Effects of Molecular Crowding and Betaine on HSPB5 Interactions, with Target Proteins Differing in the Quaternary Structure and Aggregation Mechanism
Abstract
:1. Introduction
2. Results
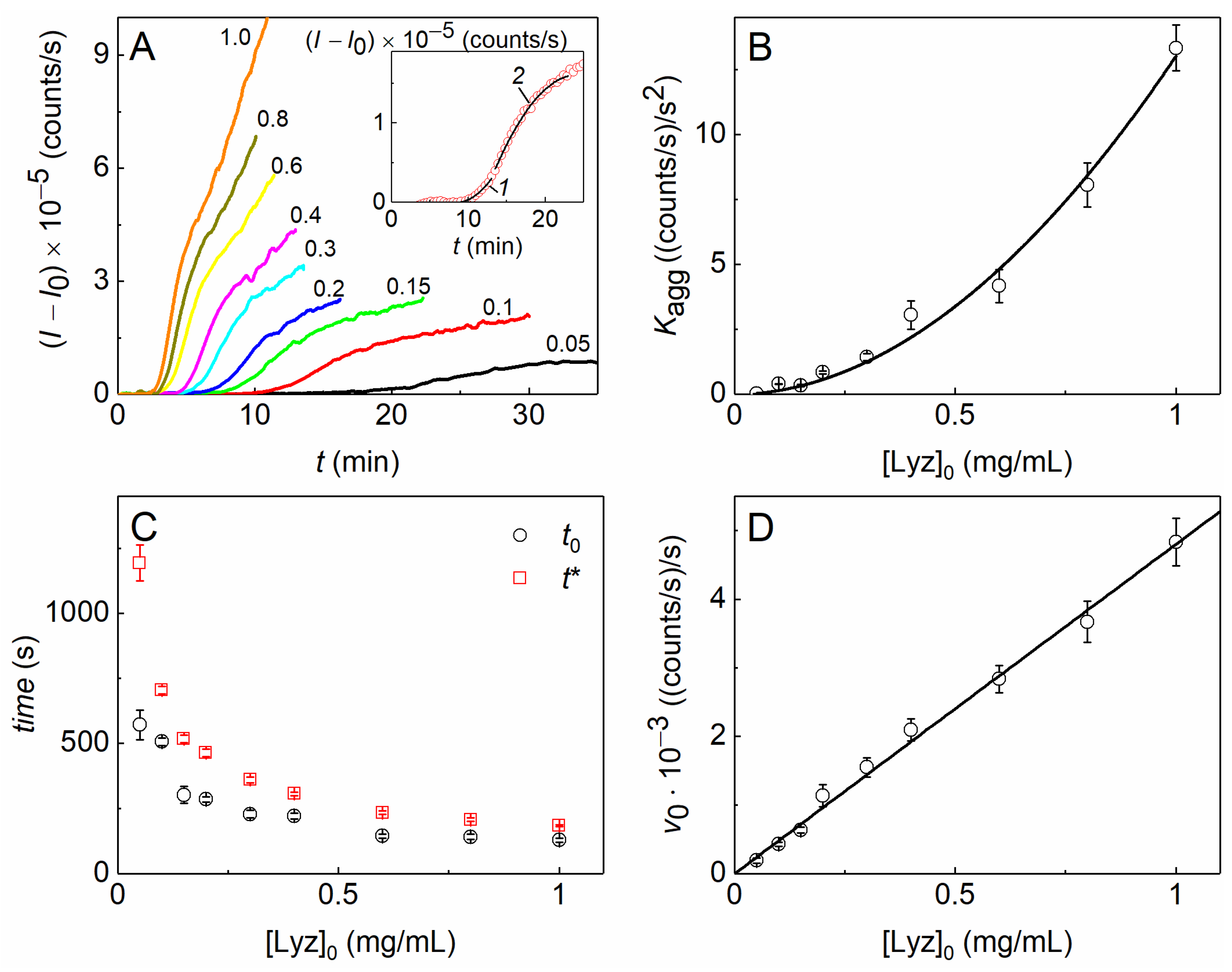
2.1. The Kinetics of DTT-Induced Aggregation of Lysozyme
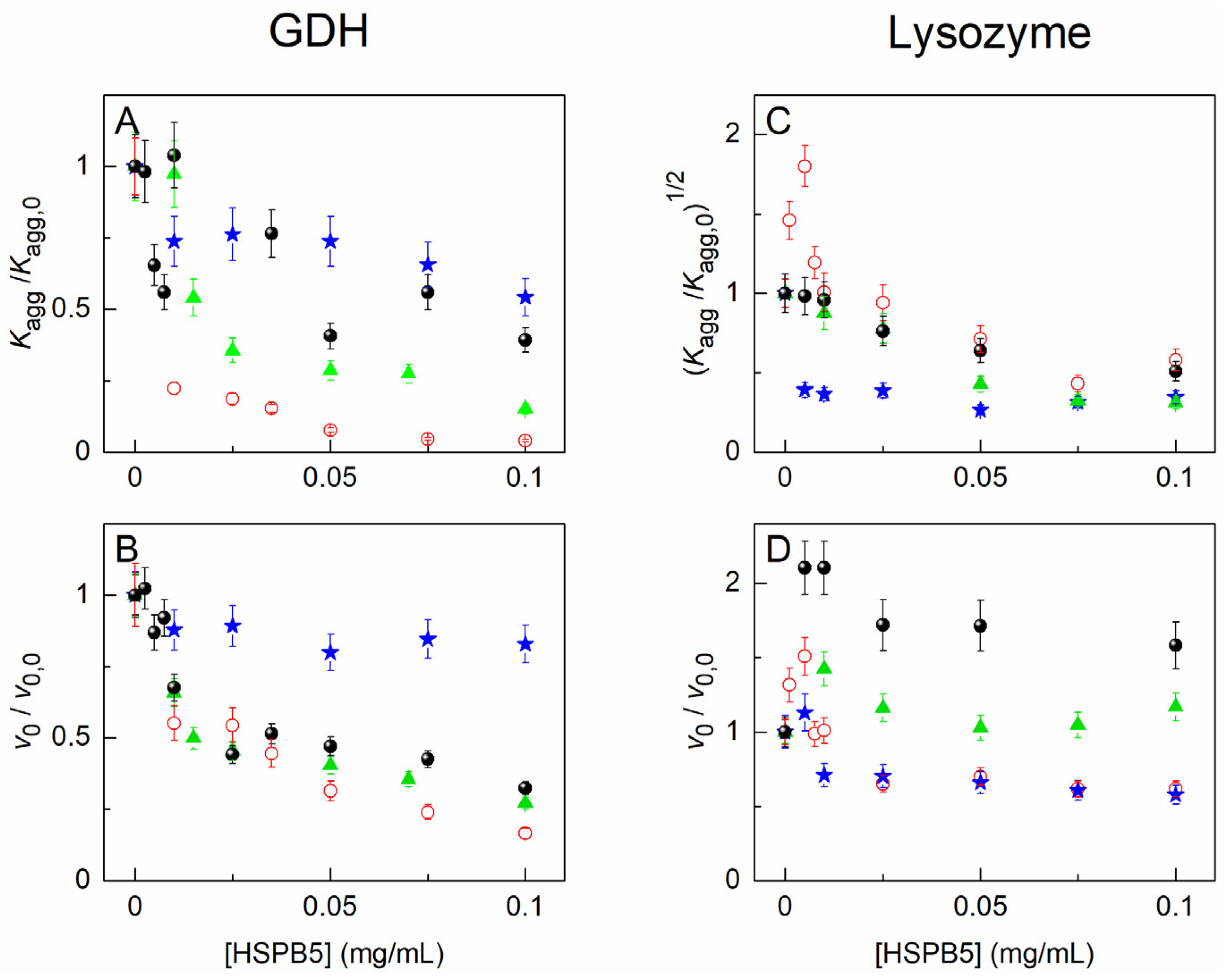
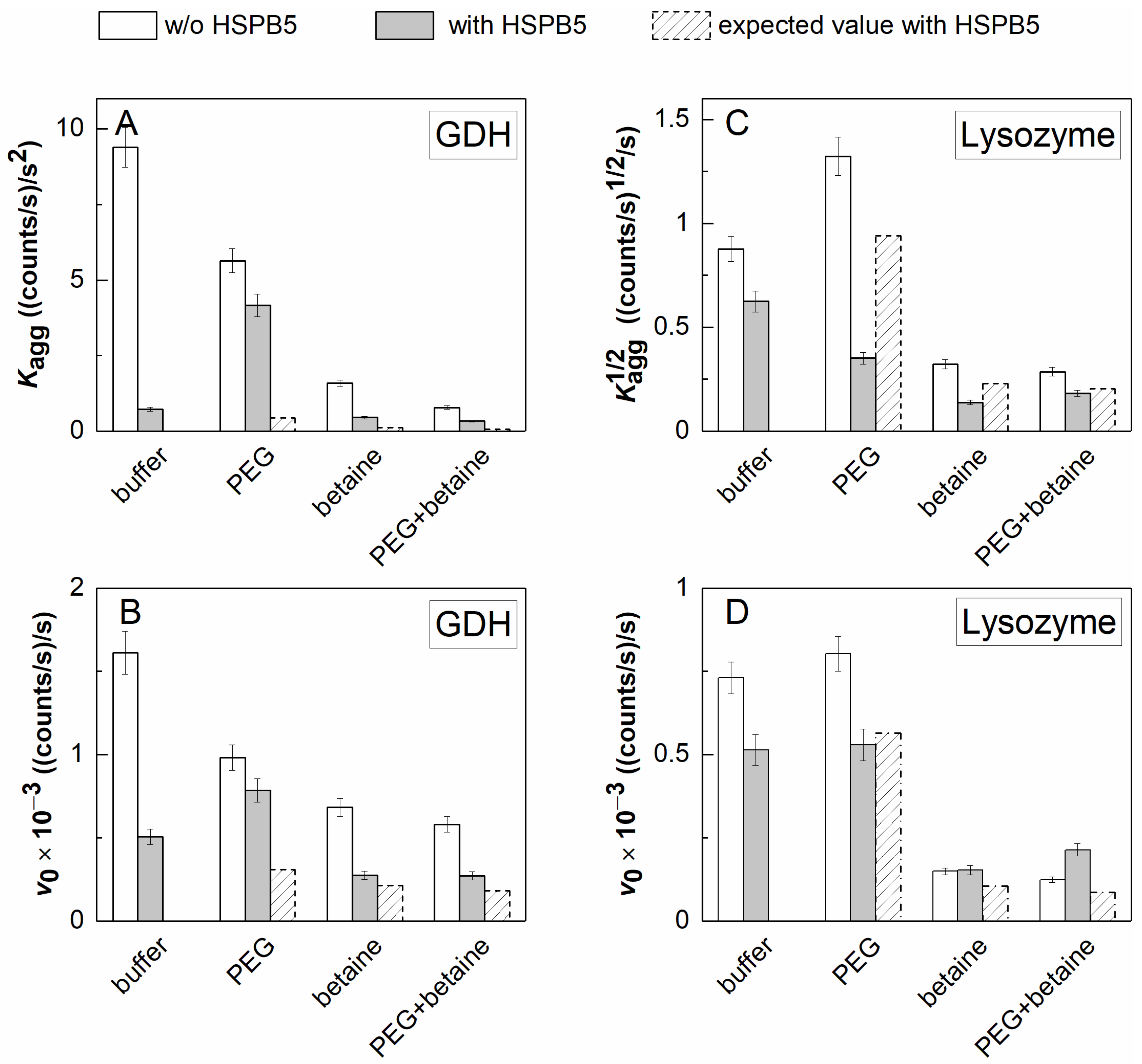
2.2. The Effect of HSPB5, Betaine, PEG, and Their Mixture on Thermal Aggregation of GDH and DTT-Induced Aggregation of Lysozyme
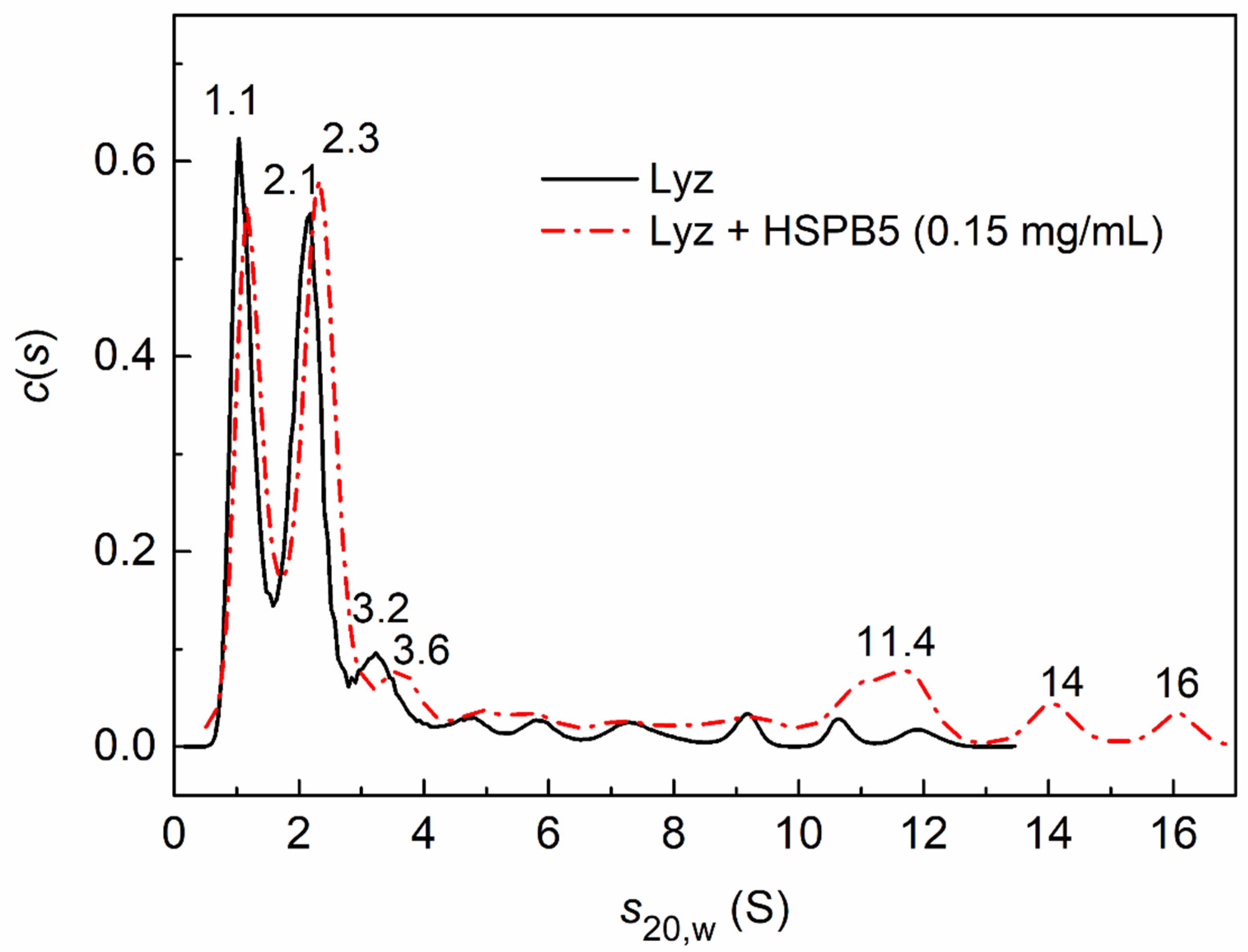
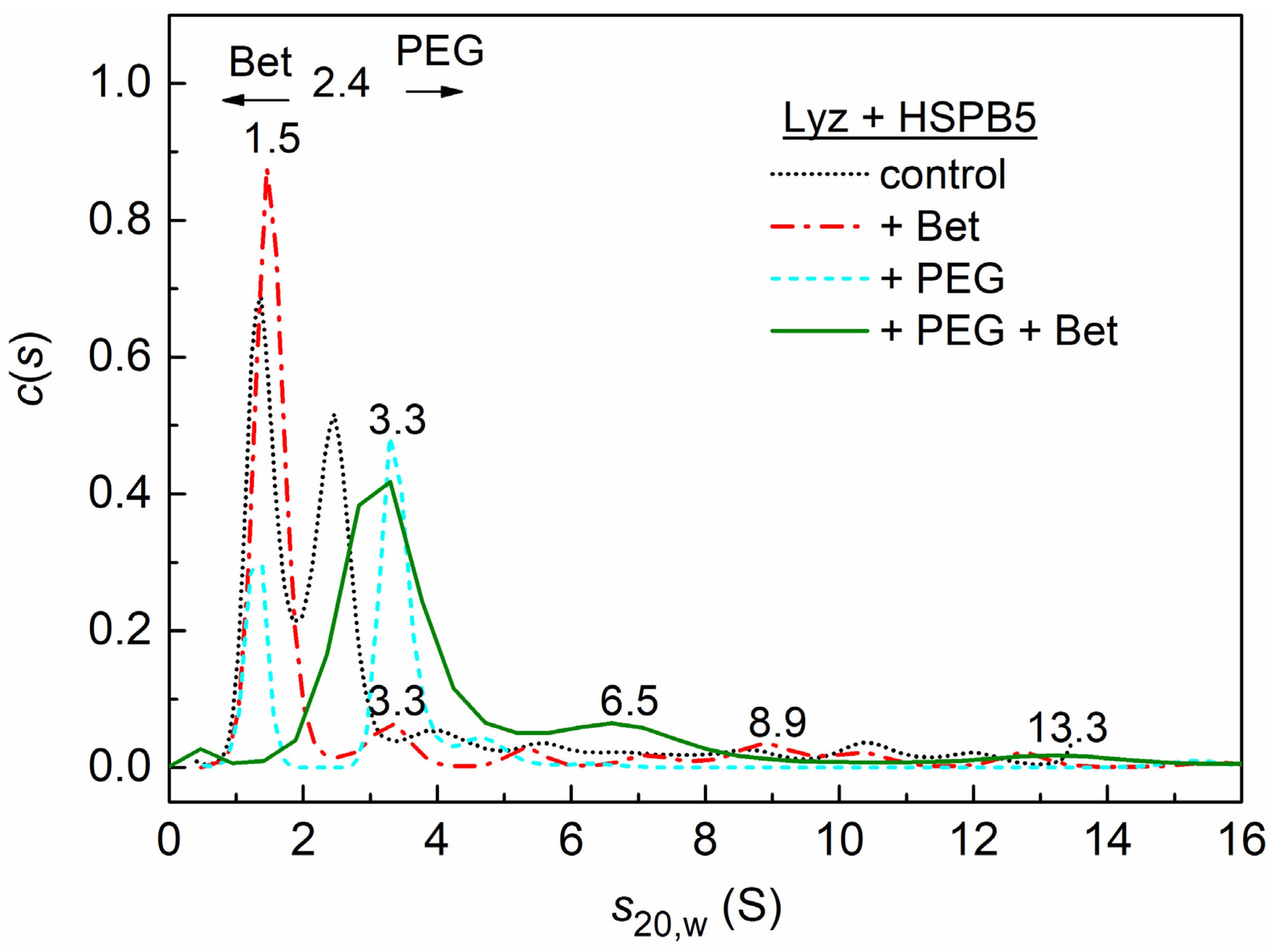
2.3. Sedimentation Velocity Analysis of HSPB5 Interaction with Lysozyme in the Presence of Betaine, PEG, and Their Mixture
2.4. Sedimentation Velocity Analysis of the Interaction of HSPB5 with GDH in the Presence of Betaine, PEG, and Their Mixture
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Protein Preparations
4.3. Dynamic Light Scattering (DLS)
4.4. Analytical Ultracentrifugation (AUC)
4.5. Densitometry and Viscosimetry
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haslbeck, M.; Vierling, E. A first line of stress defense: Small heat shock proteins and their function in protein homeostasis. J. Mol. Biol. 2015, 427, 1537–1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyedmers, J.; Mogk, A.; Bukau, B. Cellular strategies for controlling protein aggregation. Nat. Rev. Mol. Cell Biol. 2010, 11, 777–788. [Google Scholar] [CrossRef]
- Chen, B.; Retzlaff, M.; Roos, T.; Frydman, J. Cellular strategies of protein quality control. Cold Spring Harb. Perspect. Biol. 2011, 3, a004374. [Google Scholar] [CrossRef] [Green Version]
- Mymrikov, E.V.; Seit-Nebi, A.S.; Gusev, N.B. Large potentials of small heat shock proteins. Physiol. Rev. 2011, 91, 1123–1159. [Google Scholar] [CrossRef] [Green Version]
- Webster, J.M.; Darling, A.L.; Uversky, V.N.; Blair, L.J. Small heat shock proteins, big impact on protein aggregation in neurodegenerative disease. Front. Pharmacol. 2019, 10, 1047. [Google Scholar] [CrossRef] [PubMed]
- Riedl, M.; Strauch, A.; Catici, D.A.M.; Haslbeck, M. Proteinaceous transformers: Structural and functional variability of human sHsps. Int. J. Mol. Sci. 2020, 21, 5448. [Google Scholar] [CrossRef]
- Carra, S.; Alberti, S.; Benesch, J.L.P.; Boelens, W.; Buchner, J.; Carver, J.A.; Cecconi, C.; Ecroyd, H.; Gusev, N.; Hightower, L.E.; et al. Small heat shock proteins: Multifaceted proteins with important implications for life. Cell Stress Chaperones 2019, 24, 295–308. [Google Scholar] [CrossRef]
- Arrigo, A.-P. Hsp27: Novel regulator of intracellular redox state. IUBMB Life 2001, 52, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Basha, E.; O’Neill, H.; Vierling, E. Small heat shock proteins and α-crystallins: Dynamic proteins with flexible functions. Trends Biochem. Sci. 2012, 37, 106–117. [Google Scholar] [CrossRef] [Green Version]
- Hilton, G.R.; Lioe, H.; Stengel, F.; Baldwin, A.J.; Benesch, J.L.P. Small heat-shock proteins: Paramedics of the cell. In Molecular Chaperones; Topics in Current Chemistry; Jackson, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 328, pp. 69–98. [Google Scholar]
- Ciocca, D.R.; Calderwood, S.K. Heat shock proteins in cancer: Diagnostic, prognostic, Predictive, and Treatment Implications. Cell Stress Chaperones 2005, 10, 86–103. [Google Scholar] [CrossRef]
- Muranov, K.O.; Ostrovsky, M.A. Biochemistry of eye lens in the norm and in cataractogenesis. Biochemistry 2022, 87, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.R.; Lubsen, N.H.; Slingsby, C. sHSP in the eye lens: Crystallin mutations, cataract and proteostasis. Int. J. Biochem. Cell Biol. 2012, 44, 1687–1697. [Google Scholar] [CrossRef]
- Makley, L.N.; McMenimen, K.A.; DeVree, B.T.; Goldman, J.W.; McGlasson, B.N.; Rajagopal, P.; Dunyak, B.M.; McQuade, T.J.; Thompson, A.D.; Sunahara, R.; et al. Pharmacological Chaperone for α-Crystallin Partially Restores Transparency in Cataract Models. Science 2015, 350, 674–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerasimovich, E.S.; Strelkov, S.V.; Gusev, N.B. Some properties of three aB-crystallin mutants carrying point substitutions in the C-terminal domain and associated with congenital diseases. Biochimie 2017, 142, 168–178. [Google Scholar] [CrossRef]
- Nefedova, V.V.; Muranova, L.K.; Sudnitsyna, M.V.; Ryzhavskaya, A.S.; Gusev, N.B. Small heat shock proteins and distal hereditary neuropathies. Biochemistry 2015, 80, 1734–1747. [Google Scholar] [CrossRef]
- Sudnitsyna, M.V.; Mymrikov, E.V.; Seit-Nebi, A.S.; Gusev, N.B. The role of Intrinsically disordered regions in the structure and functioning of small heat shock proteins. Curr. Protein Pept. Sci. 2012, 13, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Mogk, A.; Ruger-Herreros, C.; Bukau, B. Cellular functions and mechanisms of action of small heat shock proteins. Annu. Rev. Microbiol. 2019, 73, 89–110. [Google Scholar] [CrossRef]
- Reinle, K.; Mogk, A.; Bukau, B. The diverse functions of small heat shock proteins in the proteostasis network. J. Mol. Biol. 2022, 434, 167157. [Google Scholar] [CrossRef]
- Delbecq, S.P.; Klevit, R.E. One size does not fit all: The oligomeric states of aB crystallin. FEBS Lett. 2013, 587, 1073–1080. [Google Scholar] [CrossRef] [Green Version]
- Carver, J.A.; Grosas, A.B.; Ecroyd, H.; Quinlan, R.A. The functional roles of the unstructured N- and C-terminal regions in aB-crystallin and other mammalian small heat-shock proteins. Cell Stress Chaperones 2017, 22, 627–638. [Google Scholar] [CrossRef]
- Delbecq, S.P.; Rosenbaum, J.C.; Klevit, R.E. A Mechanism of subunit recruitment in human small heat shock protein oligomers. Biochemistry 2015, 54, 4276–4284. [Google Scholar] [CrossRef] [Green Version]
- Shatov, V.M.; Strelkov, S.V.; Gusev, N.B. The heterooligomerization of human small heat shock proteins Is controlled by conserved motif located in the N-terminal domain. Int. J. Mol. Sci. 2020, 21, 4248. [Google Scholar] [CrossRef] [PubMed]
- Mchaourab, H.S.; Godar, J.A.; Stewart, P.L. Structure and mechanism of protein stability sensors: Chaperone activity of small heat shock proteins. Biochemistry 2009, 48, 3828–3837. [Google Scholar] [CrossRef] [Green Version]
- Inoue, R.; Takata, T.; Fujii, N.; Ishii, K.; Uchiyama, S.; Sato, N.; Oba, Y.; Wood, K.; Kato, K.; Fujii, N.; et al. New insight into the dynamical system of aB-crystallin oligomers. Sci. Rep. 2016, 6, 29208. [Google Scholar] [CrossRef] [Green Version]
- Sprague-Piercy, M.A.; Rocha, M.A.; Kwok, A.O.; Martin, R.W. α-Crystallins in the vertebrate eye lens: Complex oligomers and molecular chaperones. Annu. Rev. Phys. Chem. 2021, 72, 143–163. [Google Scholar] [CrossRef]
- Hochberg, G.K.A.; Benesch, J.L.P. Dynamical structure of aB-crystallin. Prog. Biophys. Mol. Biol. 2014, 115, 11–20. [Google Scholar] [CrossRef]
- Braun, N.; Zacharias, M.; Peschek, J.; Kastenmüller, A.; Zou, J.; Hanzlik, M.; Haslbeck, M.; Rappsilber, J.; Buchner, J.; Weinkauf, S. Multiple molecular architectures of the eye lens chaperone aB-crystallin elucidated by a triple hybrid approach. Proc. Natl. Acad. Sci. USA 2011, 108, 20491–20496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chebotareva, N.A.; Eronina, T.B.; Roman, S.G.; Mikhaylova, V.V.; Sluchanko, N.N.; Gusev, N.B.; Kurganov, B.I. Oligomeric state of aB-crystallin under crowded conditions. Biochem. Biophys. Res. Commun. 2019, 508, 1101–1105. [Google Scholar] [CrossRef]
- Stengel, F.; Baldwin, A.J.; Painter, A.J.; Jaya, N.; Basha, E.; Kay, L.E.; Vierling, E.; Robinson, C.V.; Benesch, J.L.P. Quaternary dynamics and plasticity underlie small heat shock protein chaperone function. Proc. Natl. Acad. Sci. USA 2010, 107, 2007–2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, J.; Carver, J.A. The multifaceted nature of aB-crystallin. Cell Stress Chaperones 2020, 25, 639–654. [Google Scholar] [CrossRef]
- Mymrikov, E.V.; Seit-Nebi, A.S.; Gusev, N.B. Heterooligomeric complexes of human small heat shock proteins. Cell Stress Chaperones 2012, 17, 157–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mymrikov, E.V.; Riedl, M.; Peters, C.; Weinkauf, S.; Haslbeck, M.; Buchner, J. Regulation of small heat-shock proteins by hetero-oligomer formation. J. Biol. Chem. 2020, 295, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Alderson, T.R.; Roche, J.; Gastall, H.Y.; Dias, D.M.; Pritišanac, I.; Ying, J.; Bax, A.; Benesch, J.L.P.; Baldwin, A.J. Local unfolding of the HSP27 monomer regulates chaperone activity. Nat. Commun. 2019, 10, 1068. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Ghosh, J.G.; Clark, J.I.; Jiang, S. Studies of aB crystallin subunit dynamics by surface plasmon resonance. Anal. Biochem. 2006, 350, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Chebotareva, N.A.; Eronina, T.B.; Sluchanko, N.N.; Kurganov, B.I. Effect of Ca2+ and Mg2+ ions on oligomeric state and chaperone-like activity of aB-crystallin in crowded media. Int. J. Biol. Macromol. 2015, 76, 86–93. [Google Scholar] [CrossRef]
- Ecroyd, H.; Meehan, S.; Horwitz, J.; Aquilina, J.A.; Benesch, J.L.P.; Robinson, C.V.; Macphee, C.E.; Carver, J.A. Mimicking phosphorylation of aB-crystallin affects Its chaperone activity. Biochem. J. 2006, 401, 129–141. [Google Scholar] [CrossRef] [Green Version]
- Jovcevski, B.; Kelly, M.A.; Rote, A.P.; Berg, T.; Gastall, H.Y.; Benesch, J.L.P.; Aquilina, J.A.; Ecroyd, H. Phosphomimics destabilize Hsp27 oligomeric assemblies and enhance chaperone activity. Chem. Biol. 2015, 22, 186–195. [Google Scholar] [CrossRef]
- Muranova, L.K.; Sudnitsyna, M.V.; Gusev, N.B. aB-Crystallin phosphorylation: Advances and problems. Biochemistry 2018, 83, 1196–1206. [Google Scholar] [CrossRef]
- Roman, S.G.; Chebotareva, N.A.; Kurganov, B.I. Anti-aggregation activity of small heat shock proteins under crowded conditions. Int. J. Biol. Macromol. 2017, 100, 97–103. [Google Scholar] [CrossRef]
- Fonin, A.V.; Darling, A.L.; Kuznetsova, I.M.; Turoverov, K.K.; Uversky, V.N. Intrinsically disordered proteins in crowded milieu: When chaos prevails within the cellular gumbo. Cell. Mol. Life Sci. 2018, 75, 3907–3929. [Google Scholar] [CrossRef]
- Kuznetsova, I.M.; Turoverov, K.K.; Uversky, V.N. What macromolecular crowding can do to a protein. Int. J. Mol. Sci. 2014, 15, 23090–23140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chebotareva, N.A.; Roman, S.G.; Borzova, V.A.; Eronina, T.B.; Mikhaylova, V.V.; Kurganov, B.I. Chaperone-like activity of HSPB5: The effects of quaternary structure dynamics and crowding. Int. J. Mol. Sci. 2020, 21, 4940. [Google Scholar] [CrossRef]
- Zimmerman, S.B.; Trach, S.O. Estimation of macromolecule concentrations and excluded volume effects for the cytoplasm of Escherichia coli. J. Mol. Biol. 1991, 222, 599–620. [Google Scholar] [CrossRef] [Green Version]
- Zimmerman, S.B.; Minton, A.P. Macromolecular crowding: Biochemical, biophysical, and physiological consequences. Annu. Rev. Biophys. Biomol. Struct. 1993, 22, 27–65. [Google Scholar] [CrossRef]
- Minton, A.P.; Wilf, J. Effect of macromolecular crowding upon the structure and function of an enzyme: Glyceraldehyde-3-phosphate dehydrogenase. Biochemistry 1981, 20, 4821–4826. [Google Scholar] [CrossRef]
- Ellis, R.J. Macromolecular crowding: Obvious but underappreciated. Trends Biochem. Sci. 2001, 26, 597–604. [Google Scholar] [CrossRef]
- Minton, A.P. The influence of macromolecular crowding and macromolecular confinement on biochemical reactions in physiological media. J. Biol. Chem. 2001, 276, 10577–10580. [Google Scholar] [CrossRef] [Green Version]
- Chebotareva, N.A.; Kurganov, B.I.; Livanova, N.B. Biochemical effects of molecular crowding. Biochemistry 2004, 69, 1239–1251. [Google Scholar] [CrossRef]
- Ellis, R.J.; Minton, A.P. Protein aggregation in crowded environments. Biol. Chem. 2006, 387, 485–497. [Google Scholar] [CrossRef]
- Zhou, H.-X.; Rivas, G.; Minton, A.P. Macromolecular crowding and confinement: Biochemical, biophysical, and potential physiological consequences. Annu. Rev. Biophys. 2008, 37, 375–397. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.J. Protein Aggregation: Opposing effects of chaperones and crowding. In Folding for the Synapse; Wyttenbach, A., O’Connor, V., Eds.; Springer: Boston, MA, USA, 2011; pp. 9–34. [Google Scholar]
- Dewavrin, J.-Y.; Abdurrahiem, M.; Blocki, A.; Musib, M.; Piazza, F.; Raghunath, M. Synergistic rate boosting of collagen fibrillogenesis in heterogeneous mixtures of crowding agents. J. Phys. Chem. B 2015, 119, 4350–4358. [Google Scholar] [CrossRef] [Green Version]
- Shah, D.; Tan, A.L.; Ramakrishnan, V.; Jiang, J.; Rajagopalan, R. Effects of polydisperse crowders on aggregation reactions: A molecular thermodynamic analysis. J. Chem. Phys. 2011, 134, 064704. [Google Scholar] [CrossRef]
- Du, F.; Zhou, Z.; Mo, Z.-Y.; Shi, J.-Z.; Chen, J.; Liang, Y. Mixed macromolecular crowding accelerates the refolding of rabbit muscle creatine kinase: Implications for protein folding in physiological environments. J. Mol. Biol. 2006, 364, 469–482. [Google Scholar] [CrossRef]
- Batra, J.; Xu, K.; Zhou, H.-X. Nonadditive effects of mixed crowding on protein stability. Proteins Struct. Funct. Bioinformatics 2009, 77, 133–138. [Google Scholar] [CrossRef] [Green Version]
- Phillip, Y.; Schreiber, G. Formation of protein complexes in crowded environments—From in vitro to in vivo. FEBS Lett. 2013, 587, 1046–1052. [Google Scholar] [CrossRef] [Green Version]
- Nakano, S.; Miyoshi, D.; Sugimoto, N. Effects of molecular crowding on the structures, interactions, and functions of nucleic acids. Chem. Rev. 2014, 114, 2733–2758. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, M.; Smith, A.E.; Pielak, G.J. Impact of reconstituted cytosol on protein stability. Proc. Natl. Acad. Sci. USA 2013, 110, 19342–19347. [Google Scholar] [CrossRef] [Green Version]
- Mittal, S.; Chowhan, R.K.; Singh, L.R. Macromolecular crowding: Macromolecules friend or foe. Biochim. Biophys. Acta 2015, 1850, 1822–1831. [Google Scholar] [CrossRef] [PubMed]
- Stepanenko, O.V.; Povarova, O.I.; Sulatskaya, A.I.; Ferreira, L.A.; Zaslavsky, B.Y.; Kuznetsova, I.M.; Turoverov, K.K.; Uversky, V.N. Protein unfolding in crowded milieu: What crowding can do to a protein undergoing unfolding? J. Biomol. Struct. Dyn. 2016, 34, 2155–2170. [Google Scholar] [CrossRef] [PubMed]
- Shahid, S.; Hassan, M.I.; Islam, A.; Ahmad, F. Size-dependent studies of macromolecular crowding on the thermodynamic stability, structure and functional activity of proteins: In vitro and in silico approaches. Biochim. Biophys. Acta 2017, 1861, 178–197. [Google Scholar] [CrossRef] [PubMed]
- Guseman, A.J.; Perez Goncalves, G.M.; Speer, S.L.; Young, G.B.; Pielak, G.J. Protein shape modulates crowding effects. Proc. Natl. Acad. Sci. USA 2018, 115, 10965–10970. [Google Scholar] [CrossRef] [Green Version]
- Sapir, L.; Harries, D. Macromolecular stabilization by excluded cosolutes: Mean field theory of crowded solutions. J. Chem. Theory Comput. 2015, 11, 3478–3490. [Google Scholar] [CrossRef] [PubMed]
- Chebotareva, N.A.; Makeeva, V.F.; Bazhina, S.G.; Eronina, T.B.; Gusev, N.B.; Kurganov, B.I. Interaction of Hsp27 with native phosphorylase kinase under crowding conditions. Macromol. Biosci. 2010, 10, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Roman, S.G.; Chebotareva, N.A.; Eronina, T.B.; Kleymenov, S.Y.; Makeeva, V.F.; Poliansky, N.B.; Muranov, K.O.; Kurganov, B.I. Does the crowded cell-like environment reduce the chaperone-like activity of α-crystallin? Biochemistry 2011, 50, 10607–10623. [Google Scholar] [CrossRef] [PubMed]
- Chebotareva, N.A.; Eronina, T.B.; Roman, S.G.; Poliansky, N.B.; Muranov, K.O.; Kurganov, B.I. Effect of crowding and chaperones on self-association, aggregation and reconstitution of apophosphorylase b. Int. J. Biol. Macromol. 2013, 60, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Sluchanko, N.N.; Chebotareva, N.A.; Gusev, N.B. Quaternary structure of human small heat shock protein HSPB6 (Hsp20) in crowded media modeled by trimethylamine N-oxide (TMAO): Effect of protein phosphorylation. Biochimie 2015, 108, 68–75. [Google Scholar] [CrossRef]
- Sharma, G.S.; Krishna, S.; Khan, S.; Dar, T.A.; Khan, K.A.; Singh, L.R. Protecting thermodynamic stability of protein: The basic paradigm against stress and unfolded protein response by osmolytes. Int. J. Biol. Macromol. 2021, 177, 229–240. [Google Scholar] [CrossRef]
- Caldas, T.; Demont-Caulet, N.; Ghazi, A.; Richarme, G.Y. 1999 Thermoprotection by glycine betaine and choline. Microbiology 1999, 145, 2543–2548. [Google Scholar] [CrossRef] [Green Version]
- Rydeen, A.E.; Brustad, E.M.; Pielak, G.J. Osmolytes and protein–protein interactions. J. Am. Chem. Soc. 2018, 140, 7441–7444. [Google Scholar] [CrossRef]
- Chebotareva, N.A.; Harding, S.E.; Winzor, D.J. Ultracentrifugal studies of the effect of molecular crowding by trimethylamine N-oxide on the self-association of muscle glycogen phosphorylase b. Eur. J. Bioch. 2001, 268, 506–513. [Google Scholar] [CrossRef]
- Sharp, K.A. Analysis of the size dependence of macromolecular crowding shows that smaller is better. Proc. Natl. Acad. Sci. USA 2015, 112, 7990–7995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahid, S.; Ahmad, F.; Hassan, M.I.; Islam, A. Relationship between protein stability and functional activity in the presence of macromolecular crowding agents alone and in mixture: An insight into stability-activity trade-off. Arch. Biochem. Biophys. 2015, 584, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Fisher, H.F. L-Glutamate Dehydrogenase from Bovine Liver. In Methods in Enzymology; Glutamate, Glutamine, Glutathione, and Related Compounds; Meister, A., Ed.; Academic Press: Cambridge, MA, USA, 1985; Volume 113, pp. 16–27. [Google Scholar]
- Schachman, H.K.; Cassman, M. Sedimentation equilibrium studies on glutamic dehydrogenase. Biochemistry 1971, 10, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Oh, J.Y.; Maeng, P.J. The NADP+-dependent glutamate dehydrogenase Gdh1 is subjected to glucose starvation-induced reversible aggregation that affects stress resistance in yeast. J. Microbiol. 2019, 57, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Liu, Z.; Fisher, H.F. The existence of a hexameric intermediate with molten-globule-like properties in the thermal denaturation of bovine-liver glutamate dehydrogenase. Biophys. Chem. 1996, 63, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Borzova, V.A.; Chebotareva, N.A.; Sluchanko, N.N.; Kleymenov, S.Y.; Markossian, K.A.; Kurganov, B.I. The mechanism of thermal aggregation of glutamate dehydrogenase. The effect of chemical chaperones. Biochimie 2022, 195, 27–38. [Google Scholar] [CrossRef]
- Morrison, H. Chapter 22—Lysozyme. In Enzyme Active Sites and their Reaction Mechanisms; Morrison, H., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 121–127. [Google Scholar]
- Guez, V.; Roux, P.; Navon, A.; Goldberg, M.E. Role of individual disulfide bonds in hen lysozyme early folding steps. Protein Sci. 2002, 11, 1136–1151. [Google Scholar] [CrossRef]
- Cao, A.; Hu, D.; Lai, L. Formation of amyloid fibrils from fully reduced hen egg white lysozyme. Protein Sci. 2004, 13, 319–324. [Google Scholar] [CrossRef] [PubMed]
- White, F.H., Jr. Studies on the relationship of disulfide bonds to the formation and maintenance of secondary structure in chicken egg white lysozyme. Biochemistry 1982, 21, 967–977. [Google Scholar] [CrossRef]
- Yang, M.; Dutta, C.; Tiwari, A. Disulfide-bond scrambling promotes amorphous aggregates in lysozyme and bovine serum Albumin. J. Phys. Chem. B 2015, 119, 3969–3981. [Google Scholar] [CrossRef] [PubMed]
- Datskevich, P.N.; Gusev, N.B. Structure and properties of chimeric small heat shock proteins containing yellow fluorescent protein attached to their C-terminal ends. Cell Stress Chaperones 2014, 19, 507–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurganov, B.I. Quantification of anti-aggregation activity of chaperones. Int. J. Biol. Macromol. 2017, 100, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Mikhaylova, V.V.; Eronina, T.B.; Chebotareva, N.A.; Shubin, V.V.; Kalacheva, D.I.; Kurganov, B.I. Effect of arginine on chaperone-like activity of HspB6 and monomeric 14-3-3ζ. Int. J. Mol. Sci. 2020, 21, 2039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haslbeck, M.; Peschek, J.; Buchner, J.; Weinkauf, S. Structure and function of α-crystallins: Traversing from in vitro to in vivo. Biochim. Biophys. Acta 2016, 1860, 149–166. [Google Scholar] [CrossRef]
- Benesch, J.L.P.; Ayoub, M.; Robinson, C.V.; Aquilina, J.A. Small heat shock protein activity is regulated by variable oligomeric substructure. J. Biol. Chem. 2008, 283, 28513–28517. [Google Scholar] [CrossRef] [Green Version]
- Aquilina, J.A.; Benesch, J.L.P.; Bateman, O.A.; Slingsby, C.; Robinson, C.V. Polydispersity of a mammalian chaperone: Mass spectrometry reveals the population of oligomers in aB-crystallin. Proc. Natl. Acad. Sci. USA 2003, 100, 10611–10616. [Google Scholar] [CrossRef] [Green Version]
- Doss, E.W.; Ward, K.A.; Koretz, J.F. Preliminary studies on the aggregation process of alpha-crystallin. Exp. Eye Res. 1997, 65, 255–266. [Google Scholar] [CrossRef]
- Gonçalves, C.C.; Sharon, I.; Schmeing, T.M.; Ramos, C.H.I.; Young, J.C. The chaperone HSPB1 prepares protein aggregates for resolubilization by HSP70. Sci. Rep. 2021, 11, 17139. [Google Scholar] [CrossRef]
- Bumagina, Z.; Gurvits, B.; Artemova, N.; Muranov, K.; Kurganov, B. Paradoxical acceleration of dithiothreitol-Induced aggregation of insulin in the presence of a chaperone. Int. J. Mol. Sci. 2010, 11, 4556–4579. [Google Scholar] [CrossRef]
- Mainz, A.; Peschek, J.; Stavropoulou, M.; Back, K.C.; Bardiaux, B.; Asami, S.; Prade, E.; Peters, C.; Weinkauf, S.; Buchner, J.; et al. The chaperone aB-crystallin uses different interfaces to capture an amorphous and an amyloid client. Nat. Struct. Mol. Biol. 2015, 22, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Mymrikov, E.V.; Daake, M.; Richter, B.; Haslbeck, M.; Buchner, J. The Chaperone Activity and Substrate Spectrum of Human Small Heat Shock Proteins. J. Biol. Chem. 2017, 292, 672–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexandrov, A.I.; Grosfeld, E.V.; Dergalev, A.A.; Kushnirov, V.V.; Chuprov-Netochin, R.N.; Tyurin-Kuzmin, P.A.; Kireev, I.I.; Ter-Avanesyan, M.D.; Leonov, S.V.; Agaphonov, M.O. Analysis of novel hyperosmotic shock response suggests ‘beads in liquid’ cytosol structure. Biol. Open 2019, 8, bio044529. [Google Scholar] [CrossRef] [Green Version]
- Nasiri, P.; Ghahramani, M.; Tavaf, Z.; Niazi, A.; Moosavi-Movahedi, A.A.; Kurganov, B.I.; Yousefi, R. The biochemical association between R157H mutation in human aB-crystallin and development of cardiomyopathy: Structural and functional analyses of the mutant protein. Biochimie 2021, 190, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Mymrikov, E.V.; Bukach, O.V.; Seit-Nebi, A.S.; Gusev, N.B. The pivotal role of the Β7 strand in the intersubunit contacts of different human small heat shock proteins. Cell Stress Chaperones 2010, 15, 365–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabbaghian, M.; Ebrahim-Habibi, A.; Nemat-Gorgani, M. Thermal aggregation of a model allosteric protein in different conformational states. Int. J. Biol. Macromol. 2009, 44, 156–162. [Google Scholar] [CrossRef]
- Spinozzi, F.; Ortore, M.G.; Sinibaldi, R.; Mariani, P.; Esposito, A.; Cinelli, S.; Onori, G. Microcalorimetric study of thermal unfolding of lysozyme in water/glycerol mixtures: An analysis by solvent exchange model. J. Chem. Phys. 2008, 129, 035101. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.H.; Schuck, P. Macromolecular size-and-shape distributions by sedimentation velocity analytical ultracentrifugation. Biophys. J. 2006, 90, 4651–4661. [Google Scholar] [CrossRef]

| Sample | γagg (%) |
|---|---|
| GDH (0.2 mg/mL) | 58 |
| GDH + Bet (1 M) | 0 |
| GDH + Bet (1 M) + PEG (25 mg/mL) | 50 |
| GDH + Bet (1 M) + PEG (25 mg/mL) + HSPB5 (0.05 mg/mL) | 0 |
| GDH + Bet (1 M) + HSPB5 (0.05 mg/mL) | 0 |
| GDH + PEG (25 mg/mL) + HSPB5 (0.05 mg/mL) | 0 |
| GDH + HSPB5 (0.05 mg/mL) | 57 |
| GDH + PEG (25 mg/mL) | 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borzova, V.A.; Roman, S.G.; Pivovarova, A.V.; Chebotareva, N.A. Effects of Molecular Crowding and Betaine on HSPB5 Interactions, with Target Proteins Differing in the Quaternary Structure and Aggregation Mechanism. Int. J. Mol. Sci. 2022, 23, 15392. https://doi.org/10.3390/ijms232315392
Borzova VA, Roman SG, Pivovarova AV, Chebotareva NA. Effects of Molecular Crowding and Betaine on HSPB5 Interactions, with Target Proteins Differing in the Quaternary Structure and Aggregation Mechanism. International Journal of Molecular Sciences. 2022; 23(23):15392. https://doi.org/10.3390/ijms232315392
Chicago/Turabian StyleBorzova, Vera A., Svetlana G. Roman, Anastasiya V. Pivovarova, and Natalia A. Chebotareva. 2022. "Effects of Molecular Crowding and Betaine on HSPB5 Interactions, with Target Proteins Differing in the Quaternary Structure and Aggregation Mechanism" International Journal of Molecular Sciences 23, no. 23: 15392. https://doi.org/10.3390/ijms232315392
APA StyleBorzova, V. A., Roman, S. G., Pivovarova, A. V., & Chebotareva, N. A. (2022). Effects of Molecular Crowding and Betaine on HSPB5 Interactions, with Target Proteins Differing in the Quaternary Structure and Aggregation Mechanism. International Journal of Molecular Sciences, 23(23), 15392. https://doi.org/10.3390/ijms232315392






