The Soluble Guanylate Cyclase Stimulator BAY 41-2272 Attenuates Transforming Growth Factor β1-Induced Myofibroblast Differentiation of Human Corneal Keratocytes
Abstract
:1. Introduction
2. Results
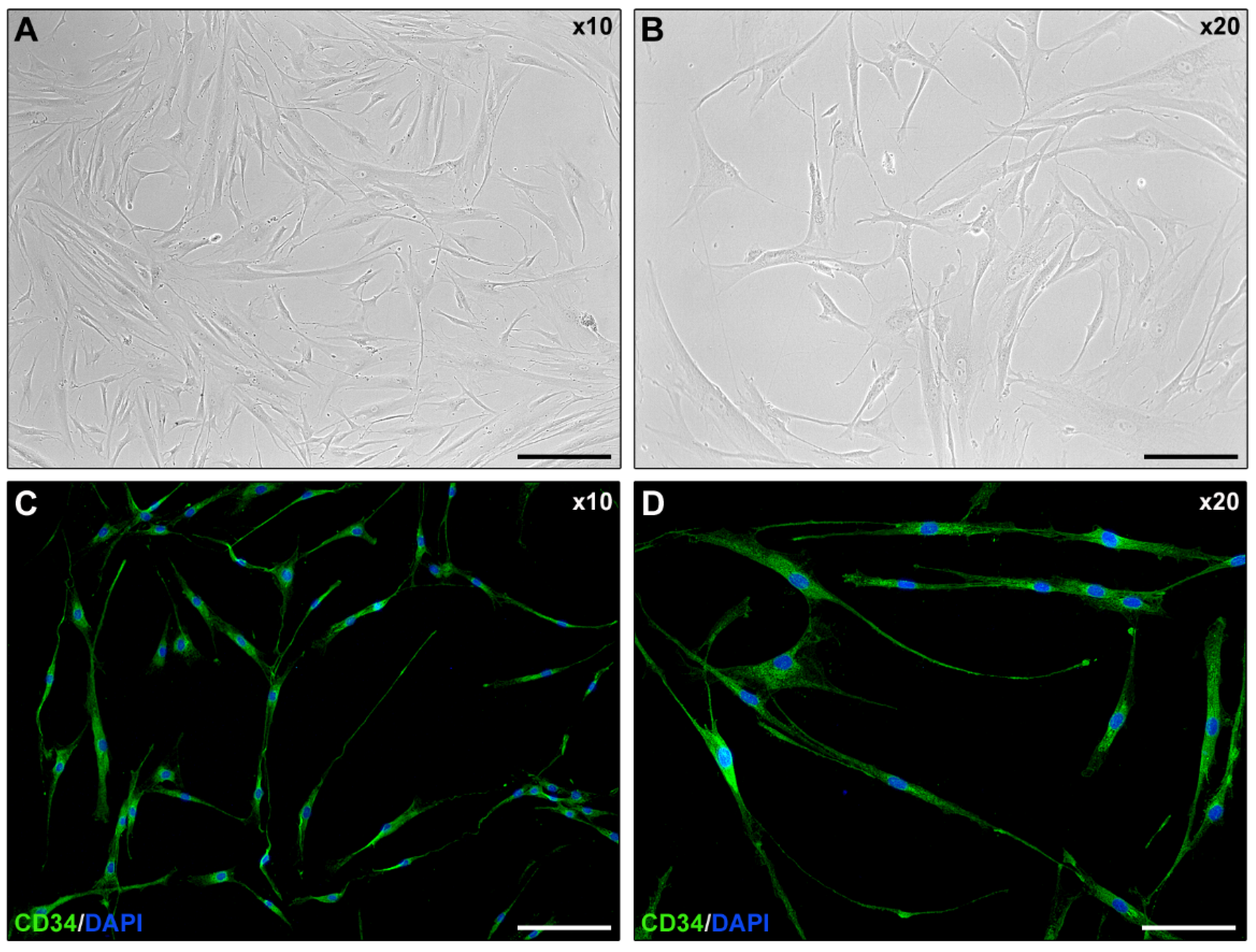
2.1. Isolated Human Corneal Keratocyte Morphology Assessment
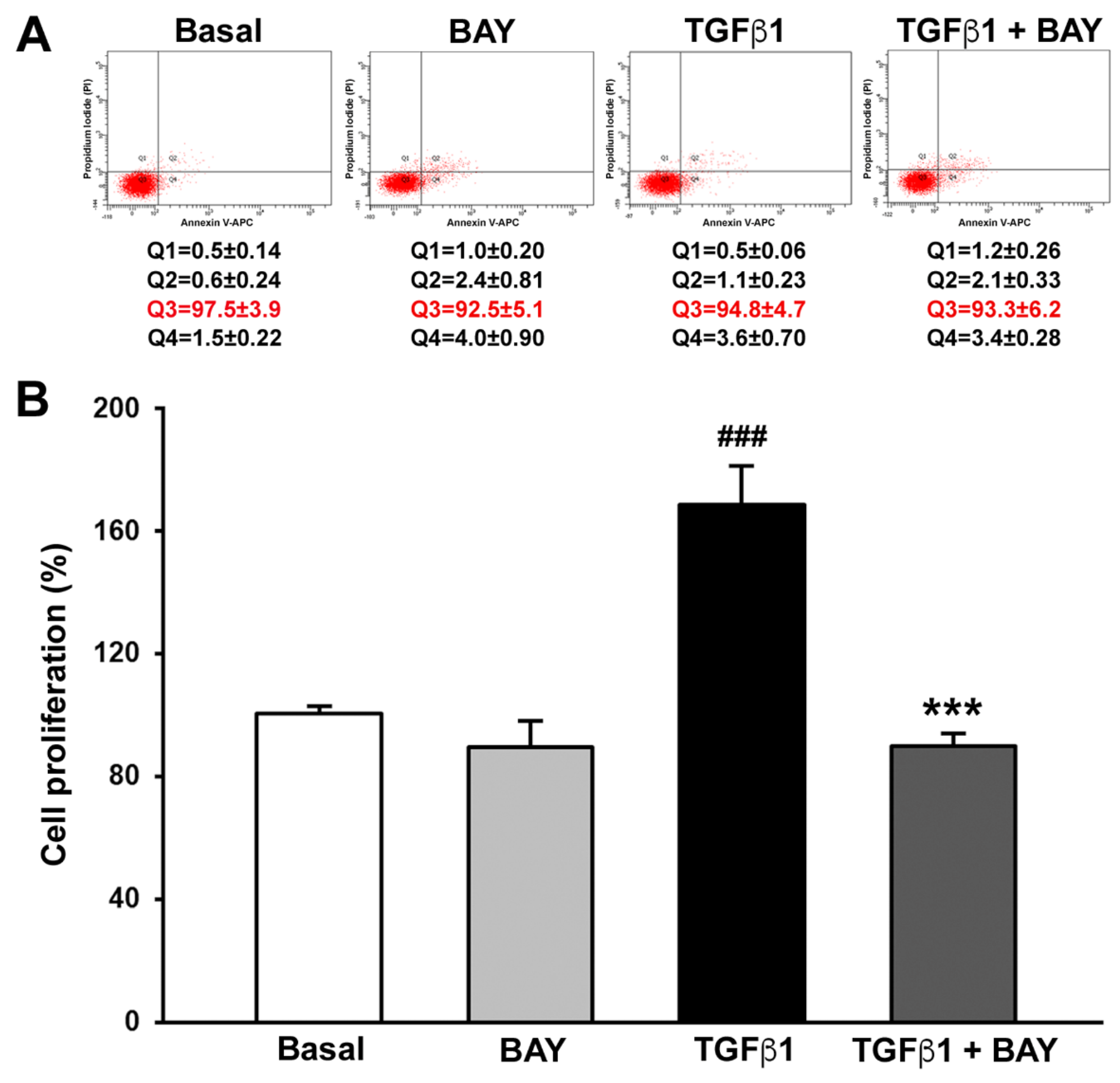
2.2. BAY 41-2272 Does Not Affect Viability and Inhibits TGFβ1-Induced Proliferation of Human Corneal Keratocytes
2.3. BAY 41-2272 Reduces TGFβ1-Induced Wound Healing Capacity of Human Corneal Keratocytes
2.4. Stimulation with BAY 41-2272 Reduces TGFβ1-Induced Invasiveness of Human Corneal Keratocytes
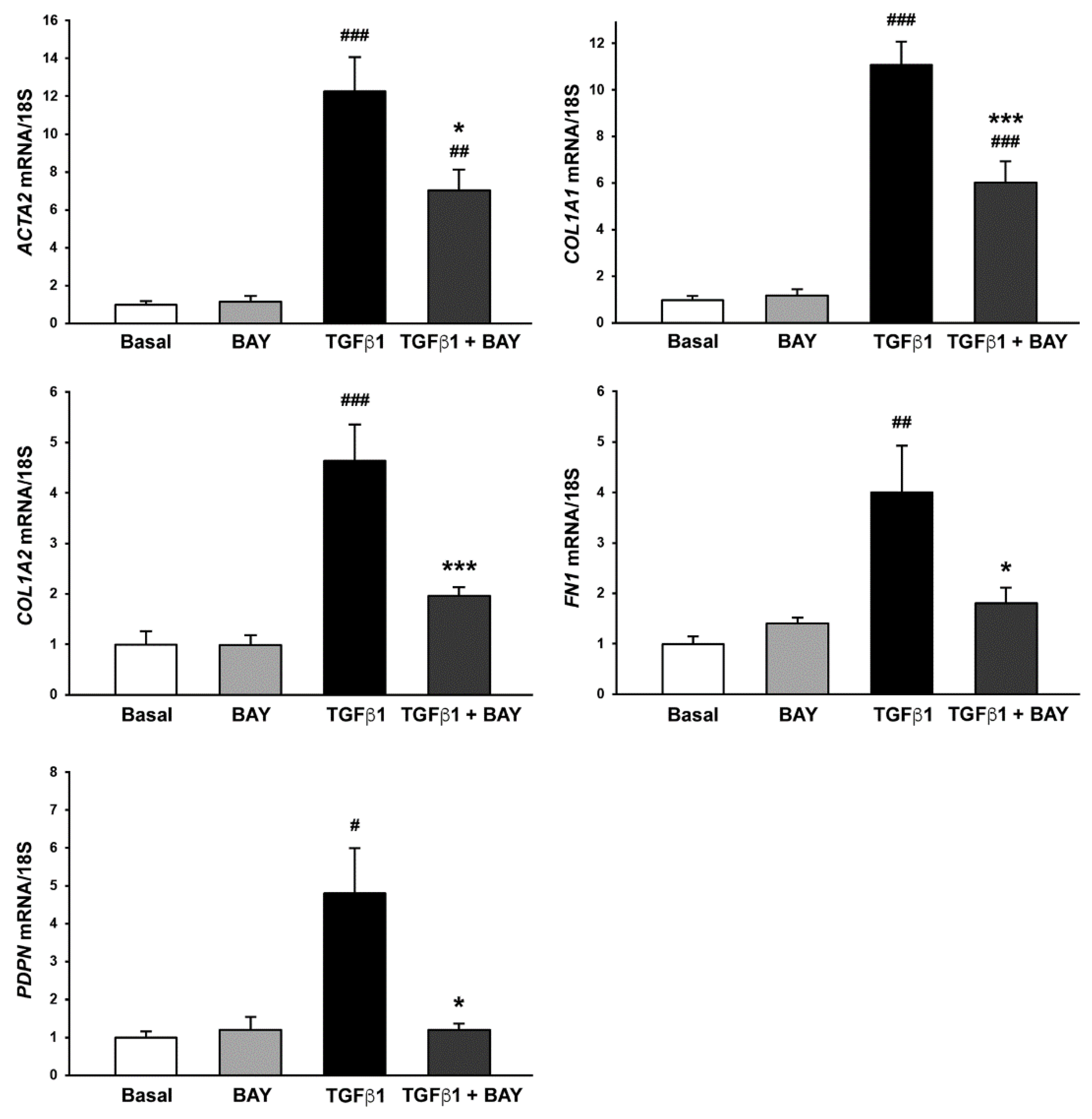
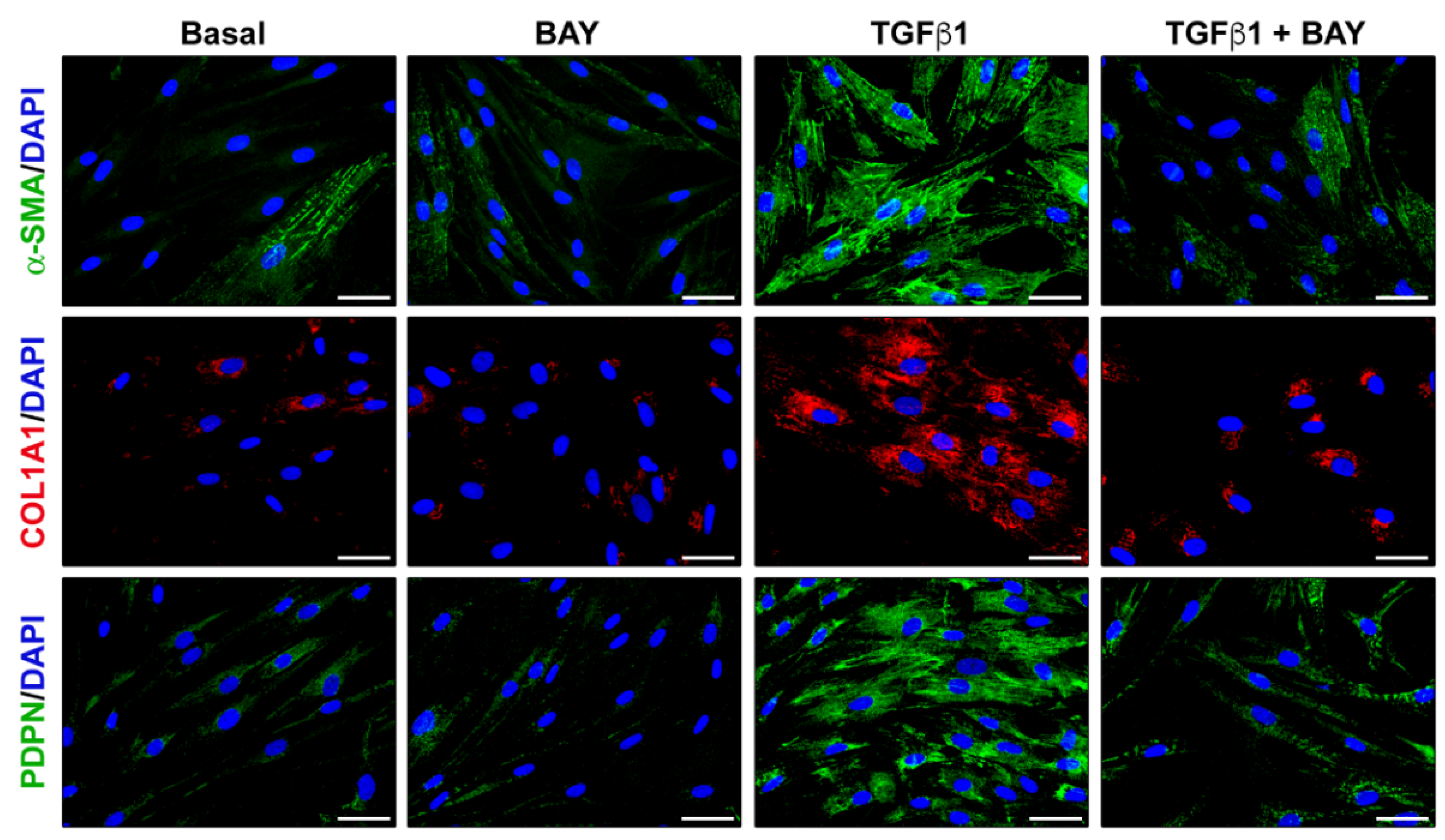
2.5. Stimulation with BAY 41-2272 Attenuates TGFβ1-Induced Myofibroblast-like Profibrotic and Contractile Phenotype of Human Corneal Keratocytes
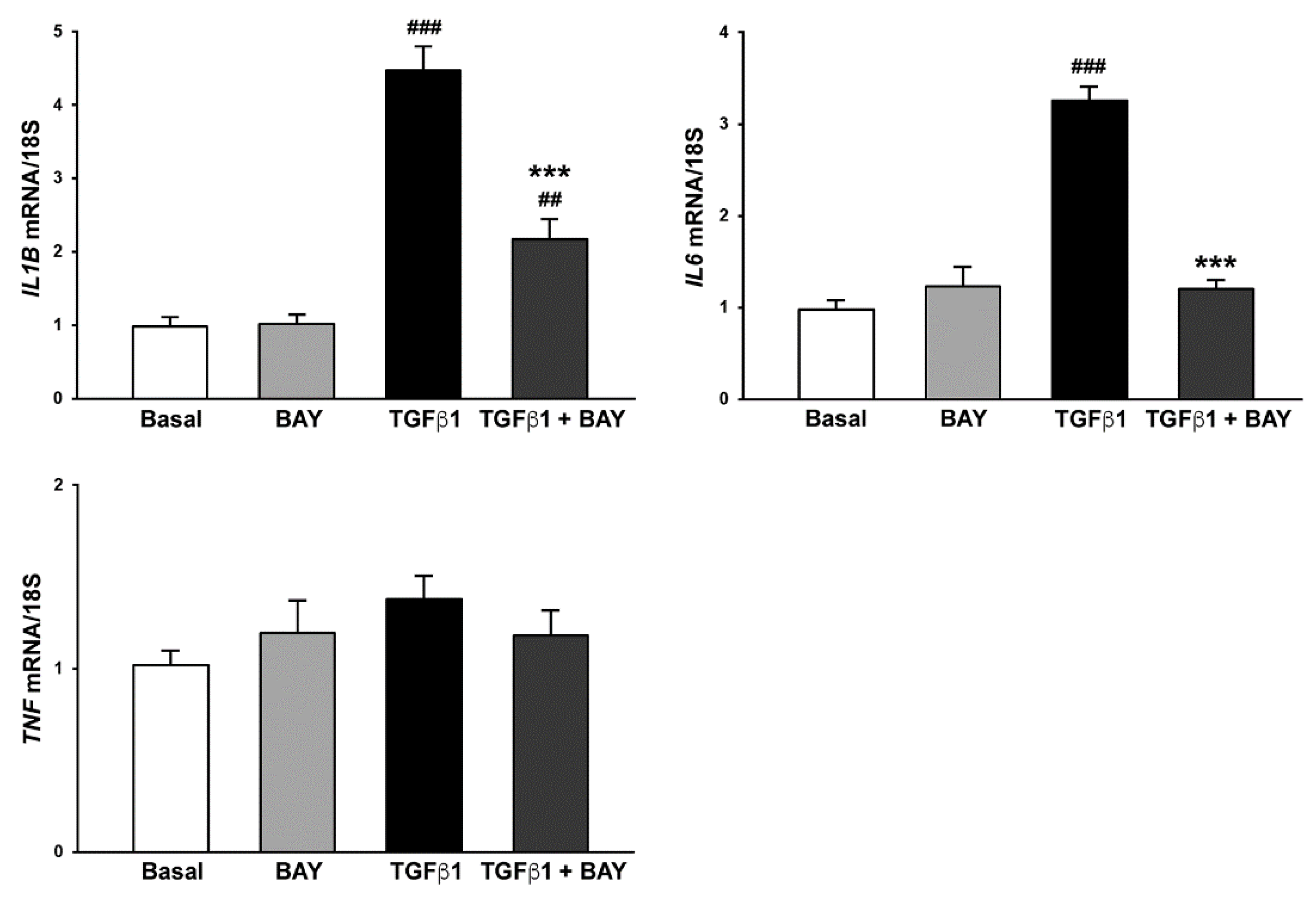
2.6. Stimulation with BAY 41-2272 Reduces TGFβ1-Induced Expression of Genes Encoding Proinflammatory Cytokines in Human Keratocytes
3. Discussion
4. Materials and Methods
4.1. Isolation of Normal Human Corneal Keratocytes
4.2. Cell Stimulation
4.3. Annexin V/PI Flow Cytometry Assay
4.4. Cell Proliferation Assay
4.5. In Vitro Wound Healing Assay
4.6. Matrigel Chemoinvasion Assay
4.7. RNA Purification, cDNA Synthesis and Quantitative Real-Time PCR
4.8. Western Blotting
4.9. Immunofluorescence
4.10. Collagen Gel Contraction Assay
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marini, M.; Mencucci, R.; Rosa, I.; Favuzza, E.; Guasti, D.; Ibba-Manneschi, L.; Manetti, M. Telocytes in normal and keratoconic human cornea: An immunohistochemical and transmission electron microscopy study. J. Cell. Mol. Med. 2017, 21, 3602–3611. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.R.; Kempuraj, D.; D’Souza, S.; Ghosh, A. Corneal stromal repair and regeneration. Prog. Retin. Eye Res. 2022, 29, 101090. [Google Scholar] [CrossRef] [PubMed]
- Català, P.; Thuret, G.; Skottman, H.; Mehta, J.S.; Parekh, M.; Ní Dhubhghaill, S.; Collin, R.W.J.; Nuijts, R.M.M.A.; Ferrari, S.; LaPointe, V.L.S.; et al. Approaches for corneal endothelium regenerative medicine. Prog. Retin. Eye Res. 2022, 87, 100987. [Google Scholar] [CrossRef]
- Kamil, S.; Mohan, R.R. Corneal stromal wound healing: Major regulators and therapeutic targets. Ocul. Surf. 2021, 19, 290–306. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.E. Corneal wound healing. Exp. Eye Res. 2020, 197, 108089. [Google Scholar] [CrossRef]
- Wilson, S.E.; Marino, G.K.; Torricelli, A.A.M.; Medeiros, C.S. Injury and defective regeneration of the epithelial basement membrane in corneal fibrosis: A paradigm for fibrosis in other organs? Matrix Biol. 2017, 64, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.E. TGF beta -1, -2 and -3 in the modulation of fibrosis in the cornea and other organs. Exp. Eye Res. 2021, 207, 108594. [Google Scholar] [CrossRef]
- Wilson, S.E. Corneal myofibroblasts and fibrosis. Exp. Eye Res. 2020, 201, 108272. [Google Scholar] [CrossRef]
- Medeiros, C.S.; Marino, G.K.; Santhiago, M.R.; Wilson, S.E. The corneal basement membranes and stromal fibrosis. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4044–4053. [Google Scholar] [CrossRef] [Green Version]
- Lightner, A.L.; Chan, T. Precision regenerative medicine. Stem Cell Res. Ther. 2021, 12, 39. [Google Scholar] [CrossRef]
- Maharajan, N.; Cho, G.W.; Choi, J.H.; Jang, C.H. Regenerative therapy using umbilical cord serum. In Vivo 2021, 35, 699–705. [Google Scholar] [CrossRef]
- Pellegrini, G.; Rama, P.; Mavilio, F.; De Luca, M. Epithelial stem cells in corneal regeneration and epidermal gene therapy. J. Pathol. 2009, 217, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Sandner, P. From molecules to patients: Exploring the therapeutic role of soluble guanylate cyclase stimulators. Biol. Chem. 2018, 399, 679–690. [Google Scholar] [CrossRef]
- Knorr, A.; Hirth-Dietrich, C.; Alonso-Alija, C.; Härter, M.; Hahn, M.; Keim, Y.; Wunder, F.; Stasch, J.P. Nitric oxide-independent activation of soluble guanylate cyclase by BAY 60-2770 in experimental liver fibrosis. Arzneimittelforschung 2008, 58, 71–80. [Google Scholar] [CrossRef]
- Xie, G.; Wang, X.; Wang, L.; Wang, L.; Atkinson, R.D.; Kanel, G.C.; Gaarde, W.A.; Deleve, L.D. Role of differentiation of liver sinusoidal endothelial cells in progression and regression of hepatic fibrosis in rats. Gastroenterology 2012, 142, 918–927. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.J.; Kuo, L.M.; Wu, Y.H.; Chang, Y.C.; Lai, K.H.; Hwang, T.L. BAY 41-2272 attenuates CTGF expression via sGC/cGMP-independent pathway in TGFβ1-activated hepatic stellate cells. Biomedicines 2020, 8, 330. [Google Scholar] [CrossRef] [PubMed]
- Masuyama, H.; Tsuruda, T.; Sekita, Y.; Hatakeyama, K.; Imamura, T.; Kato, J.; Asada, Y.; Stasch, J.P.; Kitamura, K. Pressure-independent effects of pharmacological stimulation of soluble guanylate cyclase on fibrosis in pressure-overloaded rat heart. Hypertens. Res. 2009, 32, 597–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambers, C.; Boehm, P.M.; Karabacak, Y.; Samaha, E.; Benazzo, A.; Jaksch, P.; Roth, M. Combined activation of guanylate cyclase and cyclic AMP in lung fibroblasts as a novel therapeutic concept for lung fibrosis. Biomed Res. Int. 2019, 2019, 1345402. [Google Scholar] [CrossRef]
- Zenzmaier, C.; Kern, J.; Heitz, M.; Plas, E.; Zwerschke, W.; Mattesich, M.; Sandner, P.; Berger, P. Activators and stimulators of soluble guanylate cyclase counteract myofibroblast differentiation of prostatic and dermal stromal cells. Exp. Cell Res. 2015, 338, 162–169. [Google Scholar] [CrossRef]
- Beyer, C.; Reich, N.; Schindler, S.C.; Akhmetshina, A.; Dees, C.; Tomcik, M.; Hirth-Dietrich, C.; von Degenfeld, G.; Sandner, P.; Distler, O.; et al. Stimulation of soluble guanylate cyclase reduces experimental dermal fibrosis. Ann. Rheum. Dis. 2012, 71, 1019–1026. [Google Scholar] [CrossRef]
- Beyer, C.; Zenzmaier, C.; Palumbo-Zerr, K.; Mancuso, R.; Distler, A.; Dees, C.; Zerr, P.; Huang, J.; Maier, C.; Pachowsky, M.L.; et al. Stimulation of the soluble guanylate cyclase (sGC) inhibits fibrosis by blocking non-canonical TGFβ signalling. Ann. Rheum. Dis. 2015, 74, 1408–1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dees, C.; Beyer, C.; Distler, A.; Soare, A.; Zhang, Y.; Palumbo-Zerr, K.; Distler, O.; Schett, G.; Sandner, P.; Distler, J.H. Stimulators of soluble guanylate cyclase (sGC) inhibit experimental skin fibrosis of different aetiologies. Ann. Rheum. Dis. 2015, 74, 1621–1625. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Okano, T.; Yamada, H.; Akashi, K.; Sendo, S.; Ueda, Y.; Morinobu, A.; Saegusa, J. Soluble guanylate cyclase stimulator reduced the gastrointestinal fibrosis in bleomycin-induced mouse model of systemic sclerosis. Arthritis Res. Ther. 2021, 23, 133. [Google Scholar] [CrossRef]
- Romano, E.; Rosa, I.; Fioretto, B.S.; Giuggioli, D.; Manetti, M.; Matucci-Cerinic, M. Soluble guanylate cyclase stimulation fosters angiogenesis and blunts myofibroblast-like features of systemic sclerosis endothelial cells. Rheumatology 2022, keac433, Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K. Corneal fibroblasts: Function and markers. Exp. Eye Res. 2020, 200, 108229. [Google Scholar] [CrossRef]
- Surovtseva, M.A.; Poveshchenko, O.V.; Krasner, K.Y.; Kim, I.I.; Lykov, A.P.; Bondarenko, N.A.; Shul’mina, L.A.; Trunov, A.N.; Chernykh, V.V. Morphofunctional properties of corneal stromal cells. Bull. Exp. Biol. Med. 2021, 172, 96–99. [Google Scholar] [CrossRef]
- Chang, Y.M.; Cian, A.A.; Weng, T.H.; Liang, C.M.; Pao, S.I.; Chen, Y.J. Beneficial effects of hypercapnic acidosis on the inhibition of transforming growth factor β-1-induced corneal fibrosis in vitro. Curr. Eye Res. 2021, 46, 648–656. [Google Scholar] [CrossRef]
- Chaurasia, S.S.; Kaur, H.; de Medeiros, F.W.; Smith, S.D.; Wilson, S.E. Dynamics of the expression of intermediate filaments vimentin and desmin during myofibroblast differentiation after corneal injury. Exp. Eye Res. 2009, 89, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Kim, M.; Yim, B.; Park, C.Y. Nitric oxide attenuated transforming growth factor-β induced myofibroblast differentiation of human keratocytes. Sci. Rep. 2021, 11, 8183. [Google Scholar] [CrossRef]
- Prada, J.; Schruender, S.; Ngo-Tu, T.; Baatz, H.; Hartmann, C.; Pleyer, U. Expression of tumor necrosis factor-α and interleukin-6 in corneal cells after excimer laser ablation in Wistar rats. Eye 2011, 25, 534–536. [Google Scholar] [CrossRef]
- Ebihara, N.; Matsuda, A.; Nakamura, S.; Matsuda, H.; Murakami, A. Role of the IL-6 classic- and trans-signaling pathways in corneal sterile inflammation and wound healing. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8549–8557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbosa, F.L.; Chaurasia, S.S.; Kaur, H.; de Medeiros, F.W.; Agrawal, V.; Wilson, S.E. Stromal interleukin-1 expression in the cornea after haze-associated injury. Exp. Eye Res. 2010, 91, 456–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, V.; Jaini, R.; Torricelli, A.A.; Santhiago, M.R.; Singh, N.; Ambati, B.K.; Wilson, S.E. TGFβ and PDGF-B signaling blockade inhibits myofibroblast development from both bone marrow-derived and keratocyte-derived precursor cells in vivo. Exp. Eye Res. 2014, 121, 35–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbosa, F.L.; Chaurasia, S.S.; Cutler, A.; Asosingh, K.; Kaur, H.; Medeiros, F.W.; Agrawal, V.; Wilson, S.E. Corneal myofibroblast generation from bone marrow-derived cells. Exp. Eye Res. 2010, 91, 92–96. [Google Scholar] [CrossRef] [Green Version]
- Torricelli, A.A.; Santhanam, A.; Wu, J.; Singh, V.; Wilson, S.E. The corneal fibrosis response to epithelial-stromal injury. Exp. Eye Res. 2016, 142, 110–118. [Google Scholar] [CrossRef] [Green Version]
- Stasch, J.P.; Schlossmann, J.; Hocher, B. Renal effects of soluble guanylate cyclase stimulators and activators: A review of the preclinical evidence. Curr. Opin. Pharmacol. 2015, 21, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Jester, J.V.; Ho-Chang, J. Modulation of cultured corneal keratocyte phenotype by growth factors/cytokines control in vitro contractility and extracellular matrix contraction. Exp. Eye Res. 2003, 77, 581–592. [Google Scholar] [CrossRef]
- Maruri, D.P.; Iyer, K.S.; Schmidtke, D.W.; Petroll, W.M.; Varner, V.D. Signaling downstream of focal adhesions regulates stiffness-dependent differences in the TGF-β1-mediated myofibroblast differentiation of corneal keratocytes. Front. Cell. Dev. Biol. 2022, 10, 886759. [Google Scholar] [CrossRef]
- Nazari, B.; Rice, L.M.; Stifano, G.; Barron, A.M.; Wang, Y.M.; Korndorf, T.; Lee, J.; Bhawan, J.; Lafyatis, R.; Browning, J.L. Altered dermal fibroblasts in systemic sclerosis display podoplanin and CD90. Am. J. Pathol. 2016, 186, 2650–2664. [Google Scholar] [CrossRef] [Green Version]
- Ekwall, A.K.; Eisler, T.; Anderberg, C.; Jin, C.; Karlsson, N.; Brisslert, M.; Bokarewa, M.I. The tumour-associated glycoprotein podoplanin is expressed in fibroblast-like synoviocytes of the hyperplastic synovial lining layer in rheumatoid arthritis. Arthritis Res. Ther. 2011, 13, R40. [Google Scholar] [CrossRef]
- Flores-Costa, R.; Alcaraz-Quiles, J.; Titos, E.; López-Vicario, C.; Casulleras, M.; Duran-Güell, M.; Rius, B.; Diaz, A.; Hall, K.; Shea, C.; et al. The soluble guanylate cyclase stimulator IW-1973 prevents inflammation and fibrosis in experimental non-alcoholic steatohepatitis. Br. J. Pharmacol. 2018, 175, 953–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shea, C.M.; Price, G.M.; Liu, G.; Sarno, R.; Buys, E.S.; Currie, M.G.; Masferrer, J.L. Soluble guanylate cyclase stimulator praliciguat attenuates inflammation, fibrosis, and end-organ damage in the Dahl model of cardiorenal failure. Am. J. Physiol. Ren. Physiol. 2020, 318, F148–F159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flores-Costa, R.; Duran-Güell, M.; Casulleras, M.; López-Vicario, C.; Alcaraz-Quiles, J.; Diaz, A.; Lozano, J.J.; Titos, E.; Hall, K.; Sarno, R.; et al. Stimulation of soluble guanylate cyclase exerts antiinflammatory actions in the liver through a VASP/NF-κB/NLRP3 inflammasome circuit. Proc. Natl. Acad. Sci. USA 2020, 117, 28263–28274. [Google Scholar] [CrossRef] [PubMed]
- Romano, E.; Manetti, M.; Rosa, I.; Fioretto, B.S.; Ibba-Manneschi, L.; Matucci-Cerinic, M.; Guiducci, S. Slit2/Robo4 axis may contribute to endothelial cell dysfunction and angiogenesis disturbance in systemic sclerosis. Ann. Rheum. Dis. 2018, 77, 1665–1674. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosa, I.; Fioretto, B.S.; Romano, E.; Buzzi, M.; Mencucci, R.; Marini, M.; Manetti, M. The Soluble Guanylate Cyclase Stimulator BAY 41-2272 Attenuates Transforming Growth Factor β1-Induced Myofibroblast Differentiation of Human Corneal Keratocytes. Int. J. Mol. Sci. 2022, 23, 15325. https://doi.org/10.3390/ijms232315325
Rosa I, Fioretto BS, Romano E, Buzzi M, Mencucci R, Marini M, Manetti M. The Soluble Guanylate Cyclase Stimulator BAY 41-2272 Attenuates Transforming Growth Factor β1-Induced Myofibroblast Differentiation of Human Corneal Keratocytes. International Journal of Molecular Sciences. 2022; 23(23):15325. https://doi.org/10.3390/ijms232315325
Chicago/Turabian StyleRosa, Irene, Bianca Saveria Fioretto, Eloisa Romano, Matilde Buzzi, Rita Mencucci, Mirca Marini, and Mirko Manetti. 2022. "The Soluble Guanylate Cyclase Stimulator BAY 41-2272 Attenuates Transforming Growth Factor β1-Induced Myofibroblast Differentiation of Human Corneal Keratocytes" International Journal of Molecular Sciences 23, no. 23: 15325. https://doi.org/10.3390/ijms232315325





