Resveratrol-Mediated Reversal of Doxorubicin-Induced Osteoclast Differentiation
Abstract
:1. Introduction
2. Results
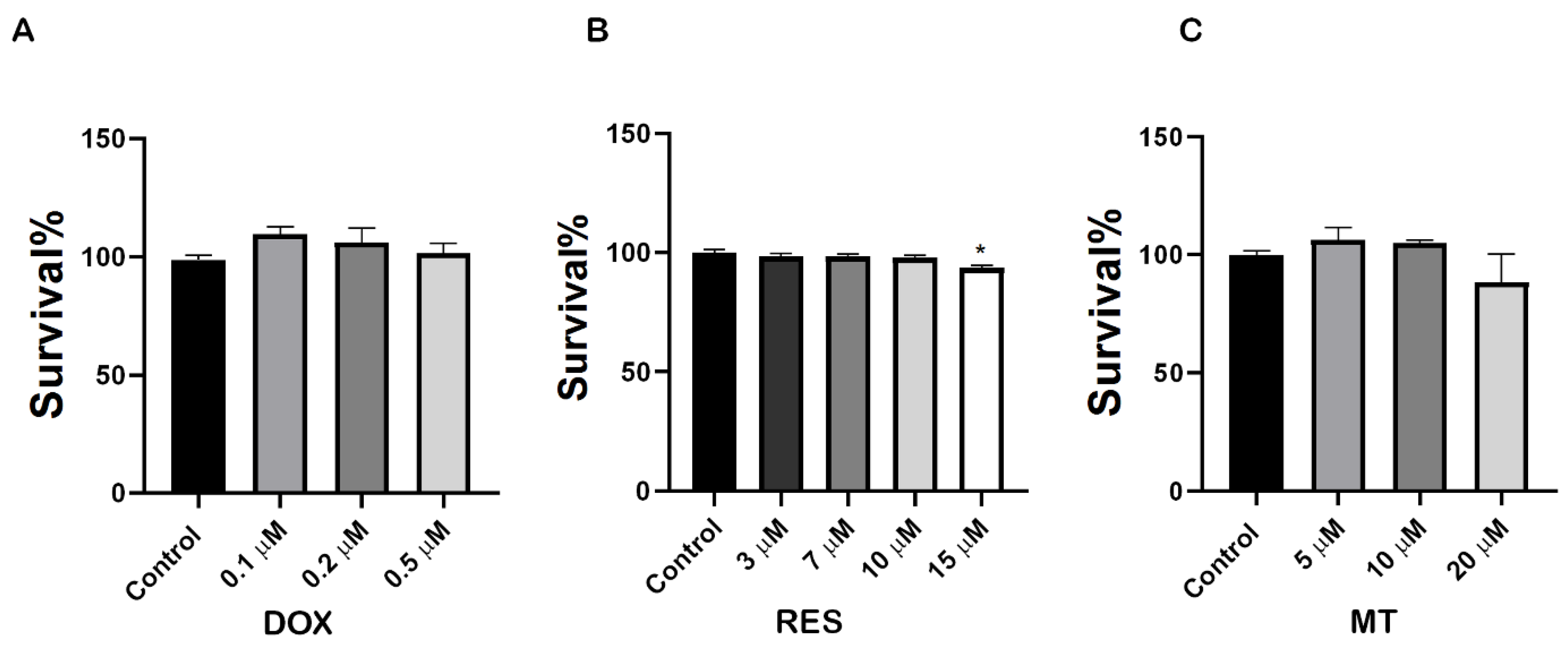
2.1. Cytotoxic Effect of Resveratrol, Doxorubicin and MitoTEMPO
2.2. Inhibition of Doxorubicin-Induced Osteoclastogenesis by RES
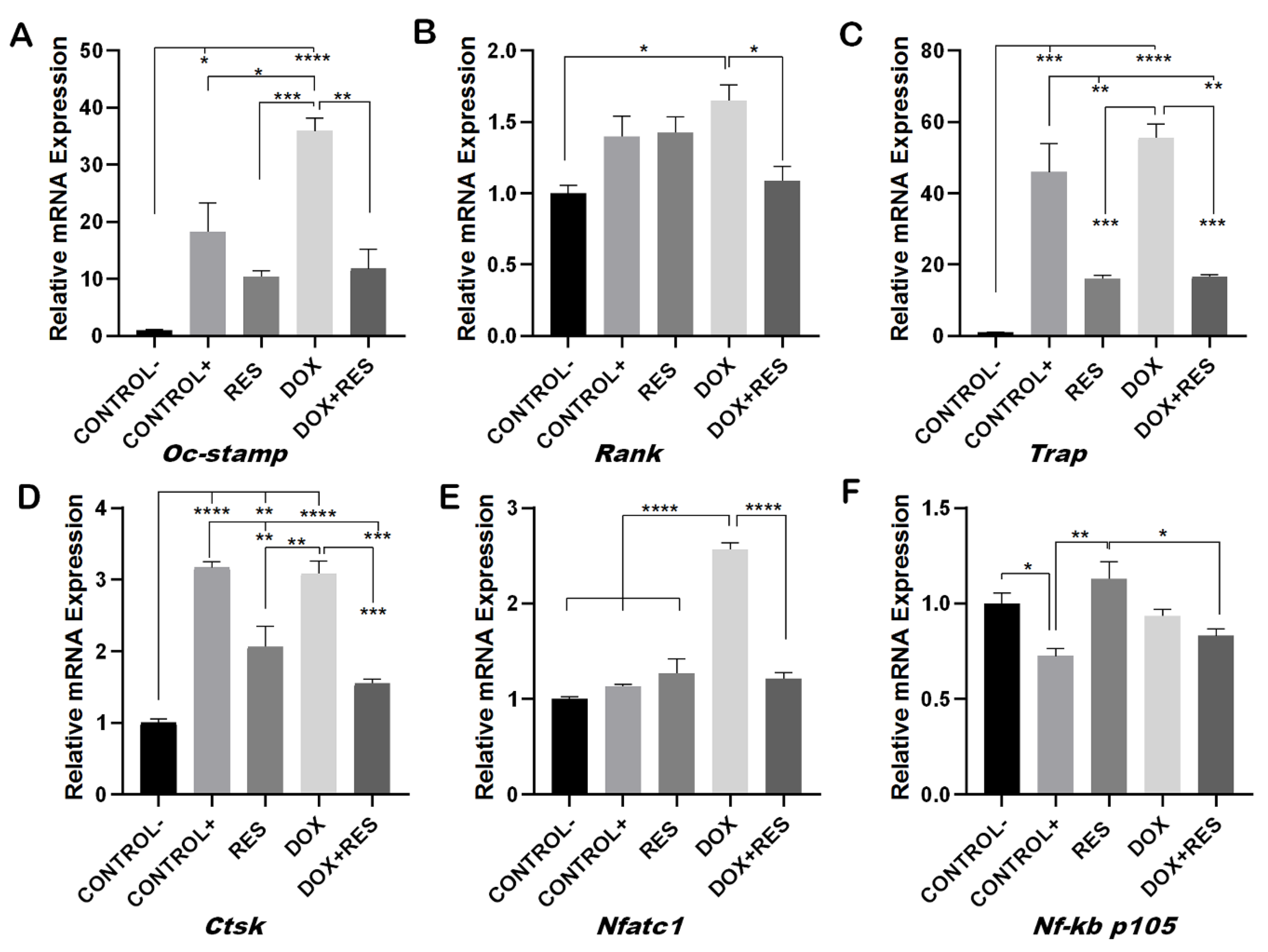
2.3. Resveratrol Inhibits Doxorubicin-Induced Osteoclast Differentiation Marker Genes
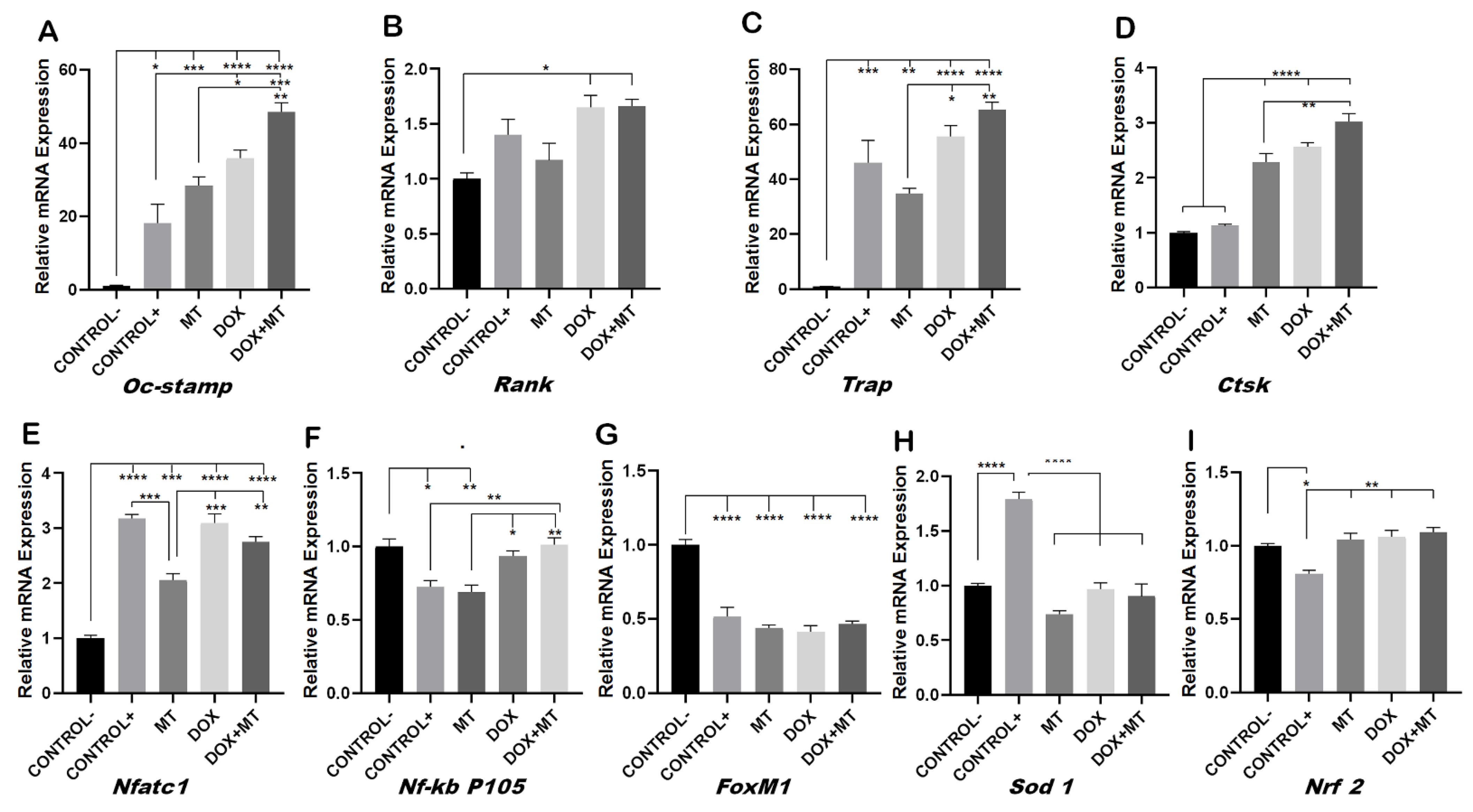
2.4. Involvement of FoxM1 on Osteoclast Differentiation and Oxidative Stress
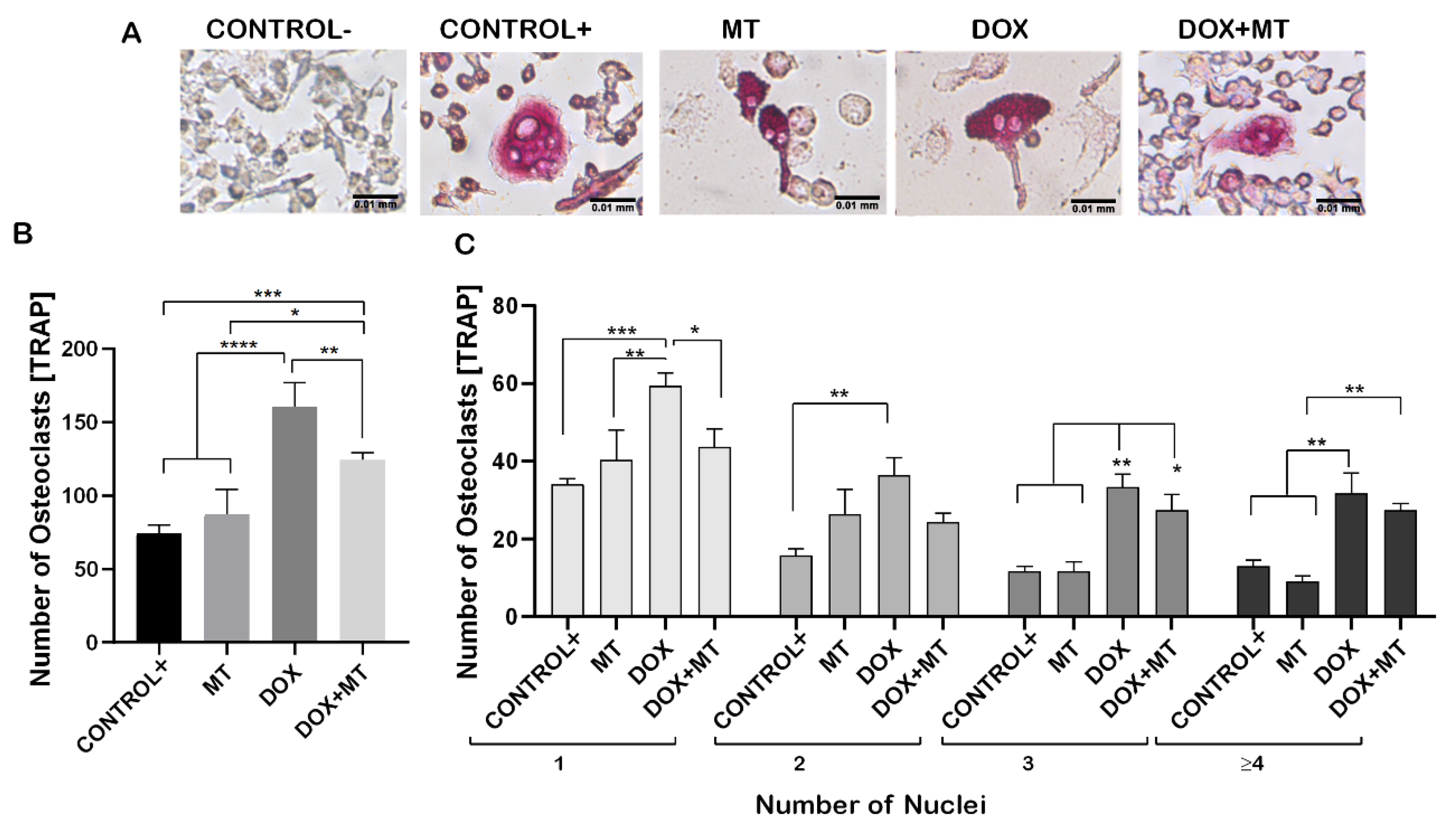
2.5. Effect of Mitochondrial Antioxidant on Doxorubicin-Induced Osteoclast Differentiation
2.6. MitoTEMPO Is Unable to Reverse Doxorubicin-Induced Osteoclast Markers Genes
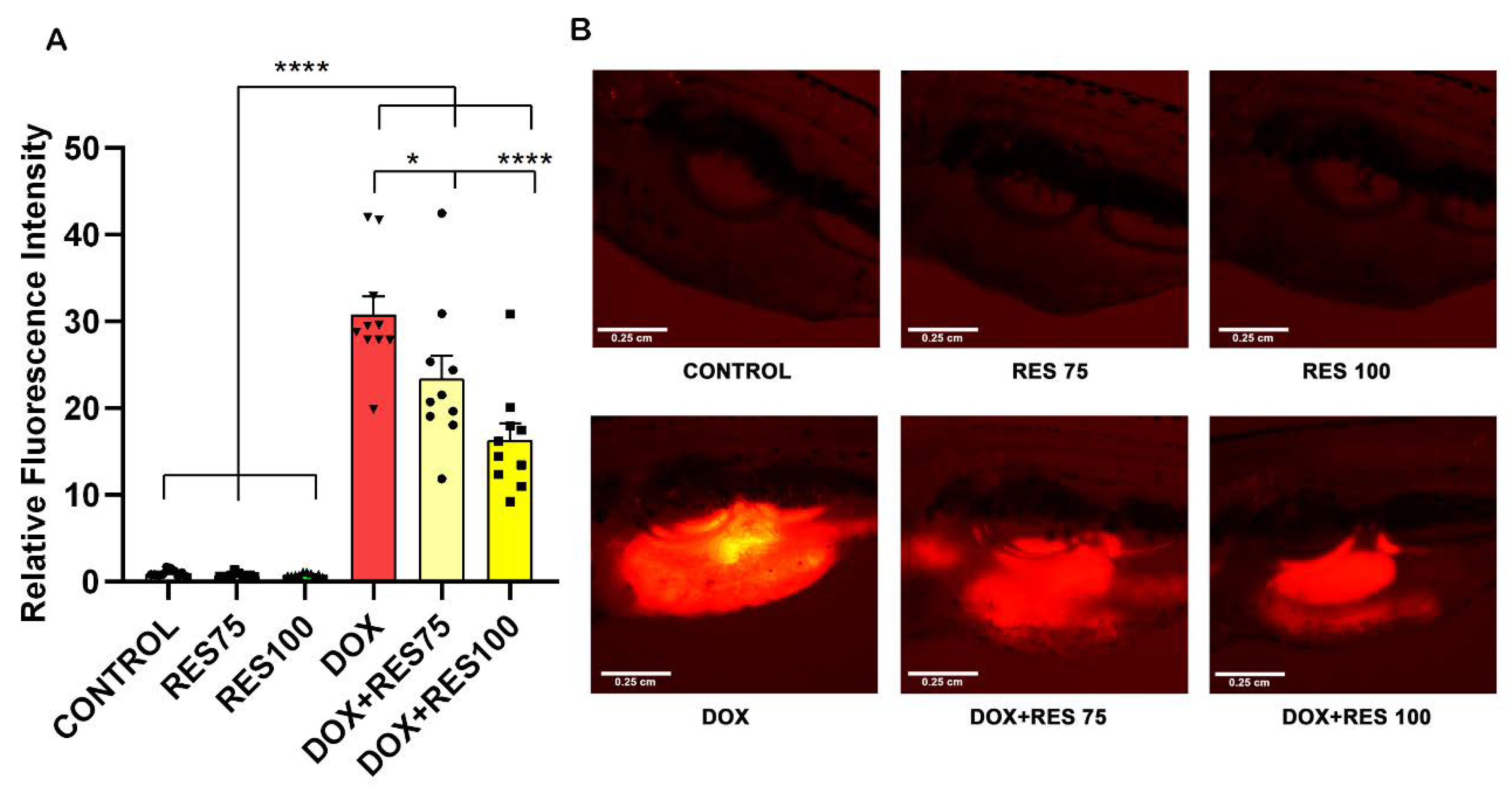
2.7. In Vivo Reversal of Doxorubicin-Induced Osteoclast Differentiation by Resveratrol
2.8. In Vivo Reversal of Doxorubicin Induced Mucositis by Resveratrol
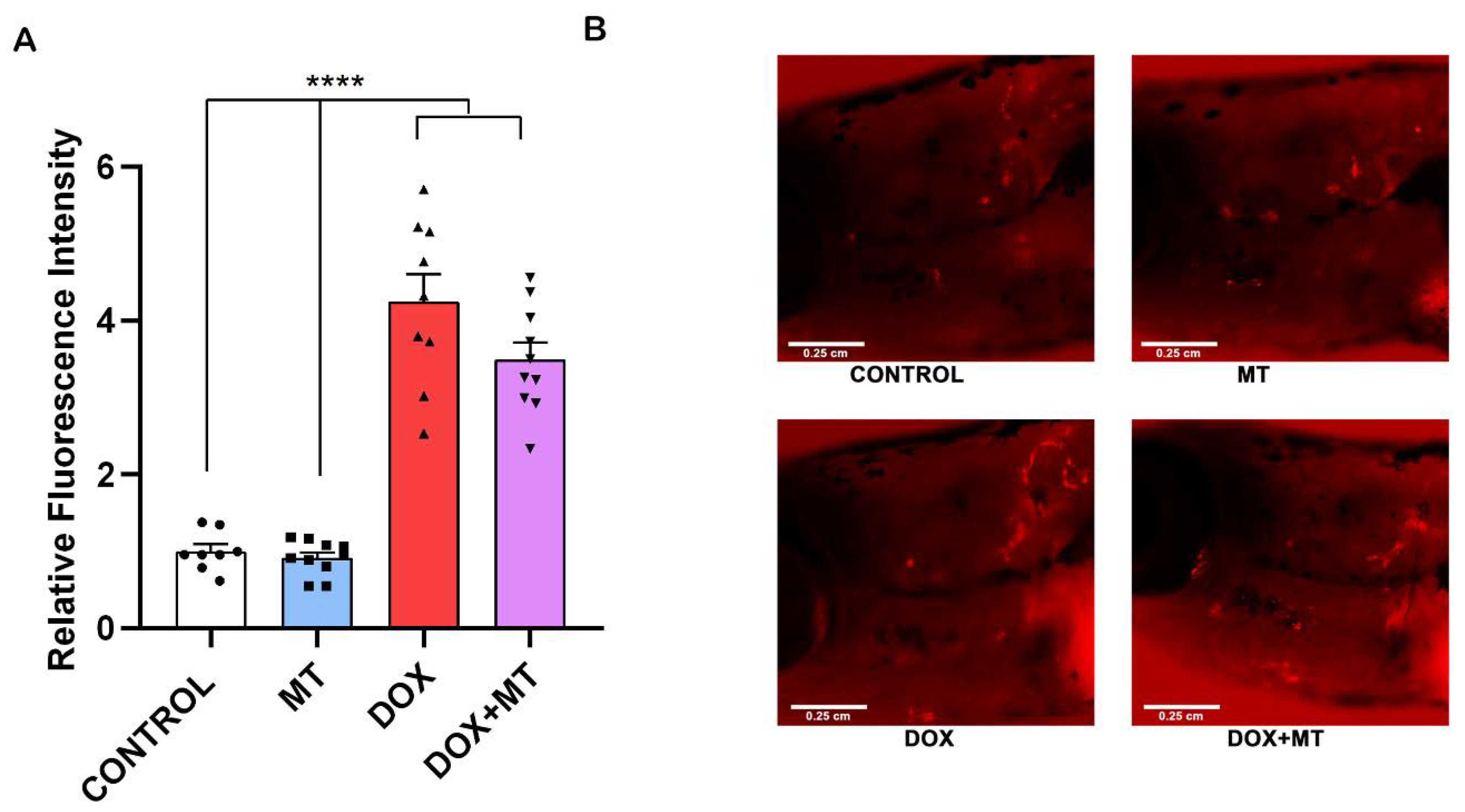
2.9. MitoTEMPO Is Unable to Reverse Doxorubicin-Induced Osteoclast Differentiation In Vivo
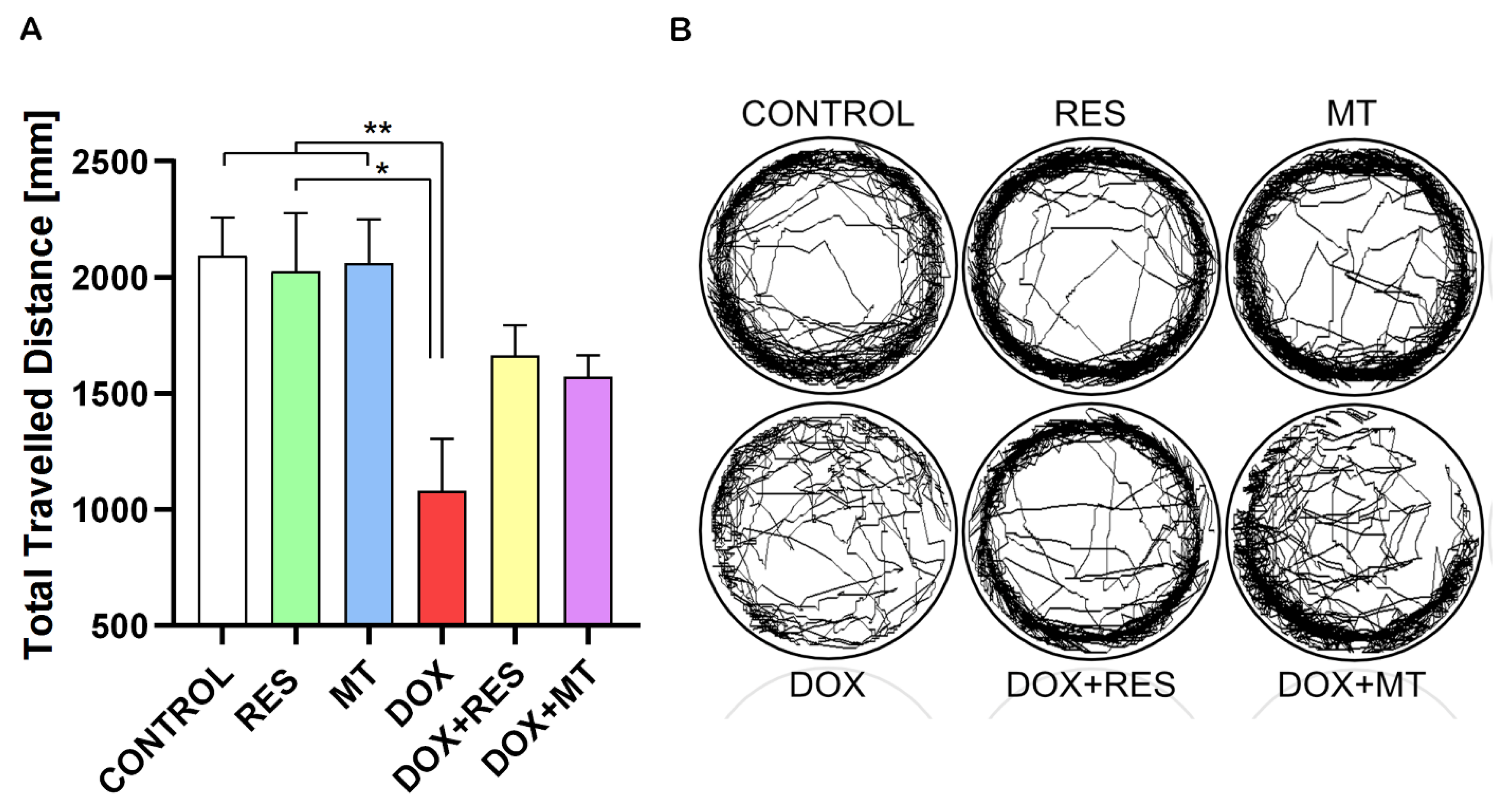
2.10. Doxorubicin Decreases Locomotor Activity of Zebrafish
3. Discussion
4. Materials and Methods
4.1. Cells and Cell Culture
4.2. XTT Assay
4.3. Osteoclast Differentiation
4.4. Tartrate-Resistant Acid Phosphatase Staining (TRAP Staining)
4.5. RT-PCR and Real-Time PCR
4.6. Osteoclast Activation In Vivo
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cappetta, D.; De Angelis, A.; Sapio, L.; Prezioso, L.; Illiano, M.; Quaini, F.; Rossi, F.; Berrino, L.; Naviglio, S.; Urbanek, K. Oxidative stress and cellular response to doxorubicin: A common factor in the complex milieu of anthracycline cardiotoxicity. Oxid. Med. Cell. Longev. 2017, 2017, 1521020. [Google Scholar] [CrossRef] [PubMed]
- Hadji, P.; Ziller, M.; Maskow, C.; Albert, U.; Kalder, M. The influence of chemotherapy on bone mineral density, quantitative ultrasonometry and bone turnover in pre-menopausal women with breast cancer. Eur. J. Cancer 2009, 45, 3205–3212. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, B.L.; Kamps, W.A.; Hartel, R.M.; Veth, R.P.H.; Sluiter, W.J.; Hoekstra, H.J. Effect of single chemotherapeutic agents on the growing skeleton of the rat. Ann. Oncol. 2000, 11, 1121–1126. [Google Scholar] [CrossRef]
- Friedlaender, G.E.; Tross, R.B.; Doganis, A.C.; Kirkwood, J.M.; Baron, R. Effects of chemotherapeutic agents on bone. I. Short-term methotrexate and doxorubicin (adriamycin) treatment in a rat model. J. Bone Jt. Surg. Ser. A 1984, 66, 602–607. [Google Scholar] [CrossRef]
- Callaway, D.A.; Jiang, J.X. Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J. Bone Miner. Metab. 2015, 33, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Thannickal, V.J.; Fanburg, B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 279, L1005-28. [Google Scholar] [CrossRef] [Green Version]
- Ha, H.; Kwak, H.B.; Lee, S.W.; Jin, H.M.; Kim, H.-M.; Kim, H.-H.; Lee, Z.H. Reactive oxygen species mediate rank signaling in osteoclasts. Exp. Cell Res. 2004, 301, 119–127. [Google Scholar] [CrossRef]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [Green Version]
- Lee, N.K.; Choi, Y.G.; Baik, J.Y.; Han, S.Y.; Jeong, D.-W.; Bae, Y.S.; Kim, N.; Lee, S.Y. A Crucial Role for Reactive Oxygen Species in RANKL-Induced Osteoclast Differentiation. Blood 2005, 106, 852–859. [Google Scholar] [CrossRef] [Green Version]
- Jiang, F.; Zhang, Y.; Dusting, G.J. NADPH oxidase-mediated redox signaling: Roles in cellular stress response, stress tolerance, and tissue repair. Pharmacol. Rev. 2011, 63, 218–242. [Google Scholar] [CrossRef]
- Hirotani, H.; Tuohy, N.A.; Woo, J.-T.; Stern, P.H.; Clipstone, N.A. The calcineurin/nuclear factor of activated T cells signaling pathway regulates osteoclastogenesis in RAW264.7 cells. J. Biol. Chem. 2004, 279, 13984–13992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Zhang, Y.; Ries, W.; Key, L. Expression of nox4 in osteoclasts. J. Cell. Biochem. 2004, 92, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Yamamoto, H.; Tominaga, K.; Masuda, K.; Kawai, T.; Teshima-Kondo, S.; Rokutan, K. NADPH oxidase-derived reactive oxygen species are essential for differentiation of a mouse macrophage cell line (RAW264.7) into osteoclasts. J. Med. Investig. 2009, 56, 33–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivasan, S.; Koenigstein, A.; Joseph, J.; Sun, L.; Kalyanaraman, B.; Zaidi, M.; Avadhani, N.G. Role of Mitochondrial Reactive Oxygen Species in Osteoclast Differentiation. In Proceedings of the Annals of the New York Academy of Sciences; Blackwell Publishing Inc.: Hoboken, NJ, USA, 2010; Volume 1192, pp. 245–252. [Google Scholar]
- Kim, K.; Kim, J.H.; Lee, J.; Jin, H.-M.; Lee, S.-H.; Fisher, D.E.; Kook, H.; Kim, K.K.; Choi, Y.; Kim, N. Nuclear factor of activated T cells c1 induces osteoclast-associated receptor gene expression during tumor necrosis factor-related activation-induced cytokine-mediated osteoclastogenesis. J. Biol. Chem. 2005, 280, 35209–35216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.J.; Carr, J.R.; Wang, Z.; Nogueira, V.; Hay, N.; Tyner, A.L.; Lau, L.F.; Costa, R.H.; Raychaudhuri, P. FoxM1, a critical regulator of oxidative stress during oncogenesis. EMBO J. 2009, 28, 2908–2918. [Google Scholar] [CrossRef] [Green Version]
- Laoukili, J.; Kooistra, M.R.H.; Brás, A.; Kauw, J.; Kerkhoven, R.M.; Morrison, A.; Clevers, H.; Medema, R.H. FoxM1 is required for execution of the mitotic programme and chromosome stability. Nat. Cell Biol. 2005, 7, 126–136. [Google Scholar] [CrossRef]
- Wang, I.C.; Chen, Y.J.; Hughes, D.; Petrovic, V.; Major, M.L.; Park, H.J.; Tan, Y.; Ackerson, T.; Costa, R.H. Forkhead box M1 regulates the transcriptional network of genes essential for mitotic progression and genes encoding the SCF (Skp2-Cks1) ubiquitin ligase. Mol. Cell. Biol. 2005, 25, 10875–10894. [Google Scholar] [CrossRef] [Green Version]
- Yao, S.; Fan, L.Y.-N.; Lam, E.W.-F. The FOXO3-FOXM1 axis: A key cancer drug target and a modulator of cancer drug resistance. Semin. Cancer Biol. 2018, 50, 77–89. [Google Scholar] [CrossRef]
- De Olano, N.; Koo, C.-Y.; Monteiro, L.J.; Pinto, P.H.; Gomes, A.R.; Aligue, R.; Lam, E.W.-F. The p38 MAPK-MK2 axis regulates E2F1 and FOXM1 expression after epirubicin treatment. Mol. Cancer Res. 2012, 10, 1189–1202. [Google Scholar] [CrossRef] [Green Version]
- Behren, A.; Mühlen, S.; Acuna Sanhueza, G.A.; Schwager, C.; Plinkert, P.K.; Huber, P.E.; Abdollahi, A.; Simon, C. phenotype-assisted transcriptome analysis identifies FOXM1 downstream from Ras-MKK3-p38 to Regulate in vitro cellular invasion. Oncogene 2010, 29, 1519–1530. [Google Scholar] [CrossRef]
- Artsi, H.; Cohen-Kfir, E.; Gurt, I.; Shahar, R.; Bajayo, A.; Kalish, N.; Bellido, T.M.; Gabet, Y.; Dresner-Pollak, R. The sirtuin1 activator SRT3025 down-regulates sclerostin and rescues ovariectomy-induced bone loss and biomechanical deterioration in female mice. Endocrinology 2014, 155, 3508–3515. [Google Scholar] [CrossRef] [PubMed]
- Su, J.-L.; Yang, C.-Y.; Zhao, M.; Kuo, M.-L.; Yen, M.-L. Forkhead proteins are critical for bone morphogenetic protein-2 regulation and anti-tumor activity of resveratrol. J. Biol. Chem. 2007, 282, 19385–19398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Momken, I.; Stevens, L.; Bergouignan, A.; Desplanches, D.; Rudwill, F.; Chery, I.; Zahariev, A.; Zahn, S.; Stein, T.P.; Sebedio, J.L.; et al. Resveratrol prevents the wasting disorders of mechanical unloading by acting as a physical exercise mimetic in the rat. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2011, 25, 3646–3660. [Google Scholar] [CrossRef]
- Kim, H.-N.; Han, L.; Iyer, S.; de Cabo, R.; Zhao, H.; O’Brien, C.A.; Manolagas, S.C.; Almeida, M. Sirtuin1 suppresses osteoclastogenesis by deacetylating FoxOs. Mol. Endocrinol. 2015, 29, 1498–1509. [Google Scholar] [CrossRef] [PubMed]
- Mercken, E.M.; Mitchell, S.J.; Martin-Montalvo, A.; Minor, R.K.; Almeida, M.; Gomes, A.P.; Scheibye-Knudsen, M.; Palacios, H.H.; Licata, J.J.; Zhang, Y.; et al. SRT2104 extends survival of male mice on a standard diet and preserves bone and muscle mass. Aging Cell 2014, 13, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Pearson, K.J.; Baur, J.A.; Lewis, K.N.; Peshkin, L.; Price, N.L.; Labinskyy, N.; Swindell, W.R.; Kamara, D.; Minor, R.K.; Perez, E.; et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008, 8, 157–168. [Google Scholar] [CrossRef] [Green Version]
- Boissy, P.; Andersen, T.L.; Abdallah, B.M.; Kassem, M.; Plesner, T.; Delaissé, J.-M. Resveratrol inhibits myeloma cell growth, prevents osteoclast formation, and promotes osteoblast differentiation. Cancer Res. 2005, 65, 9943–9952. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Andersson, G.; Lindgren, U.; Li, Y. Resveratrol prevents RANKL-induced osteoclast differentiation of murine osteoclast progenitor RAW 264.7 cells through inhibition of ros production. Biochem. Biophys. Res. Commun. 2010, 401, 356–362. [Google Scholar] [CrossRef]
- Shakibaei, M.; Buhrmann, C.; Mobasheri, A. Resveratrol-mediated SIRT-1 interactions with P300 modulate receptor activator of NF-KappaB ligand (RANKL) activation of NF-KappaB signaling and inhibit osteoclastogenesis in bone-derived cells. J. Biol. Chem. 2011, 286, 11492–11505. [Google Scholar] [CrossRef] [Green Version]
- Rubiolo, J.A.; Mithieux, G.; Vega, F.V. Resveratrol protects primary rat hepatocytes against oxidative stress damage: Activation of the Nrf2 transcription factor and augmented activities of antioxidant enzymes. Eur. J. Pharmacol. 2008, 591, 66–72. [Google Scholar] [CrossRef]
- Shin, S.M.; Cho, I.J.; Kim, S.G. Resveratrol protects mitochondria against oxidative stress through AMP-activated protein kinase-mediated glycogen synthase kinase-3beta inhibition downstream of poly(ADP-ribose)polymerase-LKB1 pathway. Mol. Pharmacol. 2009, 76, 884–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miura, S.; Saitoh, S.I.; Kokubun, T.; Owada, T.; Yamauchi, H.; Machii, H.; Takeishi, Y. Mitochondrial-targeted antioxidant maintains blood flow, mitochondrial function, and redox balance in old mice following prolonged limb ischemia. Int. J. Mol. Sci. 2017, 18, 1897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, R.; Cao, T.; Xiong, S.; Ma, J.; Fan, G.C.; Lacefield, J.C.; Lu, Y.; Le Tissier, S.; Peng, T. Therapeutic inhibition of mitochondrial reactive oxygen species with mito-TEMPO reduces diabetic cardiomyopathy. Free Radic. Biol. Med. 2016, 90, 12–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, I.S.; Kim, C. NADPH oxidase gp91(phox) contributes to RANKL-induced osteoclast differentiation by upregulating NFATc1. Sci. Rep. 2016, 6, 38014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agidigbi, T.S.; Kim, C. Reactive oxygen species in osteoclast differentiation and possible pharmaceutical targets of ros-mediated osteoclast diseases. Int. J. Mol. Sci. 2019, 20, 3576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knowles, H. Hypoxic Regulation of Osteoclast Differentiation and Bone Resorption Activity. Hypoxia 2015, 3, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Jeoung, N.H. Pyruvate dehydrogenase kinases: Therapeutic targets for diabetes and cancers. Diabetes Metab. J. 2015, 39, 188–197. [Google Scholar] [CrossRef] [Green Version]
- He, Q.; Harris, N.; Ren, J.; Han, X. Mitochondria-targeted antioxidant prevents cardiac dysfunction induced by tafazzin gene knockdown in cardiac myocytes. Oxid. Med. Cell. Longev. 2014, 2014, 654198. [Google Scholar] [CrossRef] [Green Version]
- Rana, T.; Chakrabarti, A.; Freeman, M.; Biswas, S. Doxorubicin-mediated bone loss in breast cancer bone metastases is driven by an interplay between oxidative stress and induction of TGFβ. PLoS ONE 2013, 8, e78043. [Google Scholar] [CrossRef]
- Zhou, L.; Kuai, F.; Shi, Q.; Yang, H. Doxorubicin restrains osteogenesis and promotes osteoclastogenesis in vitro. Am. J. Transl. Res. 2020, 12, 5640–5654. [Google Scholar]
- Li, Q.; Zhang, J.; Liu, D.; Liu, Y.; Zhou, Y. Force-induced decline of FOXM1 in human periodontal ligament cells contributes to osteoclast differentiation. Angle Orthod. 2019, 89, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Caetano-Lopes, J.; Henke, K.; Urso, K.; Duryea, J.; Charles, J.F.; Warman, M.L.; Harris, M.P. Unique and non-redundant function of csf1r paralogues in regulation and evolution of post-embryonic development of the zebrafish. Development 2020, 147, dev181834. [Google Scholar] [CrossRef] [PubMed]
- Halasi, M.; Pandit, B.; Wang, M.; Nogueira, V.; Hay, N.; Gartel, A.L. Combination of oxidative stress and foxm1 inhibitors induces apoptosis in cancer cells and inhibits xenograft tumor growth. Am. J. Pathol. 2013, 183, 257–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchner, M.; Park, E.; Geng, H.; Klemm, L.; Flach, J.; Passegué, E.; Schjerven, H.; Melnick, A.; Paietta, E.; Kopanja, D.; et al. Identification of FOXM1 as a therapeutic target in B-cell lineage acute lymphoblastic leukaemia. Nat. Commun. 2015, 6, 6471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, H.; Tollner, T.L.; Hu, Z.; Dai, M.; Li, X.; Guan, H.; Shan, D.; Zhang, X.; Lv, J.; Huang, C.; et al. The importance of mitochondrial metabolic activity and mitochondrial dna replication during oocyte maturation in vitro on oocyte quality and subsequent embryo developmental competence. Mol. Reprod. Dev. 2012, 79, 392–401. [Google Scholar] [CrossRef]
- Le Gal, K.; Wiel, C.; Ibrahim, M.X.; Henricsson, M.; Sayin, V.I.; Bergo, M.O. Mitochondria-targeted antioxidants MitoQ and MitoTEMPO do not influence BRAF-driven malignant melanoma and KRAS-driven lung cancer progression in mice. Antioxidants 2021, 10, 163. [Google Scholar] [CrossRef]
- Yagi, M.; Miyamoto, T.; Sawatani, Y.; Iwamoto, K.; Hosogane, N.; Fujita, N.; Morita, K.; Ninomiya, K.; Suzuki, T.; Miyamoto, K.; et al. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J. Exp. Med. 2005, 202, 345–351. [Google Scholar] [CrossRef] [Green Version]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.-L.; et al. Small molecule activators of sirtuins extend saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef]
- Gehm, B.D.; McAndrews, J.M.; Chien, P.Y.; Jameson, J.L. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc. Natl. Acad. Sci. USA 1997, 94, 14138–14143. [Google Scholar] [CrossRef] [Green Version]
- Shevde, N.K.; Bendixen, A.C.; Dienger, K.M.; Pike, J.W. Estrogens suppress RANK ligand-induced osteoclast differentiation via a stromal cell independent mechanism involving c-Jun repression. Proc. Natl. Acad. Sci. USA 2000, 97, 7829–7834. [Google Scholar] [CrossRef] [Green Version]
- Savinova, O.V.; Hoffmann, A.; Ghosh, G. The Nfkb1 and Nfkb2 proteins P105 and P100 function as the core of high-molecular-weight heterogeneous complexes. Mol. Cell 2009, 34, 591–602. [Google Scholar] [CrossRef]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.S.; Yang, Y.M.; Son, A.; Tian, Y.S.; Lee, S.I.; Kang, S.W.; Muallem, S.; Shin, D.M. RANKL-mediated reactive oxygen species pathway that induces long lasting Ca2+ oscillations essential for osteoclastogenesis. J. Biol. Chem. 2010, 285, 6913–6921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, P.J.; Parker, B.; Dimsdale, J.E.; Sadler, G.R.; Ancoli-Israel, S. The relationship between fatigue and quality of life and inflammation during anthracycline-based chemotherapy in breast cancer. Biol. Psychol. 2005, 69, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Kodama, J.; Kaito, T. Osteoclast multinucleation: Review of current literature. Int. J. Mol. Sci. 2020, 21, 5685. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.; Wu, Z.; Chu, H.Y.; Lu, J.; Lyu, A.; Liu, J.; Zhang, G. Cathepsin K: The action in and beyond bone. Front. cell Dev. Biol. 2020, 8, 433. [Google Scholar] [CrossRef]
- Medina, C.; Radomski, M.W. Role of matrix metalloproteinases in intestinal inflammation. J. Pharmacol. Exp. Ther. 2006, 318, 933–938. [Google Scholar] [CrossRef] [Green Version]
- Menzel, K.; Hausmann, M.; Obermeier, F.; Schreiter, K.; Dunger, N.; Bataille, F.; Falk, W.; Scholmerich, J.; Herfarth, H.; Rogler, G. Cathepsins B, L and D in inflammatory bowel disease macrophages and potential therapeutic effects of cathepsin inhibition in vivo. Clin. Exp. Immunol. 2006, 146, 169–180. [Google Scholar] [CrossRef]
- Kaczmarek, A.; Brinkman, B.M.; Heyndrickx, L.; Vandenabeele, P.; Krysko, D. V Severity of doxorubicin-induced small intestinal mucositis is regulated by the TLR-2 and TLR-9 pathways. J. Pathol. 2012, 226, 598–608. [Google Scholar] [CrossRef]
- Savard, J.; Liu, L.; Natarajan, L.; Rissling, M.B.; Neikrug, A.B.; He, F.; Dimsdale, J.E.; Mills, P.J.; Parker, B.A.; Sadler, G.R.; et al. Breast cancer patients have progressively impaired sleep-wake activity rhythms during chemotherapy. Sleep 2009, 32, 1155–1160. [Google Scholar] [CrossRef] [Green Version]
- Lira, F.S.; Esteves, A.M.; Pimentel, G.D.; Rosa, J.C.; Frank, M.K.; Mariano, M.O.; Budni, J.; Quevedo, J.; Dos Santos, R.V.; de Mello, M.T. Sleep pattern and locomotor activity are impaired by doxorubicin in non-tumor-bearing rats. Sleep Sci. 2016, 9, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Sanford, S.D.; Wagner, L.I.; Beaumont, J.L.; Butt, Z.; Sweet, J.J.; Cella, D. Longitudinal prospective assessment of sleep quality: Before, during, and after adjuvant chemotherapy for breast cancer. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2013, 21, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Burstone, M.S. histochemical demonstration of acid phosphatase activity in osteoclasts. J. Histochem. Cytochem. 1959, 7, 39–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pombinho, A.R.; Laizé, V.; Molha, D.M.; Marques, S.M.P.; Cancela, M.L. Development of two bone-derived cell lines from the marine teleost sparus aurata; evidence for extracellular matrix mineralization and cell-type-specific expression of matrix Gla protein and osteocalcin. Cell Tissue Res. 2004, 315, 393–406. [Google Scholar] [CrossRef]
- Viegas, M.N.; Dias, J.; Cancela, M.L.; Laizé, V. Polyunsaturated fatty acids regulate cell proliferation, extracellular matrix mineralization and gene expression in a gilthead seabream skeletal cell line. J. Appl. Ichthyol. 2012, 28, 427–432. [Google Scholar] [CrossRef]



| Gene | Sequences | |
|---|---|---|
| Beta-actin | Forward primer | CCTGACCCTGAAGTACCCCATTGA |
| Reverse primer | GTCATCTTTTCACGGTTGGCC | |
| Oc-stamp | Forward primer | TGGGCCTCCATATGACCTCGAGTAG |
| Reverse primer | TCAAAGGCTTGTAAATTGGAGGAGT | |
| Rank | Forward primer | TGCCTCTGGGAACGTGACTG |
| Reverse primer | AGGTCTGGCTGACATACACCAC | |
| Trap | Forward primer | CAGCTGTCCTGGCTCAAAA |
| Reverse primer | ACATAGCCCACACCGTTCTC | |
| Nfatc1 | Forward primer | CAAGTCCTCACCACAGGGCTCACTA |
| Reverse primer | GCGTGAGAGAGGTTCATTCTCCAAGT | |
| Ctsk | Forward primer | CTGAAGATGCTTTCCCATATGTGGG |
| Reverse primer | GCAGGCGTTGTTCTTATTCCGAG | |
| Nf-kb p105 | Forward primer | TGTCAACAGATGGCCCATACCT |
| Reverse primer | TTGTGACCAACTGAACGATAACCT | |
| Sod 1 | Forward primer | GGACAATACACAAGGCTGTACCA |
| Reverse primer | CAGTCACATTGCCCAGGTCTC | |
| Nrf 2 | Forward primer | AAAGTTCAGTCTTCACTGCCC |
| Reverse primer | TCGGTATTAAGACACTGTAATTCGG | |
| FoxM1 | Forward primer | GTCTCCTTCTGGACCATTCACC |
| Reverse primer | GCTCAGGATTGGGTCGTTTCTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poudel, S.; Martins, G.; Cancela, M.L.; Gavaia, P.J. Resveratrol-Mediated Reversal of Doxorubicin-Induced Osteoclast Differentiation. Int. J. Mol. Sci. 2022, 23, 15160. https://doi.org/10.3390/ijms232315160
Poudel S, Martins G, Cancela ML, Gavaia PJ. Resveratrol-Mediated Reversal of Doxorubicin-Induced Osteoclast Differentiation. International Journal of Molecular Sciences. 2022; 23(23):15160. https://doi.org/10.3390/ijms232315160
Chicago/Turabian StylePoudel, Sunil, Gil Martins, M. Leonor Cancela, and Paulo J. Gavaia. 2022. "Resveratrol-Mediated Reversal of Doxorubicin-Induced Osteoclast Differentiation" International Journal of Molecular Sciences 23, no. 23: 15160. https://doi.org/10.3390/ijms232315160





