Switchable Nanozyme Activity of Porphyrins Intercalated in Layered Gadolinium Hydroxide
Abstract
:1. Introduction
2. Results and Discussion
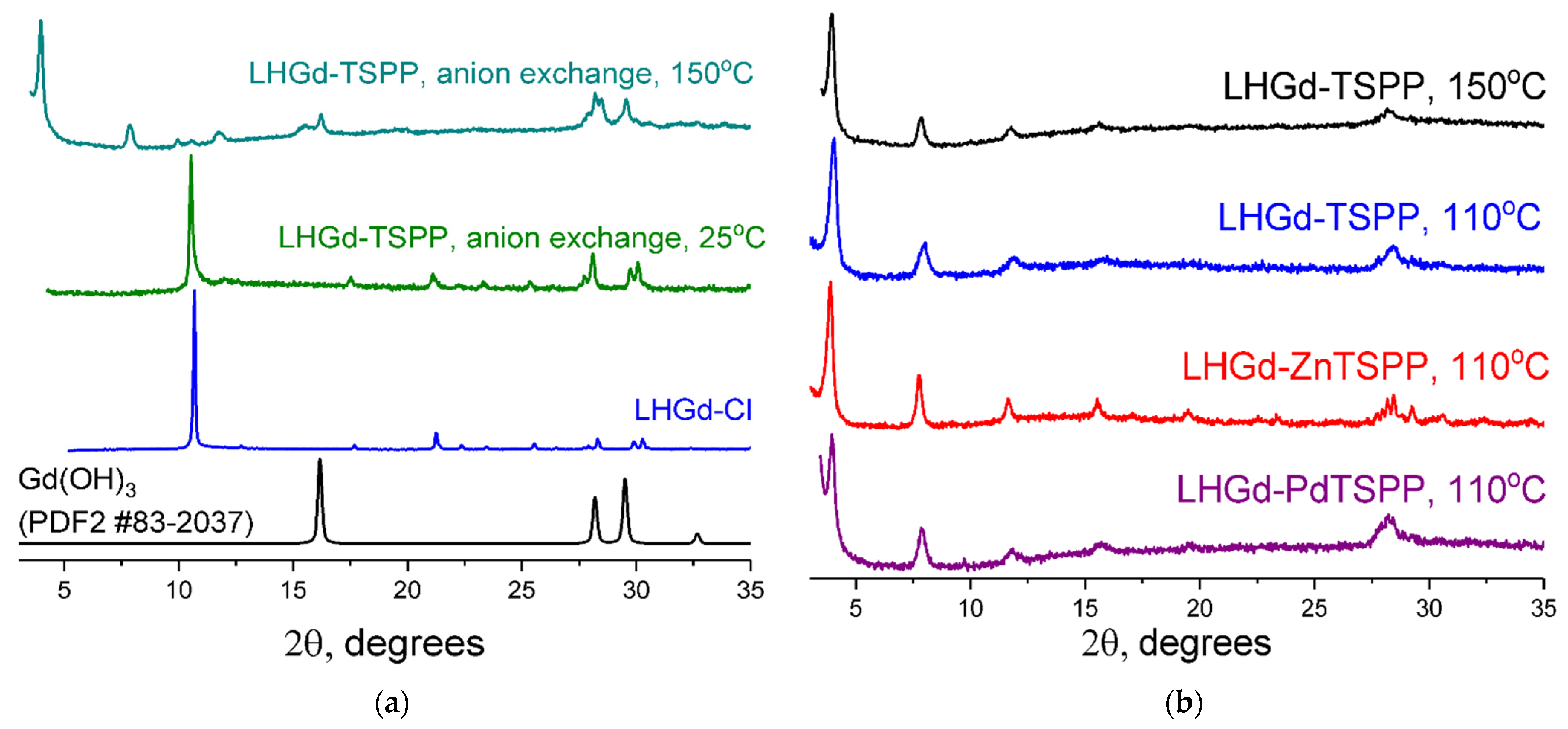
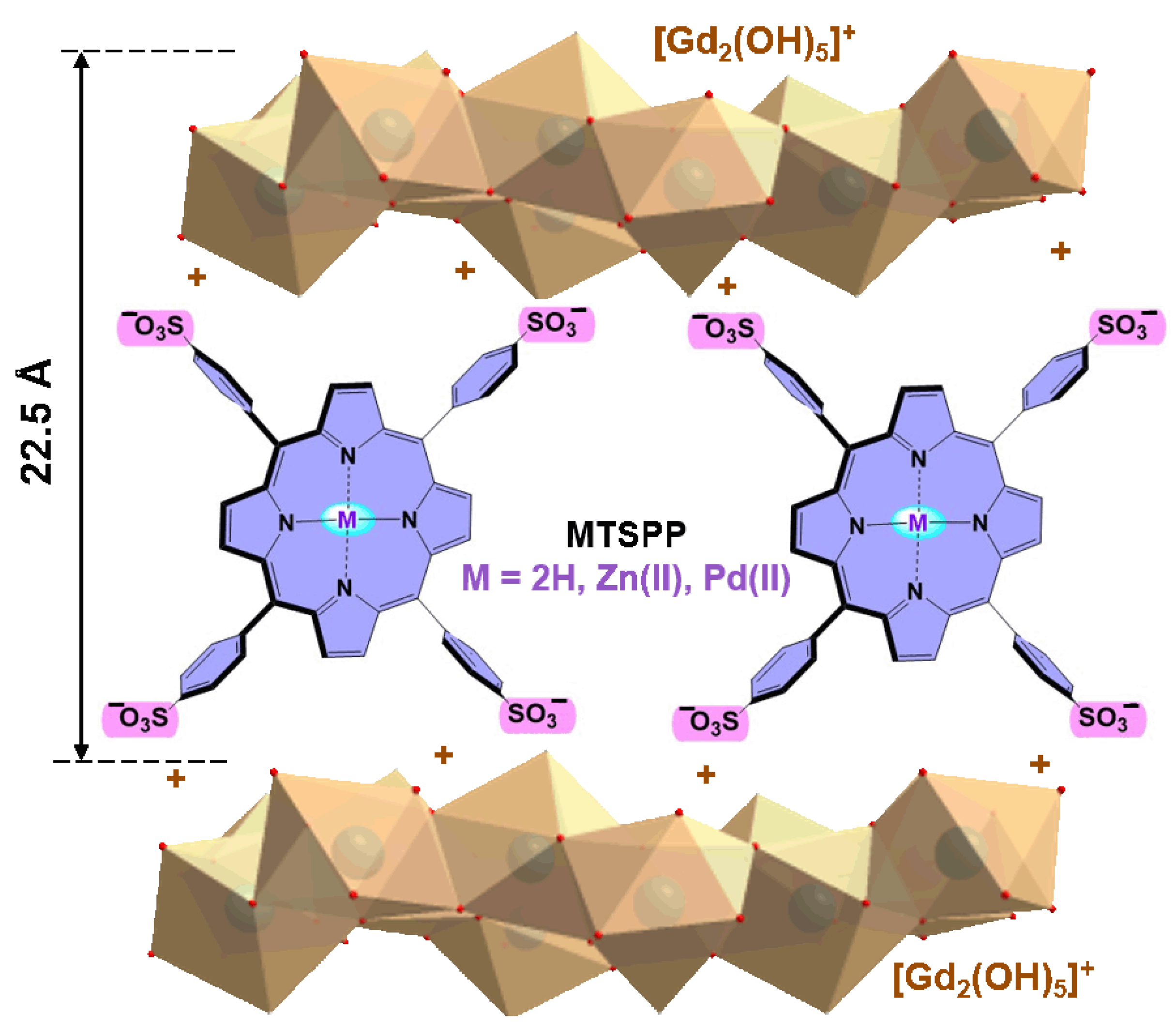
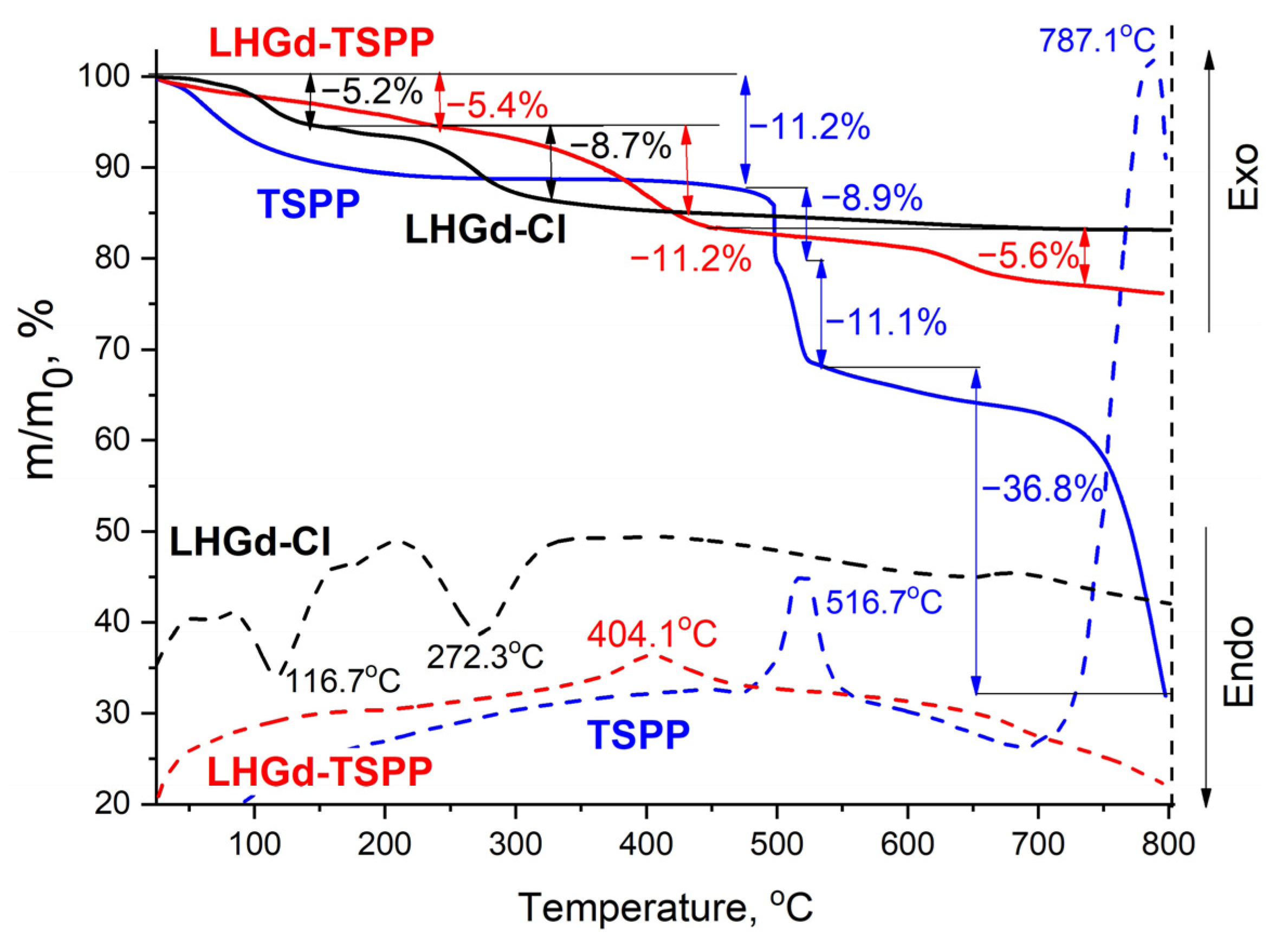
2.1. Preparation and Characterisation of LHGd Intercalated with Porphyrin
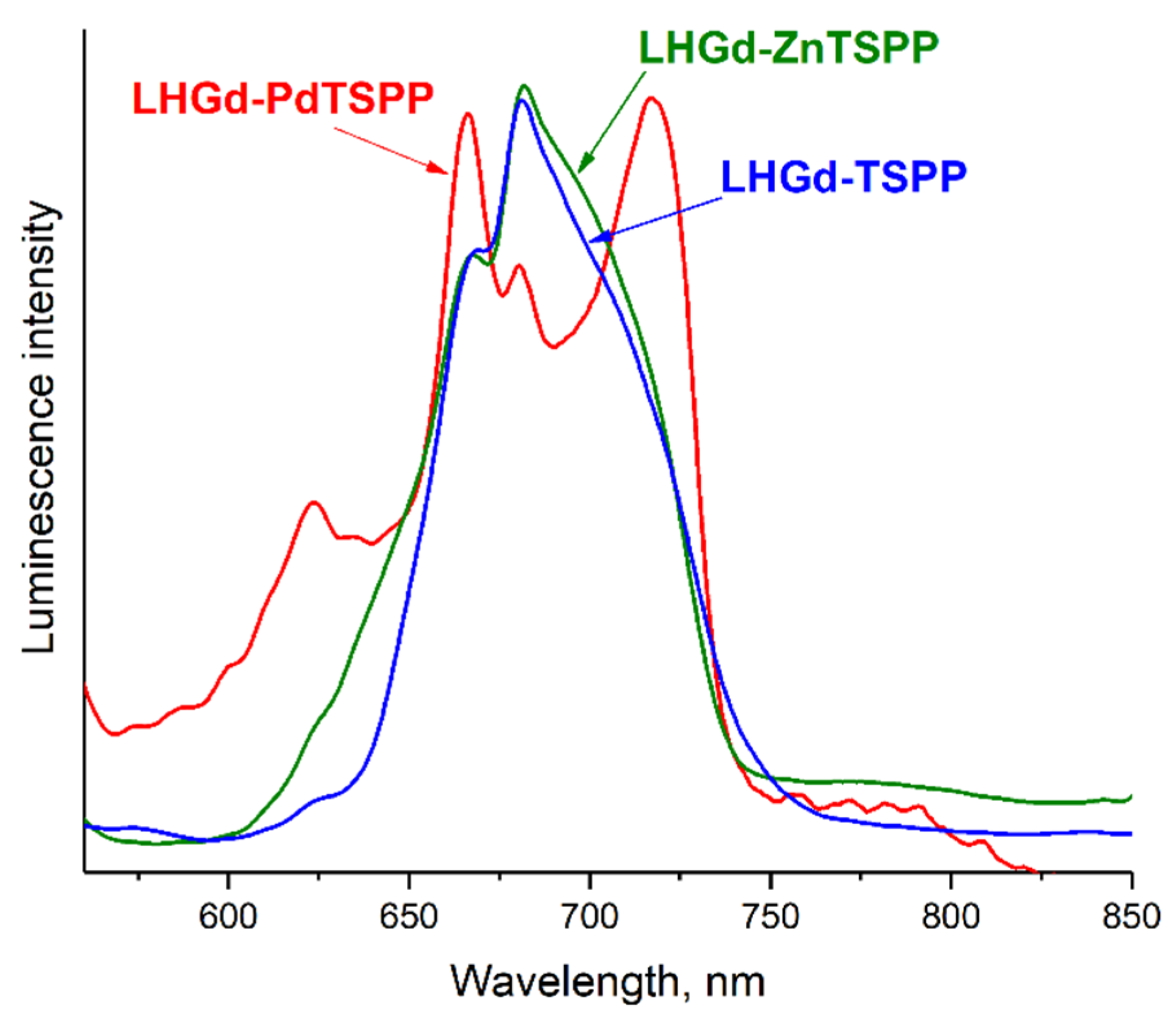
2.2. Optical Properties of the LHGd Intercalated with Porphyrin
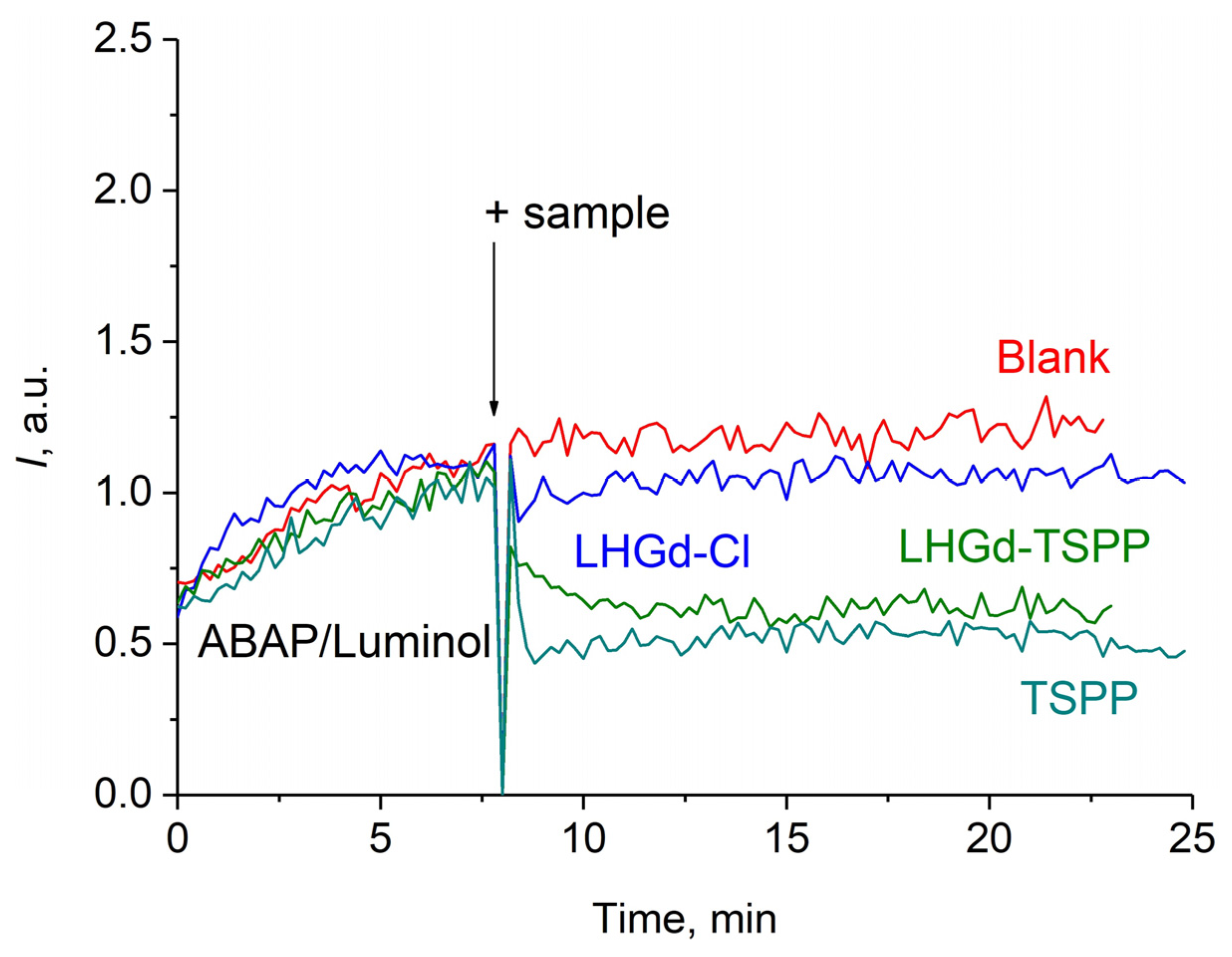
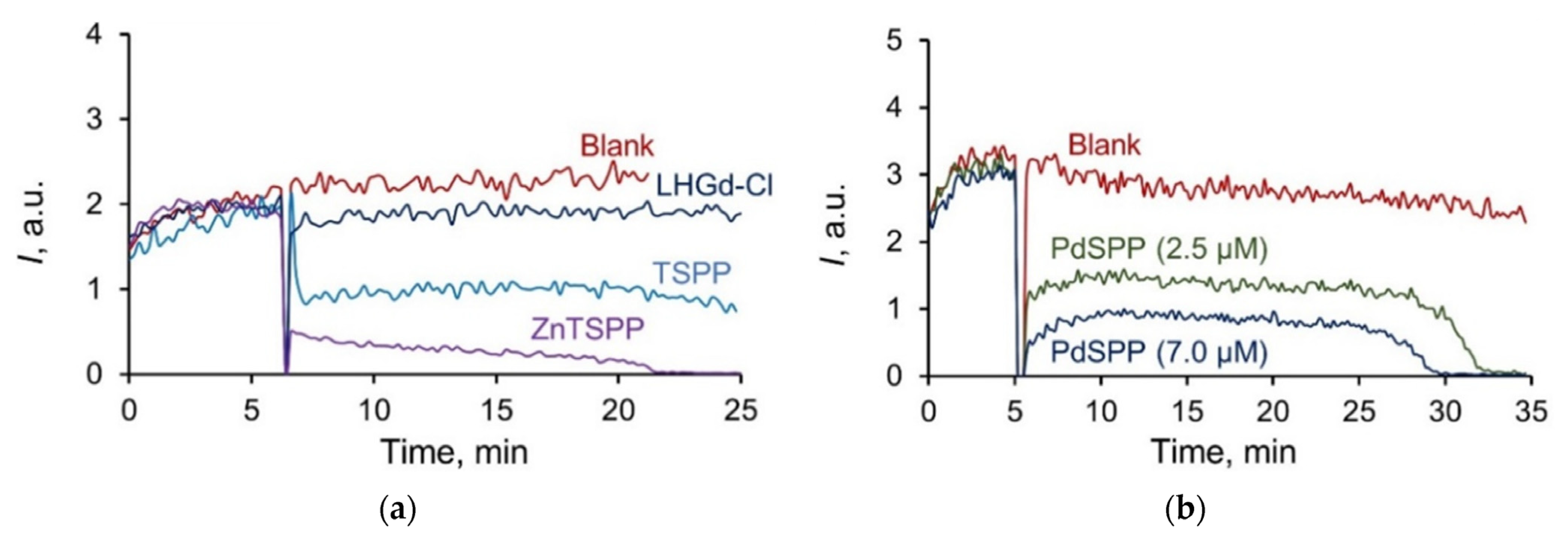
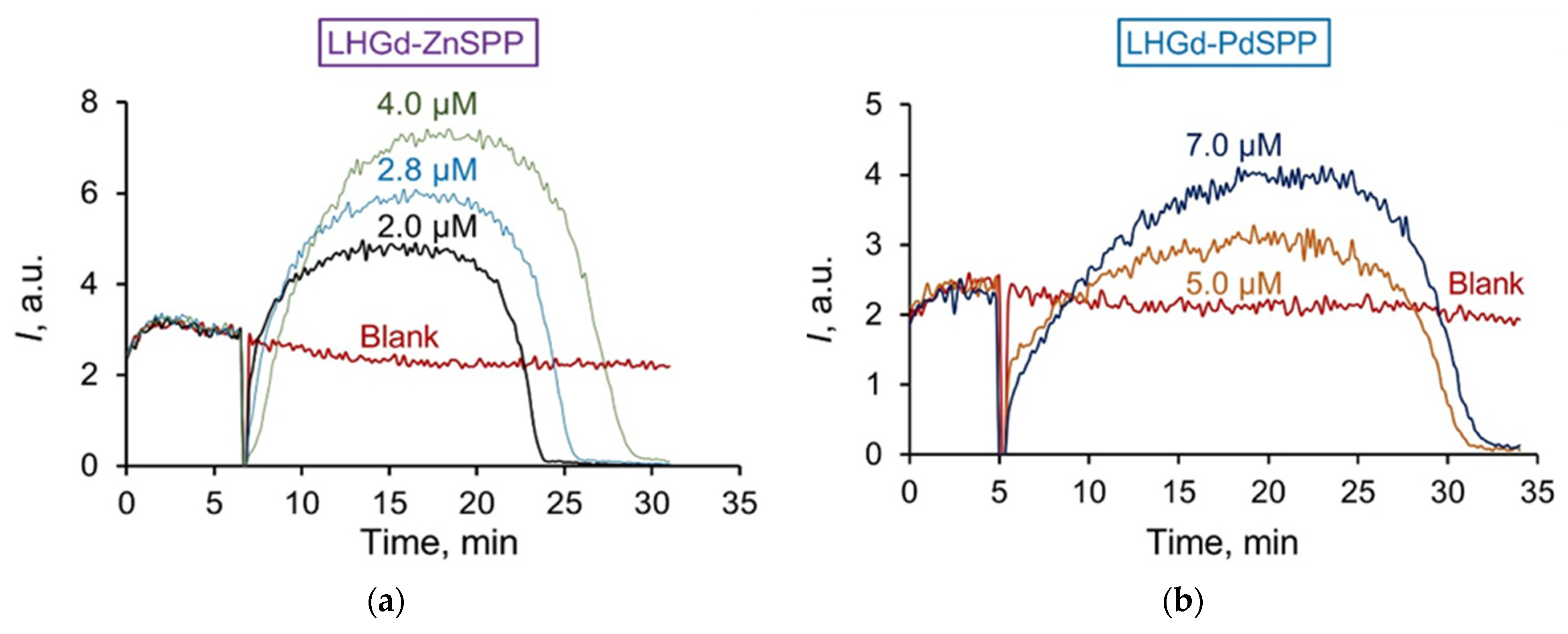
2.3. Radical Scavenging Properties of the LHGd Intercalated with TSPP, ZnTSPP, and PdTSPP
3. Materials and Methods
3.1. Materials
3.2. Porphyrin Synthesis
3.3. Gd(III) Layered Hydroxochloride (LHGd-Cl) Synthesis
3.4. Preparation of LHGd-Porphyrin Hybrid Materials (LHGd-MTSPP)
3.4.1. General Procedure for Anion Exchange Reactions
3.4.2. General Procedure for the Coprecipitation Method
3.5. Instrumental Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jouyban, A.; Amini, R. Layered Double Hydroxides as an Efficient Nanozyme for Analytical Applications. Microchem. J. 2021, 164, 105970. [Google Scholar] [CrossRef]
- Yan, X. (Ed.) Nanozymology; Nanostructure Science and Technology; Springer: Singapore, 2020; ISBN 978-981-15-1489-0. [Google Scholar]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with Enzyme-like Characteristics (Nanozymes): Next-Generation Artificial Enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wei, H.; Zhang, Z.; Wang, E.; Dong, S. Nanozyme: An Emerging Alternative to Natural Enzyme for Biosensing and Immunoassay. TrAC Trends Anal. Chem. 2018, 105, 218–224. [Google Scholar] [CrossRef]
- Wang, H.; Wan, K.; Shi, X. Recent Advances in Nanozyme Research. Adv. Mater. 2019, 31, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic Peroxidase-like Activity of Ferromagnetic Nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Derakhshankhah, H.; Jafari, S.; Salatin, S.; Dehghanian, M.; Falahati, M.; Ansari, A. Nanozyme Antioxidants as Emerging Alternatives for Natural Antioxidants: Achievements and Challenges in Perspective. Nano Today 2019, 29, 100775. [Google Scholar] [CrossRef]
- Kang, T.; Kim, Y.G.; Kim, D.; Hyeon, T. Inorganic Nanoparticles with Enzyme-Mimetic Activities for Biomedical Applications. Coord. Chem. Rev. 2020, 403, 213092. [Google Scholar] [CrossRef]
- Alizadeh, N.; Salimi, A.; Hallaj, R. Mimicking Peroxidase-like Activity of Co3O4-CeO2 Nanosheets Integrated Paper-Based Analytical Devices for Detection of Glucose with Smartphone. Sens. Actuators B Chem. 2019, 288, 44–52. [Google Scholar] [CrossRef]
- Ma, W.; Xue, Y.; Guo, S.; Jiang, Y.; Wu, F.; Yu, P.; Mao, L. Graphdiyne Oxide: A New Carbon Nanozyme. Chem. Commun. 2020, 56, 5115–5118. [Google Scholar] [CrossRef]
- Sun, H.; Zhou, Y.; Ren, J.; Qu, X. Carbon Nanozymes: Enzymatic Properties, Catalytic Mechanism, and Applications. Angew. Chemie Int. Ed. 2018, 57, 9224–9237. [Google Scholar] [CrossRef]
- Wang, L.; Hu, Z.; Wu, S.; Pan, J.; Xu, X.; Niu, X. A Peroxidase-Mimicking Zr-Based MOF Colorimetric Sensing Array to Quantify and Discriminate Phosphorylated Proteins. Anal. Chim. Acta 2020, 1121, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Wang, Q.; Liu, Q.; Deng, X.; Liu, T.; Li, D.; Zhang, X. Porphyrin-Based Porous Organic Framework: An Efficient and Stable Peroxidase-Mimicking Nanozyme for Detection of H2O2 and Evaluation of Antioxidant. Sens. Actuators B Chem. 2018, 277, 86–94. [Google Scholar] [CrossRef]
- Yang, J.; Dai, H.; Sun, Y.; Wang, L.; Qin, G.; Zhou, J.; Chen, Q.; Sun, G. 2D Material–Based Peroxidase-Mimicking Nanozymes: Catalytic Mechanisms and Bioapplications. Anal. Bioanal. Chem. 2022, 414, 2971–2989. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.G.; Slade, C.T. Structural Aspects of Layered Double Hydroxides; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–87. [Google Scholar] [CrossRef]
- Laipan, M.; Yu, J.; Zhu, R.; Smith, A.T.; He, H.; Hare, D.O.; Su, L. Functionalized Layered Double Hydroxides for Innovative Applications. Mater. Horiz. 2019, 7, 715–745. [Google Scholar] [CrossRef]
- Fujita, W.; Awaga, K. Reversible Structural Transformation and Drastic Magnetic Change in a Copper Hydroxides Intercalation Compound. J. Am. Chem. Soc. 1997, 119, 4563–4564. [Google Scholar] [CrossRef]
- Yeganeh Ghotbi, M.; Feli, B.; Azadfalah, M.; Javaheri, M. Ultra High Performance N-Doped Carbon Catalysts for the ORR Derived from the Reaction between Organic-Nitrate Anions inside a Layered Nanoreactor. RSC Adv. 2015, 5, 92577–92584. [Google Scholar] [CrossRef]
- Yapryntsev, A.D.; Baranchikov, A.E.; Ivanov, V.K. Layered Rare-Earth Hydroxides: A New Family of Anion-Exchangeable Layered Inorganic Materials. Russ. Chem. Rev. 2020, 89, 629–666. [Google Scholar] [CrossRef]
- Ning, Y.; Zhu, M.; Zhang, J.-L. Near-Infrared (NIR) Lanthanide Molecular Probes for Bioimaging and Biosensing. Coord. Chem. Rev. 2019, 399, 213028. [Google Scholar] [CrossRef]
- Yu, X.-F.; Sun, Z.; Xiang, Y.; Cao, Z.; He, D.-F.; Wang, Q.-Q. Synthesis of Highly Luminescent and Anion-Exchangeable Cerium-Doped Layered Yttrium Hydroxides for Sensing and Photofunctional Applications. Adv. Funct. Mater. 2011, 21, 4388–4396. [Google Scholar] [CrossRef]
- Musumeci, A.W.; Xu, Z.P.; Smith, S.V.; Minchin, R.F.; Martin, D.J. Layered Double Hydroxide Nanoparticles Incorporating Terbium: Applicability as a Fluorescent Probe and Morphology Modifier. J. Nanoparticle Res. 2010, 12, 111–120. [Google Scholar] [CrossRef]
- Xu, Y.; Goyanes, A.; Wang, Y.; Weston, A.J.; So, P.W.; Geraldes, C.F.; Fogg, A.M.; Basit, A.W.; Williams, G.R. Layered Gadolinium Hydroxides for Simultaneous Drug Delivery and Imaging. Dalt. Trans. 2018, 47, 3166–3177. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.-I.; Lee, K.S.; Lee, J.H.; Lee, I.S.; Byeon, S.-H. Synthesis of Colloidal Aqueous Suspensions of a Layered Gadolinium Hydroxide: A Potential MRI Contrast Agent. Dalt. Trans. 2009, 14, 2490–2495. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-M.; Liu, J.-W.; Jiang, J.-H.; Zhong, W. Cobalt Oxyhydroxide Nanoflakes with Intrinsic Peroxidase Catalytic Activity and Their Application to Serum Glucose Detection. Anal. Bioanal. Chem. 2017, 409, 4225–4232. [Google Scholar] [CrossRef]
- Kong, X.; Jin, L.; Wei, M.; Duan, X. Antioxidant Drugs Intercalated into Layered Double Hydroxide: Structure and In Vitro Release. Appl. Clay Sci. 2010, 49, 324–329. [Google Scholar] [CrossRef]
- Silion, M.; Hritcu, D.; Lisa, G.; Popa, M.I. New Hybrid Materials Based on Layered Double Hydroxides and Antioxidant Compounds. Preparation, Characterization and Release Kinetic Studies. J. Porous Mater. 2012, 19, 267–276. [Google Scholar] [CrossRef]
- Lonkar, S.P.; Kutlu, B.; Leuteritz, A.; Heinrich, G. Nanohybrids of Phenolic Antioxidant Intercalated into MgAl-Layered Double Hydroxide Clay. Appl. Clay Sci. 2013, 71, 8–14. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, X.; Wang, X.; Iqbal, A.; Yang, C.; Liu, W.; Qin, W. Carbon Dot/NiAl-Layered Double Hydroxide Hybrid Material: Facile Synthesis, Intrinsic Peroxidase-like Catalytic Activity and Its Application. RSC Adv. 2015, 5, 95495–95503. [Google Scholar] [CrossRef]
- Chen, L.; Sun, B.; Wang, X.; Qiao, F.; Ai, S. 2D Ultrathin Nanosheets of Co–Al Layered Double Hydroxides Prepared in l-Asparagine Solution: Enhanced Peroxidase-like Activity and Colorimetric Detection of Glucose. J. Mater. Chem. B 2013, 1, 2268. [Google Scholar] [CrossRef]
- Takagi, S.; Eguchi, M.; Tryk, D.; Inoue, H. Porphyrin Photochemistry in Inorganic/Organic Hybrid Materials: Clays, Layered Semiconductors, Nanotubes, and Mesoporous Materials. J. Photochem. Photobiol. C Photochem. Rev. 2006, 7, 104–126. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, F.; Linhardt, R.J. Porphyrin-Based Compounds and Their Applications in Materials and Medicine. Dye. Pigment. 2021, 188, 109136. [Google Scholar] [CrossRef]
- Senge, M.O.; Sergeeva, N.N.; Hale, K.J. Classic Highlights in Porphyrin and Porphyrinoid Total Synthesis and Biosynthesis. Chem. Soc. Rev. 2021, 50, 4730–4789. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Hong, K.I.; Lee, H.; Jang, W.D. Bioinspired Applications of Porphyrin Derivatives. Acc. Chem. Res. 2021, 54, 2249–2260. [Google Scholar] [CrossRef] [PubMed]
- Barona-Castaño, J.; Carmona-Vargas, C.; Brocksom, T.; de Oliveira, K. Porphyrins as Catalysts in Scalable Organic Reactions. Molecules 2016, 21, 310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, M.M.; Dias, L.D.; Calvete, M.J.F. Metalloporphyrins: Bioinspired Oxidation Catalysts. ACS Catal. 2018, 8, 10784–10808. [Google Scholar] [CrossRef]
- Sun, E.J.; Wang, Y.; Li, Y.S.; Bai, X.Y.; Sun, G.J.; Wang, S.S.; Chang, Y. Noncovalently Metalloporphyrins Functionalized by Graphene Oxide for Photodegradation of Methylene Blue. Russ. J. Inorg. Chem. 2021, 66, 1973–1979. [Google Scholar] [CrossRef]
- Pushpan, S.; Venkatraman, S.; Anand, V.; Sankar, J.; Parmeswaran, D.; Ganesan, S.; Chandrashekar, T. Porphyrins in Photodynamic Therapy—A Search for Ideal Photosensitizers. Curr. Med. Chem. Agents 2002, 2, 187–207. [Google Scholar] [CrossRef]
- Arnaut, L.G. Design of Porphyrin-Based Photosensitizers for Photodynamic Therapy. In Advances in Inorganic Chemistry; Elsevier Inc.: Amsterdam, The Netherlands, 2011; Volume 63, pp. 187–233. ISBN 9780123859044. [Google Scholar]
- Kou, J.; Dou, D.; Yang, L. Porphyrin Photosensitizers in Photodynamic Therapy and Its Applications. Oncotarget 2017, 8, 81591–81603. [Google Scholar] [CrossRef] [Green Version]
- Negut, C.C.; Stefan-van Staden, R.I.; van Staden, J.F. Porphyrins-as Active Materials in the Design of Sensors. An Overview. ECS J. Solid State Sci. Technol. 2020, 9, 051005. [Google Scholar] [CrossRef]
- Patel, M.; Day, B.J. Metalloporphyrin Class of Therapeutic Catalytic Antioxidants. Trends Pharmacol. Sci. 1999, 20, 359–364. [Google Scholar] [CrossRef]
- Canlica, M. A Study on Ball-Types Phthalocyanines Substituted for Carboxyl Groups: Spectroscopic, Photophysical and Photochemical Properties. Russ. J. Inorg. Chem. 2021, 66, 391–400. [Google Scholar] [CrossRef]
- Batinić-Haberle, I.; Rebouças, J.S.; Spasojević, I. Superoxide Dismutase Mimics: Chemistry, Pharmacology, and Therapeutic Potential. Antioxid. Redox Signal. 2010, 13, 877–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolyada, M.N.; Osipova, V.P.; Berberova, N.T.; Pimenov, Y.T.; Milaeva, E.R. Decline of Prooxidant Activity of Butyl and Phenyl Derivatives of Tin in the Presence of Meso-Tetrakis(3,5-Di-Tert-Butyl-4-Hydroxyphenyl)Porphyrin. Macroheterocycles 2017, 10, 57–61. [Google Scholar] [CrossRef] [Green Version]
- Antonova, N.A.; Osipova, V.P.; Kolyada, M.N.; Movchan, N.O.; Milaeva, E.R.; Pimenov, Y.T. Study of the Antioxidant Properties of Porphyrins and Their Complexes with Metals. Macroheterocycles 2010, 3, 139–144. [Google Scholar] [CrossRef] [Green Version]
- Batinic-Haberle, I.; Rajic, Z.; Benov, L. A Combination of Two Antioxidants (An SOD Mimic and Ascorbate) Produces a Pro-Oxidative Effect Forcing Escherichia Coli to Adapt Via Induction of OxyR Regulon. Anticancer. Agents Med. Chem. 2012, 11, 329–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Long, X.; Zhang, D.; Sun, Y.; Zhou, Y.; Ma, Y.; Qi, L.; Zhang, X. Layered Double Hydroxide-Hemin Nanocomposite as Mimetic Peroxidase and Its Application in Sensing. Sens. Actuators B. Chem. 2014, 192, 150–156. [Google Scholar] [CrossRef]
- Qiao, F.; Shi, W.; Dong, J.; Lv, W.; Ai, S. Functional Hybrids of Layered Double Hydroxides with Hemin: Synergistic Effect for Peroxynitrite-Scavenging Activity. RSC Adv. 2014, 4, 44614–44620. [Google Scholar] [CrossRef]
- Lang, K.; Bezdička, P.; Bourdelande, J.L.; Hernando, J.; Jirka, I.; Káfuňková, E.; Kovanda, F.; Kubát, P.; Mosinger, J.; Wagnerová, D.M. Layered Double Hydroxides with Intercalated Porphyrins as Photofunctional Materials: Subtle Structural Changes Modify Singlet Oxygen Production. Chem. Mater. 2007, 19, 3822–3829. [Google Scholar] [CrossRef]
- Park, I.Y.; Kuroda, K.; Kato, C. Preparation of a Layered Double Hydroxide–Porphyrin Intercalation Compound. Chem. Lett. 1989, 18, 2057–2058. [Google Scholar] [CrossRef]
- Lang, K.; Kubát, P.; Mosinger, J.; Bujdák, J.; Hof, M.; Janda, P.; Sýkora, J.; Iyi, N. Photoactive Oriented Films of Layered Double Hydroxides. Phys. Chem. Chem. Phys. 2008, 10, 4429–4434. [Google Scholar] [CrossRef]
- Demel, J.; Kubát, P.; Jirka, I.; Kovář, P.; Pospíšil, M.; Lang, K.; Kuba, P.; Jirka, I.; Kova, P.; Pospı, M.; et al. Inorganic-Organic Hybrid Materials: Layered Zinc Hydroxide Salts with Intercalated Porphyrin Sensitizers. J. Phys. Chem. C 2010, 114, 16321–16328. [Google Scholar] [CrossRef]
- Káfuňková, E.; Taviot-Guého, C.; Bezdička, P.; Klementová, M.; Kovář, P.; Kubát, P.; Mosinger, J.J.J.; Pospíśil, M.; Lang, K. Porphyrins Intercalated in Zn/Al and Mg/Al Layered Double Hydroxides: Properties and Structural Arrangement. Chem. Mater. 2010, 22, 2481–2490. [Google Scholar] [CrossRef]
- Jiřičková, M.; Demel, J.; Kubát, P.; Hostomský, J.; Kovanda, F.; Lang, K. Photoactive Self-Standing Films Made of Layered Double Hydroxides with Arranged Porphyrin Molecules. J. Phys. Chem. C 2011, 115, 21700–21706. [Google Scholar] [CrossRef]
- Demel, J.; Kubát, P.; Millange, F.; Marrot, J.; Císařová, I.; Lang, K. Lanthanide-Porphyrin Hybrids: From Layered Structures to Metal-Organic Frameworks with Photophysical Properties. Inorg. Chem. 2013, 52, 2779–2786. [Google Scholar] [CrossRef] [PubMed]
- Taviot-Guého, C.; Prévot, V.; Forano, C.; Renaudin, G.; Mousty, C.; Leroux, F. Tailoring Hybrid Layered Double Hydroxides for the Development of Innovative Applications. Adv. Funct. Mater. 2018, 28, 1703868. [Google Scholar] [CrossRef]
- Lukashin, A.V.; Vyacheslavov, A.S.; Vertegel, A.A.; Tret’yakov, Y.D. Synthesis of PbS/LDH Nanocomposites with the Use of the Method of Reversible Delamination of LDHs. Dokl. Chem. 2002, 385, 178–181. [Google Scholar] [CrossRef]
- Sokolov, M.R.; Enakieva, Y.Y.; Yapryntsev, A.D.; Shiryaev, A.A.; Zvyagina, A.I.; Kalinina, M.A. Intercalation of Porphyrin-Based SURMOF in Layered Eu(III) Hydroxide: An Approach Toward Symbimetic Hybrid Materials. Adv. Funct. Mater. 2020, 30, 2000681. [Google Scholar] [CrossRef]
- Tong, Z.; Shichi, T.; Zhang, G.; Takagi, K. The Intercalation of Metalloporphyrin Complex Anions into Layered Double Hydroxides. Res. Chem. Intermed. 2003, 29, 335–341. [Google Scholar] [CrossRef]
- Yapryntsev, A.D.; Bykov, A.Y.; Baranchikov, A.E.; Zhizhin, K.Y.; Ivanov, V.K.; Kuznetsov, N.T. Closo-Dodecaborate Intercalated Yttrium Hydroxide as a First Example of Boron Cluster Anion-Containing Layered Inorganic Substances. Inorg. Chem. 2017, 56, 3421–3428. [Google Scholar] [CrossRef]
- Delahaye, É.; Eyele-Mezui, S.; Bardeau, J.F.; Leuvrey, C.; Mager, L.; Rabu, P.; Rogez, G. New Layered Organic-Inorganic Magnets Incorporating Azo Dyes. J. Mater. Chem. 2009, 19, 6106–6115. [Google Scholar] [CrossRef]
- Taibi, M.; Ammar, S.; Jouini, N.; Fiévet, F.; Molinié, P.; Drillon, M. Layered Nickel Hydroxide Salts: Synthesis, Characterization and Magnetic Behaviour in Relation to the Basal Spacing. J. Mater. Chem. 2002, 12, 3238–3244. [Google Scholar] [CrossRef]
- Ju, R.; Gu, Q. Biohybrid Based on Layered Terbium Hydroxide and Applications as Drug Carrier and Biological Fluorescence Probe. Appl. Organomet. Chem. 2018, 32, e3926. [Google Scholar] [CrossRef]
- Gu, Q.-Y.; Qiu, X.; Liu, J.-J.; Fu, M.; Chao, J.-P.; Ju, R.-J.; Li, X.-T. Nanostructured Layered Terbium Hydroxide Containing NASIDs: In Vitro Physicochemical and Biological Evaluations. J. Nanosci. Nanotechnol. 2018, 18, 5320–5326. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Chen, W.; Duan, F.; Ju, R. Fabrication of a Nano-Drug Delivery System Based on Layered Rare-Earth Hydroxides Integrating Drug-Loading and Fluorescence Properties. Dalt. Trans. 2016, 45, 12137–12143. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Li, J.; Ji, L.; Ju, R.; Jin, H.; Zhang, R. Fabrication of Novel Bifunctional Nanohybrid Based on Layered Rare-Earth Hydroxide with Magnetic and Fluorescent Properties. Front. Mater. Sci. 2020, 14, 488–496. [Google Scholar] [CrossRef]
- Mamardashvili, G.M.; Lazovskiy, D.A.; Khodov, I.A.; Mamardashvili, N.Z.; Efimov, A.E. New Polyporphyrin Arrays with Controlled Fluorescence Obtained by Diaxial Sn(Iv)-Porphyrin Phenolates Chelation with Cu2+ Cation. Polymers 2021, 13, 829. [Google Scholar] [CrossRef]
- Lee, S.S.; Lee, B.I.; Kim, S.J.; Byeon, S.H.; Kang, J.K. Thermal Decomposition and Recovery Behaviors of Layered Gadolinium Hydroxychloride. Inorg. Chem. 2012, 51, 10222–10232. [Google Scholar] [CrossRef]
- Wang, X.; Molokeev, M.S.; Zhu, Q.; Li, J.-G. Controlled Hydrothermal Crystallization of Anhydrous Ln2(OH)4SO4 (Ln = Eu − Lu, Y) as a New Family of Layered Rare Earth Metal Hydroxides. Chem. –A Eur. J. 2017, 23, 16034–16043. [Google Scholar] [CrossRef] [Green Version]
- El-Refaey, A.; Shaban, S.Y.; El-Kemary, M.; El-Khouly, M.E. Spectroscopic and Thermodynamic Studies of Light Harvesting Perylenediimide Derivative—Zinc Porphyrin Complex in Aqueous Media. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 186, 132–139. [Google Scholar] [CrossRef]
- Gonçalves, P.J.; Corrêa, D.S.; Franzen, P.L.; De Boni, L.; Almeida, L.M.; Mendonça, C.R.; Borissevitch, I.E.; Zílio, S.C. Effect of Interaction with Micelles on the Excited-State Optical Properties of Zinc Porphyrins and J-Aggregates Formation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 112, 309–317. [Google Scholar] [CrossRef]
- Kalyanasundaram, K.; Neumann-Spallart, M. Photophysical and Redox Properties of Water-Soluble Porphyrins in Aqueous Media. J. Phys. Chem. 1982, 86, 5163–5169. [Google Scholar] [CrossRef]
- Tovmasyan, A.; Sheng, H.; Weitner, T.; Arulpragasam, A.; Lu, M.; Warner, D.S.; Vujaskovic, Z.; Spasojevic, I.; Batinic-Haberle, I. Design, Mechanism of Action, Bioavailability and Therapeutic Effects of Mn Porphyrin-Based Redox Modulators. Med. Princ. Pract. 2013, 22, 103–130. [Google Scholar] [CrossRef] [PubMed]
- Yoon, I.; Park, H.S.; Cui, B.C.; Li, J.Z.; Kim, J.H.; Lkhagvadulam, B.; Shim, Y.K. Photodynamic and Antioxidant Activities of Divalent Transition Metal Complexes of Methyl Pheophorbide-A. Bull. Korean Chem. Soc. 2011, 32, 2981–2987. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.; Omar, W.A.E.; El-Asmy, H.A.; Abou-Zeid, L.; Fadda, A.A. Docking Studies, Antitumor and Antioxidant Evaluation of Newly Synthesized Porphyrin and Metalloporphyrin Derivatives. Dye. Pigment. 2020, 183, 108728. [Google Scholar] [CrossRef]
- Pérez, M.J.; Cederbaum, A.I. Antioxidant and Pro-Oxidant Effects of a Manganese Porphyrin Complex against CYP2E1-Dependent Toxicity. Free Radic. Biol. Med. 2002, 33, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Batinic-Haberle, I.; Tovmasyan, A.; Spasojevic, I. Mn Porphyrin-Based Redox-Active Drugs: Differential Effects as Cancer Therapeutics and Protectors of Normal Tissue Against Oxidative Injury. Antioxid. Redox Signal. 2018, 29, 1691–1724. [Google Scholar] [CrossRef]
- Adler, A.D.; Longo, F.R.; Kampas, F.; Kim, J. On the Preparation of Metalloporphyrins. J. Inorg. Nucl. Chem. 1970, 32, 2443–2445. [Google Scholar] [CrossRef]
- Rodina, A.A.; Yapryntsev, A.D.; Churakov, A.V.; Baranchikov, A.E. Layered Rare Earth Hydroxides React with Formamide to Give [Ln(HCOO)3 · 2(HCONH2)]. Russ. J. Inorg. Chem. 2021, 66, 125–132. [Google Scholar] [CrossRef]
- Dolgopolova, E.A.; Ivanova, O.S.; Sharikov, F.Y.; Baranchikov, A.E.; Shcherbakov, A.B.; Trietyakov, Y.D. Microwave-Hydrothermal Synthesis of Gadolinium-Doped Nanocrystalline Ceria in the Presence of Hexamethylenetetramine. Russ. J. Inorg. Chem. 2012, 57, 1303–1307. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung Der Größe Und Der Inneren Struktur von Kolloidteilchen Mittels Röntgenstrahlen. Göttinger Nachr. Math. Phys. 1918, 2, 98–100. [Google Scholar]
- Wojdyr, M. Fityk: A General-Purpose Peak Fitting Program. J. Appl. Crystallogr. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]

| Material | Element Ratio | Calculated | Found |
|---|---|---|---|
| [Gd2(OH)5]4TSPP3/4Cl nH2O | Gd:S | 2.7:1 | 2.6:1 |
| [Gd2(OH)5]4PdTSPP·nH2O | Gd:Cl | ∞ | 6.6:1 |
| Gd:S | 2:1 | 2.3:1 | |
| Gd:Pd | 8:1 | 10.0:1 | |
| S:Pd | 4:1 | 4.3:1 | |
| [Gd2(OH)5]4ZnTSPP·nH2O | Gd:Cl | ∞ | 56.1:1 |
| Gd:S | 2:1 | 1.7:1 | |
| Gd:Zn | 8:1 | 7.6:1 | |
| S:Zn | 4:1 | 4.5:1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teplonogova, M.A.; Volostnykh, M.V.; Yapryntsev, A.D.; Sozarukova, M.M.; Gorbunova, Y.G.; Sheichenko, E.D.; Baranchikov, A.E.; Ivanov, V.K. Switchable Nanozyme Activity of Porphyrins Intercalated in Layered Gadolinium Hydroxide. Int. J. Mol. Sci. 2022, 23, 15373. https://doi.org/10.3390/ijms232315373
Teplonogova MA, Volostnykh MV, Yapryntsev AD, Sozarukova MM, Gorbunova YG, Sheichenko ED, Baranchikov AE, Ivanov VK. Switchable Nanozyme Activity of Porphyrins Intercalated in Layered Gadolinium Hydroxide. International Journal of Molecular Sciences. 2022; 23(23):15373. https://doi.org/10.3390/ijms232315373
Chicago/Turabian StyleTeplonogova, Maria A., Marina V. Volostnykh, Alexey D. Yapryntsev, Madina M. Sozarukova, Yulia G. Gorbunova, Ekaterina D. Sheichenko, Alexander E. Baranchikov, and Vladimir K. Ivanov. 2022. "Switchable Nanozyme Activity of Porphyrins Intercalated in Layered Gadolinium Hydroxide" International Journal of Molecular Sciences 23, no. 23: 15373. https://doi.org/10.3390/ijms232315373








