Tear Proteomics Approach to Distinguishing Primary from Secondary Sjögren’s Syndrome for Dry Eye Patients with Long-Term Instillation of Eyedrops
Abstract
:1. Introduction
2. Results
2.1. Subject Characteristics
2.2. Proteome Profiling of TF
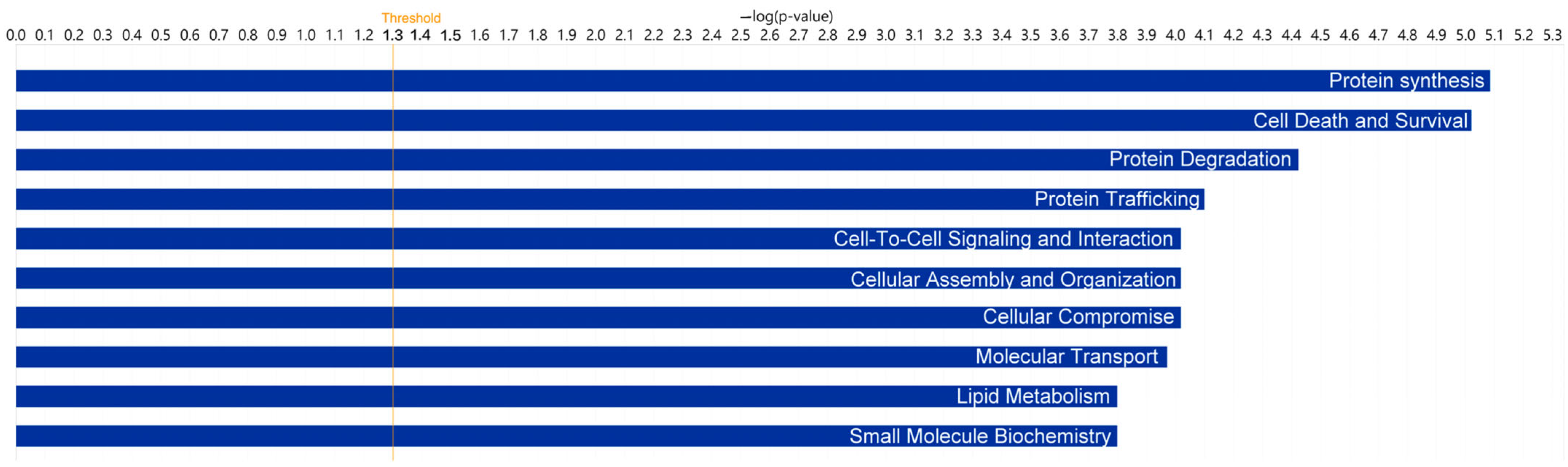
2.3. Gene Ontology Analysis in the TF of DED Patients
2.4. Interaction Network Model of Candidate Proteins Revealed a Key Regulator in DED
2.5. Verification of Candidate Biomarkers
3. Discussion
4. Materials and Methods
4.1. Enrollment of Patients and Assessment of Ocular Surface Dryness
4.2. Assessment Protocol
4.3. Meniscometry for Determining Tear Volume
4.4. Quantification of Ocular Surface Redness
4.5. Evaluation of Tear Film Stability
4.6. Sampling of Tear Fluid for Proteomics and ELISA
4.7. TMT Gel-Free Proteomics to Globally Screen Serum Proteins
4.8. Analysis for Proteomics Data
4.9. Enrichment Analysis Using Gene Ontology and Network Analysis
4.10. ELISA Validation for Selected Candidate Proteins
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef]
- Von Hohenstein-Blaul, N.T.U.; Funke, S.; Grus, F.H. Tears as a source of biomarkers for ocular and systemic diseases. Exp. Eye Res. 2013, 117, 126–137. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, F.R.; Motta, A.C.F.; Módulo, C.M.; Garcia, D.M.; Chiorini, J.A.; Louzada-Junior, P.; Rocha, E.M. Clinical and laboratory evaluation of sicca complaints: Distinctive aspects of primary, secondary and non-Sjogren syndrome. Adv. Rheumatol. 2022, 62, 23. [Google Scholar] [CrossRef] [PubMed]
- Choudhry, H.S.; Hosseini, S.; Choudhry, H.S.; Fatahzadeh, M.; Khianey, R.; Dastjerdi, M.H. Updates in diagnostics, treatments, and correlations between oral and ocular manifestations of Sjogren’s syndrome. Ocul. Surf. 2022, 26, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Taşdemir, M.; Hasan, C.; Ağbaş, A.; Kasapçopur, Ö.; Canpolat, N.; Sever, L.; Çalışkan, S. Sjögren’s syndrome associated with systemic lupus erythematosus. Türk Pediatri Arşivi 2016, 51, 166–168. [Google Scholar] [CrossRef]
- Rasmussen, A.; Radfar, L.; Lewis, D.; Grundahl, K.; Stone, D.U.; Kaufman, C.E.; Rhodus, N.L.; Segal, B.; Wallace, D.J.; Weisman, M.H.; et al. Previous diagnosis of Sjögren’s Syndrome as rheumatoid arthritis or systemic lupus erythematosus. Rheumatology 2016, 55, 1195–1201. [Google Scholar] [CrossRef] [Green Version]
- Kassan, S.S.; Moutsopoulos, H.M. Clinical manifestations and early diagnosis of Sjögren Syndrome. Arch. Intern. Med. 2004, 164, 1275–1284. [Google Scholar] [CrossRef]
- Karampatakis, V.; Konidaris, V.; Michailidou, M.; Gerofotis, A.; Daniilidis, M. Peripheral corneal ulceration associated with rheumatoid arthritis. Am. J. Case Rep. 2013, 14, 318–321. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, R.; Ishii, T.; Yoshida, M.; Takada, N.; Yokokura, S.; Shirota, Y.; Fujii, H.; Harigae, H. Ulcerative keratitis in patients with rheumatoid arthritis in the modern biologic era: A series of eight cases and literature review. Int. J. Rheum. Dis. 2017, 20, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Cryan, L.M.; O’Brien, C. Proteomics as a research tool in clinical and experimental ophthalmology. PROTEOMICS–Clin. Appl. 2008, 2, 762–775. [Google Scholar] [CrossRef]
- Grus, F.H.; Joachim, S.C.; Pfeiffer, N. Proteomics in ocular fluids. PROTEOMICS–Clin. Appl. 2007, 1, 876–888. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Beuerman, R.W. Tear analysis in ocular surface diseases. Prog. Retin. Eye Res. 2012, 31, 527–550. [Google Scholar] [CrossRef] [PubMed]
- Borges, D.D.A.; Alborghetti, M.R.; Leme, A.F.P.; Domingues, R.R.; Duarte, B.; Veiga, M.; Ferrer, M.T.; Wanzeler, A.C.V.; Arieta, C.E.L.; Alves, M. Tear proteomic profile in three distinct ocular surface diseases: Keratoconus, pterygium, and dry eye related to graft-versus-host disease. Clin. Proteom. 2020, 17, 42. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.T.; Fang, P.C.; Chao, T.L.; Chen, A.; Lai, Y.H.; Huang, Y.T.; Tseng, C.Y. Tear Proteomics Approach to Monitoring Sjögren Syndrome or Dry Eye Disease. Int. J. Mol. Sci. 2019, 20, 1932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagan, S.; Martin, E.; Enríquez-de-Salamanca, A. Tear fluid biomarkers in ocular and systemic disease: Potential use for predictive, preventive and personalised medicine. EPMA J. 2016, 7, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, J.H.; Ji, Y.W.; Hwang, H.S.; Oh, J.W.; Kim, H.C.; Lee, H.K.; Kim, K.P. Proteomic analysis of human lacrimal and tear fluid in dry eye disease. Sci. Rep. 2017, 7, 13363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aqrawi, L.A.; Galtung, H.K.; Guerreiro, E.M.; Øvstebø, R.; Thiede, B.; Utheim, T.P.; Chen, X.; Utheim, Ø.A.; Palm, Ø.; Skarstein, K.; et al. Proteomic and histopathological characterisation of sicca subjects and primary Sjögren’s syndrome patients reveals promising tear, saliva and extracellular vesicle disease biomarkers. Arthritis Res. Ther. 2019, 21, 181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilboe, I.M.; Kvien, T.K.; Uhlig, T.; Husby, G. Sicca symptoms and secondary Sjögren’s syndrome in systemic lupus erythematosus: Comparison with rheumatoid arthritis and correlation with disease variables. Ann. Rheum. Dis. 2001, 60, 1103–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Qin, Q.; Liu, B.; Fu, Y.; Lin, L.; Huang, X.; Jin, X. Clinical analysis: Aqueous-deficient and meibomian gland dysfunction in patients with primary Sjogren’s Syndrome. Front. Med. 2019, 6, 291. [Google Scholar] [CrossRef]
- Gu, Z.; Lu, Q.; Zhang, A.; Shuai, Z.W.; Liao, R. Analysis of ocular surface characteristics and incidence of dry eye disease in systemic lupus erythematosus patients without secondary Sjögren’s Syndrome. Front. Med. 2022, 9, 833995. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.; Ikeda, C.; Watanabe, S.; Oie, Y.; Soma, T.; Watanabe, H.; Maeda, N.; Nishida, K. Effect of non-invasive tear stability assessment on tear meniscus height. Acta Ophthalmol. 2015, 93, e135–e139. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, N.; Ventrella, R.; Peng, H.; Pal-Ghosh, S.; Arvanitis, C.; Rappoport, J.Z.; Mitchell, B.J.; Stepp, M.A.; Lavker, R.M.; Getsios, S. EphA2/Ephrin-A1 mediate corneal epithelial cell compartmentalization via ADAM10 regulation of EGFR signaling. Investig. Ophthalmol. Vis. Sci. 2018, 59, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Gordon, G.M.; Austin, J.S.; Sklar, A.L.; Feuer, W.J.; LaGier, A.J.; Fini, M.E. Comprehensive gene expression profiling and functional analysis of matrix metalloproteinases and TIMPs, and identification of ADAM-10 gene expression, in a corneal model of epithelial resurfacing. J. Cell. Physiol. 2011, 226, 1461–1470. [Google Scholar] [CrossRef]
- Hynne, H.; Aqrawi, L.A.; Jensen, J.L.; Thiede, B.; Palm, Ø.; Amdal, C.D.; Westgaard, K.L.; Herlofson, B.B.; Utheim, T.P.; Galtung, H.K. Proteomic profiling of saliva and tears in radiated head and neck cancer patients as compared to primary Sjögren’s Syndrome patients. Int. J. Mol. Sci. 2022, 23, 3714. [Google Scholar] [CrossRef] [PubMed]
- Wetzel, S.; Seipold, L.; Saftig, P. The metalloproteinase ADAM10: A useful therapeutic target? Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2017, 1864, 2071–2081. [Google Scholar] [CrossRef] [PubMed]
- Saftig, P.; Reiss, K. The “A Disintegrin And Metalloproteases” ADAM10 and ADAM17: Novel drug targets with therapeutic potential? Eur. J. Cell Biol. 2011, 90, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.M.; Tharakan, A.; Martin, R.K. Targeting ADAM10 in cancer and autoimmunity. Front. Immunol. 2020, 11, 499. [Google Scholar] [CrossRef] [Green Version]
- Brazzell, R.K.; Stern, M.E.; Aquavella, J.V.; Beuerman, R.W.; Baird, L. Human recombinant epidermal growth factor in experimental corneal wound healing. Investig. Ophthalmol. Vis. Sci. 1991, 32, 336–340. [Google Scholar]
- Rao, K.; Farley, W.J.; Pflugfelder, S.C. Association between high tear epidermal growth factor levels and corneal subepithelial fibrosis in dry eye conditions. Investig. Ophthalmol. Vis. Sci. 2010, 51, 844–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, H.; Bleiden, L.; de Paiva, C.S.; Farley, W.; Stern, M.E.; Pflugfelder, S.C. Tear cytokine profiles in dysfunctional tear syndrome. Am. J. Ophthalmol. 2009, 147, 198–205.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef] [PubMed]
- Aringer, M.; Costenbader, K.; Daikh, D.; Brinks, R.; Mosca, M.; Ramsey-Goldman, R.; Smolen, J.S.; Wofsy, D.; Boumpas, D.T.; Kamen, D.L.; et al. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheumatol. 2019, 71, 1400–1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bron, A.J.; Evans, V.E.; Smith, J.A. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea 2003, 22, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.; Doh, S.H.; Chung, S.K. Comparison of tear meniscus height measurements obtained with the Keratograph and Fourier domain optical coherence tomography in dry eye. Cornea 2015, 34, 1209–1213. [Google Scholar] [CrossRef]
- Downie, L.E.; Keller, P.R.; Vingrys, A.J. Assessing ocular bulbar redness: A comparison of methods. Ophthalmic Physiol. Opt. 2016, 36, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Hong, J.; Tian, L.; Cui, X.; Sun, X.; Xu, J. Assessment of bulbar redness with a newly developed Keratograph. Optom. Vis. Sci. 2015, 92, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.-H.; Fang, P.-C.; Yu, H.-J.; Lin, P.-W.; Huang, H.-M.; Kuo, M.-T. Analysis of tear film spatial instability for pediatric myopia under treatment. Sci. Rep. 2020, 10, 14789. [Google Scholar] [CrossRef]
- Markoulli, M.; Papas, E.; Petznick, A.; Holden, B. Validation of the flush Method as an alternative to basal or reflex tear collection. Curr. Eye Res. 2011, 36, 198–207. [Google Scholar] [CrossRef]



| Non-SS-DED (n = 12) | pSS-DED (n = 12) | sSS-DED (n = 12) | p Value | p Value (Trend) a | |
|---|---|---|---|---|---|
| Age | 60.75 ± 1.77 | 63.58 ± 1.38 | 59.92 ± 2.89 | 0.446 | 0.782 |
| OSDI b | 40.38 ± 20.20 | 50.24 ± 29.97 | 64.93 ± 27.06 | 0.103 | 0.036 |
| Oxford staining score | 0.010 | 0.003 | |||
| 0 | 11 (91.7) | 5 (41.7) | 3 (25.0) | ||
| 1 | 0 | 4 (33.3) | 2 (16.7) | ||
| 2 | 1 (8.3) | 1 (8.3) | 4 (33.3) | ||
| 4 | 0 | 2 (16.7) | 3 (25.0) | ||
| Tear meniscus height | 0.23 ± 0.10 | 0.16 ± 0.08 | 0.31 ± 0.33 | 0.209 | 0.337 |
| Noninvasive tear breakup time | |||||
| Measurement period | 15.74 ± 8.50 | 12.13 ± 7.77 | 9.74 ± 4.62 | 0.135 | 0.048 |
| NIKBUT First c | 9.27 ± 7.50 | 8.69 ± 6.46 | 5.62 ± 2.37 | 0.279 | 0.137 |
| NIKBUT Avg d | 11.69 ± 7.26 | 10.21 ± 6.25 | 6.85 ± 3.16 | 0.130 | 0.050 |
| Ocular surface redness | |||||
| Bulbar redness | 1.25 ± 0.41 | 1.49 ± 0.54 | 1.57 ± 0.68 | 0.357 | 0.172 |
| Area | 8.78 ± 4.65 | 8.19 ± 3.75 | 8.07 ± 4.17 | 0.906 | 0.679 |
| Bulbar temporal | 1.21 ± 0.40 | 1.58 ± 0.53 | 1.55 ± 0.67 | 0.189 | 0.133 |
| Bulbar nasal | 1.38 ± 0.65 | 1.41 ± 0.64 | 1.66 ± 0.75 | 0.545 | 0.316 |
| Limbal temporal | 0.83 ± 0.41 | 1.10 ± 0.44 | 1.18 ± 0.68 | 0.244 | 0.110 |
| Limbal nasal | 0.83 ± 0.34 | 0.70 ± 0.33 | 1.25 ± 0.79 | 0.041 | 0.063 |
| Canonical Pathway | p-Value |
|---|---|
| Acute phase response signaling | 4.93 × 10−13 |
| LXR/RXR activation | 1.69 × 10−9 |
| FXR/RXR activation | 1.95 × 10−9 |
| Coagulation system | 1.18 × 10−5 |
| Complement system | 1.33 × 10−5 |
| Canonical Pathway | p-Value |
|---|---|
| EIF2 signaling | 2.33 × 10−9 |
| Coronavirus pathogenesis pathway | 1.95 × 10−5 |
| Atherosclerosis signaling | 3.07 × 10−5 |
| IL-15 signaling | 5.53 × 10−5 |
| Caveolar-mediated endocytosis signaling | 8.59 × 10−5 |
| Non-SS-DED (n = 11) | pSS-DED (n = 12) | sSS-DED (n = 11) | p Value | p Value (Trend) | |
|---|---|---|---|---|---|
| EGF | 12.94 ± 7.13 | 3.32 ± 6.82 | 17.73 ± 18.76 | 0.023 | 0.361 |
| ADAM10 | 19.60 ± 5.05 | 21.35 ± 8.19 | 38.92 ± 27.54 | 0.018 | 0.011 |
| Albumin | 5270.98 ± 9990.46 | 21,160.28 ± 30,812.56 | 50,489.62 ± 105,118.12 | 0.243 | 0.101 |
| S100A8 | 89.70 ± 45.23 | 78.33 ± 41.13 | 136.67 ± 102.74 | 0.116 | 0.117 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, Y.-T.; Huang, Y.-T.; Yu, H.-J.; Fang, P.-C.; Kuo, M.-T. Tear Proteomics Approach to Distinguishing Primary from Secondary Sjögren’s Syndrome for Dry Eye Patients with Long-Term Instillation of Eyedrops. Int. J. Mol. Sci. 2022, 23, 15239. https://doi.org/10.3390/ijms232315239
Hsiao Y-T, Huang Y-T, Yu H-J, Fang P-C, Kuo M-T. Tear Proteomics Approach to Distinguishing Primary from Secondary Sjögren’s Syndrome for Dry Eye Patients with Long-Term Instillation of Eyedrops. International Journal of Molecular Sciences. 2022; 23(23):15239. https://doi.org/10.3390/ijms232315239
Chicago/Turabian StyleHsiao, Yu-Ting, Yu-Ting Huang, Hun-Ju Yu, Po-Chiung Fang, and Ming-Tse Kuo. 2022. "Tear Proteomics Approach to Distinguishing Primary from Secondary Sjögren’s Syndrome for Dry Eye Patients with Long-Term Instillation of Eyedrops" International Journal of Molecular Sciences 23, no. 23: 15239. https://doi.org/10.3390/ijms232315239
APA StyleHsiao, Y.-T., Huang, Y.-T., Yu, H.-J., Fang, P.-C., & Kuo, M.-T. (2022). Tear Proteomics Approach to Distinguishing Primary from Secondary Sjögren’s Syndrome for Dry Eye Patients with Long-Term Instillation of Eyedrops. International Journal of Molecular Sciences, 23(23), 15239. https://doi.org/10.3390/ijms232315239






