Antigen-Specific T Cells and SARS-CoV-2 Infection: Current Approaches and Future Possibilities
Abstract
1. Introduction
2. Mechanism of Cytokine Storm Development in COVID-19
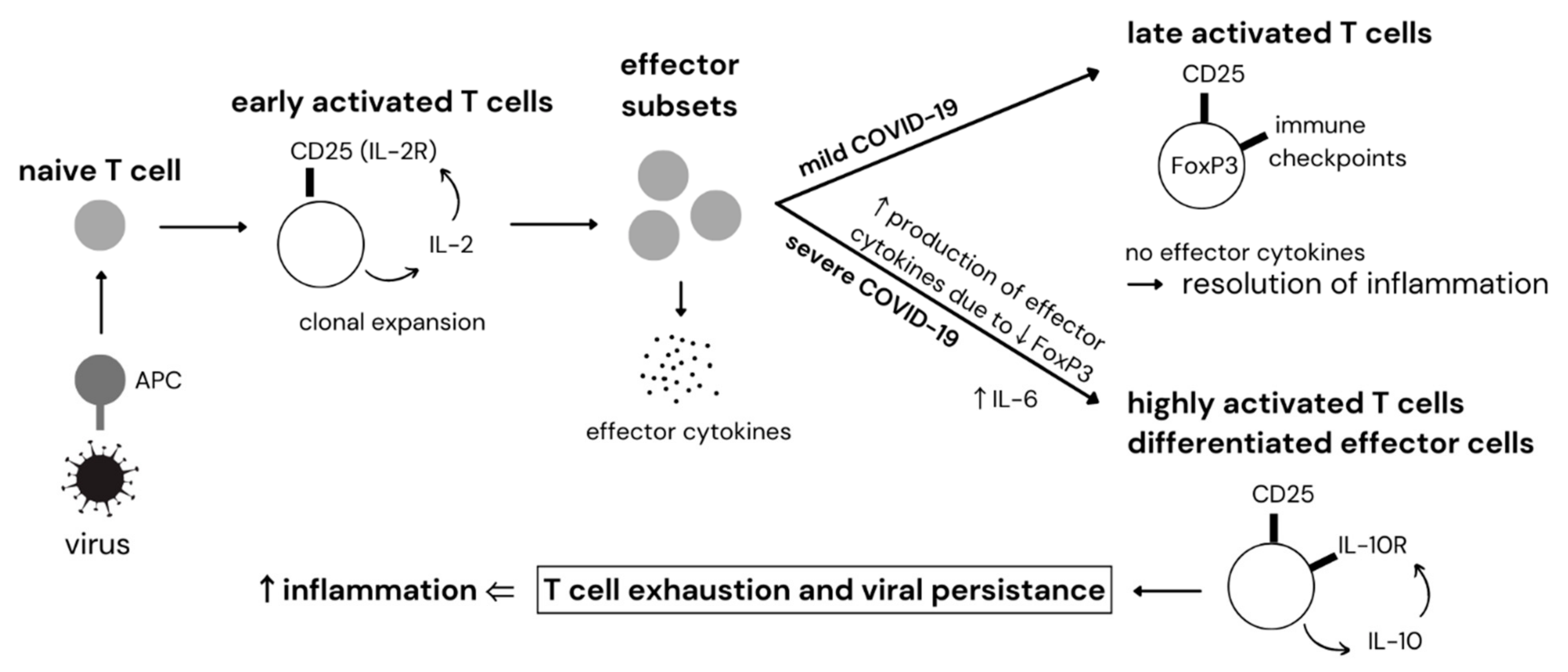
3. Dysregulation of T Cells in COVID-19
4. SARS-CoV-2-Specific T Cell Therapy for the Treatment and Prevention of Severe COVID-19 Infection
5. Large-Scale Production of SARS-CoV-2-Specific T-Cells
6. Other Potential Cell-Based Therapies for COVID-19
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADAM17 | A disintegrin and metalloproteinase 1 |
| AdV | adenovirus |
| Ang | angiotensin |
| APC | antigen-presenting cell |
| ARDS | acute respiratory distress syndrome |
| ATII | alveolar epithelial type II |
| AT1R | angiotensin II receptor type 1 |
| BALF | bronchoalveolar lavage fluid |
| BTLA | B- and T-lymphocyte attenuator |
| CAR | chimeric antigen receptor |
| CCS | cytokine capture system |
| CD25 | cluster of differentiation 25 |
| CD4 | cluster of differentiation 4 |
| CD8 | cluster of differentiation 8 |
| CMV | cytomegalovirus |
| COVID-19 | coronavirus disease 2019 |
| CST | SARS-CoV-2-specific T cell |
| CTLA-4 | cytotoxic T-lymphocyte–associated antigen 4 |
| dsRNA | double-stranded RNA |
| EBV | Epstein-Barr virus |
| EMA | European Medicines Agency |
| FDA | The United States Food and Drug Administration |
| FOXP3 | forkhead box-p3 |
| G-CSF | granulocyte colony-stimulating factor |
| GvHD | graft-versus-host disease |
| HLA | human leukocyte antigen |
| HSCT | hematopoietic stem cell transplantation |
| IFN | interferon |
| IL | interleukin |
| IL-2R | interleukin 2 receptor |
| IL-10R | interleukin 10 receptor |
| IRF | interferon regulatory factor |
| LAG-3 | lymphocyte-activation gene 3 |
| MAL | MyD88 adaptor-like protein |
| MasR | Mas receptor |
| MERS-CoV | Middle East respiratory syndrome coronavirus |
| MHC | major histocompatibility complex |
| MIS-C | multisystem inflammatory syndrome in children |
| MSC | mesenchymal stem cell |
| MyD88 | myeloid differentiation primary response protein 88 |
| N protein | nucleocapsid protein |
| NF-κB | nuclear factor kappa B |
| NK cell | natural killer cell |
| NKG2A | CD94/NK group 2 member A |
| ORF | open reading frame |
| PBMCs | peripheral blood mononuclear cells |
| PD1 | programmed cell death 1 receptor |
| RAS | renin–angiotensin system |
| RNA | ribonucleic acid |
| S protein | spike protein |
| SARS-CoV | severe acute respiratory syndrome coronavirus |
| SARS-CoV-1 | severe acute respiratory syndrome coronavirus 1 |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| scFv | single chain variable fragment |
| ssRNA | single-stranded ribonucleic acid |
| TCR | T cell receptor |
| TGF-β | transforming growth factor beta |
| Tim-3 | T cell immunoglobulin and mucin-domain containing-3 |
| TIR | Toll/interleukin receptor |
| TLR | Toll-like receptor |
| TMPRSS2 | transmembrane serine protease 2 |
| TNF-α | tumor necrosis factor α |
| TRAM | TRIF-related adaptor molecule |
| Treg | regulatory T cell |
| TRIF | TIR domain-containing adaptor-inducing interferon-β |
| VST | virus-specific T cell |
References
- Diao, B.; Wang, C.; Tan, Y.; Chen, X.; Liu, Y.; Ning, L.; Chen, L.; Li, M.; Liu, Y.; Wang, G.; et al. Reduction and Functional Exhaustion of T Cells in Patients with Coronavirus Disease 2019 (COVID-19). Front. Immunol. 2020, 11, 827. [Google Scholar] [CrossRef] [PubMed]
- Toor, S.M.; Saleh, R.; Nair, V.S.; Taha, R.Z.; Elkord, E. T-cell responses and therapies against SARS-CoV-2 infection. Immunology 2020, 162, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Manik, M.; Singh, R.K. Role of toll-like receptors in modulation of cytokine storm signaling in SARS-CoV-2-induced COVID-19. J. Med. Virol. 2021, 94, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Yin, M.; Chen, X.; Zeng, F. Clinical determinants for fatality of 44,672 patients with COVID-19. Crit. Care 2020, 24, 179. [Google Scholar] [CrossRef]
- Kuderer, N.M.; Choueiri, T.K.; Shah, D.P.; Shyr, Y.; Rubinstein, S.M.; Rivera, D.R.; Shete, S.; Hsu, C.-Y.; Desai, A.; de Lima Lopes, G., Jr.; et al. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet 2020, 395, 1907–1918. [Google Scholar] [CrossRef]
- Dai, M.; Liu, D.; Liu, M.; Zhou, F.; Li, G.; Chen, Z.; Zhang, Z.; You, H.; Wu, M.; Zheng, Q.; et al. Patients with cancer appear more vulnerable to SARS-COV-2: A multicenter study during the COVID-19 outbreak. Cancer Discov. 2020, 10, 783–791. [Google Scholar] [CrossRef]
- Sharma, A.; Bhatt, N.S.; Martin, A.S.; Abid, M.B.; Bloomquist, J.; Chemaly, R.F.; Dandoy, C.; Gauthier, J.; Gowda, L.; Perales, M.-A.; et al. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: An observational cohort study. Lancet Haematol. 2021, 8, e185–e193. [Google Scholar] [CrossRef]
- Remy, K.E.; Mazer, M.; Striker, D.A.; Ellebedy, A.H.; Walton, A.H.; Unsinger, J.; Blood, T.M.; Mudd, P.A.; Yi, D.J.; Mannion, D.A.; et al. Severe immunosuppression and not a cytokine storm characterizes COVID-19 infections. J. Clin. Investig. 2020, 5, e140329. [Google Scholar] [CrossRef]
- DiPiazza, A.T.; Graham, B.S.; Ruckwardt, T.J. T cell immunity to SARS-CoV-2 following natural infection and vaccination. Biochem. Biophys. Res. Commun. 2020, 538, 211–217. [Google Scholar] [CrossRef]
- Bollard, C.M.; Heslop, H.E. T cells for viral infections after allogeneic hematopoietic stem cell transplant. Blood 2016, 127, 3331–3340. [Google Scholar] [CrossRef]
- McLaughlin, L.P.; Bollard, C.M.; Keller, M.D. Adoptive T Cell Therapy for Epstein–Barr Virus Complications in Patients With Primary Immunodeficiency Disorders. Front. Immunol. 2018, 9, 556. [Google Scholar] [CrossRef] [PubMed]
- Muftuoglu, M.; Olson, A.; Marin, D.; Ahmed, S.; Mulanovich, V.; Tummala, S.; Chi, T.L.; Ferrajoli, A.; Kaur, I.; Li, L.; et al. Allogeneic BK Virus–Specific T Cells for Progressive Multifocal Leukoencephalopathy. N. Engl. J. Med. 2018, 379, 1443–1451. [Google Scholar] [CrossRef] [PubMed]
- Tzannou, I.; Papadopoulou, A.; Naik, S.; Leung, K.; Martinez, C.A.; Ramos, C.A.; Carrum, G.; Sasa, G.; Lulla, P.; Watanabe, A.; et al. Off-the-Shelf Virus-Specific T Cells to Treat BK Virus, Human Herpesvirus 6, Cytomegalovirus, Epstein-Barr Virus, and Adenovirus Infections After Allogeneic Hematopoietic Stem-Cell Transplantation. J. Clin. Oncol. 2017, 35, 3547–3557. [Google Scholar] [CrossRef] [PubMed]
- Leen, A.M.; Christin, A.; Myers, G.D.; Liu, H.; Cruz, C.R.; Hanley, P.J.; Kennedy-Nasser, A.A.; Leung, K.S.; Gee, A.P.; Krance, R.A.; et al. Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein-Barr virus infections after haploidentical and matched unrelated stem cell transplantation. Blood 2009, 114, 4283–4292. [Google Scholar] [CrossRef] [PubMed]
- Gerdemann, U.; Keirnan, J.M.; Katari, U.L.; Yanagisawa, R.; Christin, A.S.; Huye, L.E.; Perna, S.K.; Ennamuri, S.; Gottschalk, S.; Brenner, M.K.; et al. Rapidly Generated Multivirus-specific Cytotoxic T Lymphocytes for the Prophylaxis and Treatment of Viral Infections. Mol. Ther. 2012, 20, 1622–1632. [Google Scholar] [CrossRef]
- Gerdemann, U.; Katari, U.L.; Papadopoulou, A.; Keirnan, J.M.; Craddock, J.A.; Liu, H.; Martinez, C.A.; Kennedy-Nasser, A.; Leung, K.S.; Gottschalk, S.M.; et al. Safety and clinical efficacy of rapidly-generated trivirus-directed T cells as treatment for adenovirus, EBV, and CMV infections after allogeneic hematopoietic stem cell transplant. Mol. Ther. 2013, 21, 2113–2121. [Google Scholar] [CrossRef]
- Blyth, E.; Clancy, L.; Simms, R.; Ma, C.K.K.; Burgess, J.; Deo, S.; Byth, K.; Dubosq, M.-C.; Shaw, P.J.; Micklethwaite, K.P.; et al. Donor-derived CMV-specific T cells reduce the requirement for CMV-directed pharmacotherapy after allogeneic stem cell transplantation. Blood 2013, 121, 3745–3758. [Google Scholar] [CrossRef]
- Papadopoulou, A.; Gerdemann, U.; Katari, U.L.; Tzannou, I.; Liu, H.; Martinez, C.; Leung, K.; Carrum, G.; Gee, A.P.; Vera, J.F.; et al. Activity of Broad-Spectrum T Cells as Treatment for AdV, EBV, CMV, BKV, and HHV6 Infections after HSCT. Sci. Transl. Med. 2014, 6, 242ra83. [Google Scholar] [CrossRef]
- Heslop, H.E.; Slobod, K.S.; Pule, M.A.; Hale, G.A.; Rousseau, A.; Smith, C.A.; Bollard, C.M.; Liu, H.; Wu, M.-F.; Rochester, R.J.; et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood 2010, 115, 925–935. [Google Scholar] [CrossRef]
- Keller, M.D.; Darko, S.; Lang, H.; Ransier, A.; Lazarski, C.A.; Wang, Y.; Hanley, P.J.; Davila, B.J.; Heimall, J.R.; Ambinder, R.F.; et al. T-cell receptor sequencing demonstrates persistence of virus-specific T cells after antiviral immunotherapy. Br. J. Haematol. 2019, 187, 206–218. [Google Scholar] [CrossRef]
- Le Bert, N.; Tan, A.T.; Kunasegaran, K.; Tham, C.Y.L.; Hafezi, M.; Chia, A.; Chng, M.H.Y.; Lin, M.; Tan, N.; Linster, M.; et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 2020, 584, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Pujadas, E.; Chaudhry, F.; McBride, R.; Richter, F.; Zhao, S.; Wajnberg, A.; Nadkarni, G.; Glicksberg, B.S.; Houldsworth, J.; Cordon-Cardo, C. SARS-CoV-2 viral load predicts COVID-19 mortality. Lancet Respir. Med. 2020, 8, e70. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, L.; Mescia, F.; Turner, L.; Hanson, A.L.; Kotagiri, P.; Dunmore, B.J.; Ruffieux, H.; De Sa, A.; Huhn, O.; Morgan, M.D.; et al. Longitudinal analysis reveals that delayed bystander CD8+ T cell activation and early immune pathology distinguish severe COVID-19 from mild disease. Immunity 2021, 54, 1257–1275.e8. [Google Scholar] [CrossRef] [PubMed]
- Hadjadj, J.; Yatim, N.; Barnabei, L.; Corneau, A.; Boussier, J.; Smith, N.; Péré, H.; Charbit, B.; Bondet, V.; Chenevier-Gobeaux, C.; et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 2020, 369, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.-C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID19. Cell 2020, 181, 1036–1045.e9. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.J.B.; June, C.H. Cytokine release syndrome in severe COVID19. Science 2020, 368, 473–474. [Google Scholar] [CrossRef] [PubMed]
- Humar, A.; St Louis, P.; Mazzulli, T.; McGeer, A.; Lipton, J.; Messner, H.; Macdonald, K.S. Elevated Serum Cytokines Are Associated with Cytomegalovirus Infection and Disease in Bone Marrow Transplant Recipients. J. Infect. Dis. 1999, 179, 484–488. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Brito-Zerón, P.; López-Guillermo, A.; Khamashta, M.A.; Bosch, X. Adult haemophagocytic syndrome. Lancet 2014, 383, 1503–1516. [Google Scholar] [CrossRef]
- Basar, R.; Uprety, N.; Ensley, E.; Daher, M.; Klein, K.; Martinez, F.; Aung, F.; Shanley, M.; Hu, B.; Gokdemir, E.; et al. Generation of glucocorticoid-resistant SARS-CoV-2 T cells for adoptive cell therapy. Cell Rep. 2021, 36, 109432. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Recognition of viruses by innate immunity. Immunol. Rev. 2007, 220, 214–224. [Google Scholar] [CrossRef]
- Gibson, P.G.; Qin, L.; Puah, S.H. COVID-19 acute respiratory distress syndrome (ARDS): Clinical features and differences from typical pre-COVID-19 ARDS. Med. J. Aust. 2020, 213, 54–56.e1. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.D.; Sumeh, A.S.; Sheraz, M.; Kavitha, M.S.; Maran, B.A.V.; Rodrigues, K.F. A mini-review on the impact of COVID 19 on vital organs. Biomed. Pharmacother. 2021, 143, 112158. [Google Scholar] [CrossRef] [PubMed]
- Pierce, C.A.; Preston-Hurlburt, P.; Dai, Y.; Aschner, C.B.; Cheshenko, N.; Galen, B.; Garforth, S.J.; Herrera, N.G.; Jangra, R.K.; Morano, N.C.; et al. Immune responses to SARS-CoV-2 infection in hospitalized pediatric and adult patients. Sci. Transl. Med. 2020, 12, eabd5487. [Google Scholar] [CrossRef] [PubMed]
- Cohen, C.A.; Li, A.P.Y.; Hachim, A.; Hui, D.S.C.; Kwan, M.Y.W.; Tsang, O.T.Y.; Chiu, S.S.; Chan, W.H.; Yau, Y.S.; Kavian, N.; et al. SARS-CoV-2 specific T cell responses are lower in children and increase with age and time after infection. Nat. Commun. 2021, 12, 4678. [Google Scholar] [CrossRef] [PubMed]
- Chou, J.; Thomas, P.G.; Randolph, A.G. Immunology of SARS-CoV-2 infection in children. Nat. Immunol. 2022, 23, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Nikolopoulou, G.B.; Maltezou, H.C. COVID-19 in Children: Where do we Stand? Arch. Med. Res. 2021, 53, 1–8. [Google Scholar] [CrossRef]
- Onofrio, L.; Caraglia, M.; Facchini, G.; Margherita, V.; De Placido, S.; Buonerba, C. Toll-like receptors and COVID-19: A two-faced story with an exciting ending. Future Sci. OA 2020, 6, FSO605. [Google Scholar] [CrossRef]
- Khanmohammadi, S.; Rezaei, N. Role of Toll-like receptors in the pathogenesis of COVID-19. J. Med. Virol. 2021, 93, 2735–2739. [Google Scholar] [CrossRef]
- Choudhury, A.; Mukherjee, S. In silico studies on the comparative characterization of the interactions of SARS-CoV-2 spike glycoprotein with ACE-2 receptor homologs and human TLRs. J. Med. Virol. 2020, 92, 2105–2113. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Sharma, A.R.; Mallick, B.; Sharma, G.; Lee, S.-S.; Chakraborty, C. Immunoinformatics approach to understand molecular interaction between multi-epitopic regions of SARS-CoV-2 spike-protein with TLR4/MD-2 complex. Infect. Genet. Evol. 2020, 85, 104587. [Google Scholar] [CrossRef]
- Yu, L.; Wang, L.; Chen, S. Endogenous toll-like receptor ligands and their biological significance. J. Cell Mol. Med. 2010, 14, 2592–2603. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.; Rowe, D.C.; Barnes, B.J.; Caffrey, D.R.; Visintin, A.; Latz, E.; Monks, B.; Pitha, P.M.; Golenbock, D.T. LPS-TLR4 Signaling to IRF-3/7 and NF-κB Involves the Toll Adapters TRAM and TRIF. J. Exp. Med. 2003, 198, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A.J.; Bowie, A.G. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 2007, 7, 353–364. [Google Scholar] [CrossRef]
- Ribero, M.S.; Jouvenet, N.; Dreux, M.; Nisole, S. Interplay between SARS-CoV-2 and the type I interferon response. PLoS Pathog. 2020, 16, e1008737. [Google Scholar] [CrossRef]
- Yang, L.; Xie, X.; Tu, Z.; Fu, J.; Xu, D.; Zhou, Y. The signal pathways and treatment of cytokine storm in COVID-19. Signal Transduct. Target. Ther. 2021, 6, 255. [Google Scholar] [CrossRef]
- De Wit, E.; Van Doremalen, N.; Falzarano, D.; Munster, V.J. SARS and MERS: Recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016, 14, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Peiris, J.; Chu, C.; Cheng, V.; Chan, K.; Hung, I.; Poon, L.; Law, K.; Tang, B.; Hon, T.; Chan, C.; et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: A prospective study. Lancet 2003, 361, 1767–1772. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.; Wu, A.; To, K.F.; Lee, N.; Lam, C.W.K.; Wong, C.K.; Chan, P.; Ng, M.H.L.; Yu, L.M.; Hui, D.; et al. Haematological manifestations in patients with severe acute respiratory syndrome: Retrospective analysis. BMJ 2003, 326, 1358–1362. [Google Scholar] [CrossRef]
- Galani, I.-E.; Rovina, N.; Lampropoulou, V.; Triantafyllia, V.; Manioudaki, M.; Pavlos, E.; Koukaki, E.; Fragkou, P.C.; Panou, V.; Rapti, V.; et al. Untuned antiviral immunity in COVID-19 revealed by temporal type I/III interferon patterns and flu comparison. Nat. Immunol. 2020, 22, 32–40. [Google Scholar] [CrossRef]
- Channappanavar, R.; Perlman, S. Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017, 39, 529–539. [Google Scholar] [CrossRef]
- Aboudounya, M.M.; Heads, R.J. COVID-19 and Toll-Like Receptor 4 (TLR4): SARS-CoV-2 May Bind and Activate TLR4 to Increase ACE2 Expression, Facilitating Entry and Causing Hyperinflammation. Mediat. Inflamm. 2021, 2021, 8874339. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e278. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, M.E.; Thurman, A.; Pezzulo, A.A.; Leidinger, M.R.; Klesney-Tait, J.A.; Karp, P.H.; Tan, P.; Wohlford-Lenane, C.; McCray, P.B.; Meyerholz, D.K. Heterogeneous expression of the SARS-Coronavirus-2 receptor ACE2 in the human respiratory tract. eBioMedicine 2020, 60, 102976. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Chen, K.; Zou, J.; Han, P.; Hao, J.; Han, Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020, 14, 185–192. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2021, 23, 3–20. [Google Scholar] [CrossRef]
- Ziegler, C.G.K.; Allon, S.J.; Nyquist, S.K.; Mbano, I.M.; Miao, V.N.; Tzouanas, C.N.; Cao, Y.; Yousif, A.S.; Bals, J.; Hauser, B.M.; et al. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell 2020, 181, 1016–1035.e19. [Google Scholar] [CrossRef]
- Zhou, L.; Niu, Z.; Jiang, X.; Zhang, Z.; Zheng, Y.; Wang, Z.; Zhu, Y.; Gao, L.; Huang, H.; Wang, X.; et al. SARS-CoV-2 Targets by the pscRNA Profiling of ACE2, TMPRSS2 and Furin Proteases. iScience 2020, 23, 101744. [Google Scholar] [CrossRef]
- Bertram, S.; Heurich, A.; Lavender, H.; Gierer, S.; Danisch, S.; Perin, P.; Lucas, J.M.; Nelson, P.S.; Pöhlmann, S.; Soilleux, E.J. Influenza and SARS-Coronavirus Activating Proteases TMPRSS2 and HAT Are Expressed at Multiple Sites in Human Respiratory and Gastrointestinal Tracts. PLoS ONE 2012, 7, e35876. [Google Scholar] [CrossRef]
- Bassi, D.E.; Zhang, J.; Renner, C.; Klein-Szanto, A.J. Targeting proprotein convertases in furin-rich lung cancer cells results in decreased in vitro and in vivo growth. Mol. Carcinog. 2016, 56, 1182–1188. [Google Scholar] [CrossRef]
- Coutard, B.; Valle, C.; de Lamballerie, X.; Canard, B.; Seidah, N.G.; Decroly, E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antivir. Res. 2020, 176, 104742. [Google Scholar] [CrossRef]
- Imai, Y.; Kuba, K.; Rao, S.; Huan, Y.; Guo, F.; Guan, B.; Yang, P.; Sarao, R.; Wada, T.; Leong-Poi, H.; et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 2005, 436, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Samavati, L.; Uhal, B.D. ACE2, Much More Than Just a Receptor for SARS-COV-2. Front. Cell Infect. Microbiol. 2020, 10, 317. [Google Scholar] [CrossRef]
- Zipeto, D.; Palmeira, J.D.F.; Argañaraz, G.A.; Argañaraz, E.R. ACE2/ADAM17/TMPRSS2 Interplay May Be the Main Risk Factor for COVID-19. Front. Immunol. 2020, 11, 576745. [Google Scholar] [CrossRef]
- Gheware, A.; Ray, A.; Rana, D.; Bajpai, P.; Nambirajan, A.; Arulselvi, S.; Mathur, P.; Trikha, A.; Arava, S.; Das, P.; et al. ACE2 protein expression in lung tissues of severe COVID-19 infection. Sci. Rep. 2022, 12, 4058. [Google Scholar] [CrossRef]
- Forrester, S.J.; Booz, G.W.; Sigmund, C.D.; Coffman, T.M.; Kawai, T.; Rizzo, V.; Scalia, R.; Eguchi, S. Angiotensin II Signal Transduction: An Update on Mechanisms of Physiology and Pathophysiology. Physiol. Rev. 2018, 98, 1627–1738. [Google Scholar] [CrossRef]
- Iwasaki, M.; Saito, J.; Zhao, H.; Sakamoto, A.; Hirota, K.; Ma, D. Inflammation Triggered by SARS-CoV-2 and ACE2 Augment Drives Multiple Organ Failure of Severe COVID-19: Molecular Mechanisms and Implications. Inflammation 2020, 44, 13–34. [Google Scholar] [CrossRef]
- Ji, Y.; Liu, J.; Wang, Z.; Liu, N. Angiotensin II Induces Inflammatory Response Partly Via Toll-Like Receptor 4-Dependent Signaling Pathway in Vascular Smooth Muscle Cells. Cell Physiol. Biochem. 2009, 23, 265–276. [Google Scholar] [CrossRef]
- Sharif-Askari, N.S.; Sharif-Askari, F.S.; Alabed, M.; Temsah, M.-H.; Al Heialy, S.; Hamid, Q.; Halwani, R. Airways Expression of SARS-CoV-2 Receptor, ACE2, and TMPRSS2 Is Lower in Children Than Adults and Increases with Smoking and COPD. Mol. Ther.—Methods Clin. Dev. 2020, 18, 1–6. [Google Scholar] [CrossRef]
- Bunyavanich, S.; Do, A.; Vicencio, A. Nasal Gene Expression of Angiotensin-Converting Enzyme 2 in Children and Adults. JAMA 2020, 323, 2427–2429. [Google Scholar] [CrossRef]
- Muus, C.; Luecken, M.; Eraslan, G.; Waghray, A.; Heimberg, G.; Sikkema, L.; Kobayashi, Y.; Vaishnav, E.D.; Subramanian, A.; Smilie, C.; et al. Integrated analyses of single-cell atlases reveal age, gender, and smoking status associations with cell type-specific expression of mediators of SARS-CoV-2 viral entry and highlights inflammatory programs in putative target cells. BioRxiv 2020. [Google Scholar] [CrossRef]
- Wang, A.; Chiou, J.; Poirion, O.B.; Buchanan, J.; Valdez, M.J.; Verheyden, J.M.; Hou, X.; Kudtarkar, P.; Narendra, S.; Newsome, J.M.; et al. Single-cell multiomic profiling of human lungs reveals cell-type-specific and age-dynamic control of SARS-CoV2 host genes. eLife 2020, 9, e62522. [Google Scholar] [CrossRef] [PubMed]
- Schuler, B.A.; Habermann, A.C.; Plosa, E.J.; Taylor, C.J.; Jetter, C.; Negretti, N.M.; Kapp, M.E.; Benjamin, J.T.; Gulleman, P.; Nichols, D.S.; et al. Age-determined expression of priming protease TMPRSS2 and localization of SARS-CoV-2 in lung epithelium. J. Clin. Investig. 2021, 131, e140766. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501.e1415. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Liang, W.; Pang, J.; Xu, G.; Chen, Y.; Guo, X.; Wang, X.; Zhao, Y.; Lai, Y.; Liu, Y.; et al. Dynamics of TCR repertoire and T cell function in COVID-19 convalescent individuals. Cell Discov. 2021, 7, 89. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Al-Haidari, A.; Sun, J.; Kazi, J.U. T cell receptor (TCR) signaling in health and disease. Signal Transduct. Target. Ther. 2021, 6, 412. [Google Scholar] [CrossRef]
- Shatrova, A.N.; Mityushova, E.V.; Vassilieva, I.O.; Aksenov, N.D.; Zenin, V.V.; Nikolsky, N.N.; Marakhova, I.I. Time-Dependent Regulation of IL-2R α-Chain (CD25) Expression by TCR Signal Strength and IL-2-Induced STAT5 Signaling in Activated Human Blood T Lymphocytes. PLoS ONE 2016, 11, e0167215. [Google Scholar] [CrossRef]
- Yang, X.; Dai, T.; Zhou, X.; Qian, H.; Guo, R.; Lei, L.; Zhang, X.; Zhang, D.; Shi, L.; Cheng, Y.; et al. Naturally activated adaptive immunity in COVID-19 patients. J. Cell Mol. Med. 2020, 24, 12457–12463. [Google Scholar] [CrossRef]
- Sojka, D.K.; Bruniquel, D.; Schwartz, R.H.; Singh, N.J. IL-2 Secretion by CD4+ T Cells In Vivo Is Rapid, Transient, and Influenced by TCR-Specific Competition. J. Immunol. 2004, 172, 6136–6143. [Google Scholar] [CrossRef]
- Kalfaoglu, B.; Almeida-Santos, J.; Tye, C.A.; Satou, Y.; Ono, M. T-Cell Hyperactivation and Paralysis in Severe COVID-19 Infection Revealed by Single-Cell Analysis. Front. Immunol. 2020, 11, 589380. [Google Scholar] [CrossRef]
- Wu, Y.; Borde, M.; Heissmeyer, V.; Feuerer, M.; Lapan, A.D.; Stroud, J.; Bates, D.L.; Guo, L.; Han, A.; Ziegler, S.F.; et al. FOXP3 Controls Regulatory T Cell Function through Cooperation with NFAT. Cell 2006, 126, 375–387. [Google Scholar] [CrossRef]
- Schmidt, A.; Oberle, N.; Krammer, P.H. Molecular Mechanisms of Treg-Mediated T Cell Suppression. Front. Immunol. 2012, 3, 51. [Google Scholar] [CrossRef]
- Ono, M. Control of regulatory T-cell differentiation and function by T-cell receptor signalling and Foxp3 transcription factor complexes. Immunology 2020, 160, 24–37. [Google Scholar] [CrossRef]
- Hashimoto, M.; Kamphorst, A.O.; Im, S.J.; Kissick, H.T.; Pillai, R.N.; Ramalingam, S.S.; Araki, K.; Ahmed, R. CD8 T Cell Exhaustion in Chronic Infection and Cancer: Opportunities for Interventions. Annu. Rev. Med. 2018, 69, 301–318. [Google Scholar] [CrossRef]
- Gao, Y.; Tang, J.; Chen, W.; Li, Q.; Nie, J.; Lin, F.; Wu, Q.; Chen, Z.; Gao, Z.; Fan, H.; et al. Inflammation negatively regulates FOXP3 and regulatory T-cell function via DBC1. Proc. Natl. Acad. Sci. USA 2015, 112, E3246–E3254. [Google Scholar] [CrossRef]
- Meckiff, B.J.; Ramírez-Suástegui, C.; Fajardo, V.; Chee, S.J.; Kusnadi, A.; Simon, H.; Eschweiler, S.; Grifoni, A.; Pelosi, E.; Weiskopf, D.; et al. Imbalance of Regulatory and Cytotoxic SARS-CoV-2-Reactive CD4+ T Cells in COVID-19. Cell 2020, 183, 1340–1353.e16. [Google Scholar] [CrossRef]
- Ortutay, Z.; Oksanen, A.; Aittomäki, S.; Ortutay, C.; Pesu, M. Proprotein convertase FURIN regulates T cell receptor-induced transactivation. J. Leukoc. Biol. 2015, 98, 73–83. [Google Scholar] [CrossRef]
- Rajendiran, A.; Tenbrock, K. Regulatory T cell function in autoimmune disease. J. Transl. Autoimmun. 2021, 4, 100130. [Google Scholar] [CrossRef]
- Oft, M. Immune regulation and cytotoxic T cell activation of IL-10 agonists—Preclinical and clinical experience. Semin. Immunol. 2019, 44, 101325. [Google Scholar] [CrossRef]
- Smith, L.K.; Boukhaled, G.M.; Condotta, S.A.; Mazouz, S.; Guthmiller, J.J.; Vijay, R.; Butler, N.S.; Bruneau, J.; Shoukry, N.H.; Krawczyk, C.M.; et al. Interleukin-10 Directly Inhibits CD8+ T Cell Function by Enhancing N-Glycan Branching to Decrease Antigen Sensitivity. Immunity 2018, 48, 299–312.e5. [Google Scholar] [CrossRef]
- Ejrnaes, M.; Filippi, C.M.; Martinic, M.; Ling, E.M.; Togher, L.M.; Crotty, S.; Von Herrath, M.G. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J. Exp. Med. 2006, 203, 2461–2472. [Google Scholar] [CrossRef]
- Blackburn, S.D.; Wherry, E.J. IL-10, T cell exhaustion and viral persistence. Trends Microbiol. 2007, 15, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Erickson, J.J.; Lu, P.; Wen, S.; Hastings, A.K.; Gilchuk, P.; Joyce, S.; Shyr, Y.; Williams, J.V. Acute Viral Respiratory Infection Rapidly Induces a CD8+ T Cell Exhaustion–like Phenotype. J. Immunol. 2015, 195, 4319–4330. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhang, H.; Dauphars, D.J.; He, Y.-W. A Potential Role of Interleukin 10 in COVID-19 Pathogenesis. Trends Immunol. 2020, 42, 3–5. [Google Scholar] [CrossRef]
- De Biasi, S.; Meschiari, M.; Gibellini, L.; Bellinazzi, C.; Borella, R.; Fidanza, L.; Gozzi, L.; Iannone, A.; Tartaro, D.L.; Mattioli, M.; et al. Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat. Commun. 2020, 11, 3434. [Google Scholar] [CrossRef]
- Laing, A.G.; Lorenc, A.; del Barrio, I.D.M.; Das, A.; Fish, M.; Monin, L.; Muñoz-Ruiz, M.; McKenzie, D.R.; Hayday, T.S.; Francos-Quijorna, I.; et al. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat. Med. 2020, 26, 1623–1635. [Google Scholar] [CrossRef] [PubMed]
- Schub, D.; Klemis, V.; Schneitler, S.; Mihm, J.; Lepper, P.M.; Wilkens, H.; Bals, R.; Eichler, H.; Gärtner, B.C.; Becker, S.L.; et al. High levels of SARS-CoV-2–specific T cells with restricted functionality in severe courses of COVID-19. J. Clin. Investig. 2020, 5, e142167. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fu, B.; Zheng, X.; Wang, D.; Zhao, C.; Qi, Y.; Sun, R.; Tian, Z.; Xu, X.; Wei, H. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl. Sci. Rev. 2020, 7, 998–1002. [Google Scholar] [CrossRef]
- Schultheiß, C.; Paschold, L.; Simnica, D.; Mohme, M.; Willscher, E.; von Wenserski, L.; Scholz, R.; Wieters, I.; Dahlke, C.; Tolosa, E.; et al. Next-Generation Sequencing of T and B Cell Receptor Repertoires from COVID-19 Patients Showed Signatures Associated with Severity of Disease. Immunity 2020, 53, 442–455.e4. [Google Scholar] [CrossRef]
- Zheng, M.; Gao, Y.; Wang, G.; Song, G.; Liu, S.; Sun, D.; Xu, Y.; Tian, Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol. Immunol. 2020, 17, 533–535. [Google Scholar] [CrossRef]
- Wherry, E.J.; Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015, 15, 486–499. [Google Scholar] [CrossRef]
- Angelosanto, J.M.; Blackburn, S.D.; Crawford, A.; Wherry, E.J. Progressive Loss of Memory T Cell Potential and Commitment to Exhaustion during Chronic Viral Infection. J. Virol. 2012, 86, 8161–8170. [Google Scholar] [CrossRef] [PubMed]
- Brooks, D.G.; McGavern, D.; Oldstone, M.B. Reprogramming of antiviral T cells prevents inactivation and restores T cell activity during persistent viral infection. J. Clin. Investig. 2006, 116, 1675–1685. [Google Scholar] [CrossRef] [PubMed]
- Utzschneider, D.T.; Charmoy, M.; Chennupati, V.; Pousse, L.; Ferreira, D.P.; Calderon-Copete, S.; Danilo, M.; Alfei, F.; Hofmann, M.; Wieland, D.; et al. T Cell Factor 1-Expressing Memory-like CD8+ T Cells Sustain the Immune Response to Chronic Viral Infections. Immunity 2016, 45, 415–427. [Google Scholar] [CrossRef]
- Zheng, H.-Y.; Zhang, M.; Yang, C.-X.; Zhang, N.; Wang, X.-C.; Yang, X.-P.; Dong, X.-Q.; Zheng, Y.-T. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell Mol. Immunol. 2020, 17, 541–543. [Google Scholar] [CrossRef]
- Welch, J.L.; Xiang, J.; Chang, Q.; Houtman, J.C.D.; Stapleton, J.T. T-Cell Expression of Angiotensin-Converting Enzyme 2 and Binding of Severe Acute Respiratory Coronavirus 2. J. Infect. Dis. 2021, 225, 810–819. [Google Scholar] [CrossRef]
- Jeannet, R.; Daix, T.; Formento, R.; Feuillard, J.; François, B. Severe COVID-19 is associated with deep and sustained multifaceted cellular immunosuppression. Intensiv. Care Med. 2020, 46, 1769–1771. [Google Scholar] [CrossRef]
- Lucas, C.; Wong, P.; Klein, J.; Castro, T.B.R.; Silva, J.; Sundaram, M.; Ellingson, M.K.; Mao, T.; Oh, J.E.; Israelow, B.; et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 2020, 584, 463–469. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, L.; Du, H.; Zhang, J.; Li, Y.Y.; Qu, J.; Zhang, W.; Wang, Y.; Bao, S.; Li, Y.; et al. SARS-CoV-2 Infection in Children. N. Engl. J. Med. 2020, 382, 1663–1665. [Google Scholar] [CrossRef]
- Bai, K.; Liu, W.; Liu, C.; Fu, Y.; Hu, J.; Qin, Y.; Zhang, Q.; Chen, H.; Xu, F.; Li, C. Clinical Analysis of 25 COVID-19 Infections in Children. Pediatr. Infect. Dis. J. 2020, 39, e100–e103. [Google Scholar] [CrossRef]
- Pierce, C.A.; Sy, S.; Galen, B.; Goldstein, D.Y.; Orner, E.; Keller, M.J.; Herold, K.C.; Herold, B.C. Natural mucosal barriers and COVID-19 in children. JCI Insight 2021, 6, e148694. [Google Scholar] [CrossRef]
- Xia, H.; Shi, P.-Y. Antagonism of Type I Interferon by Severe Acute Respiratory Syndrome Coronavirus 2. J. Interf. Cytokine Res. 2020, 40, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.-H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Auto-antibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020, 370, eabd4585. [Google Scholar] [CrossRef]
- Chen, J.; Lau, Y.F.; Lamirande, E.W.; Paddock, C.D.; Bartlett, J.H.; Zaki, S.R.; Subbarao, K. Cellular Immune Responses to Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) Infection in Senescent BALB/c Mice: CD4+ T Cells Are Important in Control of SARS-CoV Infection. J. Virol. 2010, 84, 1289–1301. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.L.; Chui, P.; Lim, B.; Salto-Tellez, M. Elucidating the molecular physiopathology of acute respiratory distress syndrome in severe acute respiratory syndrome patients. Virus Res. 2009, 145, 260–269. [Google Scholar] [CrossRef]
- Wong, C.K.; Lam, C.W.K.; Wu, A.K.L.; Ip, W.K.; Lee, N.L.S.; Chan, I.H.S.; Lit, L.C.W.; Hui, D.S.C.; Chan, M.H.M.; Chung, S.S.C.; et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 2004, 136, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Baas, T.; Taubenberger, J.K.; Chong, P.Y.; Chui, P.; Katze, M.G. SARS-CoV Virus-Host Interactions and Comparative Etiologies of Acute Respiratory Distress Syndrome as Determined by Transcriptional and Cytokine Profiling of Formalin-Fixed Paraffin-Embedded Tissues. J. Interf. Cytokine Res. 2006, 26, 309–317. [Google Scholar] [CrossRef]
- Subbarao, K.; Roberts, A. Is there an ideal animal model for SARS? Trends Microbiol. 2006, 14, 299–303. [Google Scholar] [CrossRef]
- Channappanavar, R.; Zhao, J.; Perlman, S. T cell-mediated immune response to respiratory coronaviruses. Immunol. Res. 2014, 59, 118–128. [Google Scholar] [CrossRef]
- Szabo, P.A.; Dogra, P.; Gray, J.I.; Wells, S.B.; Connors, T.J.; Weisberg, S.P.; Krupska, I.; Matsumoto, R.; Poon, M.M.; Idzikowski, E.; et al. Longitudinal profiling of respiratory and systemic immune responses reveals myeloid cell-driven lung inflammation in severe COVID-19. Immunity 2021, 54, 797–814.e6. [Google Scholar] [CrossRef]
- Liao, M.; Liu, Y.; Yuan, J.; Wen, Y.; Xu, G.; Zhao, J.; Cheng, L.; Li, J.; Wang, X.; Wang, F.; et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020, 26, 842–844. [Google Scholar] [CrossRef]
- Bange, E.M.; Han, N.A.; Wileyto, P.; Kim, J.Y.; Gouma, S.; Robinson, J.; Greenplate, A.R.; Hwee, M.A.; Porterfield, F.; Owoyemi, O.; et al. CD8+ T cells contribute to survival in patients with COVID-19 and hematologic cancer. Nat. Med. 2021, 27, 1280–1289. [Google Scholar] [CrossRef] [PubMed]
- Moderbacher, C.R.; Ramirez, S.I.; Dan, J.M.; Grifoni, A.; Hastie, K.M.; Weiskopf, D.; Belanger, S.; Abbott, R.K.; Kim, C.; Choi, J.; et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell 2020, 183, 996–1012.e19. [Google Scholar] [CrossRef] [PubMed]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef]
- Le Bert, N.; Clapham, H.E.; Tan, A.T.; Ni Chia, W.; Tham, C.Y.; Lim, J.M.; Kunasegaran, K.; Tan, L.W.L.; Dutertre, C.-A.; Shankar, N.; et al. Highly functional virus-specific cellular immune response in asymptomatic SARS-CoV-2 infection. J. Exp. Med. 2021, 218, e20202617. [Google Scholar] [CrossRef]
- Tan, A.T.; Linster, M.; Tan, C.W.; Le Bert, N.; Ni Chia, W.; Kunasegaran, K.; Zhuang, Y.; Tham, C.Y.L.; Chia, A.; Smith, G.J.D.; et al. Early induction of functional SARS-CoV-2-specific T cells associates with rapid viral clearance and mild disease in COVID-19 patients. Cell Rep. 2021, 34, 108728. [Google Scholar] [CrossRef] [PubMed]
- Agha, M.E.; Blake, M.; Chilleo, C.; Wells, A.; Haidar, G. Suboptimal Response to Coronavirus Disease 2019 Messenger RNA Vaccines in Patients With Hematologic Malignancies: A Need for Vigilance in the Postmasking Era. Open Forum Infect. Dis. 2021, 8, ofab353. [Google Scholar] [CrossRef] [PubMed]
- Barrière, J.; Chamorey, E.; Adjtoutah, Z.; Castelnau, O.; Mahamat, A.; Marco, S.; Petit, E.; Leysalle, A.; Raimondi, V.; Carles, M. Impaired immunogenicity of BNT162b2 anti-SARS-CoV-2 vaccine in patients treated for solid tumors. Ann. Oncol. 2021, 32, 1053–1055. [Google Scholar] [CrossRef]
- Hagin, D.; Freund, T.; Navon, M.; Halperin, T.; Adir, D.; Marom, R.; Levi, I.; Benor, S.; Alcalay, Y.; Freund, N.T. Immunogenicity of Pfizer-BioNTech COVID-19 vaccine in patients with inborn errors of immunity. J. Allergy Clin. Immunol. 2021, 148, 739–749. [Google Scholar] [CrossRef]
- Mamez, A.-C.; Pradier, A.; Giannotti, F.; Petitpas, A.; Urdiola, M.F.; Vu, D.-L.; Masouridi-Levrat, S.; Morin, S.; Dantin, C.; Clerc-Renaud, D.; et al. Antibody responses to SARS-CoV2 vaccination in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2021, 56, 3094–3096. [Google Scholar] [CrossRef]
- Delmonte, O.M.; Bergerson, J.R.; Burbelo, P.D.; Durkee-Shock, J.R.; Dobbs, K.; Bosticardo, M.; Keller, M.D.; McDermott, D.H.; Rao, V.K.; Dimitrova, D.; et al. Antibody responses to the SARS-CoV-2 vaccine in individuals with various inborn errors of immunity. J. Allergy Clin. Immunol. 2021, 148, 1192–1197. [Google Scholar] [CrossRef]
- Apostolidis, S.A.; Kakara, M.; Painter, M.M.; Goel, R.R.; Mathew, D.; Lenzi, K.; Rezk, A.; Patterson, K.R.; Espinoza, D.A.; Kadri, J.C.; et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021, 27, 1990–2001. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.; Nicholas, S.K.; Martinez, C.A.; Leen, A.M.; Hanley, P.J.; Gottschalk, S.M.; Rooney, C.M.; Hanson, I.C.; Krance, R.A.; Shpall, E.J.; et al. Adoptive immunotherapy for primary immunodeficiency disorders with virus-specific T lymphocytes. J. Allergy Clin. Immunol. 2016, 137, 1498–1505.e1. [Google Scholar] [CrossRef] [PubMed]
- Ng, O.-W.; Chia, A.; Tan, A.T.; Jadi, R.S.; Leong, H.N.; Bertoletti, A.; Tan, Y.-J. Memory T cell responses targeting the SARS coronavirus persist up to 11 years post-infection. Vaccine 2016, 34, 2008–2014. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Alshukairi, A.N.; Baharoon, S.A.; Ahmed, W.A.; Bokhari, A.A.; Nehdi, A.M.; Layqah, L.A.; Alghamdi, M.G.; Al Gethamy, M.M.; Dada, A.M.; et al. Recovery from the Middle East respiratory syndrome is associated with antibody and T cell responses. Sci. Immunol. 2017, 2, eaan5393. [Google Scholar] [CrossRef]
- Jung, J.H.; Rha, M.-S.; Sa, M.; Choi, H.K.; Jeon, J.H.; Seok, H.; Park, D.W.; Park, S.-H.; Jeong, H.W.; Choi, W.S.; et al. SARS-CoV-2-specific T cell memory is sustained in COVID-19 convalescent patients for 10 months with successful development of stem cell-like memory T cells. Nat. Commun. 2021, 12, 4043. [Google Scholar] [CrossRef]
- Noh, J.Y.; Yang, J.-S.; Hwang, S.Y.; Hyun, H.; Seong, H.; Yoon, J.G.; Yoon, S.-Y.; Cheong, H.J.; Kim, W.J.; Park, W.-J.; et al. Duration of Humoral Immunity and Cross-Neutralizing Activity Against the Alpha, Beta, and Delta Variants After Wild-Type Severe Acute Respiratory Syndrome Coronavirus 2 Infection: A Prospective Cohort Study. J. Infect. Dis. 2022, 226, 975–978. [Google Scholar] [CrossRef]
- Sukdolak, C.; Tischer, S.; Dieks, D.; Figueiredo, C.; Goudeva, L.; Heuft, H.-G.; Verboom, M.; Immenschuh, S.; Heim, A.; Borchers, S.; et al. CMV-, EBV- and ADV-Specific T Cell Immunity: Screening and Monitoring of Potential Third-Party Donors to Improve Post-Transplantation Outcome. Biol. Blood Marrow Transplant. 2013, 19, 1480–1492. [Google Scholar] [CrossRef]
- Tischer, S.; Priesner, C.; Heuft, H.-G.; Goudeva, L.; Mende, W.; Barthold, M.; Kloeß, S.; Arseniev, L.; Aleksandrova, K.; Maecker-Kolhoff, B.; et al. Rapid generation of clinical-grade antiviral T cells: Selection of suitable T-cell donors and GMP-compliant manufacturing of antiviral T cells. J. Transl. Med. 2014, 12, 336. [Google Scholar] [CrossRef]
- Conway, S.R.; Keller, M.D.; Bollard, C.M. Cellular therapies for the treatment and prevention of SARS-CoV-2 infection. Blood 2022, 140, 208–221. [Google Scholar] [CrossRef]
- Tischer, S.; Kaireit, T.; Figueiredo, C.; Hiller, O.; Maecker-Kolhoff, B.; Geyeregger, R.; Immenschuh, S.; Blasczyk, R.; Eiz-Vesper, B. Establishment of the reversible peptide-major histocompatibility complex (pMHC) class I Histamer technology: Tool for visualization and selection of functionally active antigen-specific CD8+ T lymphocytes. Int. Immunol. 2012, 24, 561–572. [Google Scholar] [CrossRef][Green Version]
- Rauser, G.; Einsele, H.; Sinzger, C.; Wernet, D.; Kuntz, G.; Assenmacher, M.; Campbell, J.D.M.; Topp, M.S. Rapid generation of combined CMV-specific CD4+ and CD8+ T-cell lines for adoptive transfer into recipients of allogeneic stem cell transplants. Blood 2004, 103, 3565–3572. [Google Scholar] [CrossRef] [PubMed]
- Cobbold, M.; Khan, N.; Pourgheysari, B.; Tauro, S.; McDonald, D.; Osman, H.; Assenmacher, M.; Billingham, L.; Steward, C.; Crawley, C.; et al. Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA–peptide tetramers. J. Exp. Med. 2005, 202, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Committee for Advanced Therapies of the European Medicines Agency. Available online: www.ema.europa.eu/docs/en_GB/document_library/Report/2013/05/WC500143582.pdf (accessed on 9 September 2022).
- Von Rossum, A.; Krall, R.; Escalante, N.; Choy, J.C. Inflammatory Cytokines Determine the Susceptibility of Human CD8 T Cells to Fas-mediated Activation-induced Cell Death through Modulation of FasL and c-FLIPS Expression. J. Biol. Chem. 2011, 286, 21137–21144. [Google Scholar] [CrossRef]
- Leung, W.; Soh, T.G.; Linn, Y.C.; Low, J.G.; Loh, J.; Chan, M.; Chng, W.J.; Koh, L.P.; Poon, M.L.; Ng, K.P.; et al. Rapid production of clinical-grade SARS-CoV-2 specific T cells. Adv. Cell Gene Ther. 2020, 3, e101. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.S.; Fraser, A.R.; Smith, L.; Burgoyne, P.; Imlach, S.N.; Jarvis, L.M.; Turner, D.M.; Zahra, S.; Turner, M.L.; Campbell, J.D.M. Rapid GMP-Compliant Expansion of SARS-CoV-2–Specific T Cells From Convalescent Donors for Use as an Allogeneic Cell Therapy for COVID-19. Front. Immunol. 2021, 11, 598402. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, M.; Aguilar-Gallardo, C.; Montoro, J.; Francés-Gómez, C.; Latorre, V.; Luna, I.; Planelles, D.; Carrasco, M.P.; Gómez, M.D.; González-Barberá, E.M.; et al. Adoptive transfer of ex vivo expanded SARS-CoV-2-specific cytotoxic lymphocytes: A viable strategy for COVID-19 immunosuppressed patients? Transpl. Infect. Dis. 2021, 23, e13602. [Google Scholar] [CrossRef]
- García-Ríos, E.; Leivas, A.; Mancebo, F.J.; Sánchez-Vega, L.; Lanzarot, D.; Aguado, J.M.; Martínez-López, J.; Paciello, M.L.; Pérez-Romero, P. Isolation of Functional SARS-CoV-2 Antigen-Specific T-Cells with Specific Viral Cytotoxic Activity for Adoptive Therapy of COVID-19. Biomedicines 2022, 10, 630. [Google Scholar] [CrossRef]
- Bonifacius, A.; Tischer-Zimmermann, S.; Santamorena, M.M.; Mausberg, P.; Schenk, J.; Koch, S.; Barnstorf-Brandes, J.; Gödecke, N.; Martens, J.; Goudeva, L.; et al. Rapid Manufacturing of Highly Cytotoxic Clinical-Grade SARS-CoV-2-specific T Cell Products Covering SARS-CoV-2 and Its Variants for Adoptive T Cell Therapy. Front. Bioeng. Biotechnol. 2022, 10, 867042. [Google Scholar] [CrossRef]
- Franzke, A.; Piao, W.; Lauber, J.; Gatzlaff, P.; Könecke, C.; Hansen, W.; Schmitt-Thomsen, A.; Hertenstein, B.; Buer, J.; Ganser, A. G-CSF as immune regulator in T cells expressing the G-CSF receptor: Implications for transplantation and autoimmune diseases. Blood 2003, 102, 734–739. [Google Scholar] [CrossRef]
- Toh, H.C.; Sun, L.; Soe, Y.; Wu, Y.; Phoon, Y.P.; Chia, W.K.; Wu, J.; Wong, K.Y.; Tan, P. G-CSF induces a potentially tolerant gene and immunophenotype profile in T cells in vivo. Clin. Immunol. 2009, 132, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Bunse, C.E.; Borchers, S.; Varanasi, P.R.; Tischer, S.; Figueiredo, C.; Immenschuh, S.; Kalinke, U.; Koehl, U.; Goudeva, L.; Maecker-Kolhoff, B.; et al. Impaired Functionality of Antiviral T Cells in G-CSF Mobilized Stem Cell Donors: Implications for the Selection of CTL Donor. PLoS ONE 2013, 8, e77925. [Google Scholar] [CrossRef]
- Uhlin, M.; Gertow, J.; Uzunel, M.; Okas, M.; Berglund, S.; Watz, E.; Brune, M.; Ljungman, P.; Maeurer, M.; Mattsson, J. Rapid Salvage Treatment With Virus-Specific T Cells for Therapy-Resistant Disease. Clin. Infect. Dis. 2012, 55, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Leen, A.M.; Bollard, C.M.; Mendizabal, A.M.; Shpall, E.J.; Szabolcs, P.; Antin, J.H.; Kapoor, N.; Pai, S.-Y.; Rowley, S.D.; Kebriaei, P.; et al. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood 2013, 121, 5113–5123. [Google Scholar] [CrossRef] [PubMed]
- Gallot, G.; Vollant, S.; Saïagh, S.; Clémenceau, B.; Vivien, R.; Cerato, E.; Bignon, J.-D.; Ferrand, C.; Jaccard, A.; Vigouroux, S.; et al. T-cell Therapy Using a Bank of EBV-specific Cytotoxic T Cells: Lessons from a phase I/II feasibility and safety study. J. Immunother. 2014, 37, 170–179. [Google Scholar] [CrossRef]
- Withers, B.; Blyth, E.; Clancy, L.E.; Yong, A.; Fraser, C.; Burgess, J.; Simms, R.; Brown, R.; Kliman, D.; Dubosq, M.-C.; et al. Long-term control of recurrent or refractory viral infections after allogeneic HSCT with third-party virus-specific T cells. Blood Adv. 2017, 1, 2193–2205. [Google Scholar] [CrossRef] [PubMed]
- Melenhorst, J.J.; Leen, A.M.; Bollard, C.M.; Quigley, M.F.; Price, D.A.; Rooney, C.M.; Brenner, M.K.; Barrett, A.J.; Heslop, H.E. Allogeneic virus-specific T cells with HLA alloreactivity do not produce GVHD in human subjects. Blood 2010, 116, 4700–4702. [Google Scholar] [CrossRef]
- Doubrovina, E.; Oflaz-Sozmen, B.; Prockop, S.E.; Kernan, N.A.; Abramson, S.; Teruya-Feldstein, J.; Hedvat, C.; Chou, J.F.; Heller, G.; Barker, J.N.; et al. Adoptive immunotherapy with unselected or EBV-specific T cells for biopsy-proven EBV+ lymphomas after allogeneic hematopoietic cell transplantation. Blood 2012, 119, 2644–2656. [Google Scholar] [CrossRef]
- Icheva, V.; Kayser, S.; Wolff, D.; Tuve, S.; Kyzirakos, C.; Bethge, W.; Greil, J.; Albert, M.H.; Schwinger, W.; Nathrath, M.; et al. Adoptive Transfer of Epstein-Barr Virus (EBV) Nuclear Antigen 1–Specific T Cells As Treatment for EBV Reactivation and Lymphoproliferative Disorders After Allogeneic Stem-Cell Transplantation. J. Clin. Oncol. 2013, 31, 39–48. [Google Scholar] [CrossRef]
- O’Reilly, R.J.; Prockop, S.; Hasan, A.N.; Koehne, G.; Doubrovina, E. Virus-specific T-cell banks for ‘off the shelf’ adoptive therapy of refractory infections. Bone Marrow Transplant. 2016, 51, 1163–1172. [Google Scholar] [CrossRef]
- Tzannou, I.; Watanabe, A.; Naik, S.; Daum, R.; Kuvalekar, M.; Leung, K.S.; Martinez, C.; Sasa, G.; Wu, M.; Gee, A.P.; et al. “Mini” bank of only 8 donors supplies CMV-directed T cells to diverse recipients. Blood Adv. 2019, 3, 2571–2580. [Google Scholar] [CrossRef]
- Vasileiou, S.; Kuvalekar, M.; Workineh, A.; Watanabe, A.; Velazquez, Y.; Lulla, S.; Heslop, H.E.; Mooney, K.; Grimes, K.; Carrum, G.; et al. 37. Allogeneic, Off-the-Shelf, SARS-CoV-2-specific T Cells Demonstrate Reactivity Against Emerging Variant Strains. Open Forum Infect. Dis. 2021, 8, S27. [Google Scholar] [CrossRef]
- Braun, J.; Loyal, L.; Frentsch, M.; Wendisch, D.; Georg, P.; Kurth, F.; Hippenstiel, S.; Dingeldey, M.; Kruse, B.; Fauchere, F.; et al. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature 2020, 587, 270–274. [Google Scholar] [CrossRef]
- Keller, M.D.; Harris, K.M.; Jensen-Wachspress, M.A.; Kankate, V.V.; Lang, H.; Lazarski, C.A.; Durkee-Shock, J.; Lee, P.-H.; Chaudhry, K.; Webber, K.; et al. SARS-CoV-2–specific T cells are rapidly expanded for therapeutic use and target conserved regions of the membrane protein. Blood 2020, 136, 2905–2917. [Google Scholar] [CrossRef]
- Mateus, J.; Grifoni, A.; Tarke, A.; Sidney, J.; Ramirez, S.I.; Dan, J.M.; Burger, Z.C.; Rawlings, S.A.; Smith, D.M.; Phillips, E.; et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science 2020, 370, 89–94. [Google Scholar] [CrossRef]
- Goel, R.R.; Painter, M.M.; Apostolidis, S.A.; Mathew, D.; Meng, W.; Rosenfeld, A.M.; Lundgreen, K.A.; Reynaldi, A.; Khoury, D.S.; Pattekar, A.; et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science 2021, 374, abm0829. [Google Scholar] [CrossRef]
- Tarke, A.; Sidney, J.; Methot, N.; Yu, E.D.; Zhang, Y.; Dan, J.M.; Goodwin, B.; Rubiro, P.; Sutherland, A.; Wang, E.; et al. Impact of SARS-CoV-2 variants on the total CD4+ and CD8+ T cell reactivity in infected or vaccinated individuals. Cell Rep. Med. 2021, 2, 100355. [Google Scholar] [CrossRef]
- Painter, M.M.; Mathew, D.; Goel, R.R.; Apostolidis, S.A.; Pattekar, A.; Kuthuru, O.; Baxter, A.E.; Herati, R.S.; Oldridge, D.A.; Gouma, S.; et al. Rapid induction of antigen-specific CD4+ T cells is associated with coordinated humoral and cellular immunity to SARS-CoV-2 mRNA vaccination. Immunity 2021, 54, 2133–2142.e3. [Google Scholar] [CrossRef]
- Stanojevic, M.; Geiger, A.; Ostermeier, B.; Sohai, D.; Lazarski, C.; Lang, H.; Jensen-Wachspress, M.; Webber, K.; Burbelo, P.; Cohen, J.; et al. Spike-directed vaccination elicits robust spike-specific T-cell response, including to mutant strains. Cytotherapy 2021, 24, 10–15. [Google Scholar] [CrossRef]
- Mazzoni, A.; Vanni, A.; Spinicci, M.; Capone, M.; Lamacchia, G.; Salvati, L.; Coppi, M.; Antonelli, A.; Carnasciali, A.; Farahvachi, P.; et al. SARS-CoV-2 Spike-Specific CD4+ T Cell Response Is Conserved Against Variants of Concern, Including Omicron. Front. Immunol. 2022, 13, 801431. [Google Scholar] [CrossRef]
- Taborska, P.; Lastovicka, J.; Stakheev, D.; Strizova, Z.; Bartunkova, J.; Smrz, D. SARS-CoV-2 spike glycoprotein-reactive T cells can be readily expanded from COVID-19 vaccinated donors. Immun. Inflamm. Dis. 2021, 9, 1452–1467. [Google Scholar] [CrossRef]
- Grifoni, A.; Sidney, J.; Vita, R.; Peters, B.; Crotty, S.; Weiskopf, D.; Sette, A. SARS-CoV-2 human T cell epitopes: Adaptive immune response against COVID-19. Cell Host Microbe 2021, 29, 1076–1092. [Google Scholar] [CrossRef]
- Tarke, A.; Sidney, J.; Kidd, C.K.; Dan, J.M.; Ramirez, S.I.; Yu, E.D.; Mateus, J.; da Silva Antunes, R.; Moore, E.; Rubiro, P.; et al. Comprehensive analysis of T cell immunodominance and immunoprevalence of SARS-CoV-2 epitopes in COVID-19 cases. Cell Rep. Med. 2021, 2, 100204. [Google Scholar] [CrossRef]
- Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef]
- Quadeer, A.A.; Ahmed, S.F.; McKay, M.R. Landscape of epitopes targeted by T cells in 852 individuals recovered from COVID-19: Meta-analysis, immunoprevalence, and web platform. Cell Rep. Med. 2021, 2, 100312. [Google Scholar] [CrossRef]
- Hamelin, D.J.; Fournelle, D.; Grenier, J.-C.; Schockaert, J.; Kovalchik, K.A.; Kubiniok, P.; Mostefai, F.; Duquette, J.D.; Saab, F.; Sirois, I.; et al. The mutational landscape of SARS-CoV-2 variants diversifies T cell targets in an HLA-supertype-dependent manner. Cell Syst. 2021, 13, 143–157.e3. [Google Scholar] [CrossRef]
- Skelly, D.T.; Harding, A.C.; Gilbert-Jaramillo, J.; Knight, M.L.; Longet, S.; Brown, A.; Adele, S.; Adland, E.; Brown, H.; Tipton, T.; et al. Two doses of SARS-CoV-2 vaccination induce robust immune responses to emerging SARS-CoV-2 variants of concern. Nat. Commun. 2021, 12, 5061. [Google Scholar] [CrossRef]
- Kuhlmann, C.; Mayer, C.K.; Claassen, M.; Maponga, T.; Burgers, W.A.; Keeton, R.; Riou, C.; Sutherland, A.D.; Suliman, T.; Shaw, M.L.; et al. Breakthrough infections with SARS-CoV-2 omicron despite mRNA vaccine booster dose. Lancet 2022, 399, 625–626. [Google Scholar] [CrossRef]
- GeurtsvanKessel, C.H.; Geers, D.; Schmitz, K.S.; Mykytyn, A.Z.; Lamers, M.M.; Bogers, S.; Scherbeijn, S.; Gommers, L.; Sablerolles, R.S.G.; Nieuwkoop, N.N.; et al. Divergent SARS-CoV-2 Omicron–reactive T and B cell responses in COVID-19 vaccine recipients. Sci. Immunol. 2022, 7, eabo2202. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, S.; Ren, L.; Zheng, P.; Hu, X.; Jin, T.; Tan, X. Profiling CD8+ T cell epitopes of COVID-19 convalescents reveals reduced cellular immune responses to SARS-CoV-2 variants. Cell Rep. 2021, 36, 109708. [Google Scholar] [CrossRef]
- de Silva, T.I.; Liu, G.; Lindsey, B.B.; Dong, D.; Moore, S.C.; Hsu, N.S.; Shah, D.; Wellington, D.; Mentzer, A.J.; Angyal, A.; et al. The impact of viral mutations on recognition by SARS-CoV-2 specific T cells. iScience 2021, 24, 103353. [Google Scholar] [CrossRef]
- The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group; Sterne, J.A.C.; Murthy, S.; Diaz, J.V.; Slutsky, A.S.; Villar, J.; Angus, D.C.; Annane, D.; Azevedo, L.C.P.; Berwanger, O.; et al. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA 2020, 324, 1330–1341. [Google Scholar] [CrossRef]
- Li, H.; Yan, B.; Gao, R.; Ren, J.; Yang, J. Effectiveness of corticosteroids to treat severe COVID-19: A systematic review and meta-analysis of prospective studies. Int. Immunopharmacol. 2021, 100, 108121. [Google Scholar] [CrossRef]
- Braat, M.C.; Oosterhuis, B.; Koopmans, R.P.; Meewis, J.M.; Van Boxtel, C.J. Kinetic-dynamic modeling of lymphocytopenia induced by the combined action of dexamethasone and hydrocortisone in humans, after inhalation and intravenous administration of dexamethasone. J. Pharmacol. Exp. Ther. 1992, 262, 509–515. [Google Scholar]
- Ma, J.; Xie, Y.; Shi, Y.; Qin, W.; Zhao, B.; Jin, Y. Glucocorticoid-induced apoptosis requires FOXO3A activity. Biochem. Biophys. Res. Commun. 2008, 377, 894–898. [Google Scholar] [CrossRef]
- Orange, J.S. Natural killer cell deficiency. J. Allergy Clin. Immunol. 2013, 132, 515–525. [Google Scholar] [CrossRef]
- Lisco, A.; Hsu, A.P.; Dimitrova, D.; Proctor, D.M.; Mace, E.M.; Ye, P.; Anderson, M.V.; Hicks, S.N.; Grivas, C.; Hammoud, D.A.; et al. Treatment of Relapsing HPV Diseases by Restored Function of Natural Killer Cells. New Engl. J. Med. 2021, 385, 921–929. [Google Scholar] [CrossRef]
- Wilk, A.J.; Rustagi, A.; Zhao, N.Q.; Roque, J.; Martínez-Colón, G.J.; McKechnie, J.L.; Ivison, G.T.; Ranganath, T.; Vergara, R.; Hollis, T.; et al. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat. Med. 2020, 26, 1070–1076. [Google Scholar] [CrossRef]
- Wang, F.; Nie, J.; Wang, H.; Zhao, Q.; Xiong, Y.; Deng, L.; Song, S.; Ma, Z.; Mo, P.; Zhang, Y. Characteristics of Peripheral Lymphocyte Subset Alteration in COVID-19 Pneumonia. J. Infect. Dis. 2020, 221, 1762–1769. [Google Scholar] [CrossRef]
- Jiang, Y.; Wei, X.; Guan, J.; Qin, S.; Wang, Z.; Lu, H.; Qian, J.; Wu, L.; Chen, Y.; Chen, Y.; et al. COVID-19 pneumonia: CD8+ T and NK cells are decreased in number but compensatory increased in cytotoxic potential. Clin. Immunol. 2020, 218, 108516. [Google Scholar] [CrossRef]
- Osman, M.; Faridi, R.M.; Sligl, W.; Shabani-Rad, M.-T.; Dharmani-Khan, P.; Parker, A.; Kalra, A.; Tripathi, M.B.; Storek, J.; Tervaert, J.W.C.; et al. Impaired natural killer cell counts and cytolytic activity in patients with severe COVID-19. Blood Adv. 2020, 4, 5035–5039. [Google Scholar] [CrossRef]
- Littwitz, E.; Francois, S.; Dittmer, U.; Gibbert, K. Distinct roles of NK cells in viral immunity during different phases of acute Friend retrovirus infection. Retrovirology 2013, 10, 127. [Google Scholar] [CrossRef]
- Ahmed, F.; Jo, D.-H.; Lee, S.-H. Can Natural Killer Cells Be a Principal Player in Anti-SARS-CoV-2 Immunity? Front. Immunol. 2020, 11, 586765. [Google Scholar] [CrossRef]
- Van Eeden, C.; Khan, L.; Osman, M.S.; Tervaert, J.W.C. Natural Killer Cell Dysfunction and its Role in COVID-19. Int. J. Mol. Sci. 2020, 21, 6351. [Google Scholar] [CrossRef]
- Björkström, N.K.; Strunz, B.; Ljunggren, H.-G. Natural killer cells in antiviral immunity. Nat. Rev. Immunol. 2021, 22, 112–123. [Google Scholar] [CrossRef]
- Zavvar, M.; Yahyapoor, A.; Baghdadi, H.; Zargaran, S.; Assadiasl, S.; Abdolmohammadi, K.; Abooei, A.H.; Sattarian, M.R.; JalaliFarahani, M.; Zarei, N.; et al. COVID-19 immunotherapy: Treatment based on the immune cell-mediated approaches. Int. Immunopharmacol. 2022, 107, 108655. [Google Scholar] [CrossRef]
- Ma, M.T.; Badeti, S.; Chen, C.-H.; Kim, J.; Choudhary, A.; Honnen, B.; Reichman, C.; Calianese, D.; Pinter, A.; Jiang, Q.; et al. CAR-NK Cells Effectively Target SARS-CoV-2-Spike-Expressing Cell Lines In Vitro. Front. Immunol. 2021, 12, 652223. [Google Scholar] [CrossRef]
- D’Alessio, F.R.; Tsushima, K.; Aggarwal, N.R.; West, E.E.; Willett, M.H.; Britos, M.F.; Pipeling, M.R.; Brower, R.G.; Tuder, R.M.; McDyer, J.F.; et al. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J. Clin. Investig. 2009, 119, 2898–2913. [Google Scholar] [CrossRef]
- Fulton, R.B.; Meyerholz, D.K.; Varga, S.M. Foxp3+ CD4 Regulatory T Cells Limit Pulmonary Immunopathology by Modulating the CD8 T Cell Response during Respiratory Syncytial Virus Infection. J. Immunol. 2010, 185, 2382–2392. [Google Scholar] [CrossRef]
- Arpaia, N.; Green, J.A.; Moltedo, B.; Arvey, A.; Hemmers, S.; Yuan, S.; Treuting, P.M.; Rudensky, A.Y. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell 2015, 162, 1078–1089. [Google Scholar] [CrossRef]
- Wilson, J.G.; Liu, K.D.; Zhuo, H.; Caballero, L.; McMillan, M.; Fang, X.; Cosgrove, K.; Vojnik, R.; Calfee, C.S.; Lee, J.-W.; et al. Mesenchymal stem (stromal) cells for treatment of ARDS: A phase 1 clinical trial. Lancet Respir. Med. 2014, 3, 24–32. [Google Scholar] [CrossRef]
- Hu, S.; Li, J.; Xu, X.; Liu, A.; He, H.; Xu, J.; Chen, Q.; Liu, S.; Liu, L.; Qiu, H.; et al. The hepatocyte growth factor-expressing character is required for mesenchymal stem cells to protect the lung injured by lipopolysaccharide in vivo. Stem Cell Res. Ther. 2016, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Matthay, M.A.; Calfee, C.S.; Zhuo, H.; Thompson, B.T.; Wilson, J.G.; Levitt, J.E.; Rogers, A.J.; Gotts, J.E.; Wiener-Kronish, J.P.; Bajwa, E.K.; et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): A randomised phase 2a safety trial. Lancet Respir. Med. 2018, 7, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Leng, Z.; Zhu, R.; Hou, W.; Feng, Y.; Yang, Y.; Han, Q.; Shan, G.; Meng, F.; Du, D.; Wang, S.; et al. Transplantation of ACE2- Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis. 2020, 11, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Avanzini, M.A.; Mura, M.; Percivalle, E.; Bastaroli, F.; Croce, S.; Valsecchi, C.; Lenta, E.; Nykjaer, G.; Cassaniti, I.; Bagnarino, J.; et al. Human mesenchymal stromal cells do not express ACE2 and TMPRSS2 and are not permissive to SARS-CoV-2 infection. Stem Cells Transl. Med. 2020, 10, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.J.; Beaty, D.E.; Fruhwirth, L.L.; Chaves, A.P.L.; Riordan, N.H. Dodging COVID-19 infection: Low expression and localization of ACE2 and TMPRSS2 in multiple donor-derived lines of human umbilical cord-derived mesenchymal stem cells. J. Transl. Med. 2021, 19, 149. [Google Scholar] [CrossRef] [PubMed]
- Vasanthan, J.; Gurusamy, N.; Rajasingh, S.; Sigamani, V.; Kirankumar, S.; Thomas, E.L.; Rajasingh, J. Role of Human Mesenchymal Stem Cells in Regenerative Therapy. Cells 2020, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Le Burel, S.; Thepenier, C.; Boutin, L.; Lataillade, J.-J.; Peltzer, J. Effect of Mesenchymal Stromal Cells on T Cells in a Septic Context: Immunosuppression or Immunostimulation? Stem Cells Dev. 2017, 26, 1477–1489. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Y.; Li, Q.; Liu, K.; Hou, J.; Shao, C.; Wang, Y. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat. Rev. Nephrol. 2018, 14, 493–507. [Google Scholar] [CrossRef]
- Mishra, D.K.; Rocha, H.J.; Miller, R.; Kim, M.P. Immune cells inhibit the tumor metastasis in the 4D cellular lung model by reducing the number of live circulating tumor cells. Sci. Rep. 2018, 8, 16569. [Google Scholar] [CrossRef]
- Jeyaraman, M.; John, A.; Koshy, S.; Ranjan, R.; Anudeep, T.C.; Jain, R.; Swati, K.; Jha, N.K.; Sharma, A.; Kesari, K.K.; et al. Fostering mesenchymal stem cell therapy to halt cytokine storm in COVID-19. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2020, 1867, 166014. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Xu, M.; Deng, Z.; Zhao, Y.; Yang, M.; Liu, Y.; Yuan, R.; Sun, Y.; Zhang, H.; et al. Regulation of Inflammatory Cytokine Storms by Mesenchymal Stem Cells. Front. Immunol. 2021, 12, 726909. [Google Scholar] [CrossRef] [PubMed]
- Doorn, J.; Moll, G.; Le Blanc, K.; van Blitterswijk, C.; de Boer, J. Therapeutic Applications of Mesenchymal Stromal Cells: Paracrine Effects and Potential Improvements. Tissue Eng. Part B Rev. 2012, 18, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, M.E.; Fibbe, W.E. Mesenchymal Stromal Cells: Sensors and Switchers of Inflammation. Cell Stem Cell 2013, 13, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Chen, B.; Xiao, Z.; Zhao, L.; Xu, X.; Wan, X.; Jin, M.; Dai, J.; Dai, H. Paracrine factors from mesenchymal stem cells attenuate epithelial injury and lung fibrosis. Mol. Med. Rep. 2014, 11, 2831–2837. [Google Scholar] [CrossRef] [PubMed]
- Klyushnenkova, E.; Mosca, J.D.; Zernetkina, V.; Majumdar, M.K.; Beggs, K.J.; Simonetti, D.W.; Deans, R.J.; McIntosh, K.R. T cell responses to allogeneic human mesenchymal stem cells: Immunogenicity, tolerance, and suppression. J. Biomed. Sci. 2005, 12, 47–57. [Google Scholar] [CrossRef]
- Grau-Vorster, M.; Laitinen, A.; Nystedt, J.; Vives, J. HLA-DR expression in clinical-grade bone marrow-derived multipotent mesenchymal stromal cells: A two-site study. Stem Cell Res. Ther. 2019, 10, 164. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, J.; Gong, L.; Yu, D.; An, C.; Bunpetch, V.; Dai, J.; Huang, H.; Zou, X.; Ouyang, H.; et al. The Plasticity of Mesenchymal Stem Cells in Regulating Surface HLA-I. iScience 2019, 15, 66–78. [Google Scholar] [CrossRef]
- Shu, L.; Niu, C.; Li, R.; Huang, T.; Wang, Y.; Huang, M.; Ji, N.; Zheng, Y.; Chen, X.; Shi, L.; et al. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res. Ther. 2020, 11, 361. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, Y.; Luo, X.; He, X.; Zhang, Y.; Wang, J. Administration of umbilical cord mesenchymal stem cells in patients with severe COVID-19 pneumonia. Crit. Care 2020, 24, 420. [Google Scholar] [CrossRef]
- Chen, X.; Shan, Y.; Wen, Y.; Sun, J.; Du, H. Mesenchymal stem cell therapy in severe COVID-19: A retrospective study of short-term treatment efficacy and side effects. J. Infect. 2020, 81, 647–679. [Google Scholar] [CrossRef]
- Meng, F.; Xu, R.; Wang, S.; Xu, Z.; Zhang, C.; Li, Y.; Yang, T.; Shi, L.; Fu, J.; Jiang, T.; et al. Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: A phase 1 clinical trial. Signal Transduct. Target. Ther. 2020, 5, 172. [Google Scholar] [CrossRef] [PubMed]
- Lanzoni, G.; Linetsky, E.; Correa, D.; Cayetano, S.M.; Alvarez, R.A.; Kouroupis, D.; Gil, A.A.; Poggioli, R.; Ruiz, P.; Marttos, A.C.; et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: A double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl. Med. 2021, 10, 660–673. [Google Scholar] [CrossRef] [PubMed]
- Dilogo, I.H.; Aditianingsih, D.; Sugiarto, A.; Burhan, E.; Damayanti, T.; Sitompul, P.A.; Mariana, N.; Antarianto, R.D.; Liem, I.K.; Kispa, T.; et al. Umbilical Cord Mesenchymal Stromal Cells as Critical COVID-19 Adjuvant Therapy: A Randomized Controlled Trial. Stem Cells Transl. Med. 2021, 10, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Huang, H.; Lu, X.; Yan, X.; Jiang, X.; Xu, R.; Wang, S.; Zhang, C.; Yuan, X.; Xu, Z.; et al. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: A randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduct. Target. Ther. 2021, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Eiro, N.; Cabrera, J.R.; Fraile, M.; Costa, L.; Vizoso, F.J. The Coronavirus Pandemic (SARS-CoV-2): New Problems Demand New Solutions, the Alternative of Mesenchymal (Stem) Stromal Cells. Front. Cell Dev. Biol. 2020, 8, 645. [Google Scholar] [CrossRef]
- Sengupta, V.; Sengupta, S.; Lazo, A.; Woods, P.; Nolan, A.; Bremer, N. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020, 29, 747–754. [Google Scholar] [CrossRef]
- Wing, J.B.; Tanaka, A.; Sakaguchi, S. Human FOXP3+ Regulatory T Cell Heterogeneity and Function in Autoimmunity and Cancer. Immunity 2019, 50, 302–316. [Google Scholar] [CrossRef]
- Romano, M.; Fanelli, G.; Albany, C.J.; Giganti, G.; Lombardi, G. Past, Present, and Future of Regulatory T Cell Therapy in Transplantation and Autoimmunity. Front. Immunol. 2019, 10, 43. [Google Scholar] [CrossRef]
- Sledzinska, A.; Mucha, M.V.D.; Sledzi, A.; Bergerhoff, K.; Jenner, R.G.; Peggs, K.S.; Quezada, S.A.; Mucha, M.V.D.; Bergerhoff, K.; Hotblack, A.; et al. Regulatory T Cells Restrain Interleukin-2- and Blimp- 1-Dependent Acquisition of Cytotoxic Function by Article Regulatory T Cells Restrain Interleukin-2- and Blimp-1-Dependent Acquisition of Cytotoxic Function by CD4 + T Cells. Immunity 2020, 52, 151–166.e6. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef]
- Wang, F.; Hou, H.; Luo, Y.; Tang, G.; Wu, S.; Huang, M.; Liu, W.; Zhu, Y.; Lin, Q.; Mao, L.; et al. The laboratory tests and host immunity of COVID-19 patients with different severity of illness. J. Clin. Investig. 2020, 5, e137799. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Su, B.; Pang, L.; Qiao, L.; Feng, Y.; Ouyang, Y.; Guo, X.; Shi, H.; Wei, F.; Su, X.; et al. High-dimensional immune profiling by mass cytometry revealed immunosuppression and dysfunction of immunity in COVID-19 patients. Cell Mol. Immunol. 2020, 17, 650–652. [Google Scholar] [CrossRef] [PubMed]
- Rahimzadeh, M.; Naderi, N. Toward an understanding of regulatory T cells in COVID-19: A systematic review. J. Med. Virol. 2021, 93, 4167–4181. [Google Scholar] [CrossRef] [PubMed]
- Anghelina, D.; Zhao, J.; Trandem, K.; Perlman, S. Role of regulatory T cells in coronavirus-induced acute encephalitis. Virology 2009, 385, 358–367. [Google Scholar] [CrossRef]
- Gladstone, D.E.; Kim, B.S.; Mooney, R.K.; Karaba, A.H.; D’Alessio, F.R. Regulatory T Cells for Treating Patients With COVID-19 and Acute Respiratory Distress Syndrome: Two Case Reports. Ann. Intern. Med. 2020, 173, 852–853. [Google Scholar] [CrossRef]


| Clinical Trial | Therapy | Population Eligibility | Study Design | Phase of Clinical Trial | Estimated Admission | Blood Disorder Exclusion |
|---|---|---|---|---|---|---|
| NCT04457726 | Allogeneic CSTs | 1 to 90 y SARS-CoV-2 RT-PCR 1 within 72 h of enrollment | Arm A: severe COVID-19 Arm B: mild to moderate COVID-19 with high risk of progression to severe disease based on age and/ or underlying comorbidity | 2/2 (recruiting) | 18 | Not excluded unless receiving 0.5 mg/kg/d steroids |
| NCT04765449 | Partially HLA-matched banked allogeneic CSTs | ≥18 y SARS-CoV-2 RT-PCR 1 High risk of severe disease based on age and/or underlying comorbidity No supplemental O2 requirement | Arm A: Treatment with CSTs Arm B: No available HLA-matched product, monitored at home and may receive any standard of care treatment for COVID-19 | 1 (recruiting) | 24 | Included: chemotherapy for malignancy within the prior 24 mo Excluded: prior allogeneic HSCT or solid organ transplant; current chemotherapy, radiation, and/or immunosuppressive drug regimen |
| NCT04742595 | Partially HLA-matched banked allogeneic CSTs | ≥18 y Immunocompromised with cancer SARS-CoV-2 RT-PCR 1 within 2 wk of enrollment Presence of respiratory symptoms | Patients receive CSTs on day 1 and treatment may repeat every 14 d at investigators’ discretion. | Early 1 (recruiting) | 16 | No (required for inclusion) |
| NCT04762186 | Allogeneic CSTs | Maximum 14 d between symptom onset and enrollment WHO score 5 or 4 with one additional risk factor for progression | Phase I: dose finding phase Phase II: randomized pilot study comparing CST treatment at dose determined in phase I to current institutional standard of care treatment for COVID-19 | 1/2 (recruiting) | 12 | No (inclusion criteria as risk factor for severe disease) |
| NCT04896606 | Family derived HLAmatched Allogeneic CSTs | 18 to 65 y SARS-CoV-2 RT-PCR 1 Hospitalized for mild to moderate COVID-19 disease Risk of progression based on underlying comorbidity HLA-matched family related donor with recent SARS-CoV-2 infection at least 10 d from symptom onset available | Experimental arm: Patients receive family donor derived CSTs up to five times every 2 wk along with standard of care. Active Comparator: Standard of care alone. | 2/2 (recruiting) | 50 | No (inclusion criteria as risk factor for severe disease) |
| NCT05141058 | HSCT donor-derived allogeneic CSTs | ≥12 y and <80 y ≥28 d and <4 wk after allogeneic HSCT SARS-CoV-2 RT-PCR negative | Arm A: Adults (≥18 to <80 y) will receive a single infusion of CSTs at escalating doses for prophylaxis against SARS-CoV-2 infection. Arm B: Children (≥12 and <18 y) will receive a single infusion of CSTs at escalating doses for prophylaxis against SARS-CoV-2. | 2/2 (recruiting) | 24 | No (required for inclusion) |
| NCT04351659 | Blood donation from convalescent donor | 21 to 65 y history of COVID-19 with documented positive test for SARS-CoV-2 in the past; recovered from COVID-19 and is now suitable for blood donation, fulfilling all standard blood donor criteria; Negative test for SARS-CoV-2 currently | Patients receive blood donation from convalescent donor (1 unit) | 1 (recruiting) | 8 | Yes (Do not meet the standard criteria for blood cell donation) |
| NCT05447013 | CoV-2-STs generated from COVID-19recovered donors | 18 to 80 yHospitalized patients, SARS-CoV-2 PCR positive, within 8 days from the onset of the symptoms who have: Pneumonia or/and SatO2 ≤ 94% on room air or/and respiratory rate ≥ 24 breaths/min AND Lymphopenia CD3+ ≤ 650/μL or/and ALC ≤ 1000/microl AND Increased values of D-dimers (≥2Χ) or/and ferritin (>1000 ng/mL) or/and CRP (≥3Χ) or/and LDH (≥2Χ) | Experimental: For Phase II: Arm A Standard of care (SOC) and Coronavirus-specific T cells (CoV-2-STs) Active Comparator: For Phase II: Arm B Standard of care (SOC) | 2/2 (recruiting) | 182 | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nova, Z.; Zemanek, T.; Botek, N. Antigen-Specific T Cells and SARS-CoV-2 Infection: Current Approaches and Future Possibilities. Int. J. Mol. Sci. 2022, 23, 15122. https://doi.org/10.3390/ijms232315122
Nova Z, Zemanek T, Botek N. Antigen-Specific T Cells and SARS-CoV-2 Infection: Current Approaches and Future Possibilities. International Journal of Molecular Sciences. 2022; 23(23):15122. https://doi.org/10.3390/ijms232315122
Chicago/Turabian StyleNova, Zuzana, Tomas Zemanek, and Norbert Botek. 2022. "Antigen-Specific T Cells and SARS-CoV-2 Infection: Current Approaches and Future Possibilities" International Journal of Molecular Sciences 23, no. 23: 15122. https://doi.org/10.3390/ijms232315122
APA StyleNova, Z., Zemanek, T., & Botek, N. (2022). Antigen-Specific T Cells and SARS-CoV-2 Infection: Current Approaches and Future Possibilities. International Journal of Molecular Sciences, 23(23), 15122. https://doi.org/10.3390/ijms232315122








