–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Ortega, M.; Rodriguez-Vita, J.; Sanchez-Lopez, E.; Carvajal, G.; Egido, J. TGF-β signaling in vascular fibrosis. Cardiovasc. Res. 2007, 74, 196–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Vita, J.; Sánchez-López, E.; Esteban, V.; Rupérez, M.; Egido, J.; Ruiz-Ortega, M. Angiotensin II Activates the Smad Pathway in Vascular Smooth Muscle Cells by a Transforming Growth Factor-β–Independent Mechanism. Circulation 2005, 111, 2509–2517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rupérez, M.; Lorenzo, O.; Blanco-Colio, L.M.; Esteban, V.; Egido, J.; Ruiz-Ortega, M. Connective Tissue Growth Factor Is a Mediator of Angiotensin II–Induced Fibrosis. Circulation 2003, 108, 1499–1505. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues-Diez, R.R.; Garcia-Redondo, A.B.; Orejudo, M.; Rodrigues-Diez, R.; Briones, A.M.; Bosch-Panadero, E.; Kery, G.; Pato, J.; Ortiz, A.; Salaices, M.; et al. The C-Terminal Module IV of Connective Tissue Growth Factor, Through EGFR/Nox1 Signaling, Activates the NF-κB Pathway and Proinflammatory Factors in Vascular Smooth Muscle Cells. Antioxid. Redox Signal. 2015, 22, 29–47. [Google Scholar] [CrossRef] [Green Version]
- Ponticos, M. Connective tissue growth factor (CCN2) in blood vessels. Vasc. Pharmacol. 2013, 58, 189–193. [Google Scholar] [CrossRef]
- Ivkovic, S.; Yoon, B.S.; Popoff, S.N.; Safadi, F.F.; Libuda, D.E.; Stephenson, R.C.; Daluiski, A.; Lyons, K. Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development 2003, 130, 2779–2791. [Google Scholar] [CrossRef] [Green Version]
- Meng, Y.; Tian, C.; Liu, L.; Wang, L.; Chang, Q. Elevated expression of connective tissue growth factor, osteopontin and increased collagen content in human ascending thoracic aortic aneurysms. Vascular 2013, 22, 20–27. [Google Scholar] [CrossRef]
- Rodrigues-Díez, R.R.; Tejera-Muñoz, A.; Esteban, V.; Steffensen, L.B.; Rodrigues-Díez, R.; Orejudo, M.; Rayego-Mateos, S.; Falke, L.L.; Cannata-Ortiz, P.; Ortiz, A.; et al. CCN2 (Cellular Communication Network Factor 2) Deletion Alters Vascular Integrity and Function Predisposing to Aneurysm Formation. Hypertension 2022, 79, E42–E55. [Google Scholar] [CrossRef]
- Ostberg, N.; Zafar, M.; Ziganshin, B.; Elefteriades, J. The Genetics of Thoracic Aortic Aneurysms and Dissection: A Clinical Perspective. Biomolecules 2020, 10, 182. [Google Scholar] [CrossRef] [Green Version]
- Pinard, A.; Jones, G.T.; Milewicz, D.M. Genetics of Thoracic and Abdominal Aortic Diseases. Circ. Res. 2019, 124, 588–606. [Google Scholar] [CrossRef]
- Ogata, T.; Shibamura, H.; Tromp, G.; Sinha, M.; Goddard, K.A.; Sakalihasan, N.; Limet, R.; MacKean, G.L.; Arthur, C.; Sueda, T.; et al. Genetic analysis of polymorphisms in biologically relevant candidate genes in patients with abdominal aortic aneurysms. J. Vasc. Surg. 2005, 41, 1036–1042. [Google Scholar] [CrossRef] [Green Version]
- Jabłońska, A.; Zagrapan, B.; Neumayer, C.; Eilenberg, W.; Scheuba, A.; Brostjan, C.; Demyanets, S.; Klinger, M.; Nanobachvili, J.; Huk, I. Polymorphisms in the IL-6 and TNF-α gene are associated with an increased risk of abdominal aortic aneurysm. Int. J. Cardiol. 2020, 329, 192–197. [Google Scholar] [CrossRef]
- Dessein, A.; Chevillard, C.; Arnaud, V.; Hou, X.; Hamdoun, A.A.; Dessein, H.; He, H.; Abdelmaboud, S.A.; Luo, X.; Li, J.; et al. Variants of CTGF are associated with hepatic fibrosis in Chinese, Sudanese, and Brazilians infected with Schistosomes. J. Exp. Med. 2009, 206, 2321–2328. [Google Scholar] [CrossRef]
- Ahmad, A.; Askari, S.; Befekadu, R.; Hahn-Strömberg, V. Investigating the association between polymorphisms in connective tissue growth factor and susceptibility to colon carcinoma. Mol. Med. Rep. 2014, 11, 2493–2503. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Carter, R.E.; Jaffa, M.A.; Nakerakanti, S.; Lackland, D.; Lopes-Virella, M.; Trojanowska, M.; Luttrell, L.; Jaffa, A.A. The DCCT/EDIC Study Group Genetic variant in the promoter of connective tissue growth factor gene confers susceptibility to nephropathy in type 1 diabetes. J. Med. Genet. 2010, 47, 391–397. [Google Scholar] [CrossRef]
- Klay, D.; van der Vis, J.J.; Roothaan, S.M.; Nguyen, T.Q.; Grutters, J.C.; Goldschmeding, R.; van Moorsel, C.H.M. Connective Tissue Growth Factor Single Nucleotide Polymorphisms in (Familial) Pulmonary Fibrosis and Connective Tissue Disease Associated Interstitial Lung Disease. Lung 2021, 199, 659–666. [Google Scholar] [CrossRef]
- Fonseca, C.; Lindahl, G.E.; Ponticos, M.; Sestini, P.; Renzoni, E.A.; Holmes, A.M.; Spagnolo, P.; Pantelidis, P.; Leoni, P.; McHugh, N.; et al. A Polymorphism in the CTGF Promoter Region Associated with Systemic Sclerosis. N. Engl. J. Med. 2007, 357, 1210–1220. [Google Scholar] [CrossRef] [Green Version]
- Bujak, K.; Lejawa, M.; Gąsior, M.; Osadnik, T. The CTGF gene -945 G/C polymorphism is associated with target lesion revascularization for in-stent restenosis. Exp. Mol. Pathol. 2020, 118, 104598. [Google Scholar] [CrossRef]
- Szabó, Z.; Magga, J.; Alakoski, T.; Ulvila, J.; Piuhola, J.; Vainio, L.; Kivirikko, K.I.; Vuolteenaho, O.; Ruskoaho, H.; Lipson, K.; et al. Connective Tissue Growth Factor Inhibition Attenuates Left Ventricular Remodeling and Dysfunction in Pressure Overload–Induced Heart Failure. Hypertension 2014, 63, 1235–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontes, M.S.; Kessler, E.L.; van Stuijvenberg, L.; Brans, M.A.; Falke, L.L.; Kok, B.; Leask, A.; van Rijen, H.V.; Vos, M.A.; Goldschmeding, R.; et al. CTGF knockout does not affect cardiac hypertrophy and fibrosis formation upon chronic pressure overload. J. Mol. Cell. Cardiol. 2015, 88, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Gravning, J.; Ørn, S.; Kaasbøll, O.J.; Martinov, V.N.; Manhenke, C.; Dickstein, K.; Edvardsen, T.; Attramadal, H.; Ahmed, M.S. Myocardial Connective Tissue Growth Factor (CCN2/CTGF) Attenuates Left Ventricular Remodeling after Myocardial Infarction. PLoS ONE 2012, 7, e52120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaasbøll, O.J.; Moe, I.T.; Ahmed, M.S.; Stang, E.; Hagelin, E.M.V.; Attramadal, H. CTGF/CCN2 Postconditioning Increases Tolerance of Murine Hearts towards Ischemia-Reperfusion Injury. PLoS ONE 2016, 11, e0149000. [Google Scholar] [CrossRef]
- Chaqour, B. Caught between a “Rho” and a hard place: Are CCN1/CYR61 and CCN2/CTGF the arbiters of microvascular stiffness? J. Cell Commun. Signal. 2019, 14, 21–29. [Google Scholar] [CrossRef]
- Oh, C.-D.; Yasuda, H.; Zhao, W.; Henry, S.P.; Zhang, Z.; Xue, M.; De Crombrugghe, B.; Chen, D. SOX9 directly Regulates CTGF/CCN2 Transcription in Growth Plate Chondrocytes and in Nucleus Pulposus Cells of Intervertebral Disc. Sci. Rep. 2016, 6, 29916. [Google Scholar] [CrossRef] [Green Version]
- Miyashita, N.; Horie, M.; Suzuki, H.I.; Saito, M.; Mikami, Y.; Okuda, K.; Boucher, R.C.; Suzukawa, M.; Hebisawa, A.; Saito, A.; et al. FOXL1 Regulates Lung Fibroblast Function via Multiple Mechanisms. Am. J. Respir. Cell Mol. Biol. 2020, 63, 831–842. [Google Scholar] [CrossRef]
- Raza, S.; Jokl, E.; Pritchett, J.; Martin, K.; Su, K.; Simpson, K.; Birchall, L.; Mullan, A.F.; Athwal, V.S.; Doherty, D.T.; et al. SOX9 is required for kidney fibrosis and activates NAV3 to drive renal myofibroblast function. Sci. Signal. 2021, 14, eabb4282. [Google Scholar] [CrossRef]
- Gajjala, P.R.; Kasam, R.K.; Soundararajan, D.; Sinner, D.; Huang, S.K.; Jegga, A.G.; Madala, S.K. Dysregulated overexpression of Sox9 induces fibroblast activation in pulmonary fibrosis. JCI Insight 2021, 6, e152503. [Google Scholar] [CrossRef]
- Orriols, M.; Varona, S.; Martí-Pàmies, I.; Galán, M.; Guadall, A.; Escudero, J.R.; Martín-Ventura, J.L.; Camacho, M.; Vila, L.; Martínez-González, J.; et al. Down-regulation of Fibulin-5 is associated with aortic dilation: Role of inflammation and epigenetics. Cardiovasc. Res. 2016, 110, 431–442. [Google Scholar] [CrossRef]
- Kaasbøll, O.J.; Gadicherla, A.K.; Wang, J.-H.; Monsen, V.T.; Hagelin, E.M.V.; Dong, M.-Q.; Attramadal, H. Connective tissue growth factor (CCN2) is a matricellular preproprotein controlled by proteolytic activation. J. Biol. Chem. 2018, 293, 17953–17970. [Google Scholar] [CrossRef] [Green Version]
- Writing Group Members; Hiratzka, L.F.; Bakris, G.L.; Beckman, J.; Bersin, R.M.; Carr, V.F.; Casey, D.; Eagle, K.A.; Hermann, L.K.; Isselbacher, E.M.; et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Soc. Circulation 2010, 121, e266–e369. [Google Scholar] [CrossRef] [Green Version]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [Green Version]
- Lancellotti, P.; Tribouilloy, C.; Hagendorff, A.; Moura, L.; Popescu, B.A.; Agricola, E.; Monin, J.-L.; Pierard, L.A.; Badano, L.; Zamorano, J.L.; et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 1: Aortic and pulmonary regurgitation (native valve disease). Eur. Heart J.-Cardiovasc. Imaging 2010, 11, 223–244. [Google Scholar] [CrossRef] [Green Version]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Evangelista, A.; Griffin, B.P.; Iung, B.; Otto, C.M.; Pellikka, P.A.; Quiñones, M. Echocardiographic Assessment of Valve Stenosis: EAE/ASE Recommendations for Clinical Practice. J. Am. Soc. Echocardiogr. 2009, 22, 1–23. [Google Scholar] [CrossRef]
- Loeys, B.L.; Dietz, H.C.; Braverman, A.C.; Callewaert, B.L.; De Backer, J.; Devereux, R.B.; Hilhorst-Hofstee, Y.; Jondeau, G.; Faivre, L.; Milewicz, D.M.; et al. The revised Ghent nosology for the Marfan syndrome. J. Med. Genet. 2010, 47, 476–485. [Google Scholar] [CrossRef] [Green Version]
- Ehlers-Danlos Syndromes: Revised Nosology, Villefranche, 1997. Ehlers-Danlos National Foundation (USA) and Ehlers-Danlos Support Group (UK)-PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/9557891/ (accessed on 6 June 2022).
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redon, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cífková, R.; De Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC Practice Guidelines for the Management of Arterial Hypertension. Blood Press. 2013, 23, 3–16. [Google Scholar] [CrossRef]
- Stone, N.J.; Robinson, J.G.; Lichtenstein, A.H.; Merz, C.N.B.; Blum, C.B.; Eckel, R.H.; Goldberg, A.C.; Gordon, D.; Levy, D.; Lloyd-Jones, D.M.; et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63, 2889–2934. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2010, 33, S62–S69. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2012; Available online: http://www.R-project.org (accessed on 13 October 2022).



| Variable | Controls N = 297 | TAA N = 69 | p-Value |
|---|---|---|---|
| Sex | 0.001 | ||
| Male | 177 (59.6) | 56 (81.2) | |
| Female | 120 (40.4) | 13 (18.8) | |
| Age, years | 69.0 [60.0, 77.0] | 58.0 [44.0, 67.0] | <0.001 |
| Smoking status | 0.035 | ||
| Never | 196 (66.0) | 39 (56.5) | |
| Current | 69 (23.2) | 26 (37.7) | |
| Former | 32 (10.8) | 4 (5.8) | |
| Dyslipidemia | 140 (47.1) | 26 (37.7) | 0.198 |
| Hypertension | 183 (61.6) | 30 (43.5) | 0.009 |
| Diabetes mellitus | 89 (30.0) | 3 (4.3) | <0.001 |
| Aortic valve | 0.002 | ||
| Normal functioning | 64 (21.5) | 11 (15.9) | |
| Stenosis | 183 (61.6) | 32 (46.4) | |
| Regurgitation | 36 (12.1) | 21 (30.4) | |
| Mixed | 14 (4.71) | 5 (7.25) | |
| Bicuspid aortic valve | 72 (24.2) | 59 (85.5) | <0.001 |
| Polymorphism | Total (nN = 366) † | Controls (N = 297) | TAA (N = 69) | OR (95% CI) | p-Value |
|---|---|---|---|---|---|
| CTGF_rs6918698 | |||||
| CC | 100 (27.3) | 85 (28.6) | 15 (21.7) | - | |
| CG | 172 (47.0) | 135 (45.5) | 37 (53.6) | 1.55 (0.82–3.08) | 0.190 |
| GG | 94 (25.7) | 77 (25.9) | 17 (24.6) | 1.25 (0.58–2.70) | 0.563 |
| C allele | 372 (50.8) | 305 (51.3) | 67 (48.6) | - | |
| G allele | 360 (49.2) | 289 (48.7) | 71 (51.4) | 1.12 (0.77–1.62) | 0.554 |
| CTGF_rs9402373 | |||||
| CC | 244 (66.7) | 202 (68.9) | 42 (62.7) | - | |
| CG | 104 (28.4) | 80 (27.3) | 24 (35.8) | 1.44 (0.81–2.52) | 0.203 |
| GG | 12 (3.3) | 11 (3.8) | 1 (1.5) | 0.437 (0.02–2.34) | 0.434 |
| C allele | 592 (82.2) | 484 (82.6) | 108 (80.6) | - | |
| G allele | 128 (17.8) | 102 (17.4) | 26 (19.4) | 1.14 (0.70–1.82) | 0.586 |
| CTGF_rs12526196 | |||||
| TT | 303 (82.8) | 250 (85.3) | 53 (79.1) | - | |
| TC | 53 (14.5) | 41 (14.0) | 12 (17.9) | 1.38 (0.66–2.74) | 0.372 |
| CC | 4 (1.1) | 2 (0.7) | 2 (3.0) | 4.72 (0.56–40.03) | 0.125 |
| T allele | 659 (91.5) | 541 (92.3) | 118 (88.1) | - | |
| C allele | 61 (8.5) | 45 (7.7) | 16 (11.9) | 1.63 (0.87–2.93) | 0.113 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tejera-Muñoz, A.; Rodríguez, I.; Del Río-García, Á.; Mohamedi, Y.; Martín, M.; Chiminazzo, V.; Suárez-Álvarez, B.; López-Larrea, C.; Ruiz-Ortega, M.; Rodrigues-Díez, R.R. The CCN2 Polymorphism rs12526196 Is a Risk Factor for Ascending Thoracic Aortic Aneurysm. Int. J. Mol. Sci. 2022, 23, 15406. https://doi.org/10.3390/ijms232315406
Tejera-Muñoz A, Rodríguez I, Del Río-García Á, Mohamedi Y, Martín M, Chiminazzo V, Suárez-Álvarez B, López-Larrea C, Ruiz-Ortega M, Rodrigues-Díez RR. The CCN2 Polymorphism rs12526196 Is a Risk Factor for Ascending Thoracic Aortic Aneurysm. International Journal of Molecular Sciences. 2022; 23(23):15406. https://doi.org/10.3390/ijms232315406
Chicago/Turabian StyleTejera-Muñoz, Antonio, Isabel Rodríguez, Álvaro Del Río-García, Yamina Mohamedi, María Martín, Valentina Chiminazzo, Beatriz Suárez-Álvarez, Carlos López-Larrea, Marta Ruiz-Ortega, and Raúl R. Rodrigues-Díez. 2022. "The CCN2 Polymorphism rs12526196 Is a Risk Factor for Ascending Thoracic Aortic Aneurysm" International Journal of Molecular Sciences 23, no. 23: 15406. https://doi.org/10.3390/ijms232315406
APA StyleTejera-Muñoz, A., Rodríguez, I., Del Río-García, Á., Mohamedi, Y., Martín, M., Chiminazzo, V., Suárez-Álvarez, B., López-Larrea, C., Ruiz-Ortega, M., & Rodrigues-Díez, R. R. (2022). The CCN2 Polymorphism rs12526196 Is a Risk Factor for Ascending Thoracic Aortic Aneurysm. International Journal of Molecular Sciences, 23(23), 15406. https://doi.org/10.3390/ijms232315406