Mechanisms Involved in the Neurotoxicity and Abuse Liability of Nitrous Oxide: A Narrative Review
Abstract
1. Introduction
2. Neurotoxicity of N2O
2.1. Acute Neurotoxicity
2.2. Chronic Neurotoxicity
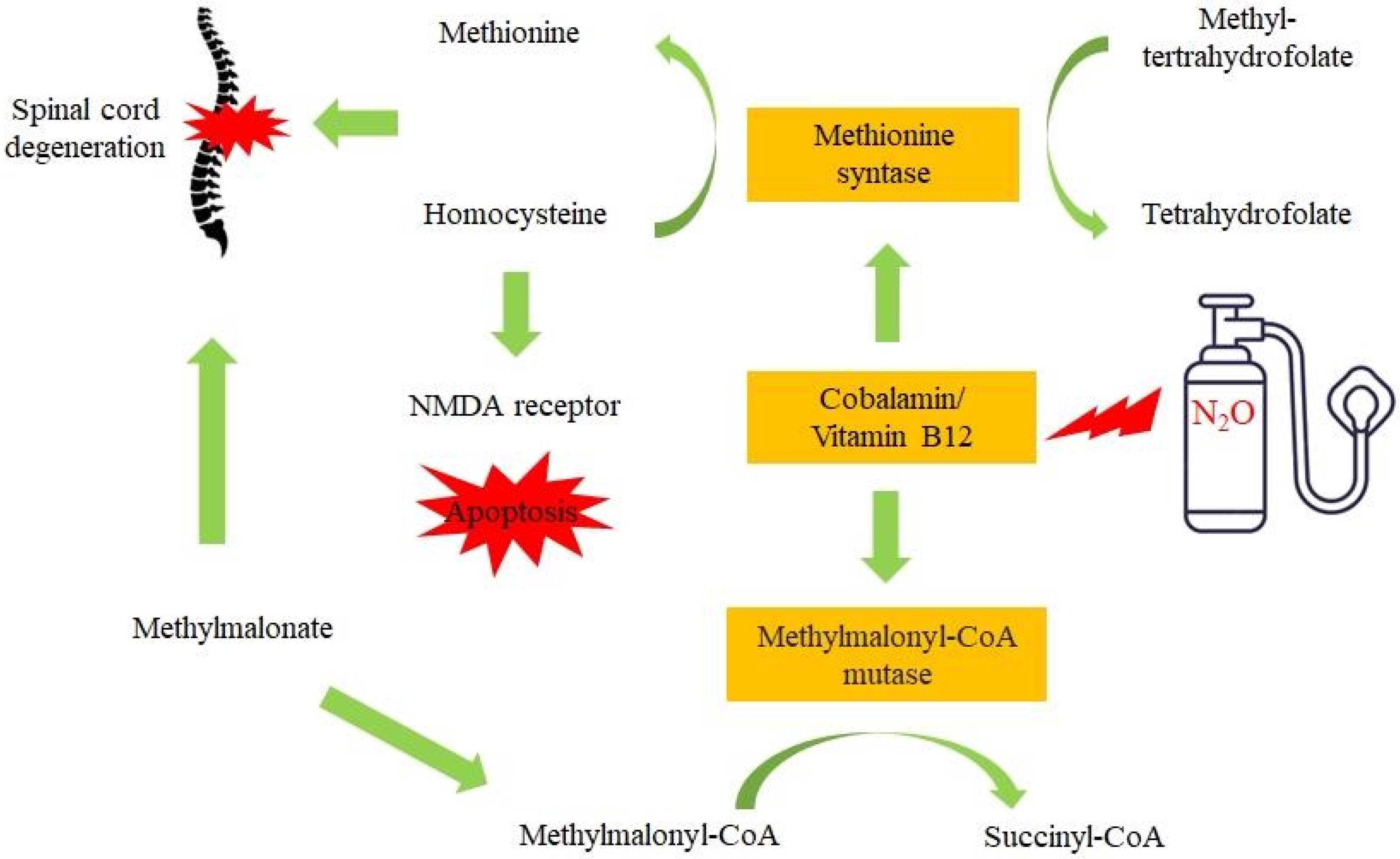
3. The Molecular Mechanisms behind N2O Neurotoxicity
4. From Incidental Use to N2O Abuse (Binging)
5. Dependence Liability
5.1. Human Data
5.2. Animal Studies
6. Molecular Mechanisms of N2O Abuse and Dependence
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Van Amsterdam, J.G.C.; Nabben, T.; van den Brink, W. Increasing Recreational Nitrous Oxide Use: Should We Worry? A Narrative Review. J. Psychopharmacol. 2022, 36, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Global Drug Survey. Global Drug Survey 2019 Executive Summary. Available online: https://www.globaldrugsurvey.com/wp-content/themes/globaldrugsurvey/results/GDS2019-Exec-Summary.pdf (accessed on 16 August 2022).
- Office for National Statistics. Drug Misuse in England and Wales: Year Ending March 2020. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/crimeandjustice/articles/drugmisuseinenglandandwales/yearendingmarch2020 (accessed on 12 July 2021).
- Forrester, M. Nitrous Oxide Misuse Reported to Two United States Data Systems during 2000–2019. J. Addict. Dis. 2021, 39, 46–53. [Google Scholar] [CrossRef]
- Perino, J.; Tournier, M.; Mathieu, C.; Letinier, L.; Peyré, A.; Perret, G.; Pereira, E.; Fourrier-Réglat, A.; Pollet, C.; Fatseas, M.; et al. Psychoactive Substance Use among Students: A Cross-Sectional Analysis. Fundam. Clin. Pharmacol. 2022, 36, 908–914. [Google Scholar] [CrossRef]
- Nabben, T.; Weijs, J.; van Amsterdam, J. Problematic Use of Nitrous Oxide by Young Moroccan-Dutch Adults. Int. J. Environ. Res. Public Health 2021, 18, 5574. [Google Scholar] [CrossRef]
- van Amsterdam, J.; Nabben, T.; van den Brink, W. Recreational Nitrous Oxide Use: Prevalence and Risks. Regul. Toxicol. Pharmacol. 2015, 73, 790–796. [Google Scholar] [CrossRef] [PubMed]
- van Riel, A.J.H.P.; Hunault, C.C.; van den Hengel-Koot, I.S.; Nugteren-van Lonkhuyzen, J.J.; de Lange, D.W.; Hondebrink, L. Alarming Increase in Poisonings from Recreational Nitrous Oxide Use after a Change in EU-Legislation, Inquiries to the Dutch Poisons Information Center. Int. J. Drug Policy 2022, 100, 103519. [Google Scholar] [CrossRef]
- Bethmont, A.; Harper, C.; Chan, B.; Dawson, A.; McAnulty, J. Increasing Illicit Use of Nitrous Oxide in Presentations to NSW Emergency Departments. Med. J. Aust. 2019, 211, 429–429.e1. [Google Scholar] [CrossRef] [PubMed]
- Redmond, J.; Cruse, B.; Kiers, L. Nitrous Oxide-Induced Neurological Disorders: An Increasing Public Health Concern. Intern. Med. J. 2022, 52, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Li, L.; Ma, X.; Li, S.; Xue, Y.; Yan, P.; Chen, M.; Wu, J. Recreational Nitrous Oxide Abuse: Prevalence, Neurotoxicity, and Treatment. Neurotox. Res. 2021, 39, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Dufayet, L.; Caré, W.; Laborde-Casterot, H.; Chouachi, L.; Langrand, J.; Vodovar, D. Possible Impact of the COVID-19 Pandemic on the Recreational Use of Nitrous Oxide in the Paris Area, France. Rev. Med. Interne 2022, 43, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Marcus, E. Nitrous Nation: A Party Drug Endures. The New York Times, 30 January 2021; 1–6. [Google Scholar]
- Swart, G.; Blair, C.; Lu, Z.; Yogendran, S.; Offord, J.; Sutherland, E.; Barnes, S.; Palavra, N.; Cremer, P.; Bolitho, S.; et al. Nitrous Oxide-Induced Myeloneuropathy. Eur. J. Neurol. 2021, 28, 3938–3944. [Google Scholar] [CrossRef] [PubMed]
- Garakani, A.; Jaffe, R.J.; Savla, D.; Welch, A.K.; Protin, C.A.; Bryson, E.O.; McDowell, D.M. Neurologic, Psychiatric, and Other Medical Manifestations of Nitrous Oxide Abuse: A Systematic Review of the Case Literature. Am. J. Addict. 2016, 25, 358–369. [Google Scholar] [CrossRef]
- Marsden, P.; Sharma, A.A.; Rotella, J.A. Review Article: Clinical Manifestations and Outcomes of Chronic Nitrous Oxide Misuse: A Systematic Review. Emerg. Med. Australas. 2022, 34, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Oussalah, A.; Julien, M.; Levy, J.; Hajjar, O.; Franczak, C.; Stephan, C.; Laugel, E.; Wandzel, M.; Filhine-Tresarrieu, P.; Green, R.; et al. Global Burden Related to Nitrous Oxide Exposure in Medical and Recreational Settings: A Systematic Review and Individual Patient Data Meta-Analysis. J. Clin. Med. 2019, 8, 551. [Google Scholar] [CrossRef] [PubMed]
- Vollhardt, R.; Mazoyer, J.; Bernardaud, L.; Haddad, A.; Jaubert, P.; Coman, I.; Manceau, P.; Mongin, M.; Degos, B. Neurological Consequences of Recreational Nitrous Oxide Abuse during SARS-CoV-2 Pandemic. J. Neurol. 2022, 269, 1921–1926. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Qiao, Y.; Li, W.; Fang, X.; Gao, H.; Zheng, D.; Ma, Y. Analysis of Clinical Characteristics and Prognostic Factors in 110 Patients with Nitrous Oxide Abuse. Brain Behav. 2022, 12, e2533. [Google Scholar] [CrossRef]
- ANSES. Nitrous Oxide Poisoning on the Increase; ANSES: Maisons-Alfort, France, 2021. [Google Scholar]
- Lin, J.P.; Gao, S.Y.; Lin, C.C. The Clinical Presentations of Nitrous Oxide Users in an Emergency Department. Toxics 2022, 10, 112. [Google Scholar] [CrossRef]
- Micallef, J.; Mallaret, M.; Lapeyre-Mestre, M.; Daveluy, A.; Victorri-Vigneau, C.; Peyrière, H.; Debruyne, D.; Deheul, S.; Bordet, R.; Chevallier, C.; et al. Warning on Increased Serious Health Complications Related to Non-Medical Use of Nitrous Oxide. Therapie 2021, 76, 478–479. [Google Scholar] [CrossRef]
- Wu, G.; Wang, S.; Wang, T.; Han, J.; Yu, A.; Feng, C.; Wang, Y.; Liu, S. Neurological and Psychological Characteristics of Young Nitrous Oxide Abusers and Its Underlying Causes During the COVID-19 Lockdown. Front. Public Health 2022, 10, 854977. [Google Scholar] [CrossRef]
- Kamboj, S.K.; Zhao, H.; Troebinger, L.; Piazza, G.; Cawley, E.; Hennessy, V.; Iskandar, G.; Das, R.K. Rewarding Subjective Effects of the NMDAR Antagonist Nitrous Oxide (Laughing Gas) Are Moderated by Impulsivity and Depressive Symptoms in Healthy Volunteers. Int. J. Neuropsychopharmacol. 2021, 24, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Gillman, M.A. Opioid Properties of Nitrous Oxide and Ketamine Contribute to Their Antidepressant Actions. Int. J. Neuropsychopharmacol. 2021, 24, 892–893. [Google Scholar] [CrossRef] [PubMed]
- Einsiedler, M.; Voulleminot, P.; Demuth, S.; Kalaaji, P.; Bogdan, T.; Gauer, L.; Reschwein, C.; Nadaj-Pakleza, A.; de Sèze, J.; Kremer, L.; et al. A Rise in Cases of Nitrous Oxide Abuse: Neurological Complications and Biological Findings. J. Neurol. 2021, 269, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Ba, F.; Bi, G.; Guo, Y.; Gao, Y.; Li, W. The Sharp Rise of Neurological Disorders Associated with Recreational Nitrous Oxide Use in China: A Single-Center Experience and a Brief Review of Chinese Literature. J. Neurol. 2020, 267, 422–429. [Google Scholar] [CrossRef]
- Winstock, A.; Ferris, J. Nitrous Oxide Causes Peripheral Neuropathy in a Dose Dependent Manner among Recreational Users. J. Psychopharmacol. 2020, 34, 229–236. [Google Scholar] [CrossRef]
- Kaar, S.J.; Ferris, J.; Waldron, J.; Devaney, M.; Ramsey, J.; Winstock, A.R. Up: The Rise of Nitrous Oxide Abuse. An International Survey of Contemporary Nitrous Oxide Use. J. Psychopharmacol. 2016, 30, 395–401. [Google Scholar] [CrossRef]
- Patel, K.K.; Mejia Munne, J.C.; Gunness, V.R.N.; Hersey, D.; Alshafai, N.; Sciubba, D.; Nasser, R.; Gimbel, D.; Cheng, J.; Nouri, A. Subacute Combined Degeneration of the Spinal Cord Following Nitrous Oxide Anesthesia: A Systematic Review of Cases. Clin. Neurol. Neurosurg. 2018, 173, 163–168. [Google Scholar] [CrossRef]
- Gao, H.; Li, W.; Ren, J.; Dong, X.; Ma, Y.; Zheng, D. Clinical and MRI Differences Between Patients With Subacute Combined Degeneration of the Spinal Cord Related vs. Unrelated to Recreational Nitrous Oxide Use: A Retrospective Study. Front. Neurol. 2021, 12, 626174. [Google Scholar] [CrossRef]
- Dang, X.T.; Nguyen, T.X.; Nguyen, T.T.H.; Ha, H.T. Nitrous Oxide-Induced Neuropathy among Recreational Users in Vietnam. Int. J. Environ. Res. Public Health 2021, 18, 6230. [Google Scholar] [CrossRef]
- Lan, S.Y.; Kuo, C.Y.; Chou, C.C.; Kong, S.S.; Hung, P.C.; Tsai, H.Y.; Chen, Y.C.; Lin, J.J.; Chou, I.J.; Lin, K.L. Recreational Nitrous Oxide Abuse Related Subacute Combined Degeneration of the Spinal Cord in Adolescents—A Case Series and Literature Review. Brain Dev. 2019, 41, 428–435. [Google Scholar] [CrossRef]
- Paulus, M.C.; Wijnhoven, A.M.; Maessen, G.C.; Blankensteijn, S.R.; van der Heyden, M.A.G. Does Vitamin B12 Deficiency Explain Psychiatric Symptoms in Recreational Nitrous Oxide Users? A Narrative Review. Clin. Toxicol. 2021, 59, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Stockton, L.; Simonsen, C.; Seago, S. Nitrous Oxide-Induced Vitamin B12 Deficiency. Proc. Bayl. Univ. Med. Cent. 2017, 30, 171–172. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hathout, L.; El-Saden, S. Nitrous Oxide-Induced B₁₂ Deficiency Myelopathy: Perspectives on the Clinical Biochemistry of Vitamin B₁₂. J. Neurol. Sci. 2011, 301, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Toohey, J.I. Vitamin B12 and Methionine Synthesis: A Critical Review. Is Nature’s Most Beautiful Cofactor Misunderstood? Biofactors 2006, 26, 45–57. [Google Scholar] [CrossRef]
- Richardson, P.G. Peripheral Neuropathy Following Nitrous Oxide Abuse. Emerg. Med. Australas. 2010, 22, 88–90. [Google Scholar] [CrossRef]
- Landgrave-Gómez, J.; Mercado-Gómez, O.; Guevara-Guzmán, R. Epigenetic Mechanisms in Neurological and Neurodegenerative Diseases. Front. Cell. Neurosci. 2015, 9, 58. [Google Scholar] [CrossRef]
- Miller, A.; Korem, M.; Almog, R.; Galboiz, Y. Vitamin B12, Demyelination, Remyelination and Repair in Multiple Sclerosis. J. Neurol. Sci. 2005, 233, 93–97. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Klein, J.P. Subacute Combined Degeneration from Nitrous Oxide Use. N. Engl. J. Med. 2022, 387, 832. [Google Scholar] [CrossRef]
- Check, L.; Abdelsayed, N.; Figueroa, G.; Ragunathan, A.; Faris, M. Subacute Combined Degeneration of the Cervical Spine Secondary to Inhaled Nitrous-Oxide-Induced Cobalamin Deficiency. Cureus 2022, 14, e21214. [Google Scholar] [CrossRef]
- Narasimhan, P.; Sklar, R.; Murrell, M.; Swanson, R.A.; Sharp, F.R. Methylmalonyl-CoA Mutase Induction by Cerebral Ischemia and Neurotoxicity of the Mitochondrial Toxin Methylmalonic Acid. J. Neurosci. 1996, 16, 7336–7346. [Google Scholar] [CrossRef]
- Fernandes, C.G.; Borges, C.G.; Seminotti, B.; Amaral, A.U.; Knebel, L.A.; Eichler, P.; De Oliveira, A.B.; Leipnitz, G.; Wajner, M. Experimental Evidence That Methylmalonic Acid Provokes Oxidative Damage and Compromises Antioxidant Defenses in Nerve Terminal and Striatum of Young Rats. Cell. Mol. Neurobiol. 2011, 31, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Okun, J.G.; Hörster, F.; Farkas, L.M.; Feyh, P.; Hinz, A.; Sauer, S.; Hoffmann, G.F.; Unsicker, K.; Mayatepek, E.; Kölker, S. Neurodegeneration in Methylmalonic Aciduria Involves Inhibition of Complex II and the Tricarboxylic Acid Cycle, and Synergistically Acting Excitotoxicity. J. Biol. Chem. 2002, 277, 14674–14680. [Google Scholar] [CrossRef] [PubMed]
- Savage, S.; Ma, D. The Neurotoxicity of Nitrous Oxide: The Facts and “Putative” Mechanisms. Brain Sci. 2014, 4, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Abushik, P.A.; Niittykoski, M.; Giniatullina, R.; Shakirzyanova, A.; Bart, G.; Fayuk, D.; Sibarov, D.A.; Antonov, S.M.; Giniatullin, R. The Role of NMDA and MGluR5 Receptors in Calcium Mobilization and Neurotoxicity of Homocysteine in Trigeminal and Cortical Neurons and Glial Cells. J. Neurochem. 2014, 129, 264–274. [Google Scholar] [CrossRef]
- Oomens, T.; Riezebos, R.K.; Amoroso, G.; Kuipers, R.S. Case Report of an Acute Myocardial Infarction after High-Dose Recreational Nitrous Oxide Use: A Consequence of Hyperhomocysteinaemia? Eur. Heart J. Case Rep. 2021, 5, ytaa557. [Google Scholar] [CrossRef]
- Jevtović-Todorović, V.; Todorović, S.M.; Mennerick, S.; Powell, S.; Dikranian, K.; Benshoff, N.; Zorumski, C.F.; Olney, J.W. Nitrous Oxide (Laughing Gas) Is an NMDA Antagonist, Neuroprotectant and Neurotoxin. Nat. Med. 1998, 4, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Abraini, J.H.; David, H.N.; Lemaire, M. Potentially Neuroprotective and Therapeutic Properties of Nitrous Oxide and Xenon. Ann. N. Y. Acad. Sci. 2005, 1053, 289–300. [Google Scholar] [CrossRef]
- Mohsenzadegan, M.; Kourosh Arami, M.; Oshaghi, M.; Sedigh Maroufi, S. A Review of the Effects of the Anesthetic Gas Nitrous Oxide on the Immune System; a Starting Point for Future Experiences. Immunopharmacol. Immunotoxicol. 2020, 42, 179–186. [Google Scholar] [CrossRef]
- Emmanouil, D.E.; Quock, R.M. Advances in Understanding the Actions of Nitrous Oxide. Anesth. Prog. 2007, 54, 9–18. [Google Scholar] [CrossRef]
- Malamed, S.F.; Clark, M.S. Nitrous Oxide-Oxygen: A New Look at a Very Old Technique. J. Calif. Dent. Assoc. 2003, 31, 397–403. [Google Scholar]
- Balster, R.L.; Cruz, S.L.; Howard, M.O.; Dell, C.A.; Cottler, L.B. Classification of Abused Inhalants. Addiction 2009, 104, 878–882. [Google Scholar] [CrossRef]
- APA (American Psychiatric Association). Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Gillman, M.A. Nitrous Oxide, an Opioid Addictive Agent. Review of the Evidence. Am. J. Med. 1986, 81, 97–102. [Google Scholar] [CrossRef]
- Gillman, M.A.; Lichtigfeld, F.J. Pharmacology of Psychotropic Analgesic Nitrous Oxide as a Multipotent Opioid Agonist. Int. J. Neurosci. 1994, 76, 5–12. [Google Scholar] [CrossRef]
- Zacny, J.P.; Klafta, J.M.; Coalson, D.W.; Marks, S.; Young, C.J.; Klock, P.A.; Toledano, A.Y.; Jordan, N.; Apfelbaum, J.L. The Reinforcing Effects of Brief Exposures to Nitrous Oxide in Healthy Volunteers. Drug Alcohol Depend. 1996, 42, 197–200. [Google Scholar] [CrossRef]
- Dohrn, C.S.; Lichtor, J.L.; Coalson, D.W.; Flemming, D.; Zacny, J.P. Reinforcing Effects of Extended Inhalation of a Low Nitrous Oxide Concentration in Humans. Pharmacol. Biochem. Behav. 1993, 46, 927–932. [Google Scholar] [CrossRef]
- Fidalgo, M.; Prud’homme, T.; Allio, A.; Bronnec, M.; Bulteau, S.; Jolliet, P.; Victorri-Vigneau, C. Nitrous Oxide: What Do We Know about Its Use Disorder Potential? Results of the French Monitoring Centre for Addiction Network Survey and Literature Review. Subst. Abus. 2019, 40, 33–42. [Google Scholar] [CrossRef]
- Ramsay, D.S.; Leroux, B.G.; Rothen, M.; Prall, C.W.; Fiset, L.O.; Woods, S.C. Nitrous Oxide Analgesia in Humans: Acute and Chronic Tolerance. Pain 2005, 114, 19–28. [Google Scholar] [CrossRef]
- Ickowicz, S.; Brar, R.; Nolan, S. Case Study: Naltrexone for the Treatment of Nitrous Oxide Use. J. Addict. Med. 2020, 14, e277–e279. [Google Scholar] [CrossRef]
- Garg, A.; Sinha, P.; Kumar, P.; Prakash, O. Use of Naltrexone in Ketamine Dependence. Addict. Behav. 2014, 39, 1215–1216. [Google Scholar] [CrossRef]
- Ramsay, D.S.; Watson, C.H.; Leroux, B.G.; Prall, C.W.; Kaiyala, K.J. Conditioned Place Aversion and Self-Administration of Nitrous Oxide in Rats. Pharmacol. Biochem. Behav. 2003, 74, 623–633. [Google Scholar] [CrossRef]
- Tracy, M.E.; Slavova-Hernandez, G.G.; Shelton, K.L. Assessment of Reinforcement Enhancing Effects of Toluene Vapor and Nitrous Oxide in Intracranial Self-Stimulation. Psychopharmacology 2014, 231, 1339–1350. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.W.; Grubman, J.; Weiss, B. Nitrous Oxide Self-Administration by the Squirrel Monkey. J. Pharmacol. Exp. Ther. 1977, 202, 491–499. [Google Scholar] [PubMed]
- Rupreht, J.; Ukponmwan, O.E.; Dworacek, B.; Admiraal, P.V.; Dzoljic, M.R. Enkephalinase Inhibition Prevented Tolerance to Nitrous Oxide Analgesia in Rats. Acta Anaesthesiol. Scand. 1984, 28, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Dzoljic, M.; Rupreht, J.; Erdmann, W.; Stijnen, T.H.; van Briemen, L.J.; Dzoljic, M.R. Behavioral and Electrophysiological Aspects of Nitrous Oxide Dependence. Brain Res. Bull. 1994, 33, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Harper, M.H.; Winter, P.M.; Johnson, B.H.; Koblin, D.D.; Eger IInd, E.I. Withdrawal Convulsions in Mice Following Nitrous Oxide. Anesth. Analg. 1980, 59, 19–21. [Google Scholar] [CrossRef]
- Rupreht, J.; Dworacek, B.; Ducardus, R.; Schmitz, P.I.; Dzoljic, M.R. The Involvement of the Central Cholinergic and Endorphinergic Systems in the Nitrous Oxide Withdrawal Syndrome in Mice. Anesthesiology 1983, 58, 524–526. [Google Scholar] [CrossRef]
- Milne, B.; Cervenko, F.W.; Jhamandas, K.H. Physical Dependence on Nitrous Oxide in Mice: Resemblance to Alcohol but Not to Opiate Withdrawal. Can. Anaesth. Soc. J. 1981, 28, 46–50. [Google Scholar] [CrossRef]
- Dzoljic, M.R.; Haffmans, J.; Rupreht, J.; Adolfs, M.J.P.; Dzoljic, M.M.; Cappendijk, S.L.T. Decrease of Beta-Endorphin in the Brain of Rats Following Nitrous Oxide Withdrawal. Drug Metabol. Drug Interact. 1991, 9, 139–148. [Google Scholar] [CrossRef]
- Benturquia, N.; Le Marec, T.; Scherrmann, J.M.; Noble, F. Effects of Nitrous Oxide on Dopamine Release in the Rat Nucleus Accumbens and Expectation of Reward. Neuroscience 2008, 155, 341–344. [Google Scholar] [CrossRef]
- Gillman, M.A. Analgesic (Sub Anesthetic) Nitrous Oxide Interacts with the Endogenous Opiod System: A Review of the Evidence. Life Sci. 1986, 39, 1209–1221. [Google Scholar] [CrossRef]
- Gillman, M.A.; Lichtigfeld, F.J. Opioid Properties of Psychotropic Analgesic Nitrous Oxide (Laughing Gas). Perspect. Biol. Med. 1994, 38, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Maze, M.; Sanders, R.D.; Weimann, J. Biologic Effects of Nitrous Oxide: A Mechanistic and Toxicologic Review. Anesthesiology 2008, 109, 707–722. [Google Scholar] [CrossRef]
- Maze, M.; Fujinaga, M. Recent Advances in Understanding the Actions and Toxicity of Nitrous Oxide. Anaesthesia 2000, 55, 311–314. [Google Scholar] [CrossRef]
- Smith, D.J.; Bouchal, R.L.; DeSanctis, C.A.; Monroe, P.J.; Amedro, J.B.; Perrotti, J.M.; Crisp, T. Properties of the Interaction between Ketamine and Opiate Binding Sites in Vivo and in Vitro. Neuropharmacology 1987, 26, 1253–1260. [Google Scholar] [CrossRef]
- Smith, P.B.; Welch, S.P.; Martin, B.R. Interactions between Δ9-Tetrahydrocannabinol and Kappa Opioids in Mice. J. Pharmacol. Exp. Ther. 1994, 268, 1381–1387. [Google Scholar]
- Hynes, M.D.; Berkowitz, B.A. Catecholamine Mechanisms in the Stimulation of Mouse Locomotor Activity by Nitrous Oxide and Morphine. Eur. J. Pharmacol. 1983, 90, 109–114. [Google Scholar] [CrossRef]
- Berkowitz, B.A.; Finck, A.D.; Hynes, M.D.; Ngai, S.H. Tolerance to Nitrous Oxide Analgesia in Rats and Mice. Anesthesiology 1979, 51, 309–312. [Google Scholar] [CrossRef]
- Emmanouil, D.E.; Dickens, A.S.; Heckert, R.W.; Ohgami, Y.; Chung, E.; Han, S.; Quock, R.M. Nitrous Oxide-Antinociception Is Mediated by Opioid Receptors and Nitric Oxide in the Periaqueductal Gray Region of the Midbrain. Eur. Neuropsychopharmacol. 2008, 18, 194–199. [Google Scholar] [CrossRef]
- Quock, R.M.; Kouchich, F.J.; Liang-Fu, T. Influence of Nitrous Oxide upon Regional Brain Levels of Methionine-Enkephalin-like Immunoreactivity in Rats. Brain Res. Bull. 1986, 16, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Zuniga, J.R.; Joseph, S.A.; Knigge, K.M. The Effects of Nitrous Oxide on the Central Endogenous Pro-Opiomelanocortin System in the Rat. Brain Res. 1987, 420, 57–65. [Google Scholar] [CrossRef]
- Narita, M.; Funada, M.; Suzuki, T. Regulations of Opioid Dependence by Opioid Receptor Types. Pharmacol. Ther. 2001, 89, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Tobias, J.D. Tolerance, Withdrawal, and Physical Dependency after Long-Term Sedation and Analgesia of Children in the Pediatric Intensive Care Unit. Crit. Care Med. 2000, 28, 2122–2132. [Google Scholar] [CrossRef] [PubMed]
- Kretschmer, B.D. Modulation of the Mesolimbic Dopamine System by Glutamate: Role of NMDA Receptors. J. Neurochem. 1999, 73, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Mathé, J.M.; Nomikos, G.G.; Schilström, B.; Svensson, T.H. Non-NMDA Excitatory Amino Acid Receptors in the Ventral Tegmental Area Mediate Systemic Dizocilpine (MK-801) Induced Hyperlocomotion and Dopamine Release in the Nucleus Accumbens. J. Neurosci. Res. 1998, 51, 583–592. [Google Scholar] [CrossRef]
- Kegeles, L.S.; Martinez, D.; Kochan, L.D.; Hwang, D.R.; Huang, Y.; Mawlawi, O.; Suckow, R.F.; Van Heertum, R.L.; Laruelle, M. NMDA Antagonist Effects on Striatal Dopamine Release: Positron Emission Tomography Studies in Humans. Synapse 2002, 43, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Richardson, K.J.; Shelton, K.L. N-Methyl-D-Aspartate Receptor Channel Blocker-like Discriminative Stimulus Effects of Nitrous Oxide Gas. J. Pharmacol. Exp. Ther. 2015, 352, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Izumi, Y.; Hsu, F.-F.; Conway, C.R.; Nagele, P.; Mennerick, S.J.; Zorumski, C.F. Nitrous Oxide, a Rapid Antidepressant, Has Ketamine-like Effects on Excitatory Transmission in the Adult Hippocampus. Biol. Psychiatry 2022, 92, 964–972. [Google Scholar] [CrossRef]
- van Amsterdam, J.; van den Brink, W. Nitrous Oxide-Induced Reproductive Risks: Should Recreational Nitrous Oxide Users Worry? J. Psychopharmacol. 2022, 36, 951–955. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunt, T.M.; van den Brink, W.; van Amsterdam, J. Mechanisms Involved in the Neurotoxicity and Abuse Liability of Nitrous Oxide: A Narrative Review. Int. J. Mol. Sci. 2022, 23, 14747. https://doi.org/10.3390/ijms232314747
Brunt TM, van den Brink W, van Amsterdam J. Mechanisms Involved in the Neurotoxicity and Abuse Liability of Nitrous Oxide: A Narrative Review. International Journal of Molecular Sciences. 2022; 23(23):14747. https://doi.org/10.3390/ijms232314747
Chicago/Turabian StyleBrunt, Tibor M., Wim van den Brink, and Jan van Amsterdam. 2022. "Mechanisms Involved in the Neurotoxicity and Abuse Liability of Nitrous Oxide: A Narrative Review" International Journal of Molecular Sciences 23, no. 23: 14747. https://doi.org/10.3390/ijms232314747
APA StyleBrunt, T. M., van den Brink, W., & van Amsterdam, J. (2022). Mechanisms Involved in the Neurotoxicity and Abuse Liability of Nitrous Oxide: A Narrative Review. International Journal of Molecular Sciences, 23(23), 14747. https://doi.org/10.3390/ijms232314747





