Easy and Effective Method for Extracting and Purifying Wolbachia Genomic DNA
Abstract
:1. Introduction
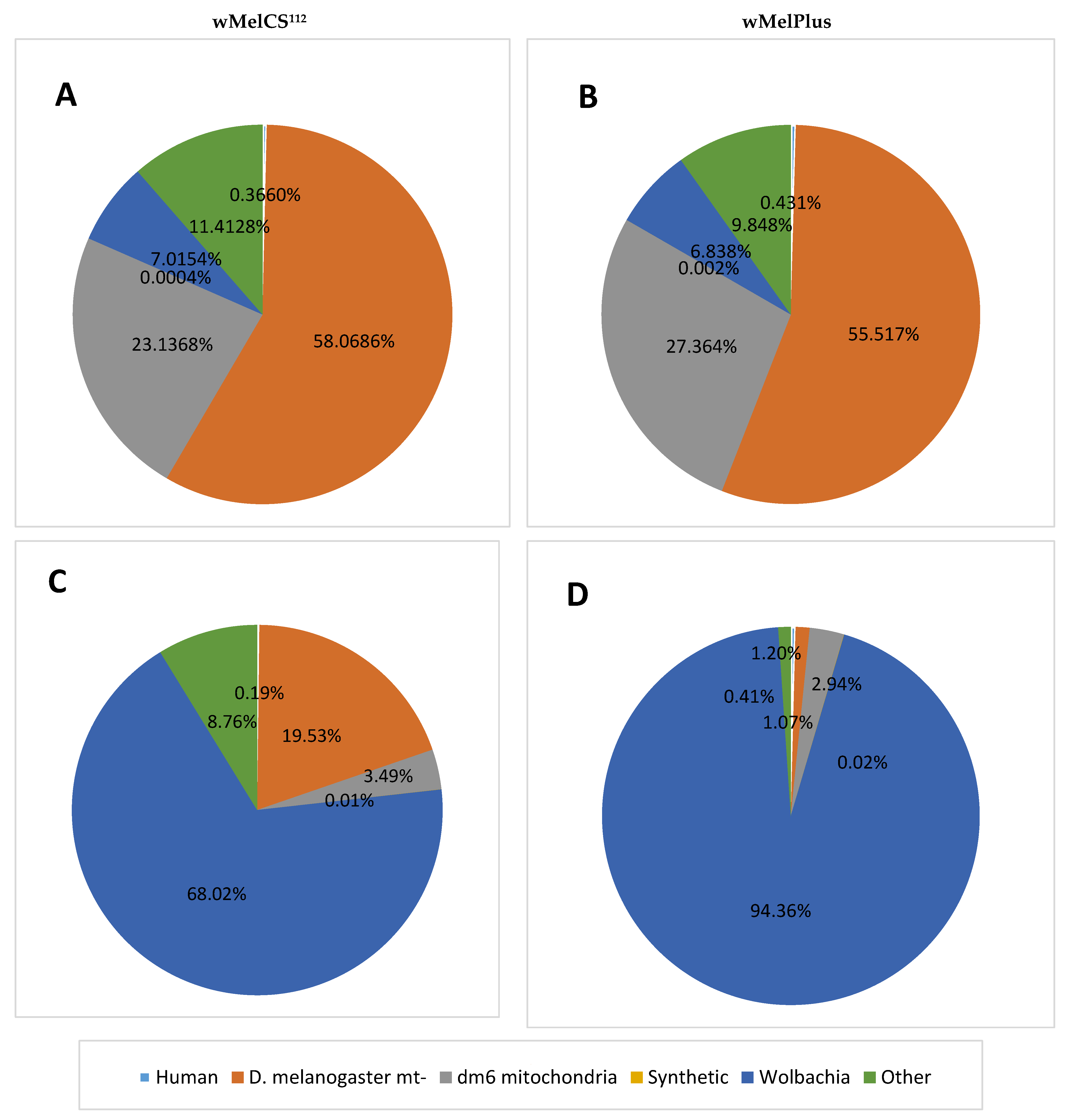
2. Results and Discussion
3. Materials and Methods
3.1. Drosophila Lines and Rearing
3.2. DNA Preparation and Sequencing
3.2.1. Wolbachia DNA Extraction from Drosophila Ovaries
3.2.2. Wolbachia DNA Extraction from a Whole Drosophila Imago
3.2.3. Genome Library Construction and Sequencing
3.3. Contamination, Quality Control and Trimming of the Sequenced Data
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zug, R.; Hammerstein, P. Still a host of hosts for Wolbachia: Analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS ONE 2012, 7, e38544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourtzis, K.; O’Neill, S. WolbachiaInfections and Arthropod Reproduction. BioScience 1998, 48, 287–293. [Google Scholar] [CrossRef] [Green Version]
- McGraw, E.A.; O’Neill, S.L. Evolution of Wolbachia pipientis transmission dynamics in insects. Trends Microbiol. 1999, 7, 297–302. [Google Scholar] [CrossRef]
- Stouthamer, R.; Breeuwer, J.A.J.; Hurst, G.D.D. Wolbachia pipientis: Microbial manipulator of Arthropod reproduction. Annu. Rev. Microbiol. 1999, 53, 71–102. [Google Scholar] [CrossRef]
- Hedges, L.M.; Brownlie, J.C.; O’Neill, S.L.; Johnson, K.N. Wolbachia and virus protection in insects. Science 2008, 322, 702. [Google Scholar] [CrossRef]
- Teixeira, L.; Ferreira, A.; Ashburner, M. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol. 2008, 6, 2753–2763. [Google Scholar] [CrossRef] [Green Version]
- Osborne, S.E.; Iturbe-Ormaetxe, I.; Brownlie, J.C.; O’Neill, S.L.; Johnson, K.N. Antiviral protection and the importance of Wolbachia density and tissue tropism in Drosophila simulans. Appl. Environ. Microbiol. 2012, 78, 6922–6929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Driver, C.; Georgiou, A.; Georgiou, G. The contribution by mitochondrially induced oxidative damage to aging in Drosophila melanogaster. Biogerontology 2004, 5, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.A.; Hercus, M.; Dagher, H. Population dynamics of the Wolbachia infection causing cytoplasmic incompatibility in Drosophila melanogaster. Genetics 1998, 148, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Fry, A.J.; Palmer, M.R.; Rand, D.M. Variable fitness effects of Wolbachia infection in Drosophila melanogaster. Heredity 2004, 93, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Toivonen, J.M.; Walker, G.A.; Martinez-Diaz, P.; Bjedov, I.; Driege, Y.; Jacobs, H.T.; Gems, D.; Partridge, L. No influence of Indy on lifespan in Drosophila after correction for genetic and cytoplasmic background effects. PLoS Genet. 2007, 3, e95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.V.; Foster, J.M.; Tzertzinis, G.; Ono, M.; Bandi, C.; Slatko, B.E.; O’Neill, S.L. Determination of Wolbachia genome size by pulsed-field gel electrophoresis. J. Bacteriol. 2001, 183, 2219–2225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mavingui, P.; Van, V.T.; Labeyrie, E.; Rances, E.; Vavre, F.; Simonet, P. Efficient procedure for purification of obligate intracellular Wolbachia pipientis and representative amplification of its genome by multiple-displacement amplification. Appl. Environ. Microbiol. 2005, 71, 6910–6917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klasson, L.; Walker, T.; Sebaihia, M.; Sanders, M.J.; Quail, M.A.; Lord, A.; Sanders, S.; Earl, J.; O’Neill, S.L.; Thomson, N.; et al. Genome evolution of Wolbachia strain wPip from the Culex pipiens group. Mol. Biol. Evol. 2008, 25, 1877–1887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iturbe-Ormaetxe, I.; Woolfit, M.; Rances, E.; Duplouy, A.; O’Neill, S.L. A simple protocol to obtain highly pure Wolbachia endosymbiont DNA for genome sequencing. J. Microbiol. Methods 2011, 84, 134–136. [Google Scholar] [CrossRef] [PubMed]
- Ellegaard, K.M.; Klasson, L.; Näslund, K.; Bourtzis, K.; Andersson, S.G.E. Comparative Genomics of Wolbachia and the Bacterial Species Concept. PLoS Genet. 2013, 9, e1003381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burdina, E.V.; Bykov, R.A.; Menshanov, P.N.; Ilinsky, Y.Y.; Gruntenko, N.E. Unique Wolbachia strain wMelPlus increases heat stress resistance in Drosophila melanogaster. Arch. Insect. Biochem. Physiol. 2021, 106, e21776. [Google Scholar] [CrossRef] [PubMed]
| RefSeq Assembly Accession | Synonym | Species | Description |
|---|---|---|---|
| GCF_016584405.1 | wMelCS_b | Wolbachia endosymbiont of Drosophila melanogaster | Wolbachia reference genome |
| GCF_000001215.4 | dm6 | Drosophila melanogaster | Release 6 plus ISO1 mitochondrial genome |
| GCF_000001405.39 | hg38 | Homo sapiens | Genome Reference Consortium Human Build 38 patch release 13 (GRCh38.p13) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreenkova, O.V.; Shishkina, O.D.; Klimenko, A.I.; Korenskaia, A.E.; Bobrovskikh, M.A.; Shatskaya, N.V.; Vasiliev, G.V.; Gruntenko, N.E. Easy and Effective Method for Extracting and Purifying Wolbachia Genomic DNA. Int. J. Mol. Sci. 2022, 23, 15315. https://doi.org/10.3390/ijms232315315
Andreenkova OV, Shishkina OD, Klimenko AI, Korenskaia AE, Bobrovskikh MA, Shatskaya NV, Vasiliev GV, Gruntenko NE. Easy and Effective Method for Extracting and Purifying Wolbachia Genomic DNA. International Journal of Molecular Sciences. 2022; 23(23):15315. https://doi.org/10.3390/ijms232315315
Chicago/Turabian StyleAndreenkova, Olga V., Olga D. Shishkina, Alexandra I. Klimenko, Aleksandra E. Korenskaia, Margarita A. Bobrovskikh, Natalja V. Shatskaya, Gennady V. Vasiliev, and Nataly E. Gruntenko. 2022. "Easy and Effective Method for Extracting and Purifying Wolbachia Genomic DNA" International Journal of Molecular Sciences 23, no. 23: 15315. https://doi.org/10.3390/ijms232315315






