Himatanthus bracteatus-Composed In Situ Polymerizable Hydrogel for Wound Healing
Abstract
:1. Introduction
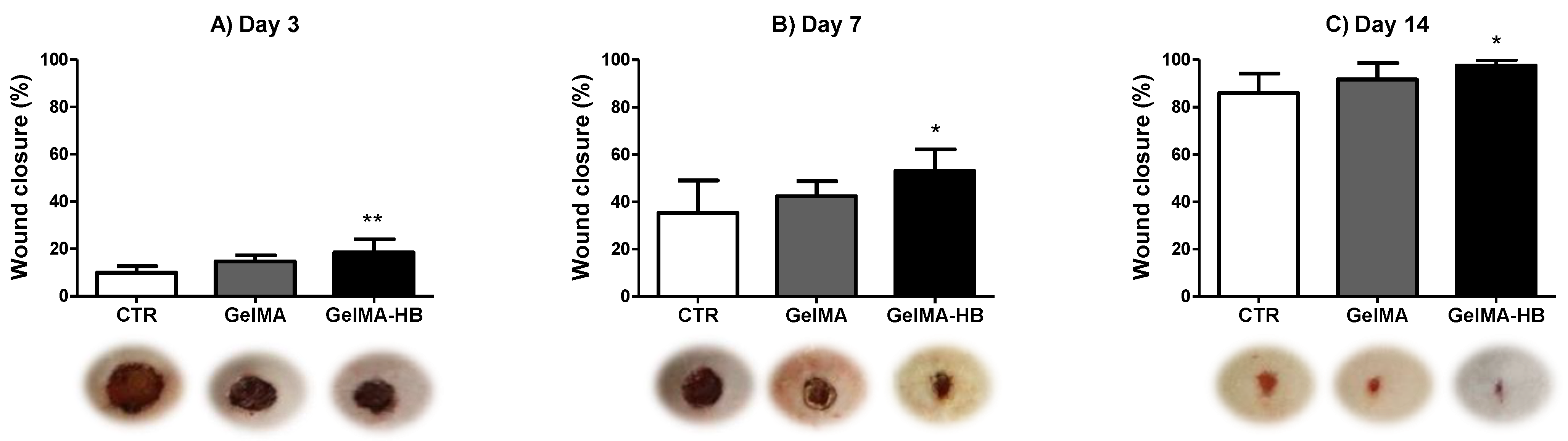
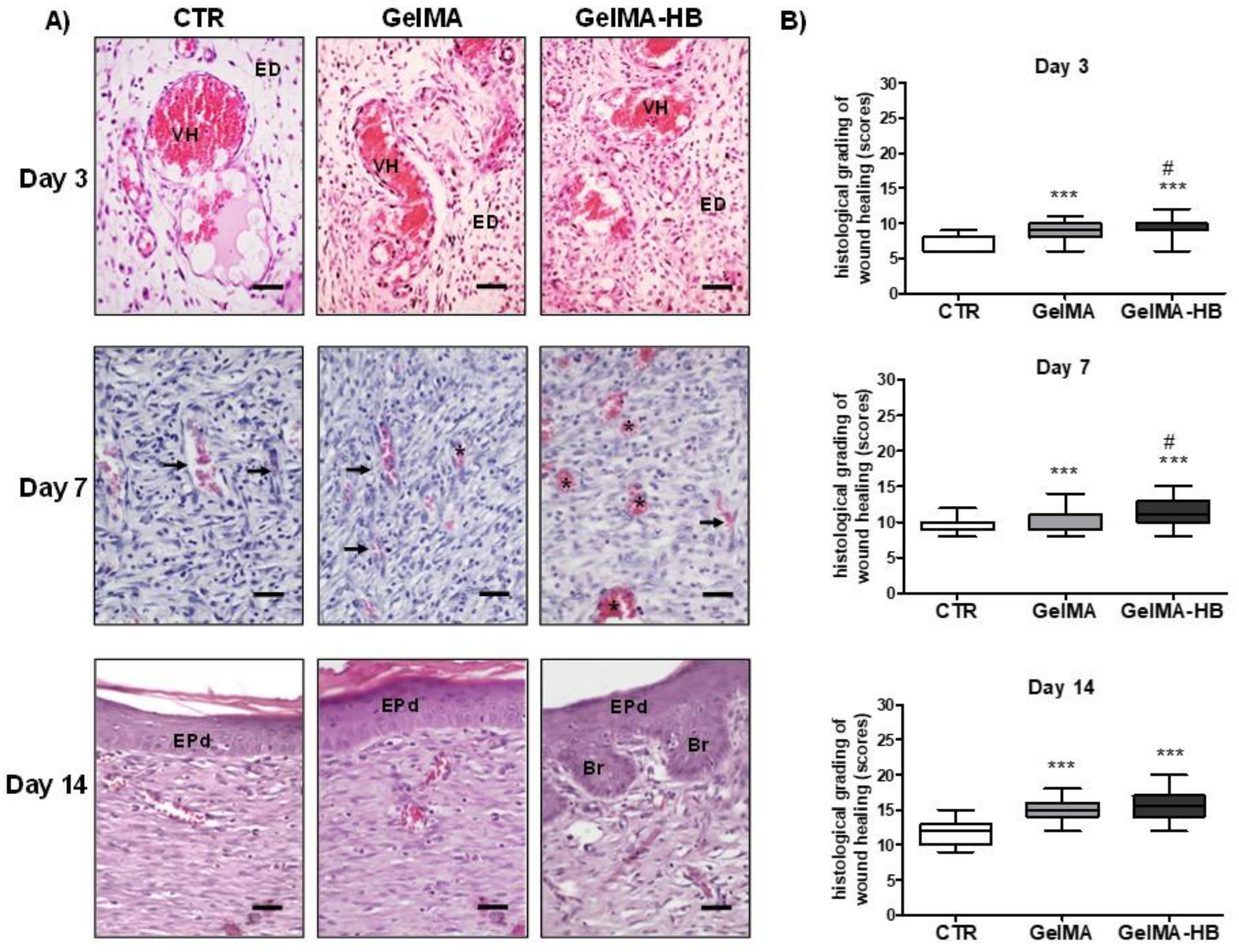
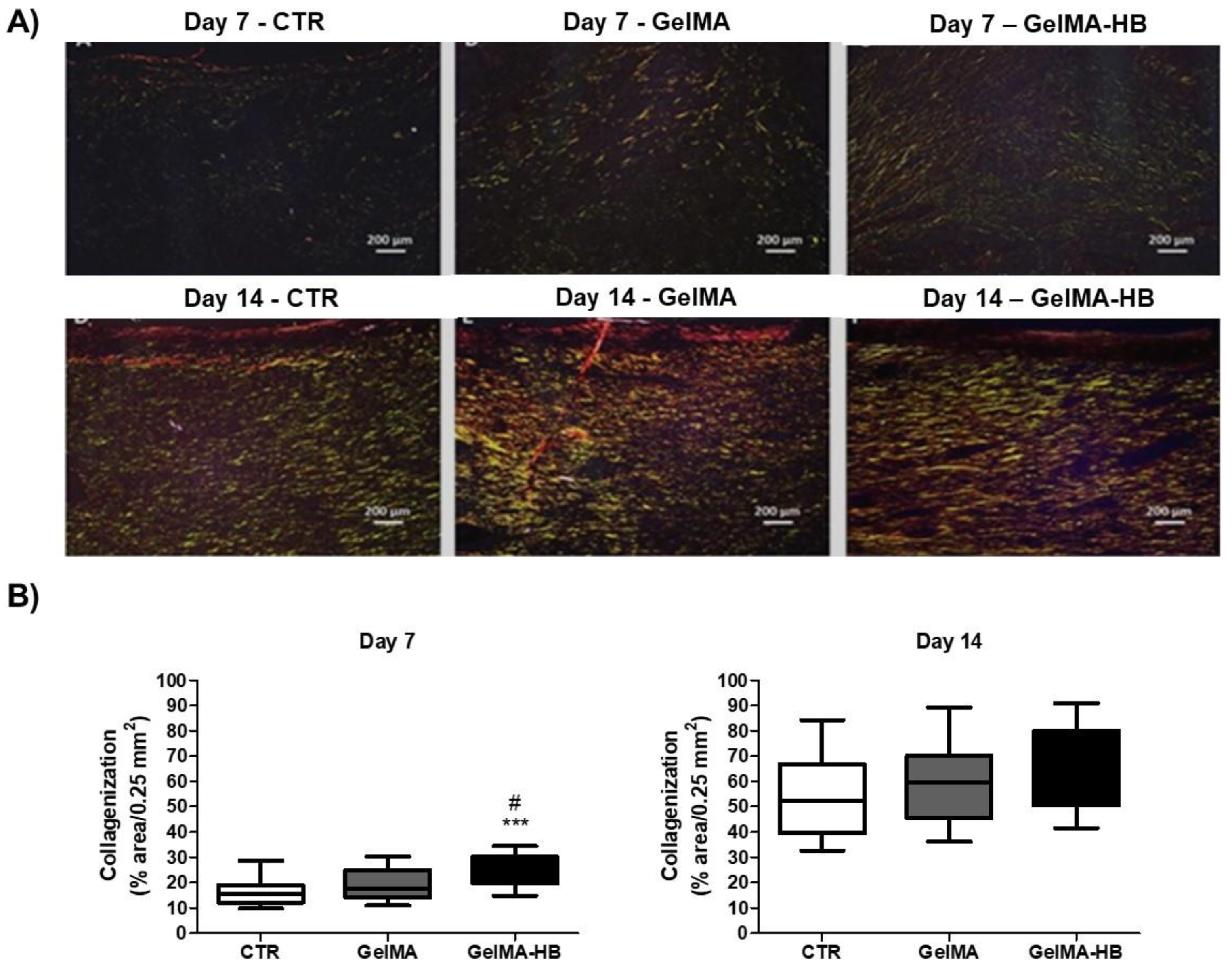
2. Results
3. Discussion
4. Material and Methods
4.1. Ethanolic Extract of H. bracteatus (EEHB)
4.2. Preparation and Characterization of Hydrogels
4.2.1. Pre-Polymer Preparation
4.2.2. Hydrogel Formulations (Photopolymerization Step)
4.2.3. Characterization of Hydrogels
4.3. Biological Assays
4.3.1. Ethics Issues
4.3.2. Anti-Inflammatory Activity of EEHB
4.3.3. Wound-Healing Assay GelMA Formulations
4.3.4. Assessment of Wound Closure Rates (WCR)
4.3.5. Pathological Analysis of the Healing Tissues
- i.
- ii.
- Immunohistochemical analysis of myofibroblast differentiation (MFd) and microvascular density (MVd): Histological sections were mounted on glass slides previously salinized, deparaffinized in xylol, and washed in decreasing concentrations of ethyl alcohol (100%, 95%, 90%, 80%, and 70%). Blocking of endogenous peroxidase activity was performed with 3% hydrogen peroxide and methyl alcohol (10 min in a dark room). Then, the procedure of immunodetection of the researched antigens was carried out by means of incubation with the primary antibodies, as described in Table 2.
- iii.
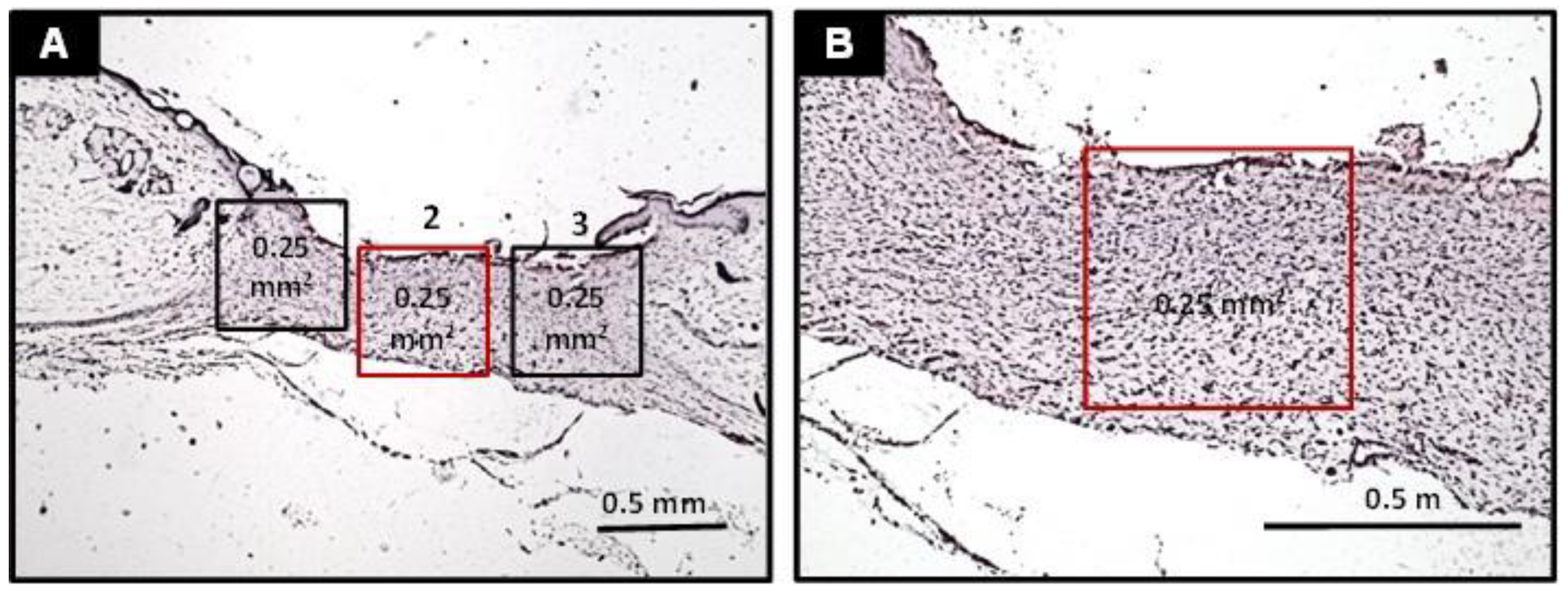
- Assessment of collagen fiber content: the analysis of collagen fibers was carried out in three histological sections of each animal stained in Sirius red and analyzed under polarized light. Collagen fibers were classified into type III or type I according to their birefringence pattern (green and yellow/red, respectively). The morphological features (stretched/wavy, thin/thick, short/long) and architectural arrangement (reticular, parallel or fascicle) of the fibers were also observed. Therefore, three histological fields (100× magnification, 0.25 mm2) of each histological section were photomicrographed, and the percentage of the area containing collagen fibers was obtained using the software Image J®.
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, P.H.; Huang, B.S.; Horng, H.C.; Yeh, C.C.; Chen, Y.J. Wound healing. J. Chin. Med. Assoc. 2018, 81, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Wallace, H.A.; Basehore, B.M.; Zito, P.M. Wound Healing Phases. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Wernick, B.; Nahirniak, P.; Stawicki, S.P. Impaired Wound Healing. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Li, J.; Zhang, Y.P.; Kirsner, R.S. Angiogenesis in wound repair: Angiogenic growth factors and the extracellular matrix. Microsc. Res. Tech. 2003, 60, 107–114. [Google Scholar] [CrossRef] [PubMed]
- DiPietro, L.A. Angiogenesis and wound repair: When enough is enough. J. Leukoc. Biol. 2016, 100, 979–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veith, A.P.; Henderson, K.; Spencer, A.; Sligar, A.D.; Baker, A.B. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv. Drug Deliv. Rev. 2019, 146, 97–125. [Google Scholar] [CrossRef] [PubMed]
- Darby, I.A.; Laverdet, B.; Bonté, F.; Desmoulière, A. Fibroblasts and myofibroblasts in wound healing. Clin. Cosmet. Investig. Dermatol. 2014, 7, 301–311. [Google Scholar] [CrossRef] [Green Version]
- Chitturi, R.T.; Balasubramaniam, A.M.; Parameswar, R.A.; Kesavan, G.; Haris, K.T.M.; Mohideen, K. The role of myofibroblasts in wound healing, contraction and its clinical implications in cleft palate repair. J. Int. Oral Health 2015, 7, 75–80. [Google Scholar]
- Darwin, E.; Tomic-Canic, M. Healing Chronic Wounds: Current Challenges and Potential Solutions. Curr. Dermatol. Rep. 2018, 7, 296–302. [Google Scholar] [CrossRef]
- Sharma, A.; Khanna, S.; Kaur, G.; Singh, I. Medicinal plants and their components for wound healing applications. Future J. Pharm. Sci. 2021, 7, 53. [Google Scholar] [CrossRef]
- Thangapazham, R.L.; Sharad, S.; Maheshwari, R.K. Phytochemicals in Wound Healing. Adv. Wound Care 2016, 5, 230–241. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, N.; Wong, S.K.; Mohamed, I.N.; Mohamed, N.; Chin, K.Y.; Ima-Nirwana, S.; Shuid, A.N. Wound Healing Properties of Selected Natural Products. Int. J. Environ. Res. Public Health 2018, 15, 2360. [Google Scholar] [CrossRef] [Green Version]
- da Silva, M.L.; Stehmann, J.R.; Serafim, M.S.M.; Vale, V.V.; Gontijo, D.C.; Brandão, G.C.; Kroon, E.G.; de Oliveira, A.B. Himatanthus bracteatus stem extracts present anti-flavivirus activity while an isolated sesquiterpene glucoside present only anti-Zika virus activity in vitro. Nat. Prod. Res. 2021, 35, 3161–3165. [Google Scholar] [CrossRef] [PubMed]
- Lucetti, D.L.; Lucetti, E.C.; Bandeira, M.A.; Veras, H.N.; Silva, A.H.; Leal, L.K.; Lopes, A.A.; Alves, V.C.; Silva, G.S.; Brito, G.A.; et al. Anti-inflammatory effects and possible mechanism of action of lupeol acetate isolated from Himatanthus drasticus (Mart.) Plumel. J. Inflamm. 2010, 7, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Miranda, A.L.; Silva, J.R.; Rezende, C.M.; Neves, J.S.; Parrini, S.C.; Pinheiro, M.L.; Cordeiro, M.C.; Tamborini, E.; Pinto, A.C. Anti-inflammatory and analgesic activities of the latex containing triterpenes from Himatanthus sucuuba. Planta Med. 2000, 66, 284–286. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.R.A.; Rezende, C.M.; Pinto, A.C.; Amaral, A.C.F. Cytotoxicity and antibacterial studies of iridoids and phenolic compounds isolated from the latex of Himatanthus sucuuba. Afr. J. Biotechnol. 2010, 43, 7357–7360. [Google Scholar]
- Ferreira, J.L.P.; Amaral, A.C.F.; Araújo, R.B.; Carvalho, J.R.; Proença, C.E.B.; Fraga, S.A.P.M.; Silva, J.R.A. Pharmacognostical comparasion of three species of Himatanthus. Int. J. Bot. 2009, 5, 171–175. [Google Scholar] [CrossRef] [Green Version]
- Tundis, R.; Loizzo, M.R.; Menichini, F.; Statti, G.A.; Menichini, F. Biological and pharmacological activities of iridoids: Recent developments. Mini-Rev. Med. Chem. 2008, 8, 399–420. [Google Scholar] [CrossRef]
- Sivamani, R.K.; Ma, B.R.; Wehrli, L.N.; Maverakis, E. Phytochemicals and Naturally Derived Substances for Wound Healing. Adv. Wound Care 2012, 1, 213–217. [Google Scholar] [CrossRef] [Green Version]
- Hajialyani, M.; Tewari, D.; Sobarzo-Sánchez, E.; Nabavi, S.M.; Farzaei, M.H.; Abdollahi, M. Natural product-based nanomedicines for wound healing purposes: Therapeutic targets and drug delivery systems. Int. J. Nanomed. 2018, 13, 5023–5043. [Google Scholar] [CrossRef] [Green Version]
- Shedoeva, A.; Leavesley, D.; Upton, Z.; Fan, C. Wound Healing and the Use of Medicinal Plants. Evid.-Based Complement. Altern. Med. 2019, 2019, 2684108. [Google Scholar] [CrossRef] [Green Version]
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef] [Green Version]
- Tiscar-González, V.; Menor-Rodríguez, M.J.; Rabadán-Sainz, C.; Fraile-Bravo, M.; Styche, T.; Valenzuela-Ocaña, F.J.; Muñoz-García, L. Clinical and Economic Impact of Wound Care Using a Polyurethane Foam Multilayer Dressing. Adv. Ski. Wound Care 2021, 34, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehman, S.R.U.; Augustine, R.; Zahid, A.A.; Ahmed, R.; Tariq, M.; Hasan, A. Reduced Graphene Oxide Incorporated GelMA Hydrogel Promotes Angiogenesis For Wound Healing Applications. Int. J. Nanomed. 2019, 14, 9603–9617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.; Yu, Z.; Li, Y.; Wang, Y.; Li, Q.; Han, D. GelMA combined with sustained release of HUVECs derived exosomes for promoting cutaneous wound healing and facilitating skin regeneration. J. Mol. Histol. 2020, 51, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.M.S.; Mano, J.F. Extremely strong and tough hydrogels as prospective candidates for tissue repair—A review. Eur. Polym. J. 2015, 72, 344–364. [Google Scholar] [CrossRef]
- Jin, S.G.; Yousaf, A.M.; Kim, K.S.; Kim, D.W.; Kim, D.S.; Kim, J.K.; Yong, C.S.; Youn, Y.S.; Kim, J.O.; Choi, H.G. Influence of hydrophilic polymers on functional properties and wound healing efficacy of hydrocolloid based wound dressings. Int. J. Pharm. 2016, 501, 160–166. [Google Scholar] [CrossRef]
- Shin, H.; Olsen, B.D.; Khademhosseini, A. The mechanical properties and cytotoxicity of cell-laden double-network hydrogels based on photocrosslinkable gelatin and gellan gum biomacromolecules. Biomaterials 2012, 33, 3143–3152. [Google Scholar] [CrossRef] [Green Version]
- Fathi, A.; Lee, S.; Breen, A.; Shirazi, A.N.; Valtchev, P.; Dehghani, F. Enhancing the mechanical properties and physical stability of biomimetic polymer hydrogels for micro-patterning and tissue engineering applications. Eur. Polym. J. 2014, 59, 161–170. [Google Scholar] [CrossRef]
- Hutson, C.B.; Nichol, J.W.; Aubin, H.; Bae, H.; Yamanlar, S.; Al-Haque, S.; Koshy, S.T.; Khademhosseini, A. Synthesis and characterization of tunable poly(ethylene glycol): Gelatin methacrylate composite hydrogels. Tissue Eng. Part A 2011, 17, 1713–1723. [Google Scholar] [CrossRef]
- Chen, L.; Mirza, R.; Kwon, Y.; DiPietro, L.A.; Koh, T.J. The murine excisional wound model: Contraction revisited. Wound Repair Regen. 2015, 23, 874–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alavarse, A.C.; Frachini, E.C.G.; da Silva, R.L.C.G.; Lima, V.H.; Shavandi, A.; Petri, D.F.S. Crosslinkers for polysaccharides and proteins: Synthesis conditions, mechanisms, and crosslinking efficiency, a review. Int. J. Biol. Macromol. 2022, 202, 558–596. [Google Scholar] [CrossRef] [PubMed]
- Gould, L.; Li, W.W. Defining complete wound closure: Closing the gap in clinical trials and practice. Wound Repair Regen. 2019, 27, 201–224. [Google Scholar] [CrossRef] [PubMed]
- Cañedo-Dorantes, L.; Cañedo-Ayala, M. Skin Acute Wound Healing: A Comprehensive Review. Int. J. Inflamm. 2019, 2019, 3706315. [Google Scholar] [CrossRef] [PubMed]
- D’Urso, M.; Kurniawan, N.A. Mechanical and Physical Regulation of Fibroblast–Myofibroblast Transition: From Cellular Mechanoresponse to Tissue Pathology. Front. Bioeng. Biotechnol. 2020, 8, 609653. [Google Scholar] [CrossRef]
- Almeida, B.M.; Nascimento, M.F.; Pereira-Filho, R.N.; Melo, G.C.; Santos, J.C.; Oliveira, C.R.; Gomes, M.Z.; Lima, S.O.; Albuquerque-Júnior, R.L. Immunohistochemical profile of stromal constituents and lymphoid cells over the course of wound healing in murine model. Acta Cir. Bras. 2014, 29, 596–602. [Google Scholar] [CrossRef] [Green Version]
- Nagle, S.M.; Waheed, A.; Wilbraham, S.C. Wound Assessment. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- van de Vyver, M.; Boodhoo, K.; Frazier, T.; Hamel, K.; Kopcewicz, M.; Levi, B.; Maartens, M.; Machcinska, S.; Nunez, J.; Pagani, C.; et al. Histology Scoring System for Murine Cutaneous Wounds. Stem Cells Dev. 2021, 30, 1141–1152. [Google Scholar] [CrossRef]
- Bochaton-Piallat, M.-L.; Gabbiani, G.; Hinz, B. The myofibroblast in wound healing and fibrosis: Answered and unanswered questions. F1000Research 2 2016, 5, 752. [Google Scholar] [CrossRef] [Green Version]
- Koga, M.; Kuramochi, M.; Karim, M.R.; Izawa, T.; Kuwamura, M.; Yamate, J. Immunohistochemical characterization of myofibroblasts appearing in isoproterenol-induced rat myocardial fibrosis. J. Vet. Med. Sci. 2019, 81, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Gerarduzzi, C.; Di Battista, J.A. Myofibroblast repair mechanisms post-inflammatory response: A fibrotic perspective. Inflamm. Res. 2017, 66, 451–465. [Google Scholar] [CrossRef]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibb, A.A.; Lazaropoulos, M.P.; Elrod, J.W. Myofibroblasts and Fibrosis: Mitochondrial and Metabolic Control of Cellular Differentiation. Circ. Res. 2020, 127, 427–447. [Google Scholar] [CrossRef]
- Monika, P.; Waiker, P.V.; Chandraprabha, M.N.; Rangarajan, A.; Murthy, K.N.C. Myofibroblast progeny in wound biology and wound healing studies. Wound Repair Regen. 2021, 29, 531–547. [Google Scholar] [CrossRef] [PubMed]
- Alhajj, M.; Goyal, A. Physiology, Granulation Tissue. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Nassiri, F.; Cusimano, M.D.; Scheithauer, B.W.; Rotondo, F.; Fazio, A.; Yousef, G.M.; Syro, L.V.; Kovacs, K.; Lloyd, R.V. Endoglin (CD105): A review of its role in angiogenesis and tumor diagnosis, progression and therapy. Anticancer. Res. 2011, 31, 2283–2290. [Google Scholar] [PubMed]
- Semenza, G.L. Vasculogenesis, angiogenesis, and arteriogenesis: Mechanisms of blood vessel formation and remodeling. J. Cell. Biochem. 2007, 102, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, R.J.; Yates, C.C.; Rodgers, M.E.; Du, X.; Wells, A. IP-10 induces dissociation of newly formed blood vessels. J. Cell Sci. 2009, 122, 2064–2077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wietecha, M.S.; Chen, L.; Ranzer, M.J.; Anderson, K.; Ying, C.; Patel, T.B.; DiPietro, L.A. Sprouty2 downregulates angiogenesis during mouse skin wound healing. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H459–H467. [Google Scholar] [CrossRef] [Green Version]
- Ding, R.; Yin, Y.-L.; Jiang, L.-H. Reactive Oxygen Species-Induced TRPM2-Mediated Ca2+ Signalling in Endothelial Cells. Antioxidants 2021, 10, 718. [Google Scholar] [CrossRef]
- Khan, S.Y.; Awad, E.M.; Oszwald, A.; Mayr, M.; Yin, X.; Waltenberger, B.; Stuppner, H.; Lipovac, M.; Uhrin, P.; Breuss, J.M. Premature senescence of endothelial cells upon chronic exposure to TNFα can be prevented by N-acetyl cysteine and plumericin. Sci. Rep. 2017, 7, 39501. [Google Scholar] [CrossRef] [Green Version]
- Critser, P.J.; Kreger, S.T.; Voytik-Harbin, S.L.; Yoder, M.C. Collagen matrix physical properties modulate endothelial colony forming cell-derived vessels in vivo. Microvasc. Res. 2010, 80, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Whittington, C.F.; Yoder, M.C.; Voytik-Harbin, S.L. Collagen-polymer guidance of vessel network formation and stabilization by endothelial colony forming cells in vitro. Macromol. Biosci. 2013, 13, 1135–1149. [Google Scholar] [CrossRef] [Green Version]
- Korntner, S.; Lehner, C.; Gehwolf, R.; Wagner, A.; Grütz, M.; Kunkel, N.; Tempfer, H.; Traweger, A. Limiting angiogenesis to modulate scar formation. Adv. Drug Deliv. Rev. 2019, 146, 170–189. [Google Scholar] [CrossRef] [PubMed]
- Volk, S.W.; Wang, Y.; Mauldin, E.A.; Liechty, K.W.; Adams, S.L. Diminished type III collagen promotes myofibroblast differentiation and increases scar deposition in cutaneous wound healing. Cells Tissues Organs 2011, 194, 25–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos, C.A.; Lima, B.S.; Trindade, G.G.G.; Souza, E.; Mota, D.S.A.; Heimfarth, L.; Quintans, J.S.S.; Quintans-Júnior, L.J.; Sussuchi, E.M.; Sarmento, V.H.V.; et al. Anti-hyperalgesic and anti-inflammatory effects of citral with β-cyclodextrin and hydroxypropyl-β-cyclodextrin inclusion complexes in animal models. Life Sci. 2019, 229, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Zelice da Cruz de Moraes, S.; Shan, A.; Oliveira Melo, M.A.; Pereira da Silva, J.; Rocha Santos Passos, F.; de Souza Graça, A.; Araújo, B.S.; Quintans, J.S.S.; Quintans Júnior, L.J.; Oliveira Barreto, E.; et al. Antinociceptive and anti-inflammatory effect of Poincianella pyramidalis (Tul.) L.P. Queiroz. J. Ethnopharmacol. 2020, 254, 112563. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, P. Assessment of the histological state of the healing wound. Plast. Aesthetic Res. 2015, 2, 239–242. [Google Scholar] [CrossRef]




| Score System | Histological Criteria | Histological Staining and Analytical Method |
|---|---|---|
| plenty—1, moderate—2, a few—3 | Inflammatory infiltrate | Light microscopy (HE) |
| profound—1, moderate—2, scanty—3, absent—4 | Amount of granulation tissue | Light microscopy (HE) |
| vertical—1, mixed—2, horizontal—3 | Orientation of collagen fibers | Polarized light (sirius red) |
| reticular—1, mixed—2, fascicle—3 | Pattern of collagenization | Polarized light (sirius red) |
| profound—1, moderate—2, minimum—3, absent—4 | Amount of early collagen (type III) | Polarized light (sirius red) |
| profound—1, moderate—2, minimum—3 | Amount of mature collagen (type I) | Polarized light (sirius red) |
| Antigen | Target Cell | Clone | Dilution | Incubation |
|---|---|---|---|---|
| α-SMA | Myofibroblasts | 1A4 (Dako) | 1:100 | 30 min |
| CD105 | Endothelial cells | SN6H (Dako) | 1:500 | Overnight (18 h) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Almeida, B.M.; dos Santos, I.D.D.; de Carvalho, F.M.A.; Correa, L.C.; Cunha, J.L.S.; Dariva, C.; Severino, P.; Cardoso, J.C.; Souto, E.B.; de Albuquerque-Júnior, R.L.C. Himatanthus bracteatus-Composed In Situ Polymerizable Hydrogel for Wound Healing. Int. J. Mol. Sci. 2022, 23, 15176. https://doi.org/10.3390/ijms232315176
de Almeida BM, dos Santos IDD, de Carvalho FMA, Correa LC, Cunha JLS, Dariva C, Severino P, Cardoso JC, Souto EB, de Albuquerque-Júnior RLC. Himatanthus bracteatus-Composed In Situ Polymerizable Hydrogel for Wound Healing. International Journal of Molecular Sciences. 2022; 23(23):15176. https://doi.org/10.3390/ijms232315176
Chicago/Turabian Stylede Almeida, Bernadeth M., Izabella D. Dorta dos Santos, Felipe M. A. de Carvalho, Luana C. Correa, John L. S. Cunha, Claudio Dariva, Patricia Severino, Juliana C. Cardoso, Eliana B. Souto, and Ricardo L. C. de Albuquerque-Júnior. 2022. "Himatanthus bracteatus-Composed In Situ Polymerizable Hydrogel for Wound Healing" International Journal of Molecular Sciences 23, no. 23: 15176. https://doi.org/10.3390/ijms232315176







