Dihydrotanshinone I Inhibits the Lung Metastasis of Breast Cancer by Suppressing Neutrophil Extracellular Traps Formation
Abstract
:1. Introduction
2. Results
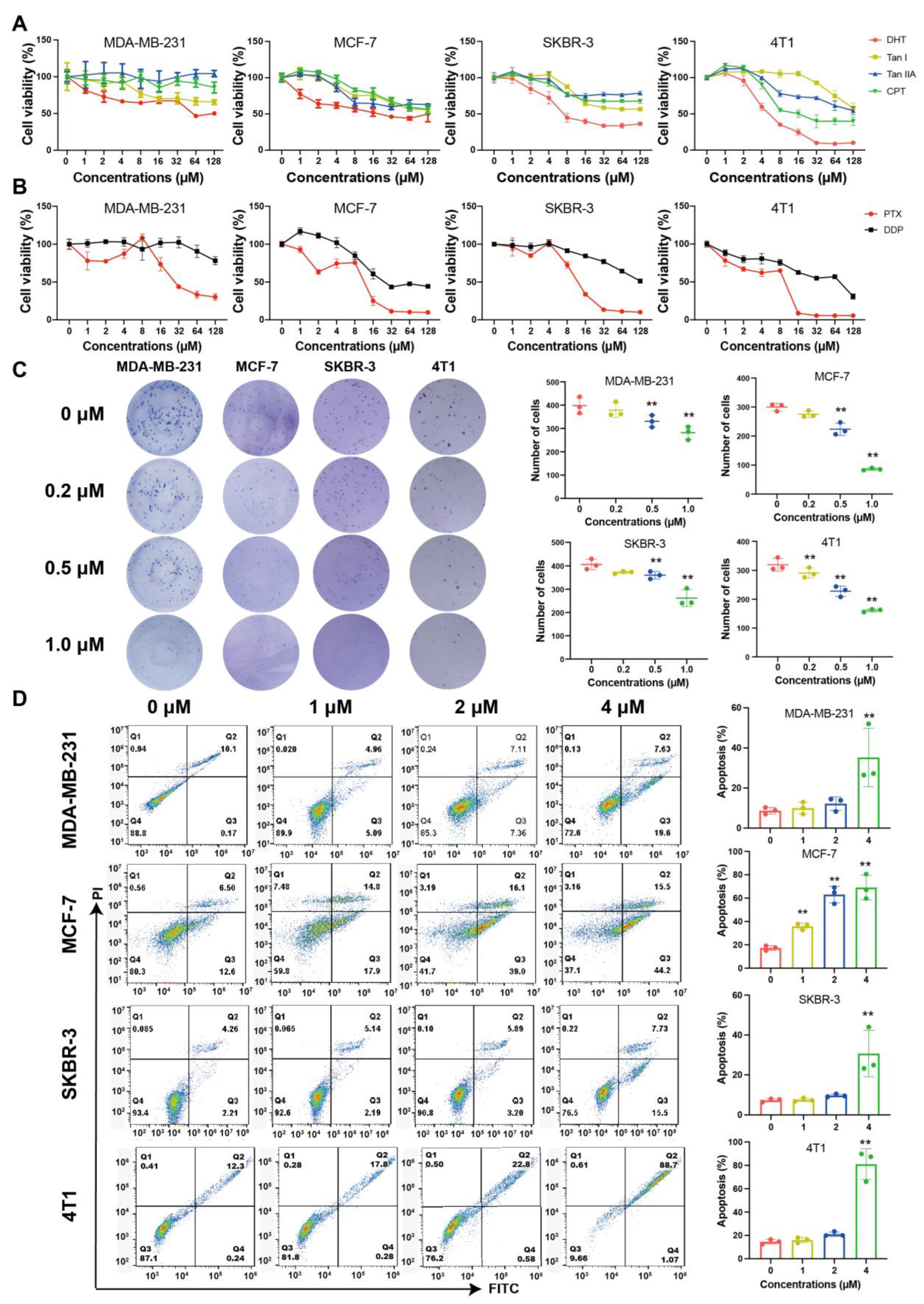
2.1. Tanshinones Inhibited Proliferation and Clonogenicity of BC Cells
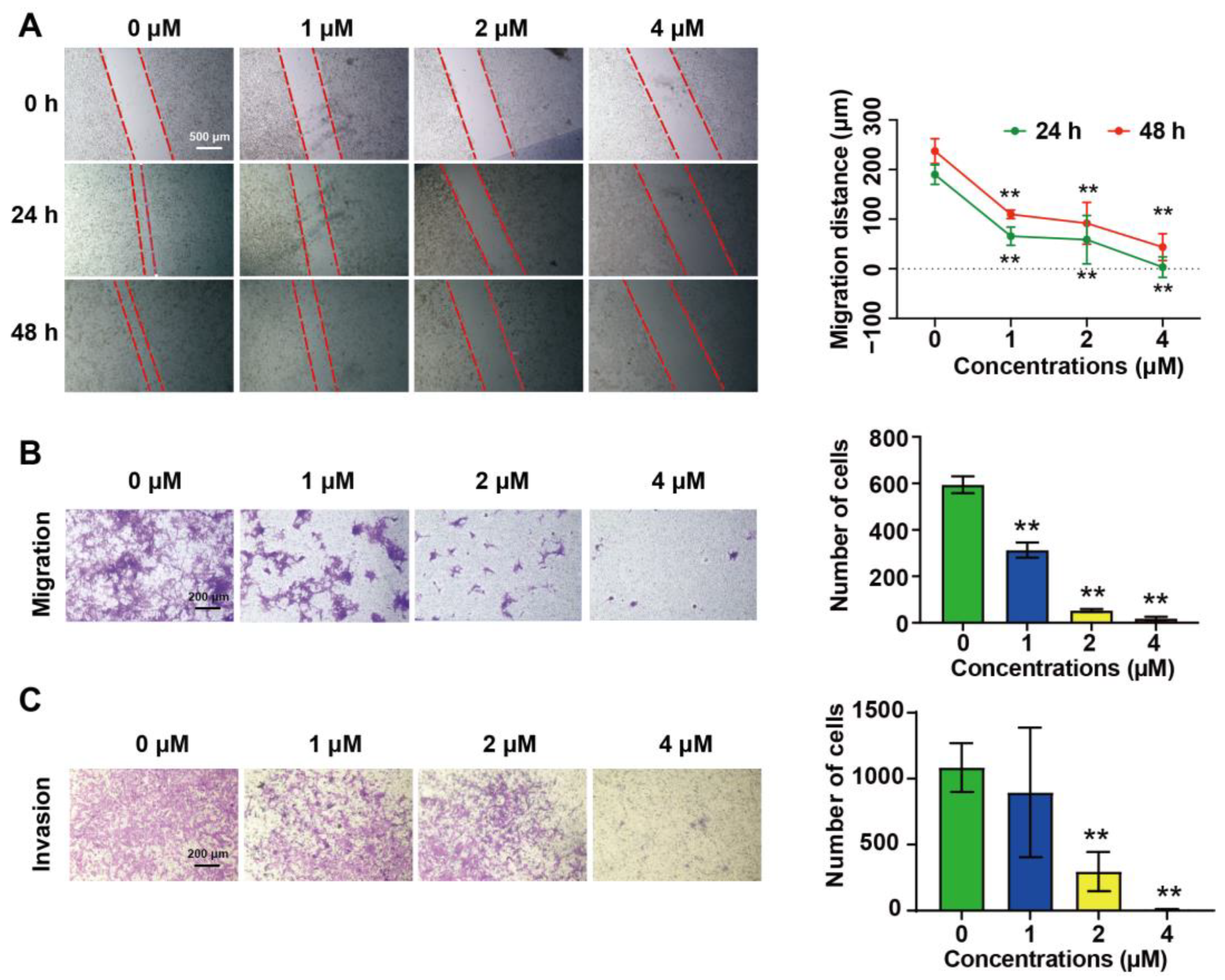
2.2. DHT Inhibited the Migration and Invasion of 4T1 Cells
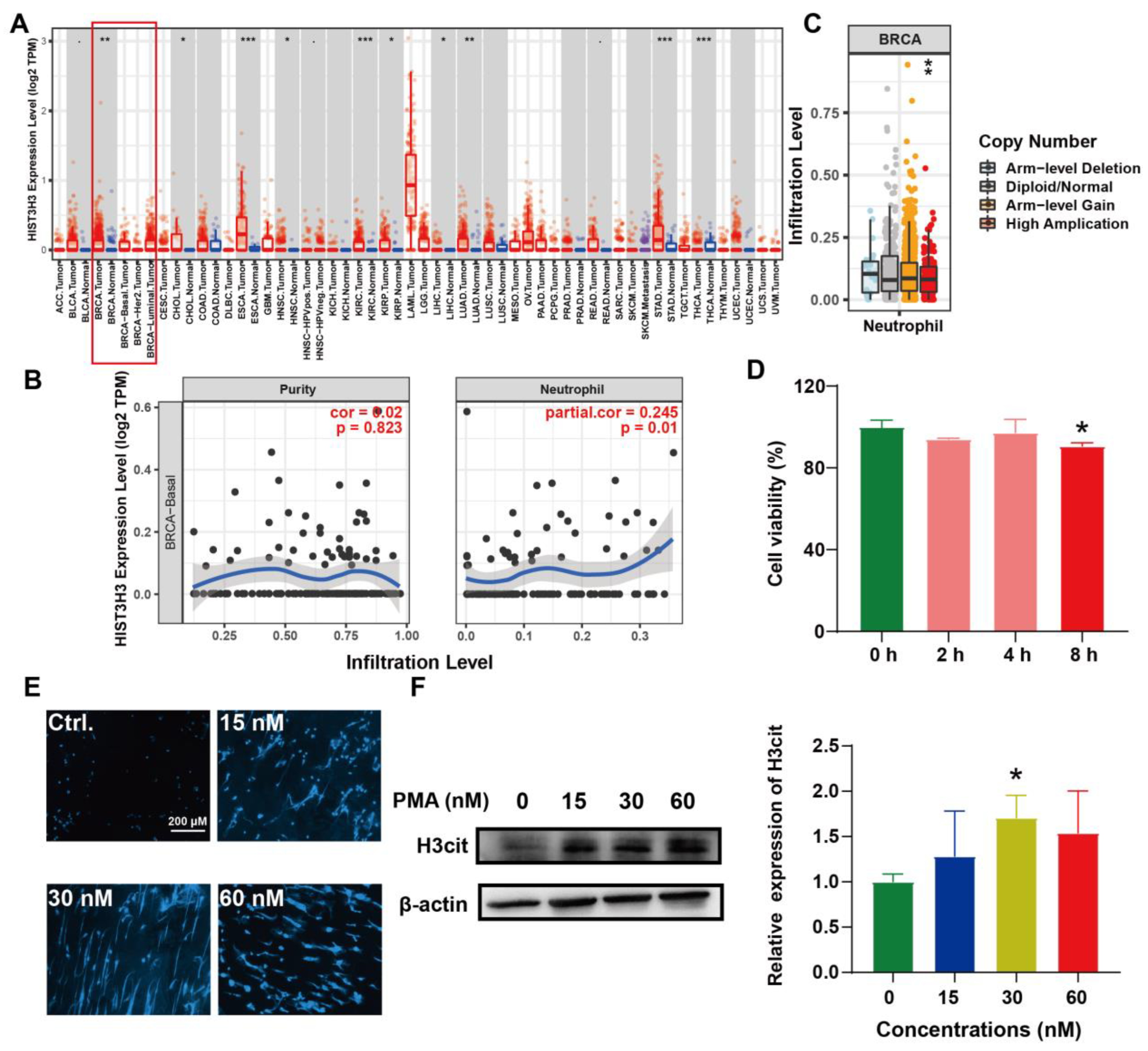
2.3. Induction of NETs and Its Correlation with BC
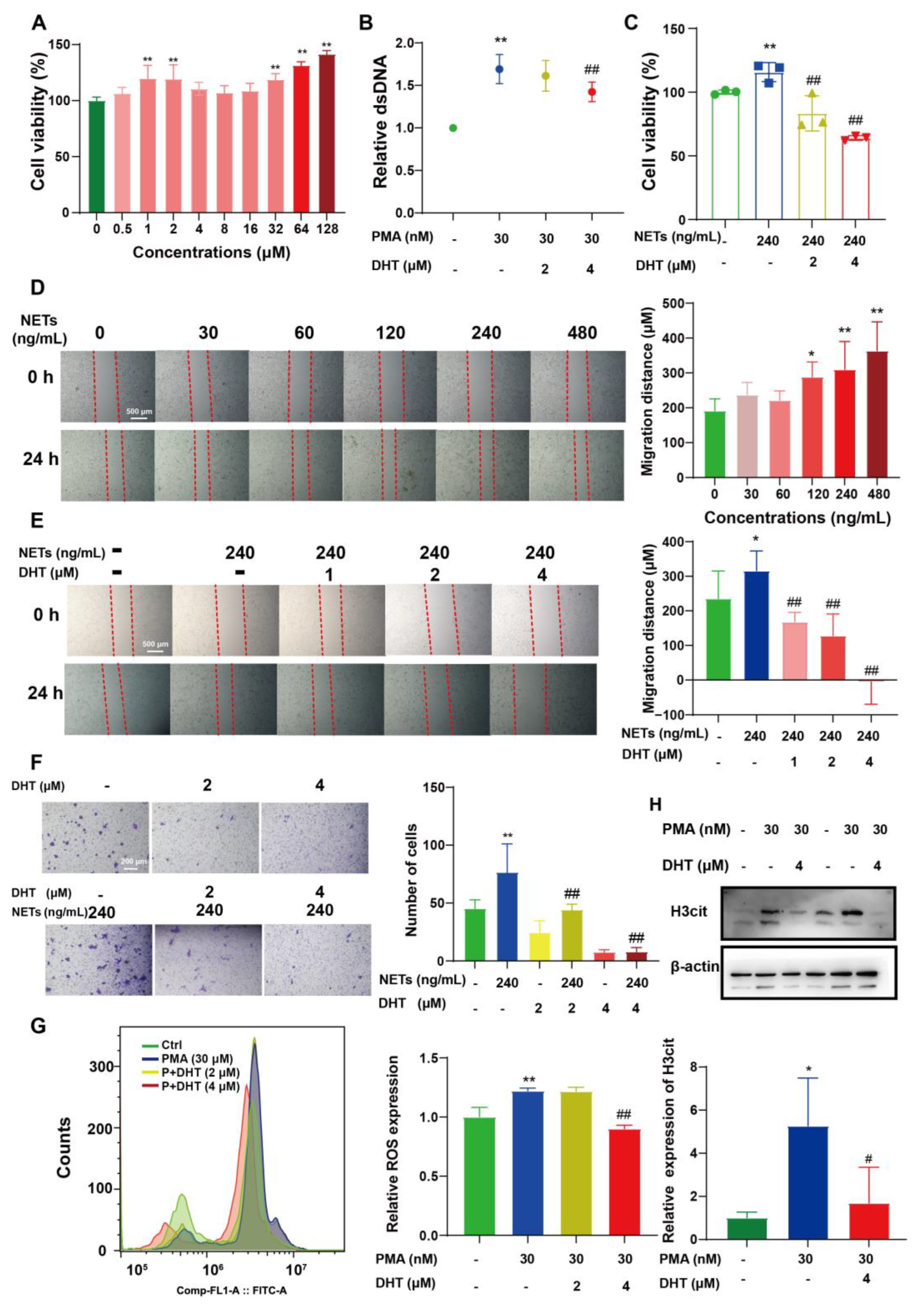
2.4. DHT Reversed NETs-Induced Proliferation, Migration, and ROS Production of 4T1 Cells
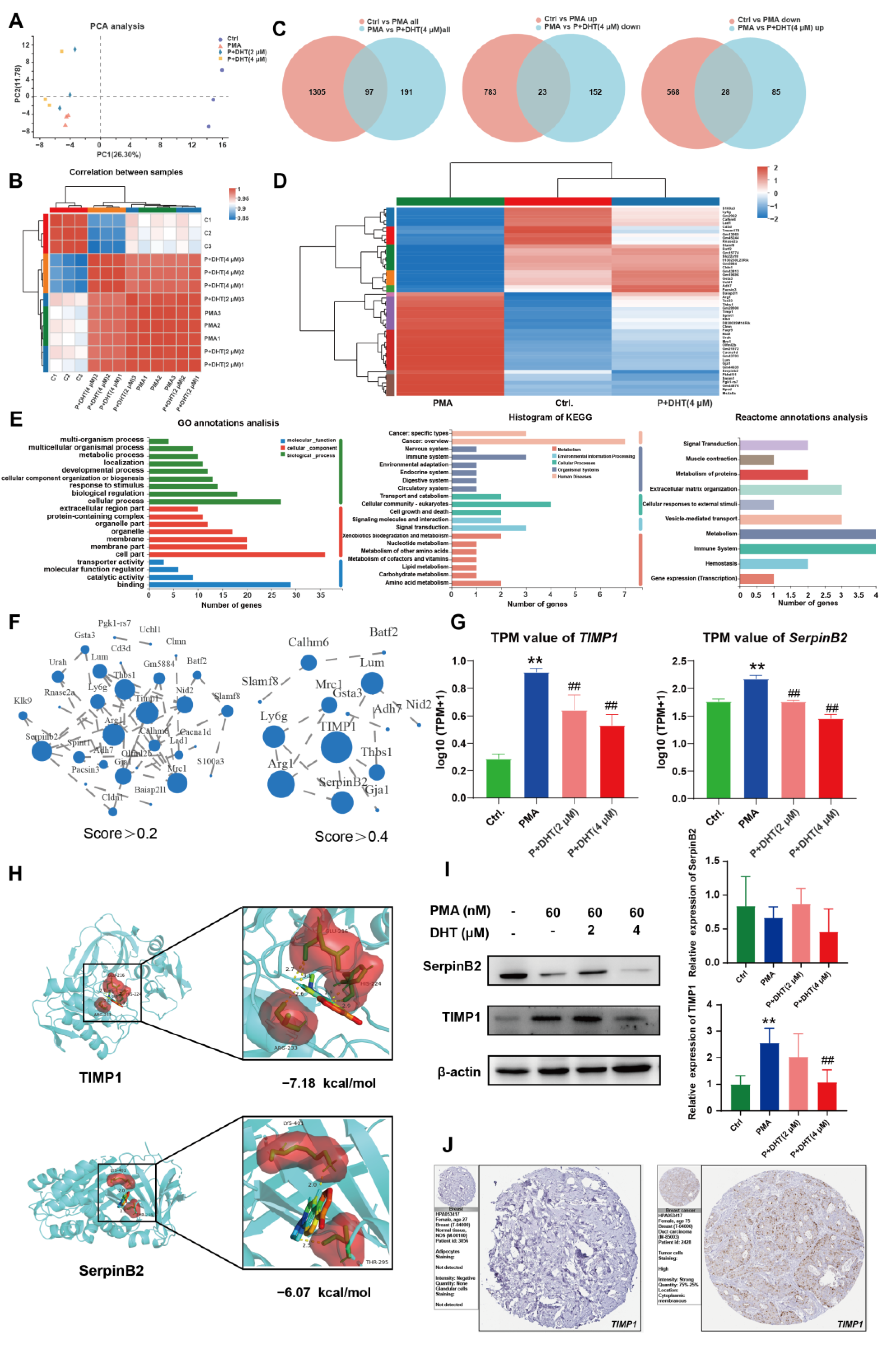
2.5. DHT Suppressed the NETs Formation by Restraining TIMP1 Expression
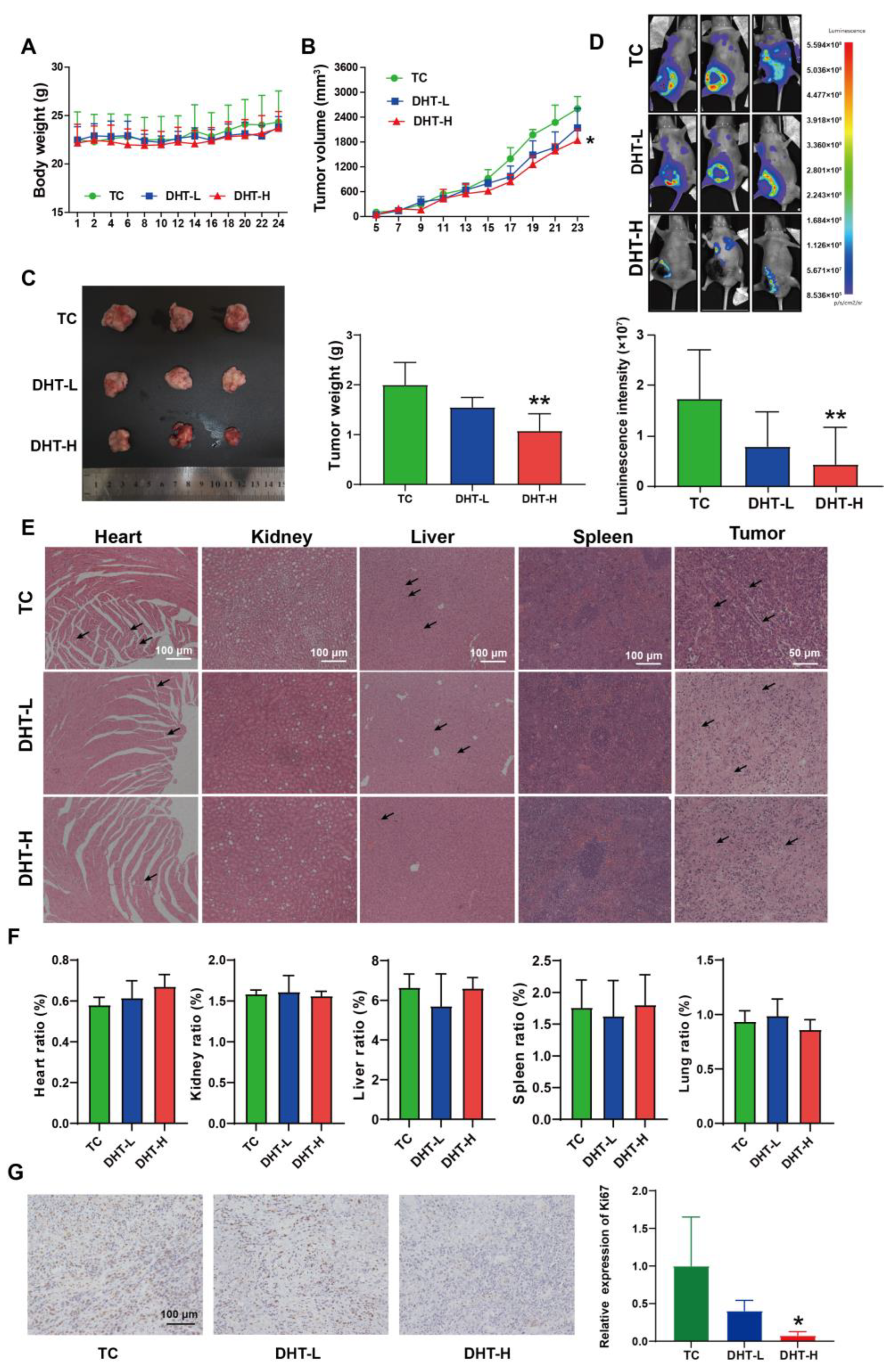
2.6. DHT Inhibited Tumor Growth and Lung Metastasis in 4T1 Tumor-Bearing Nude Mice
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. MTT Assay
4.4. Colony Formation Assay
4.5. Wound Healing and Transwell Assays
4.6. Apoptosis Analysis
4.7. Extraction of PMNs and NETs
4.8. ROS Analysis
4.9. Hoechst 33342 Staining
4.10. dsDNA Test
4.11. Western Blotting
4.12. Animal Experiments
4.13. Hematoxylin and Eosin Staining
4.14. Immunofluorescence Analysis
4.15. Immunohistochemistry
4.16. Molecular Docking
4.17. TIMER Database Analysis
4.18. RNA Sequencing
4.19. Bioinformatics Analysis
4.20. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat Rev Dis Prim. 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Poortmans, P.; Morrow, M.; Denkert, C.; Curigliano, G. Breast cancer. Lancet 2021, 397, 1750–1769. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Adams, S.; Rugo, H.; Schneeweiss, A.; Barrios, C.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.; Shaw Wright, G.; et al. Atezolizumab and nab-Paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef] [PubMed]
- Tulotta, C.; Ottewell, P. The role of IL-1B in breast cancer bone metastasis. Endocr. Relat. Cancer 2018, 25, R421–R434. [Google Scholar] [CrossRef] [Green Version]
- Pentheroudakis, G.; Fountzilas, G.; Bafaloukos, D.; Koutsoukou, V.; Pectasides, D.; Skarlos, D.; Samantas, E.; Kalofonos, H.; Gogas, H.; Pavlidis, N. Metastatic breast cancer with liver metastases: A registry analysis of clinicopathologic, management and outcome characteristics of 500 women. Breast Cancer Res. Treat. 2006, 97, 237–244. [Google Scholar] [CrossRef]
- Németh, T.; Sperandio, M.; Mócsai, A. Neutrophils as emerging therapeutic targets. Nat. Rev. Drug Discov. 2020, 19, 253–275. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Pittman, K.; Kubes, P. Damage-associated molecular patterns control neutrophil recruitment. J. Innate. Immun. 2013, 5, 315–323. [Google Scholar] [CrossRef]
- Lehman, H.; Segal, B. The role of neutrophils in host defense and disease. J. Allergy Clin. Immunol. Pr. 2020, 145, 1535–1544. [Google Scholar] [CrossRef]
- Carnevale, S.; Ghasemi, S.; Rigatelli, A.; Jaillon, S. The complexity of neutrophils in health and disease: Focus on cancer. Semin. Immunol. 2020, 48, 101409. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Zychlinsky, A. Neutrophil extracellular traps: Is immunity the second function of chromatin? J. Cell Biol. 2012, 198, 773–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, S.; Mihalache, C.; Kozlowski, E.; Schmid, I.; Simon, H. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009, 16, 1438–1444. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Franco, M.; León-Rodríguez, E.; Sevilla-González, M. Could neutrophil extracellular traps be the new prognostic markers of cancer? Rev. Invest. Clin. 2019, 71, 365–368. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Wysocki, R.; Amoozgar, Z.; Maiorino, L.; Fein, M.; Jorns, J.; Schott, A.; Kinugasa-Katayama, Y.; Lee, Y.; Won, N.; et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci. Transl. Med. 2016, 8, 361ra138. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Wang, Y.; Shi, M.; Maoz, I.; Gao, X.; Sun, M.; Yuan, T.; Li, K.; Zhou, W.; Guo, X.; et al. SmbHLH60 and SmMYC2 antagonistically regulate phenolic acids and anthocyanins biosynthesis in Salvia miltiorrhiza. J. Adv. Res. 2022; in press. [Google Scholar] [CrossRef]
- Qian, J.; Xu, X.; Su, J.; Zeng, W.; Han, B.; Hao, X.; Kai, G. A strategy for effective recovery of salvianolic acid A from Salvia miltiorrhiza (Danshen) through multiple interactions. Compos. B Eng. 2022, 231, 109563. [Google Scholar] [CrossRef]
- Dai, H.; Li, X.; Li, X.; Bai, L.; Li, Y.; Xue, M. Coexisted components of Salvia miltiorrhiza enhance intestinal absorption of cryptotanshinone via inhibition of the intestinal P-gp. Phytomedicine 2012, 19, 1256–1262. [Google Scholar] [CrossRef]
- Sun, C.; Han, B.; Zhai, Y.; Zhao, H.; Li, X.; Qian, J.; Hao, X.; Liu, Q.; Shen, J.; Kai, G. Dihydrotanshinone I inhibits ovarian tumor growth by activating oxidative stress through Keap1-mediated Nrf2 ubiquitination degradation. Free Radic. Biol. Med. 2022, 180, 220–235. [Google Scholar] [CrossRef]
- Chen, X.; Yu, J.; Zhong, B.; Lu, J.; Lu, J.; Li, S.; Lu, Y. Pharmacological activities of dihydrotanshinone I, a natural product from Salvia miltiorrhiza Bunge. Pharm. Res. 2019, 145, 104254. [Google Scholar] [CrossRef]
- Tsai, S.L.; Suk, F.M.; Wang, C.I.; Liu, D.Z.; Hou, W.C.; Lin, P.J.; Hung, L.F.; Liang, Y.C. Anti-tumor potential of 15,16-dihydrotanshinone I against breast adenocarcinoma through inducing G1 arrest and apoptosis. Biochem. Pharm. 2007, 74, 1575–1586. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.L.; Choi, H.S.; Kim, J.H.; Jeong, D.K.; Kim, K.S.; Lee, D.S. Dihydrotanshinone-induced NOX5 activation inhibits breast cancer stem cell through the ROS/Stat3 signaling pathway. Oxid. Med. Cell. Longev. 2019, 2019, 9296439. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, H.; Tan, S.; Dong, Q.; Fan, X.; Wang, Y.; Zhang, H.; He, J. The role of neutrophil extracellular traps in cancer progression, metastasis and therapy. Exp. Hematol. Oncol. 2022, 11, 99. [Google Scholar] [CrossRef]
- Trayes, K.; Cokenakes, S. Breast cancer treatment. Am. Fam. Physician. 2021, 104, 171–178. [Google Scholar] [PubMed]
- Chen, X.; Lu, P.; Wu, Y.; Wang, D.D.; Zhou, S.Y.; Yang, S.J.; Shen, H.Y.; Zhang, X.H.; Zhao, J.H.; Tang, J.H. MiRNAs-mediated cisplatin resistance in breast cancer. Tumor. Biol. 2016, 37, 12905–12913. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Han, B.; Li, X.; Sun, C.; Zhai, Y.; Li, M.; Jiang, M.; Zhang, W.; Liang, Y.; Kai, G. Salvia miltiorrhiza in breast cancer treatment: A review of its phytochemistry, derivatives, nanoparticles, and potential mechanisms. Front. Pharm. 2022, 13, 872085. [Google Scholar] [CrossRef]
- Schoeps, B.; Eckfeld, C.; Prokopchuk, O.; Böttcher, J.; Häußler, D.; Steiger, K.; Demir, I.; Knolle, P.; Soehnlein, O.; Jenne, D.; et al. TIMP1 triggers neutrophil extracellular trap formation in pancreatic cancer. Cancer Res. 2021, 81, 3568–3579. [Google Scholar] [CrossRef]
- Masucci, M.; Minopoli, M.; Del Vecchio, S.; Carriero, M. The emerging role of neutrophil extracellular traps (NETs) in tumor progression and metastasis. Front. Immunol. 2020, 11, 1749. [Google Scholar] [CrossRef]
- Xiao, Y.; Cong, M.; Li, J.; He, D.; Wu, Q.; Tian, P.; Wang, Y.; Yang, S.; Liang, C.; Liang, Y.; et al. Cathepsin C promotes breast cancer lung metastasis by modulating neutrophil infiltration and neutrophil extracellular trap formation. Cancer Cell 2021, 39, 423–437.e427. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Q.; Zhang, X.; Liu, X.; Zhou, B.; Chen, J.; Huang, D.; Li, J.; Li, H.; Chen, F.; et al. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature 2020, 583, 133–138. [Google Scholar] [CrossRef]
- Vorobjeva, N.; Chernyak, B. NETosis: Molecular mechanisms, role in physiology and pathology. Biochemistry 2020, 85, 1178–1190. [Google Scholar] [CrossRef] [PubMed]
- Würtz, S.; Schrohl, A.; Sørensen, N.; Lademann, U.; Christensen, I.; Mouridsen, H.; Brünner, N. Tissue inhibitor of metalloproteinases-1 in breast cancer. Endocr. Relat. Cancer 2005, 12, 215–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Würtz, S.; Schrohl, A.; Mouridsen, H.; Brünner, N. TIMP-1 as a tumor marker in breast cancer--an update. Acta Oncol. 2008, 47, 580–590. [Google Scholar] [CrossRef]
- Grünwald, B.; Harant, V.; Schaten, S.; Frühschütz, M.; Spallek, R.; Höchst, B.; Stutzer, K.; Berchtold, S.; Erkan, M.; Prokopchuk, O.; et al. Pancreatic premalignant lesions secrete tissue inhibitor of metalloproteinases-1, which activates hepatic stellate cells via CD63 signaling to create a premetastatic niche in the liver. Gastroenterology 2016, 151, 1011–1024.e1017. [Google Scholar] [CrossRef] [Green Version]
- Ding, W.; Zhou, X.; Jiang, G.; Xu, W.; Long, S.; Xiao, F.; Liao, Y.; Liu, J. Identification of prognostic biomarkers of glioblastoma based on multidatabase integration and its correlation with immune-infiltration cells. J. Oncol. 2022, 2022, 3909030. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Suk Kim, H.; Ki Choi, S.; Hye Hwang, E.; Woo, J.; Suk Ryu, H.; Kim, K.; Moon, A.; Kyung Moon, W. microRNA-200c/141 upregulates SerpinB2 to promote breast cancer cell metastasis and reduce patient survival. Oncotarget 2017, 8, 32769–32782. [Google Scholar] [CrossRef] [Green Version]
- Du, Q.Q.; Tang, M.; Huang, L.L.; Zhao, R.; Yan, C.; Li, Y.; Pan, X.D. The antitumor activity and mechanism of MCL3 in G422 glioblastoma. World J. Tradit. Chin. Med. 2020, 6, 353–361. [Google Scholar]
- Wu, H.; Lin, J.; Liu, Y.; Chen, H.; Hsu, K.; Lin, S.; Peng, K.; Lin, K.; Hsieh, C.; Chen, D. Luteolin suppresses androgen receptor-positive triple-negative breast cancer cell proliferation and metastasis by epigenetic regulation of MMP9 expression via the AKT/mTOR signaling pathway. Phytomedicine 2021, 81, 153437. [Google Scholar] [CrossRef]
- Fischer, A.; Jacobson, K.; Rose, J.; Zeller, R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008, 2008, pdb.prot4986. [Google Scholar] [CrossRef]
- Li, T.; Fan, J.; Wang, B.; Traugh, N.; Chen, Q.; Liu, J.; Li, B.; Liu, X. TIMER: A web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. 2017, 77, e108–e110. [Google Scholar] [CrossRef]
- Pontén, F.; Jirström, K.; Uhlen, M. The Human Protein Atlas--a tool for pathology. J. Pathol. 2008, 216, 387–393. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Liang, Y.; Sun, C.; Zhai, Y.; Li, X.; Jiang, M.; Yang, R.; Li, X.; Shu, Q.; Kai, G.; et al. Dihydrotanshinone I Inhibits the Lung Metastasis of Breast Cancer by Suppressing Neutrophil Extracellular Traps Formation. Int. J. Mol. Sci. 2022, 23, 15180. https://doi.org/10.3390/ijms232315180
Zhao H, Liang Y, Sun C, Zhai Y, Li X, Jiang M, Yang R, Li X, Shu Q, Kai G, et al. Dihydrotanshinone I Inhibits the Lung Metastasis of Breast Cancer by Suppressing Neutrophil Extracellular Traps Formation. International Journal of Molecular Sciences. 2022; 23(23):15180. https://doi.org/10.3390/ijms232315180
Chicago/Turabian StyleZhao, Huan, Yi Liang, Chengtao Sun, Yufei Zhai, Xuan Li, Mi Jiang, Ruiwen Yang, Xiaojuan Li, Qijin Shu, Guoyin Kai, and et al. 2022. "Dihydrotanshinone I Inhibits the Lung Metastasis of Breast Cancer by Suppressing Neutrophil Extracellular Traps Formation" International Journal of Molecular Sciences 23, no. 23: 15180. https://doi.org/10.3390/ijms232315180
APA StyleZhao, H., Liang, Y., Sun, C., Zhai, Y., Li, X., Jiang, M., Yang, R., Li, X., Shu, Q., Kai, G., & Han, B. (2022). Dihydrotanshinone I Inhibits the Lung Metastasis of Breast Cancer by Suppressing Neutrophil Extracellular Traps Formation. International Journal of Molecular Sciences, 23(23), 15180. https://doi.org/10.3390/ijms232315180





