Identification of the Role of TGR5 in the Regulation of Leydig Cell Homeostasis
Abstract
1. Introduction
2. Results
2.1. TGR5 Was Expressed in Mouse Leydig Cells
2.2. TGR5 mRNA Expression Was Is Regulated by the LH/CG Signaling Pathway in Mouse Leydig Cells
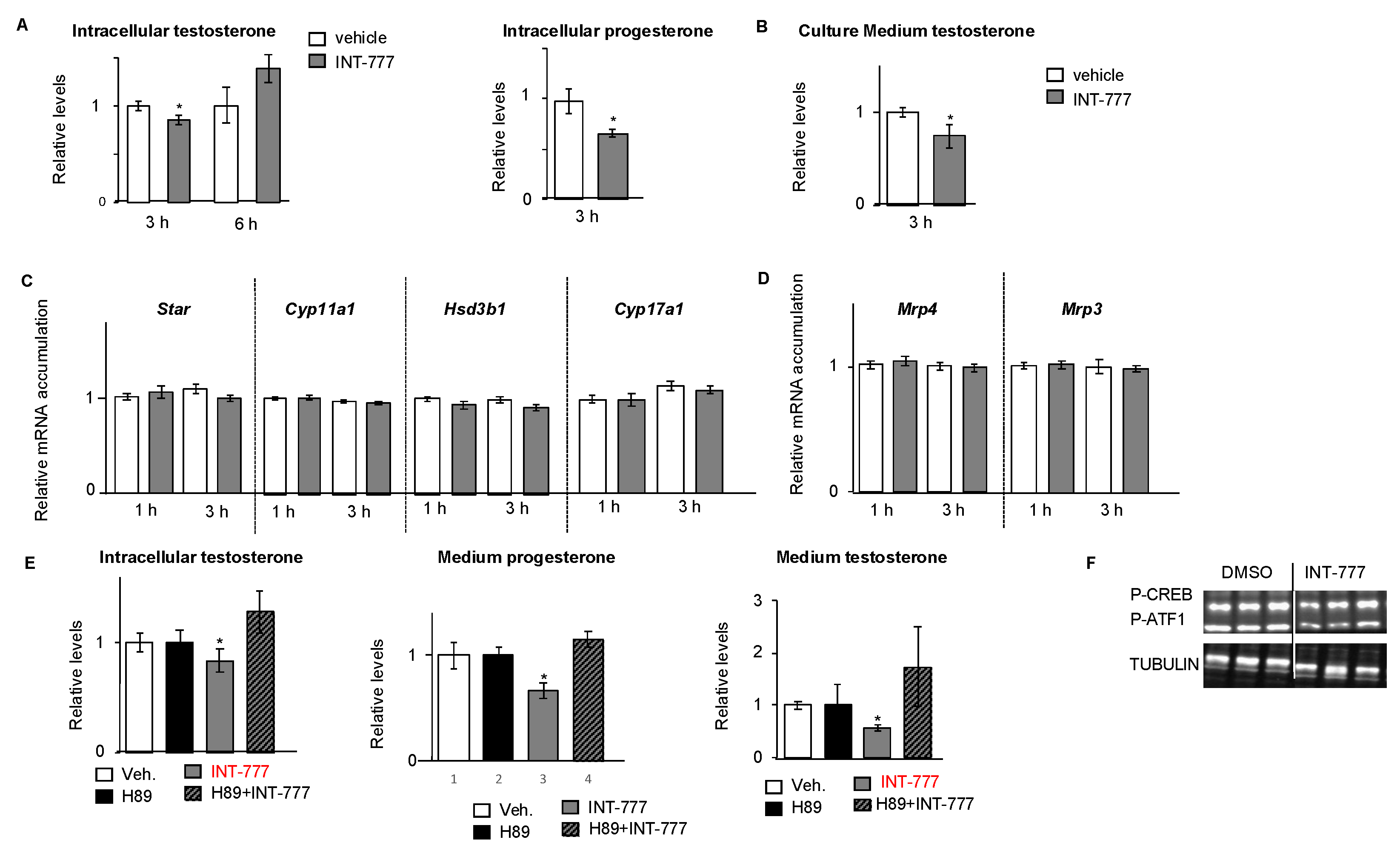
2.3. TGR5 Activation Regulated Testosterone Production in mLTC1 Cells
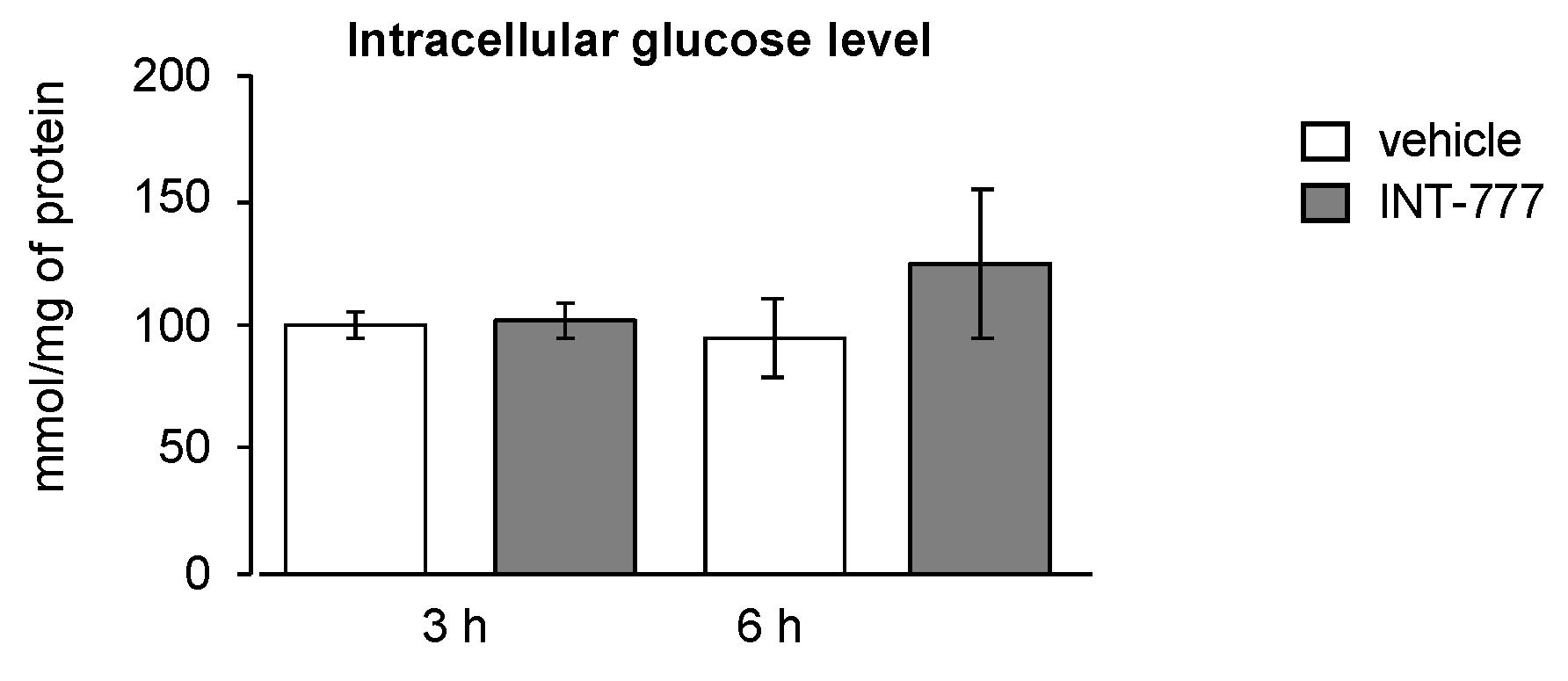
2.4. TGR5 Did Not Regulate Glucose Homeostasis in Mouse Leydig Cells
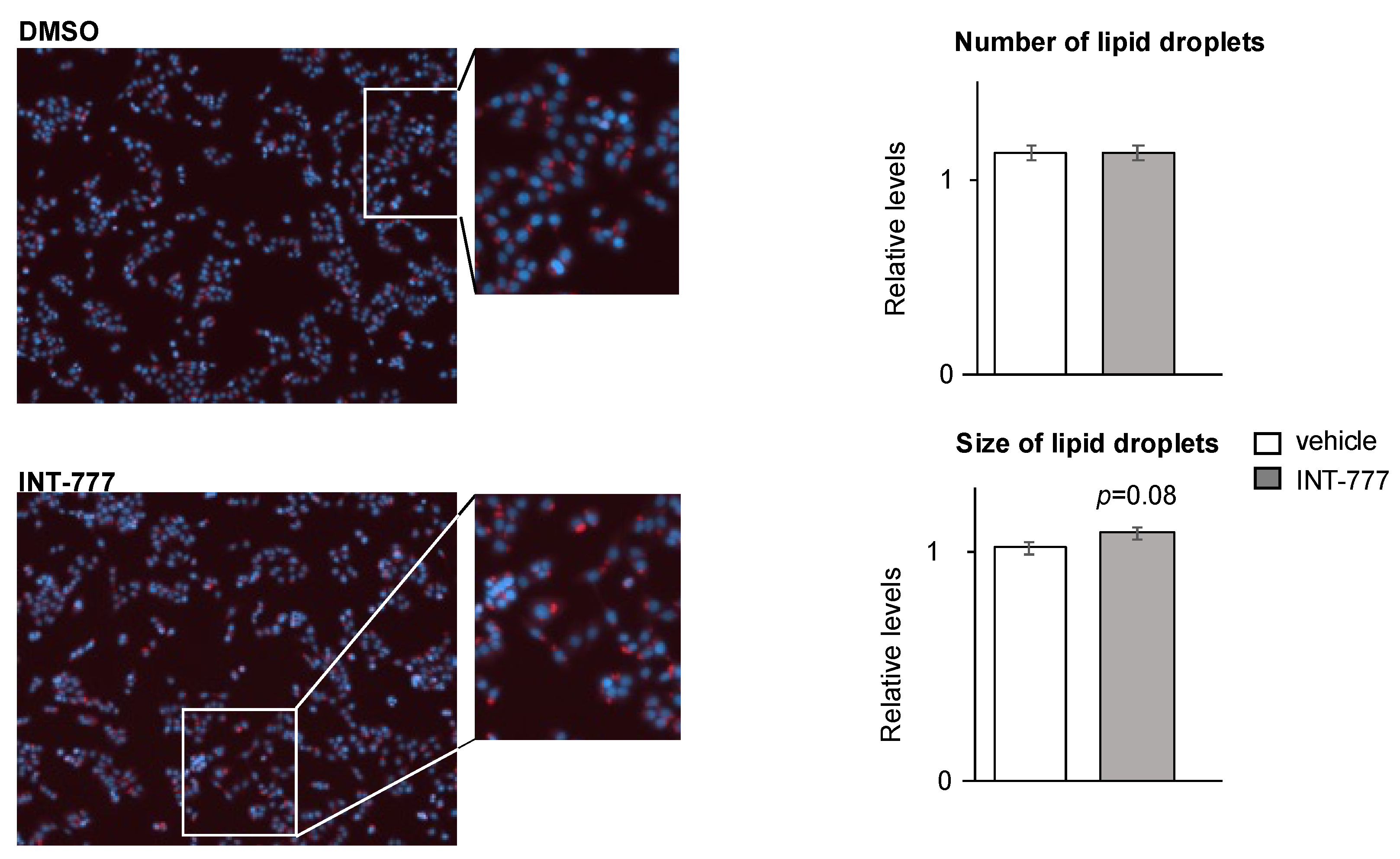
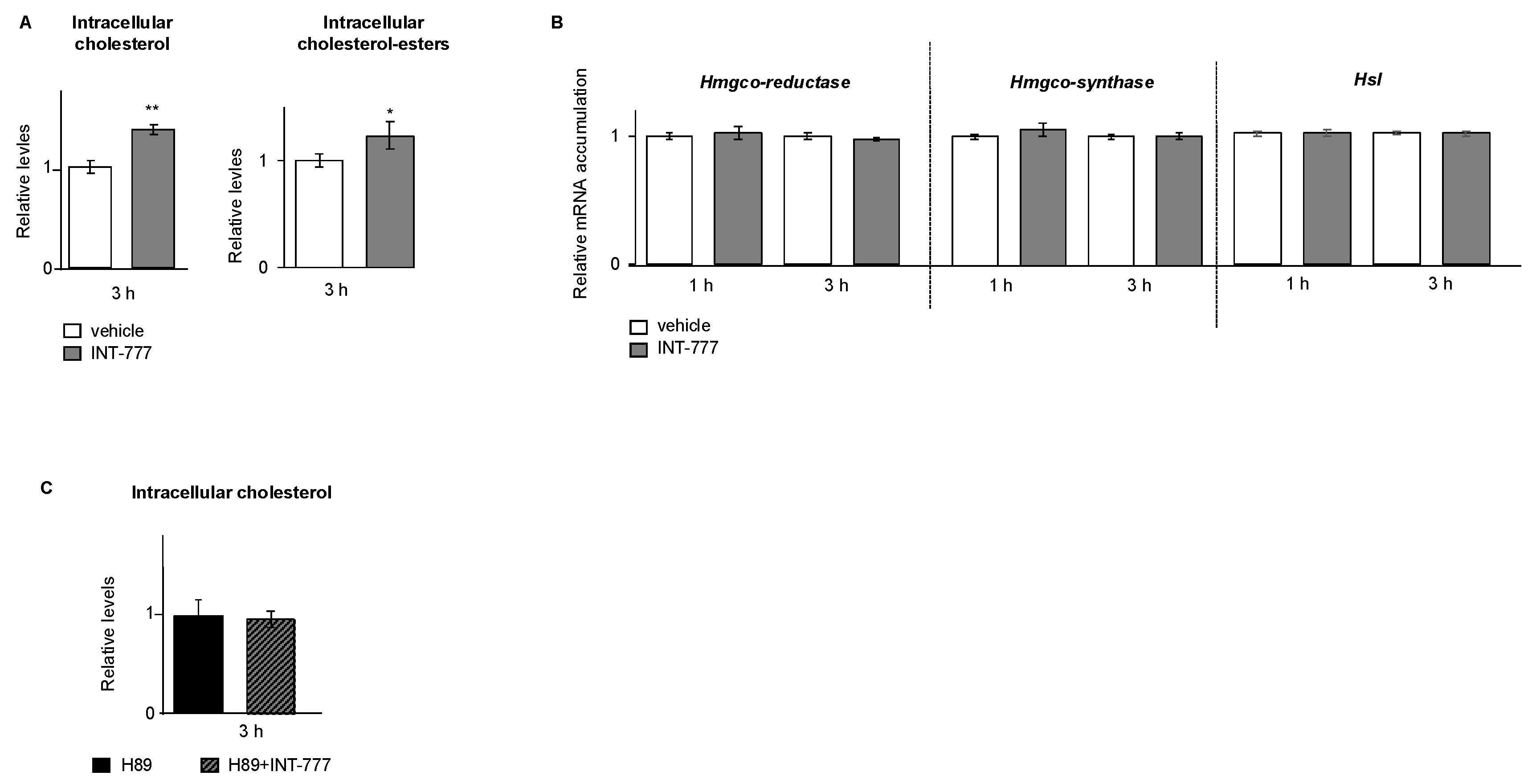
2.5. TGR5 Regulated Lipid Homeostasis in mLTC1 Mouse Leydig Cells
2.6. TGR5 Regulated Testosterone Production in Mouse Primary Leydig Cells
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Interstitial Cell Enrichment
4.3. Cell Line Approaches
4.4. Real-Time RT-qPCR
4.5. Nile-Red-O Staining
4.6. Glucose, Cholesterol, Cholesterol Ester, and Triglyceride Measurements
4.7. Testosterone Measurements
4.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Papadopoulos, V.; Baraldi, M.; Guilarte, T.R.; Knudsen, T.B.; Lacapère, J.-J.; Lindemann, P.; Norenberg, M.D.; Nutt, D.; Weizman, A.; Zhang, M.-R.; et al. Translocator Protein (18 kDa): New Nomenclature for the Peripheral-Type Benzodiazepine Receptor Based on Its Structure and Molecular Function. Trends Pharmacol. Sci. 2006, 27, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Stocco, D.M. The Role of the StAR Protein in Steroidogenesis: Challenges for the Future. J. Endocrinol. 2000, 164, 247–253. [Google Scholar] [CrossRef]
- Midzak, A.; Papadopoulos, V. Steroidogenesis: The Classics and Beyond. Steroids 2015, 103, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Sèdes, L.; Martinot, E.; Baptissart, M.; Baron, S.; Caira, F.; Beaudoin, C.; Volle, D.H. Bile Acids and Male Fertility: From Mouse to Human? Mol. Asp. Med. 2017, 56, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Holota, H.; Thiouard, L.; Monrose, M.; Garcia, M.; De Haze, A.; Saru, J.; Caira, F.; Beaudoin, C.; Volle, D. FXRα Modulates Leydig Cell Endocrine Function in Mouse. Mol. Cell. Endocrinol. 2020, 518, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Volle, D.H.; Duggavathi, R.; Magnier, B.C.; Houten, S.M.; Cummins, C.L.; Lobaccaro, J.-M.A.; Verhoeven, G.; Schoonjans, K.; Auwerx, J. The Small Heterodimer Partner Is a Gonadal Gatekeeper of Sexual Maturation in Male Mice. Genes Dev. 2007, 21, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Baptissart, M.; Martinot, E.; Vega, A.; Sédes, L.; Rouaisnel, B.; de Haze, A.; Baron, S.; Schoonjans, K.; Caira, F.; Volle, D.H. Bile Acid-FXRα Pathways Regulate Male Sexual Maturation in Mice. Oncotarget 2016, 7, 19468–19482. [Google Scholar] [CrossRef]
- Baptissart, M.; Sèdes, L.; Holota, H.; Thirouard, L.; Martinot, E.; de Haze, A.; Rouaisnel, B.; Caira, F.; Beaudoin, C.; Volle, D.H. Multigenerational Impacts of Bile Exposure Are Mediated by TGR5 Signaling Pathways. Sci. Rep. 2018, 8, 16875. [Google Scholar] [CrossRef]
- Baptissart, M.; Vega, A.; Martinot, E.; Pommier, A.J.; Houten, S.M.; Marceau, G.; de Haze, A.; Baron, S.; Schoonjans, K.; Lobaccaro, J.-M.A.; et al. Bile Acids Alter Male Fertility through G-Protein-Coupled Bile Acid Receptor 1 Signaling Pathways in Mice. Hepatology 2014, 60, 1054–1065. [Google Scholar] [CrossRef]
- Thirouard, L.; Holota, H.; Monrose, M.; Garcia, M.; de Haze, A.; Damon-Soubeyrand, C.; Renaud, Y.; Saru, J.-P.; Perino, A.; Schoonjans, K.; et al. Identification of a Crosstalk among TGR5, GLIS2, and TP53 Signaling Pathways in the Control of Undifferentiated Germ Cell Homeostasis and Chemoresistance. Adv. Sci. 2022, 9, e2200626. [Google Scholar] [CrossRef]
- Kaprara, A.; Huhtaniemi, I.T. The Hypothalamus-Pituitary-Gonad Axis: Tales of Mice and Men. Metab. Clin. Exp. 2018, 86, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Holota, H.; Thirouard, L.; Garcia, M.; Monrose, M.; de Haze, A.; Saru, J.-P.; Caira, F.; Beaudoin, C.; Volle, D.H. Fxralpha Gene Is a Target Gene of HCG Signaling Pathway and Represses HCG Induced Steroidogenesis. J. Steroid Biochem. Mol. Biol. 2019, 194, 105460. [Google Scholar] [CrossRef] [PubMed]
- Vega, A.; Martinot, E.; Baptissart, M.; De Haze, A.; Saru, J.; Baron, S.; Caira, F.; Schoonjans, K.; Lobaccaro, J.; Volle, D. Identification of the Link between the Hypothalamo-Pituitary Axis and the Testicular Orphan Nuclear Receptor NR0B2 in Male Mice. Endocrinology 2014. submitted. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Romero-Ramírez, L.; Mey, J. Taurolithocholic Acid but Not Tauroursodeoxycholic Acid Rescues Phagocytosis Activity of Bone Marrow-derived Macrophages under Inflammatory Stress. J. Cell Physiol. 2022, 237, 1455–1470. [Google Scholar] [CrossRef] [PubMed]
- Perino, A.; Velázquez-Villegas, L.A.; Bresciani, N.; Sun, Y.; Huang, Q.; Fénelon, V.S.; Castellanos-Jankiewicz, A.; Zizzari, P.; Bruschetta, G.; Jin, S.; et al. Central Anorexigenic Actions of Bile Acids Are Mediated by TGR5. Nat. Metab. 2021, 3, 595–603. [Google Scholar] [CrossRef]
- Li, C.Y.; Basit, A.; Gupta, A.; Gáborik, Z.; Kis, E.; Prasad, B. Major Glucuronide Metabolites of Testosterone Are Primarily Transported by MRP2 and MRP3 in Human Liver, Intestine and Kidney. J. Steroid Biochem. Mol. Biol. 2019, 191, 105350. [Google Scholar] [CrossRef]
- Morgan, J.A.; Cheepala, S.B.; Wang, Y.; Neale, G.; Adachi, M.; Nachagari, D.; Leggas, M.; Zhao, W.; Boyd, K.; Venkataramanan, R.; et al. Deregulated Hepatic Metabolism Exacerbates Impaired Testosterone Production in Mrp4-Deficient Mice. J. Biol. Chem. 2012, 287, 14456–14466. [Google Scholar] [CrossRef]
- Masyuk, T.V.; Masyuk, A.I.; Lorenzo Pisarello, M.; Howard, B.N.; Huang, B.Q.; Lee, P.-Y.; Fung, X.; Sergienko, E.; Ardecky, R.J.; Chung, T.D.Y.; et al. TGR5 Contributes to Hepatic Cystogenesis in Rodents with Polycystic Liver Diseases through Cyclic Adenosine Monophosphate/Gαs Signaling. Hepatology 2017, 66, 1197–1218. [Google Scholar] [CrossRef]
- Ahn, S.W.; Gang, G.-T.; Tadi, S.; Nedumaran, B.; Kim, Y.D.; Park, J.H.; Kweon, G.R.; Koo, S.-H.; Lee, K.; Ahn, R.-S.; et al. Phosphoenolpyruvate Carboxykinase and Glucose-6-Phosphatase Are Required for Steroidogenesis in Testicular Leydig Cells. J. Biol. Chem. 2012, 287, 41875–41887. [Google Scholar] [CrossRef]
- Thomas, C.; Gioiello, A.; Noriega, L.; Strehle, A.; Oury, J.; Rizzo, G.; Macchiarulo, A.; Yamamoto, H.; Mataki, C.; Pruzanski, M.; et al. TGR5-Mediated Bile Acid Sensing Controls Glucose Homeostasis. Cell Metab. 2009, 10, 167–177. [Google Scholar] [CrossRef]
- Holter, M.M.; Chirikjian, M.K.; Govani, V.N.; Cummings, B.P. TGR5 Signaling in Hepatic Metabolic Health. Nutrients 2020, 12, 2598. [Google Scholar] [CrossRef] [PubMed]
- Pols, T.W.H.; Noriega, L.G.; Nomura, M.; Auwerx, J.; Schoonjans, K. The Bile Acid Membrane Receptor TGR5: A Valuable Metabolic Target. Dig. Dis. 2011, 29, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.-J.; Azhar, S.; Kraemer, F.B. Lipid Droplets and Steroidogenic Cells. Exp. Cell Res. 2016, 340, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Sedes, L.; Thirouard, L.; Maqdasy, S.; Garcia, M.; Caira, F.; Lobaccaro, J.-M.A.; Beaudoin, C.; Volle, H. David Cholesterol: A Gatekeeper of Male Fertility? Front. Endocrinol. 2018, 9, 369. [Google Scholar] [CrossRef]
- Donepudi, A.C.; Boehme, S.; Li, F.; Chiang, J.Y.L. G Protein-Coupled Bile Acid Receptor Plays a Key Role in Bile Acid Metabolism and Fasting-Induced Hepatic Steatosis. Hepatology 2017, 65, 813–827. [Google Scholar] [CrossRef]
- Strauss, J.F.; Kallen, C.B.; Christenson, L.K.; Watari, H.; Devoto, L.; Arakane, F.; Kiriakidou, M.; Sugawara, T. The Steroidogenic Acute Regulatory Protein (StAR): A Window into the Complexities of Intracellular Cholesterol Trafficking. Recent Prog. Horm. Res. 1999, 54, 369–394; discussion 394–395. [Google Scholar]
- Martinot, E.; Sèdes, L.; Baptissart, M.; Lobaccaro, J.-M.; Caira, F.; Beaudoin, C.; Volle, D.H. Bile Acids and Their Receptors. Mol. Asp. Med. 2017, 56, 2–9. [Google Scholar] [CrossRef]
- Manna, P.R.; Dyson, M.T.; Eubank, D.W.; Clark, B.J.; Lalli, E.; Sassone-Corsi, P.; Zeleznik, A.J.; Stocco, D.M. Regulation of Steroidogenesis and the Steroidogenic Acute Regulatory Protein by a Member of the CAMP Response-Element Binding Protein Family. Mol. Endocrinol. 2002, 16, 184–199. [Google Scholar] [CrossRef] [PubMed]
- King, S.R.; LaVoie, H.A. Gonadal Transactivation of STARD1, CYP11A1 and HSD3B. Front. Biosci. (Landmark Ed.) 2012, 17, 824–846. [Google Scholar] [CrossRef]
- Bateman, M.E.; Strong, A.L.; McLachlan, J.A.; Burow, M.E.; Bunnell, B.A. The Effects of Endocrine Disruptors on Adipogenesis and Osteogenesis in Mesenchymal Stem Cells: A Review. Front. Endocrinol. 2016, 7, 171. [Google Scholar] [CrossRef]
- De Coster, S.; van Larebeke, N. Endocrine-Disrupting Chemicals: Associated Disorders and Mechanisms of Action. J. Environ. Public Health 2012, 2012, 713696. [Google Scholar] [CrossRef] [PubMed]
- Haverinen, E.; Fernandez, M.F.; Mustieles, V.; Tolonen, H. Metabolic Syndrome and Endocrine Disrupting Chemicals: An Overview of Exposure and Health Effects. Int. J. Environ. Res. Public Health 2021, 18, 13047. [Google Scholar] [CrossRef] [PubMed]
- Martinot, E.; Baptissart, M.; Véga, A.; Sèdes, L.; Rouaisnel, B.; Vaz, F.; Saru, J.-P.; de Haze, A.; Baron, S.; Caira, F.; et al. Bile Acid Homeostasis Controls CAR Signaling Pathways in Mouse Testis through FXRalpha. Sci. Rep. 2017, 7, 42182. [Google Scholar] [CrossRef]
- Kawamata, Y.; Fujii, R.; Hosoya, M.; Harada, M.; Yoshida, H.; Miwa, M.; Fukusumi, S.; Habata, Y.; Itoh, T.; Shintani, Y.; et al. A G Protein-Coupled Receptor Responsive to Bile Acids. J. Biol. Chem. 2003, 278, 9435–9440. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.D.; Filliatreau, L.; Klett, D.; Hai, N.V.; Duong, N.T.; Combarnous, Y. Effect of Soluble Adenylyl Cyclase (ADCY10) Inhibitors on the LH-Stimulated CAMP Synthesis in Mltc-1 Leydig Cell Line. Int. J. Mol. Sci. 2021, 22, 4641. [Google Scholar] [CrossRef]




| Gene Name | Forward | Reverse |
|---|---|---|
| Actin | TCATCACTATTGGCAACGAGC | AGTTTCATGGATGCCACAGG |
| Lhcgr | AGCTAATGCCTTTGACAACC | GATGGACTCATTATTCATCC |
| Star | TGTCAAGGAGATCAAGGTCCT | CGATAGGACCTGGTTGATGAT |
| Cyp11a1 | CTGCCTCCAGACTTCTTTCG | TTCTTGAAGGGCAGCTTGTT |
| Hsd3b1 | ATGGTCTGCCTGGGAATGAC | ACTGCAGGAGGTCAGAGCT |
| Cyp17a1 | CCTGATACGAAGCACTTCTCG | CCAGGACCCCAAGTGTGTTCT |
| Shp | CGATCCTCTTCAACCCAGATG | AGGGCTCCAAGACTTCACACA |
| Dax-1 | GTCCAGGCCATCAAGAGTTTC | CAGCTTTGCACAGAGCATCTC |
| Acc | GCCTTTCACATGAGATCCAGC | CTGCAATACCATTGTTGGCGA |
| Fas | AAGCGGTCTGGAAAGCTGAA | AGGCTGGGTTGATACCTCCA |
| Srebp1c | GAACAGACACTGGCCGAGAT | GAGGCCAGAGAAGCAGAAGAG |
| HmgcoA-reductase | ACAGAAACTCCACGTGACGA | TTCAGCAGTGCTTTCTCCGT |
| HmgcoA-Synthase | GCAAAAAGATCCGTGCCCAG | GTCATTCAGGAACATCCGAGC |
| Mrp3 | AGTCTTCGGGAGTGCTCATCA | AGGATTTGTGTCAAGATTCTCCG |
| Mrp4 | TTAGATGGGCCTCTGGTTCT | GCCCACAATTCCAACCTTT |
| Dgat1 | TGGCTGCATTTCAGATTGAG | GCTGGGAAGCAGATGATTGT |
| Dgat2 | CTTCCTGGTGCTAGGAGTGGC | GCTGGATGGGAAAGTAGTCTCGG |
| Hsl | CATATCCGCTCTCCAGTTGACC | CCTATCTTCTCCATCGACTACTCC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holota, H.; De Haze, A.; Martinot, E.; Monrose, M.; Saru, J.-P.; Caira, F.; Beaudoin, C.; Volle, D.H. Identification of the Role of TGR5 in the Regulation of Leydig Cell Homeostasis. Int. J. Mol. Sci. 2022, 23, 15398. https://doi.org/10.3390/ijms232315398
Holota H, De Haze A, Martinot E, Monrose M, Saru J-P, Caira F, Beaudoin C, Volle DH. Identification of the Role of TGR5 in the Regulation of Leydig Cell Homeostasis. International Journal of Molecular Sciences. 2022; 23(23):15398. https://doi.org/10.3390/ijms232315398
Chicago/Turabian StyleHolota, Hélène, Angélique De Haze, Emmanuelle Martinot, Melusine Monrose, Jean-Paul Saru, Françoise Caira, Claude Beaudoin, and David H. Volle. 2022. "Identification of the Role of TGR5 in the Regulation of Leydig Cell Homeostasis" International Journal of Molecular Sciences 23, no. 23: 15398. https://doi.org/10.3390/ijms232315398
APA StyleHolota, H., De Haze, A., Martinot, E., Monrose, M., Saru, J.-P., Caira, F., Beaudoin, C., & Volle, D. H. (2022). Identification of the Role of TGR5 in the Regulation of Leydig Cell Homeostasis. International Journal of Molecular Sciences, 23(23), 15398. https://doi.org/10.3390/ijms232315398






