Impact of Sulfated Hyaluronan on Bone Metabolism in Diabetic Charcot Neuroarthropathy and Degenerative Arthritis
Abstract
1. Introduction
2. Results
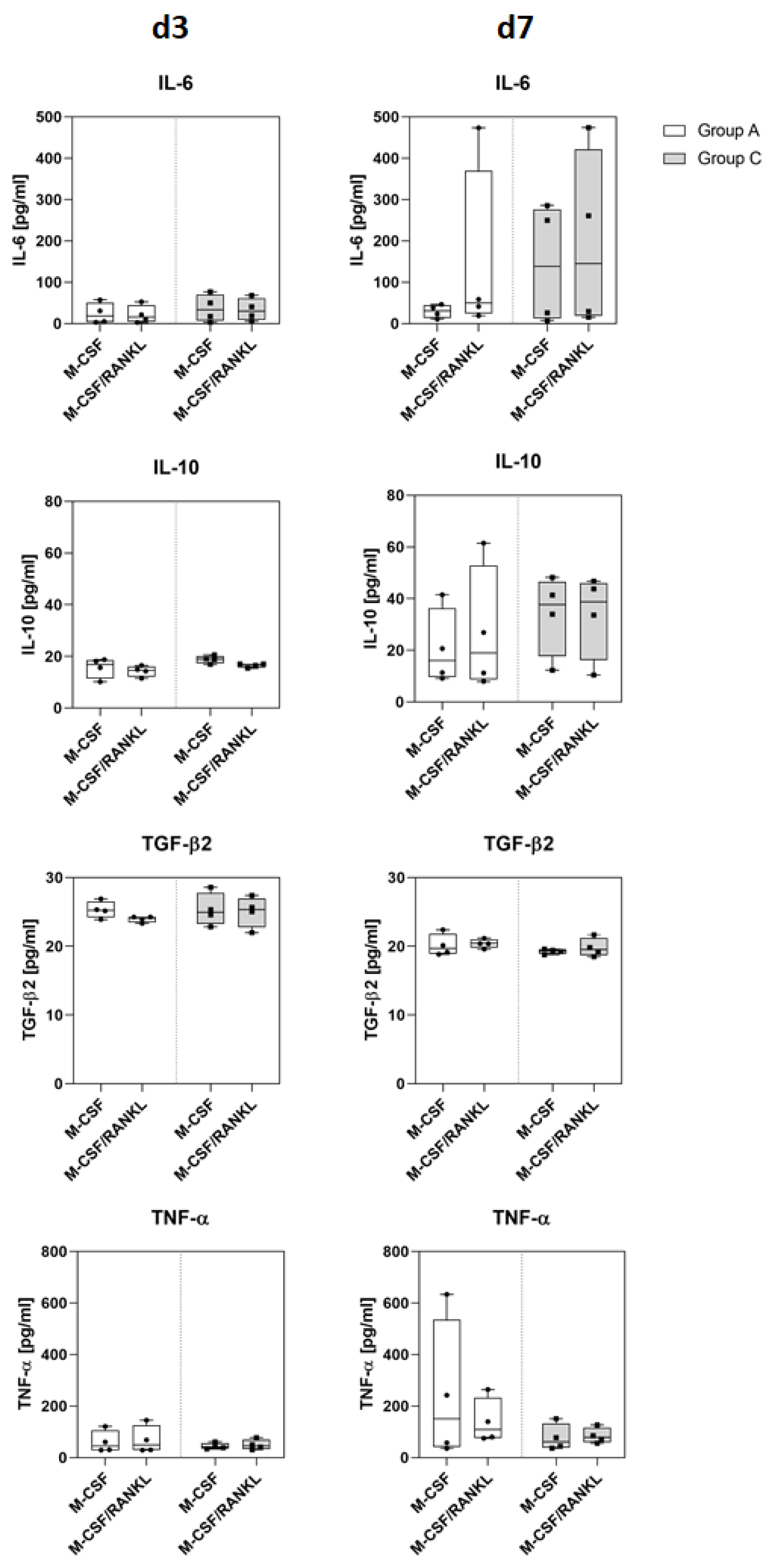
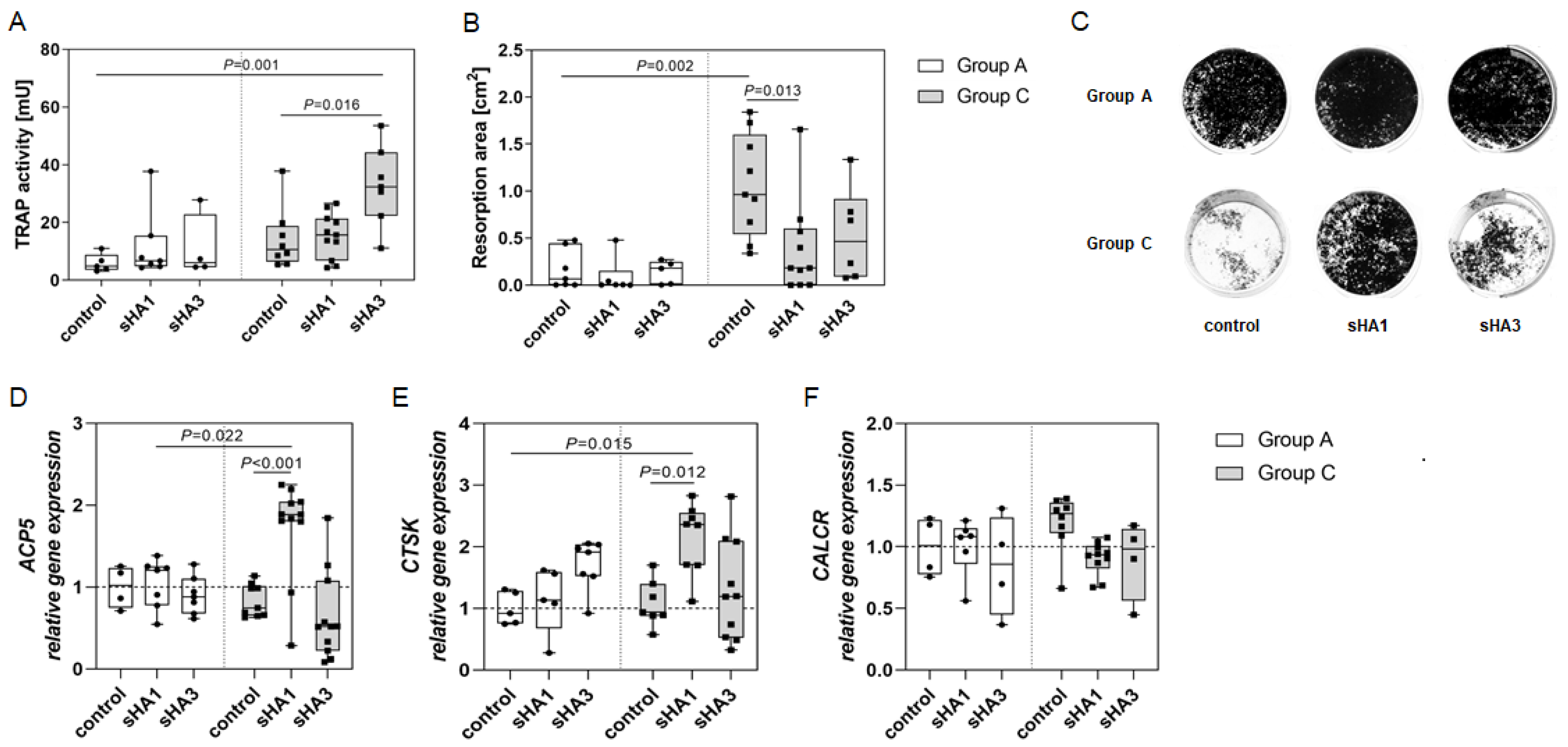
2.1. Osteoclasts
2.2. Osteoblast Gene Expression
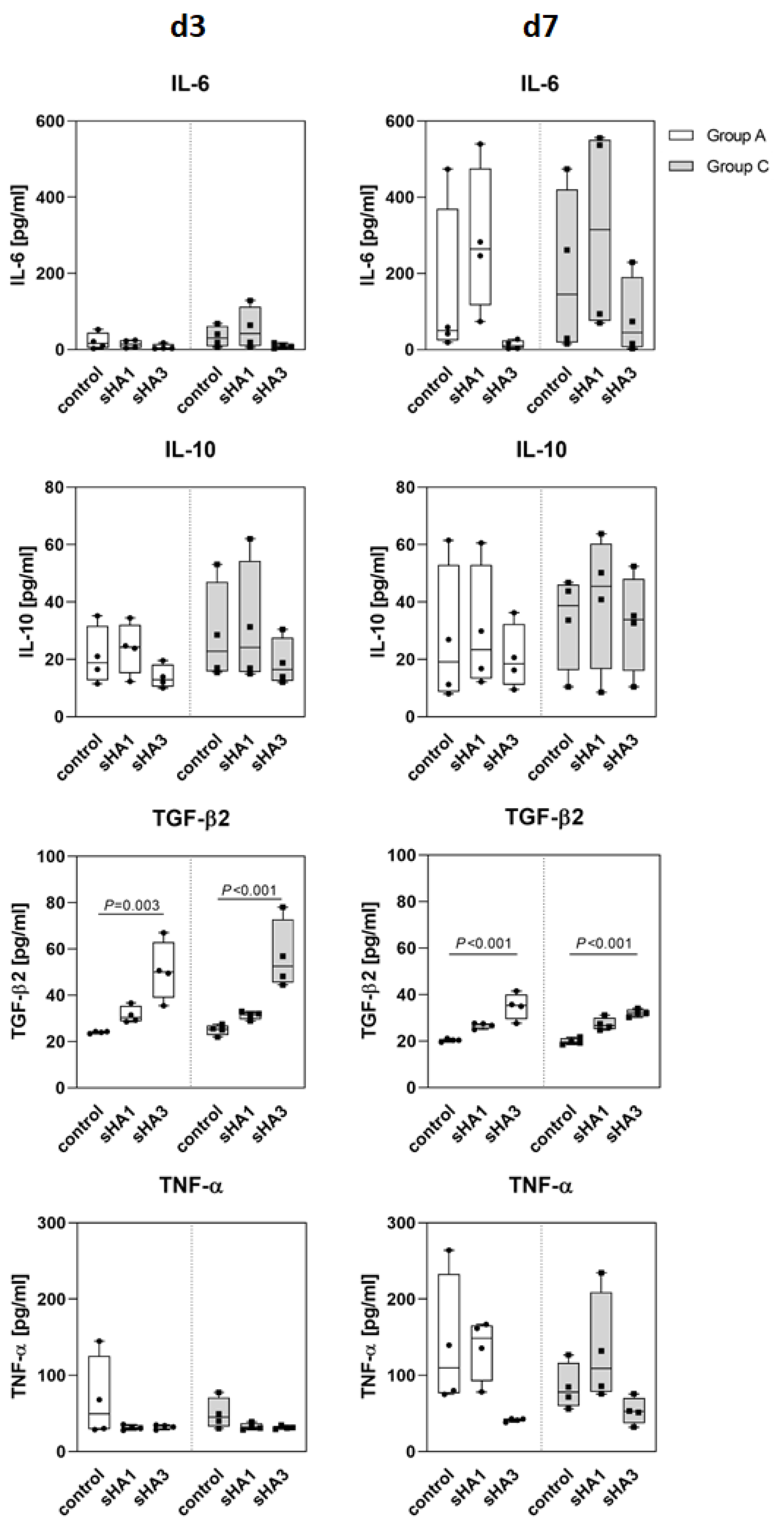
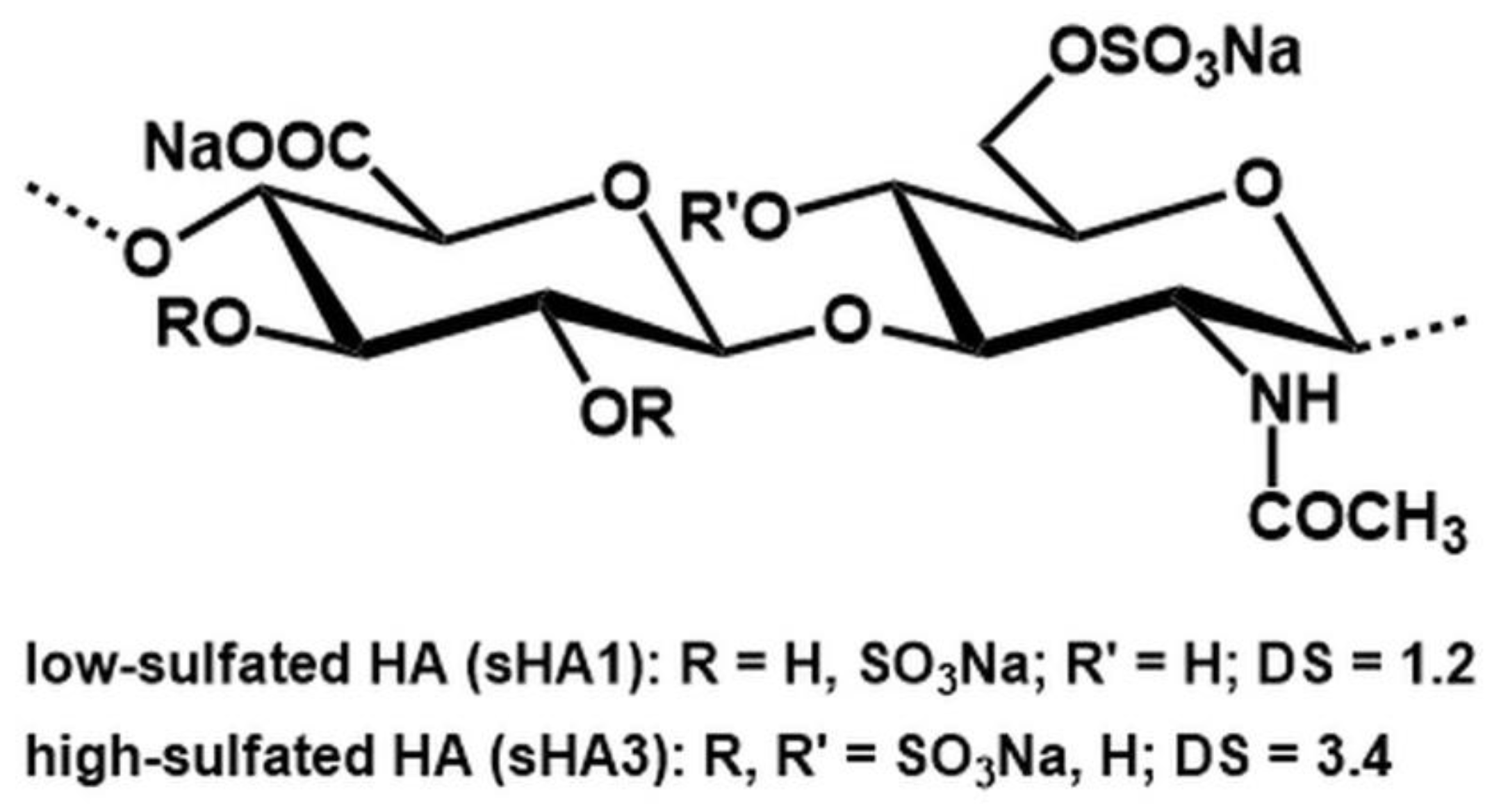
2.3. Effect of Sulfated Hyaluronan Derivatives on Osteoclasts and Osteoblasts
3. Discussion
4. Materials and Methods
4.1. Patients and Sampling
4.2. Cell Culture
4.3. Osteoclast Characterization
4.4. Osteoblast Characterization
4.5. sGAG Synthesis
4.6. RNA Isolation, Reverse Transcription and Real-Time qPCR
4.7. Determination of Cytokine Release
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dardari, D. An overview of Charcot’s neuroarthropathy. J. Clin. Transl. Endocrinol. 2020, 22, 100239. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, R.; Bilal, A.; Petrova, N.; Boddhu, K.; Manu, C.; Vas, P.; Bates, M.; Corcoran, B.; Reichert, I.; Mulholland, N.; et al. The Role of Bone Scintigraphy with SPECT/CT in the Characterization and Early Diagnosis of Stage 0 Charcot Neuroarthropathy. J. Clin. Med. 2020, 9, 4123. [Google Scholar] [CrossRef] [PubMed]
- Rammelt, S. Management of Acute Hindfoot Fractures in Diabetics. 16 April 2016. Available online: https://link.springer.com/chapter/10.1007/978-3-319-27623-6_7 (accessed on 13 July 2022).
- Amadou, C.; Carlier, A.; Amouyal, C.; Bourron, O.; Aubert, C.; Couture, T.; Fourniols, E.; Ha Van, G.; Rouanet, S.; Hartemann, A. Five-year mortality in patients with diabetic foot ulcer during 2009–2010 was lower than expected. Diabetes Metab. 2020, 46, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Jeffcoate, W.J.; Game, F.; Cavanagh, P.R. The role of proinflammatory cytokines in the cause of neuropathic osteoarthropathy (acute Charcot foot) in diabetes. Lancet 2005, 366, 2058–2061. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Busse, B.; Eastell, R.; Ferrari, S.; Frost, M.; Müller, R.; Burden, A.M.; Rivadeneira, F.; Napoli, N.; Rauner, M. Bone fragility in diabetes: Novel concepts and clinical implications. Lancet Diabetes Endocrinol. 2022, 10, 207–220. [Google Scholar] [CrossRef]
- Rossi, F.; Tortora, C.; Paoletta, M.; Marrapodi, M.M.; Argenziano, M.; Di Paola, A.; Pota, E.; Di Pinto, D.; Di Martino, M.; Iolascon, G. Osteoporosis in Childhood Cancer Survivors: Physiopathology, Prevention, Therapy and Future Perspectives. Cancers 2022, 14, 4349. [Google Scholar] [CrossRef]
- Rossi, F.; Di Paola, A.; Pota, E.; Argenziano, M.; Di Pinto, D.; Marrapodi, M.M.; Di Leva, C.; Di Martino, M.; Tortora, C. Biological Aspects of Inflamm-Aging in Childhood Cancer Survivors. Cancers 2021, 13, 4933. [Google Scholar] [CrossRef]
- Jansen, R.B.; Christensen, T.M.; Bülow, J.; Rørdam, L.; Jørgensen, N.R.; Svendsen, O.L. Markers of Local Inflammation and Bone Resorption in the Acute Diabetic Charcot Foot. J. Diabetes Res. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef]
- Schultz, G.S.; Wysocki, A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009, 17, 153–162. [Google Scholar] [CrossRef]
- Hempel, U.; Preissler, C.; Vogel, S.; Möller, S.; Hintze, V.; Becher, J.; Schnabelrauch, M.; Rauner, M.; Hofbauer, L.C.; Dieter, P. Artificial extracellular matrices with oversulfated glycosaminoglycan derivatives promote the differentiation of osteoblast-precursor cells and premature osteoblasts. Biomed Res. Int. 2014, 2014, 938368. [Google Scholar] [CrossRef]
- Vogel, S.; Arnoldini, S.; Möller, S.; Schnabelrauch, M.; Hempel, U. Sulfated hyaluronan alters fibronectin matrix assembly and promotes osteogenic differentiation of human bone marrow stromal cells. Sci. Rep. 2016, 6, 36418. [Google Scholar] [CrossRef] [PubMed]
- Förster, Y.; Schulze, S.; Penk, A.; Neuber, C.; Möller, S.; Hintze, V.; Scharnweber, D.; Schnabelrauch, M.; Pietzsch, J.; Huster, D.; et al. The influence of different artificial extracellular matrix implant coatings on the regeneration of a critical size femur defect in rats. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 116, 111157. [Google Scholar] [CrossRef] [PubMed]
- Picke, A.-K.; Salbach-Hirsch, J.; Hintze, V.; Rother, S.; Rauner, M.; Kascholke, C.; Möller, S.; Bernhardt, R.; Rammelt, S.; Pisabarro, M.T.; et al. Sulfated hyaluronan improves bone regeneration of diabetic rats by binding sclerostin and enhancing osteoblast function. Biomaterials 2016, 96, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Salbach-Hirsch, J.; Kraemer, J.; Rauner, M.; Samsonov, S.A.; Pisabarro, M.T.; Moeller, S.; Schnabelrauch, M.; Scharnweber, D.; Hofbauer, L.C.; Hintze, V. The promotion of osteoclastogenesis by sulfated hyaluronan through interference with osteoprotegerin and receptor activator of NF-κB ligand/osteoprotegerin complex formation. Biomaterials 2013, 34, 7653–7661. [Google Scholar] [CrossRef] [PubMed]
- Shafieyan, Y.; Tiedemann, K.; Goulet, A.; Komarova, S.; Quinn, T.M. Monocyte proliferation and differentiation to osteoclasts is affected by density of collagen covalently bound to a poly(dimethyl siloxane) culture surface. J. Biomed. Mater. Res. 2012, 100, 1573–1581. [Google Scholar] [CrossRef]
- Pennypacker, B.L.; Chen, C.M.; Zheng, H.; Shih, M.-S.; Belfast, M.; Samadfam, R.; Duong, L.T. Inhibition of Cathepsin K Increases Modeling-Based Bone Formation, and Improves Cortical Dimension and Strength in Adult Ovariectomized Monkeys: Odanacatib stimulates cortical bone formation in ovariectomized monkeys. J. Bone Miner. Res. 2014, 29, 1847–1858. [Google Scholar] [CrossRef]
- Charles, J.F.; Aliprantis, A.O. Osteoclasts: More than ‘bone eaters’. Trends Mol. Med. 2014, 20, 449–459. [Google Scholar] [CrossRef]
- Tatara, Y.; Suto, S.; Itoh, K. Novel roles of glycosaminoglycans in the degradation of type I collagen by cathepsin K. Glycobiology 2017, 27, 1089–1098. [Google Scholar] [CrossRef]
- Mascarenhas, J.V.; Jude, E.B. The Charcot Foot as a Complication of Diabetic Neuropathy. Curr. Diabetes Rep. 2014, 14, 561. [Google Scholar] [CrossRef]
- Torregrossa, M.; Kakpenova, A.; Simon, J.C.; Franz, S. Modulation of macrophage functions by ECM-inspired wound dressings—A promising therapeutic approach for chronic wounds. Biol. Chem. 2021, 402, 1289–1307. [Google Scholar] [CrossRef] [PubMed]
- Devlin, R.D.; Reddy, S.V.; Savino, R.; Ciliberto, G.; Roodman, G.D. IL-6 mediates the effects of IL-1 or TNF, but not PTHrP or 1,25(OH)2D3, on osteoclast-like cell formation in normal human bone marrow cultures. J. Bone Miner. Res. 1998, 13, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Baumhauer, J.F.; O’Keefe, R.J.; Schon, L.C.; Pinzur, M.S. Cytokine-induced osteoclastic bone resorption in charcot arthropathy: An immunohistochemical study. Foot Ankle Int. 2006, 27, 797–800. [Google Scholar] [CrossRef]
- Koehler, L.; Samsonov, S.; Rother, S.; Vogel, S.; Köhling, S.; Moeller, S.; Schnabelrauch, M.; Rademann, J.; Hempel, U.; Pisabarro, M.T.; et al. Sulfated Hyaluronan Derivatives Modulate TGF-β1:Receptor Complex Formation: Possible Consequences for TGF-β1 Signaling. Sci. Rep. 2017, 7, 1210. [Google Scholar] [CrossRef]
- Liedtke, D.; Hofmann, C.; Jakob, F.; Klopocki, E.; Graser, S. Tissue-Nonspecific Alkaline Phosphatase-A Gatekeeper of Physiological Conditions in Health and a Modulator of Biological Environments in Disease. Biomolecules 2020, 10, 1648. [Google Scholar] [CrossRef] [PubMed]
- Starup-Linde, J.; Vestergaard, P. Biochemical bone turnover markers in diabetes mellitus—A systematic review. Bone 2016, 82, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Park, B.H.; Kim, H.K.; Park, T.S.; Baek, H.S. Hypoxia decreases Runx2/Cbfa1 expression in human osteoblast-like cells. Mol. Cell. Endocrinol. 2002, 192, 197–203. [Google Scholar] [CrossRef]
- Marin, C.; Luyten, F.P.; Van der Schueren, B.; Kerckhofs, G.; Vandamme, K. The Impact of Type 2 Diabetes on Bone Fracture Healing. Front. Endocrinol. 2018, 9, 6. [Google Scholar] [CrossRef]
- Hoff, P.; Gaber, T.; Schmidt-Bleek, K.; Sentürk, U.; Tran, C.L.; Blankenstein, K.; Lütkecosmann, S.; Bredahl, J.; Schüler, H.J.; Simon, P.; et al. Immunologically restricted patients exhibit a pronounced inflammation and inadequate response to hypoxia in fracture hematomas. Immunol. Res. 2011, 51, 116–122. [Google Scholar] [CrossRef]
- Villafán-Bernal, J.R.; Sánchez-Enríquez, S.; Muñoz-Valle, J.F. Molecular modulation of osteocalcin and its relevance in diabetes (Review). Int. J. Mol. Med. 2011, 28, 283–293. [Google Scholar]
- Patti, A.; Gennari, L.; Merlotti, D.; Dotta, F.; Nuti, R. Endocrine Actions of Osteocalcin. Int. J. Endocrinol. 2013, 2013, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gronbach, M.; Mitrach, F.; Lidzba, V.; Müller, B.; Möller, S.; Rother, S.; Salbach-Hirsch, J.; Hofbauer, L.C.; Schnabelrauch, M.; Hintze, V.; et al. Scavenging of Dickkopf-1 by macromer-based biomaterials covalently decorated with sulfated hyaluronan displays pro-osteogenic effects. Acta Biomater. 2020, 114, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Coombe, D.R. Biological implications of glycosaminoglycan interactions with haemopoietic cytokines. Immunol. Cell Biol. 2008, 86, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Kuschert, G.S.; Coulin, F.; Power, C.A.; Proudfoot, A.E.; Hubbard, R.E.; Hoogewerf, A.J.; Wells, T.N. Glycosaminoglycans interact selectively with chemokines and modulate receptor binding and cellular responses. Biochemistry 1999, 38, 12959–12968. [Google Scholar] [CrossRef] [PubMed]
- Etich, J.; Leßmeier, L.; Rehberg, M.; Sill, H.; Zaucke, F.; Netzer, C.; Semler, O. Osteogenesis imperfecta—Pathophysiology and therapeutic options. Mol. Cell. Pediatr. 2020, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Araki, Y.; Mimura, T. Matrix Metalloproteinase Gene Activation Resulting from Disordred Epigenetic Mechanisms in Rheumatoid Arthritis. Int. J. Mol. Sci. 2017, 18, 905. [Google Scholar] [CrossRef]
- Kunkemoeller, B.; Kyriakides, T.R. Redox Signaling in Diabetic Wound Healing Regulates Extracellular Matrix Deposition. Antioxid. Redox Signal. 2017, 27, 823–838. [Google Scholar] [CrossRef]
- Schulze, S.; Wehrum, D.; Dieter, P.; Hempel, U. A supplement-free osteoclast-osteoblast co-culture for pre-clinical application. J Cell. Physiol. 2018, 233, 4391–4400. [Google Scholar] [CrossRef]
- Kunze, R.; Rösler, M.; Möller, S.; Schnabelrauch, M.; Riemer, T.; Hempel, U.; Dieter, P. Sulfated hyaluronan derivatives reduce the proliferation rate of primary rat calvarial osteoblasts. Glycoconj. J. 2010, 27, 151–158. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]



| Group A (Arthritis) | Group C (Charcot) | |
|---|---|---|
| Number | 26 | 17 |
| Age (years) | 56.1 ± 2.7 | 62.5 ± 2.2 |
| Sex (women to men ratio) | 14/12 | 2/15 |
| C-reactive protein (mg/L) | 4.1 ± 0.98 | 8.45 ± 1.97 |
| Serum glucose (mmol/L) | 5.73 ± 0.49 | 9.54 ± 1.29 |
| HbA1c (%) | n.d. | 6.7 ± 0.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulze, S.; Neuber, C.; Möller, S.; Hempel, U.; Hofbauer, L.C.; Schaser, K.-D.; Pietzsch, J.; Rammelt, S. Impact of Sulfated Hyaluronan on Bone Metabolism in Diabetic Charcot Neuroarthropathy and Degenerative Arthritis. Int. J. Mol. Sci. 2022, 23, 15146. https://doi.org/10.3390/ijms232315146
Schulze S, Neuber C, Möller S, Hempel U, Hofbauer LC, Schaser K-D, Pietzsch J, Rammelt S. Impact of Sulfated Hyaluronan on Bone Metabolism in Diabetic Charcot Neuroarthropathy and Degenerative Arthritis. International Journal of Molecular Sciences. 2022; 23(23):15146. https://doi.org/10.3390/ijms232315146
Chicago/Turabian StyleSchulze, Sabine, Christin Neuber, Stephanie Möller, Ute Hempel, Lorenz C. Hofbauer, Klaus-Dieter Schaser, Jens Pietzsch, and Stefan Rammelt. 2022. "Impact of Sulfated Hyaluronan on Bone Metabolism in Diabetic Charcot Neuroarthropathy and Degenerative Arthritis" International Journal of Molecular Sciences 23, no. 23: 15146. https://doi.org/10.3390/ijms232315146
APA StyleSchulze, S., Neuber, C., Möller, S., Hempel, U., Hofbauer, L. C., Schaser, K.-D., Pietzsch, J., & Rammelt, S. (2022). Impact of Sulfated Hyaluronan on Bone Metabolism in Diabetic Charcot Neuroarthropathy and Degenerative Arthritis. International Journal of Molecular Sciences, 23(23), 15146. https://doi.org/10.3390/ijms232315146






