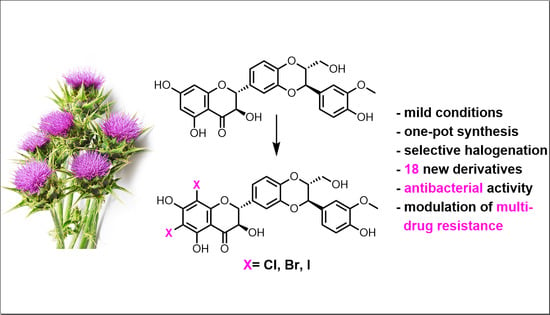
Selectively Halogenated Flavonolignans—Preparation and Antibacterial Activity
Abstract
1. Introduction
2. Results and Discussion
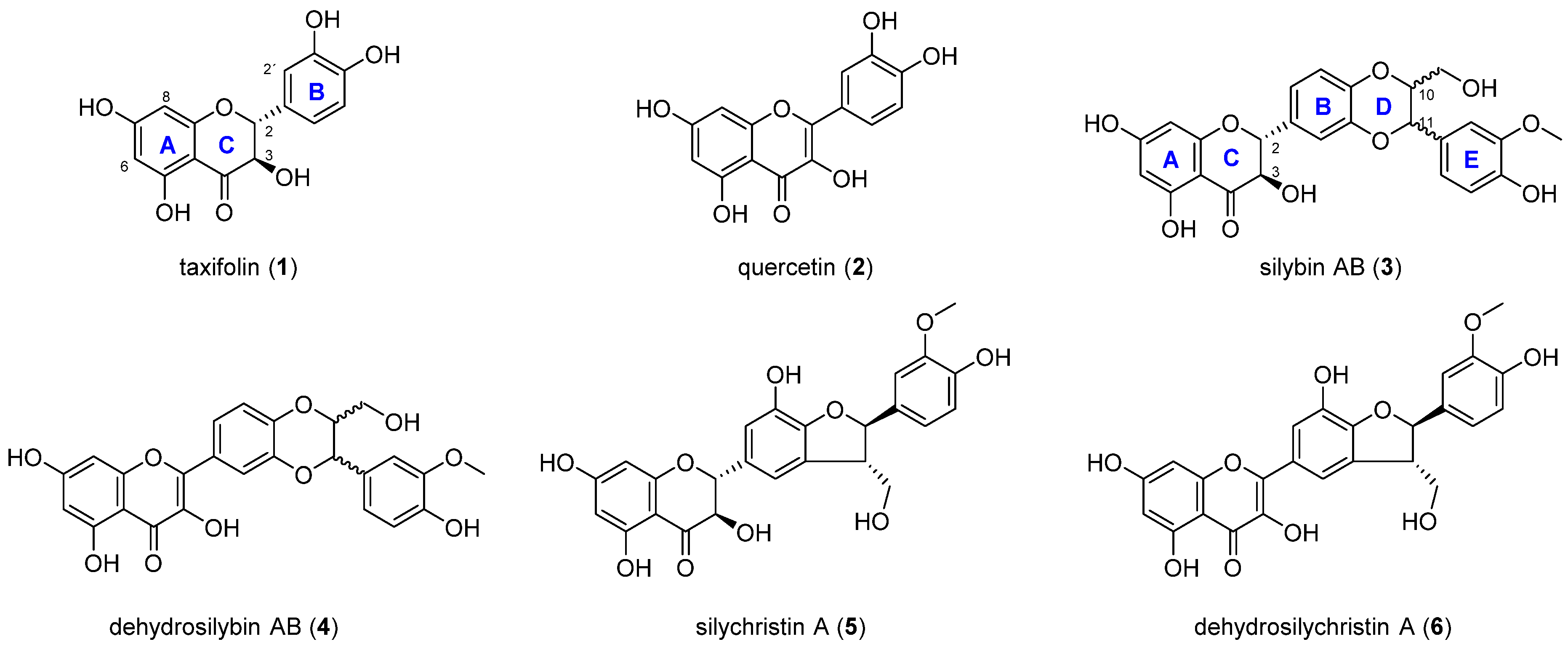
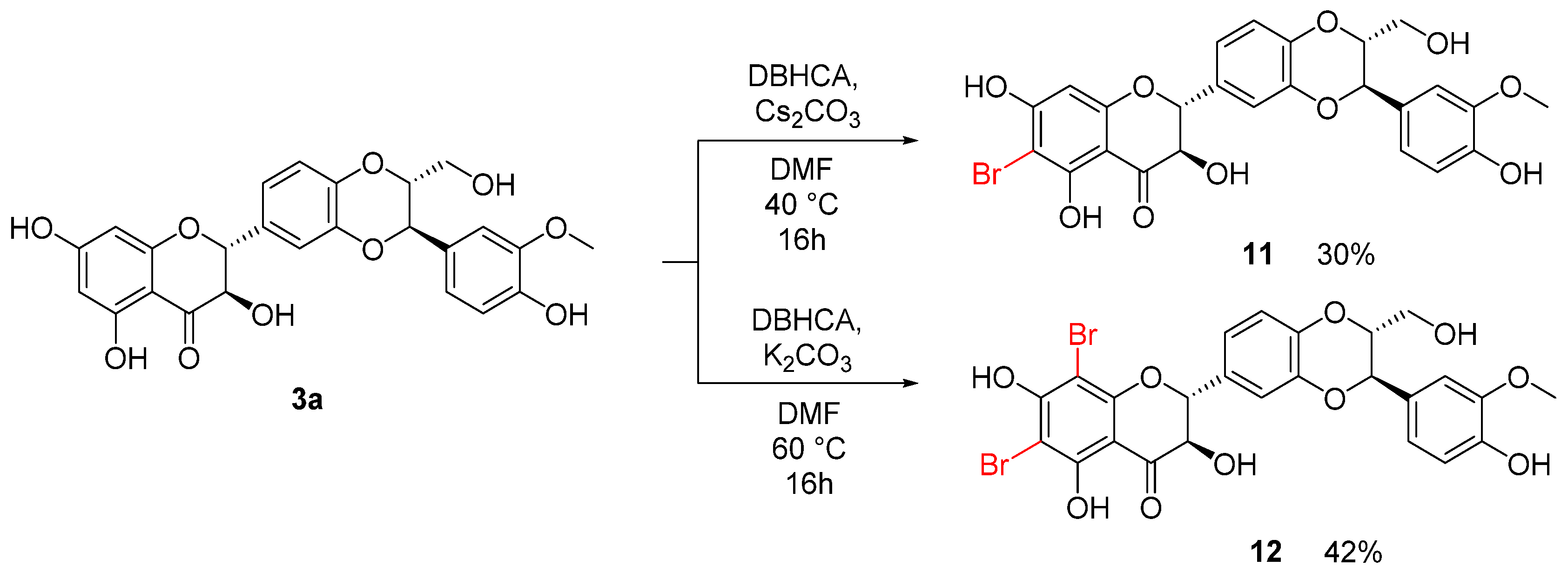
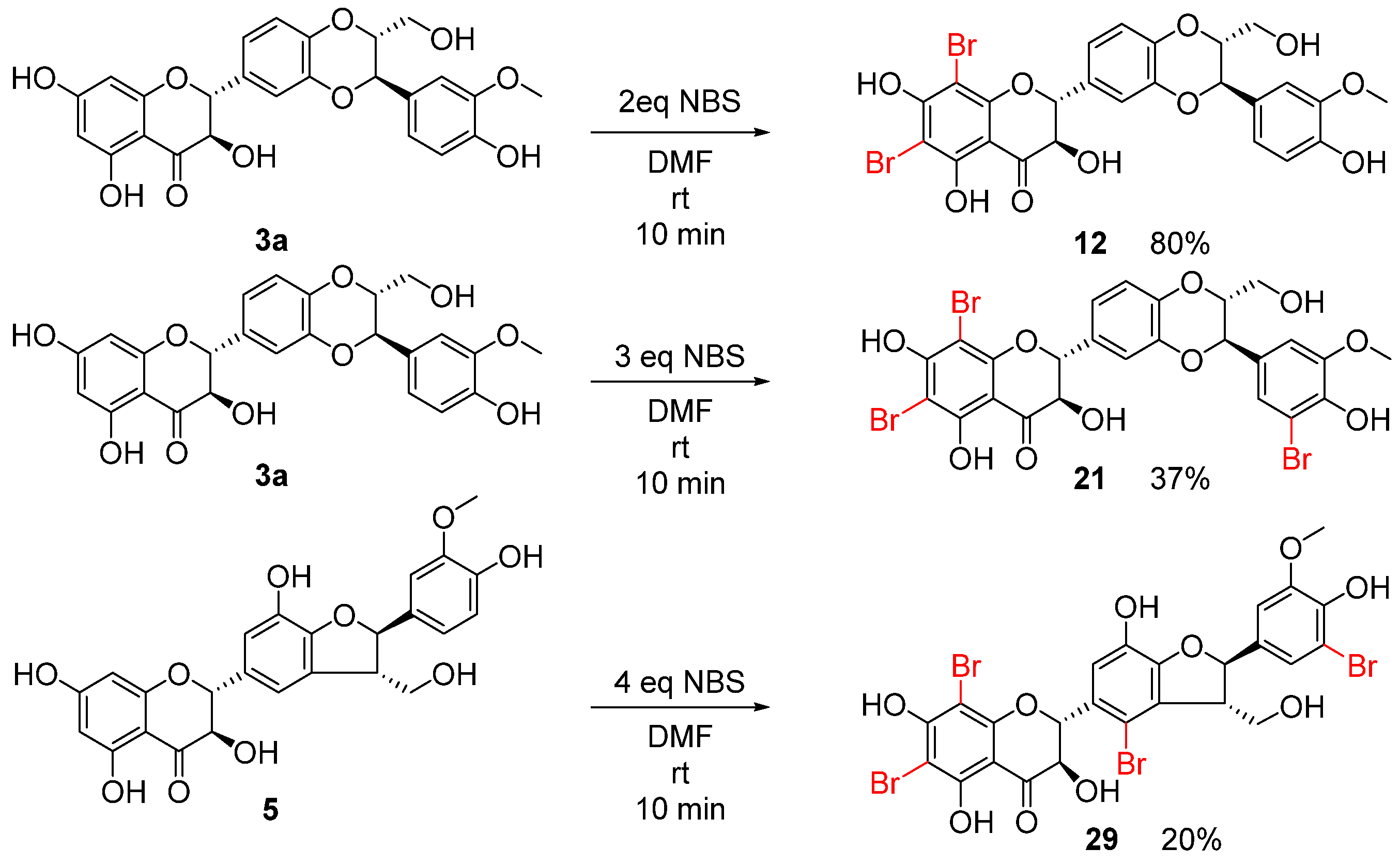
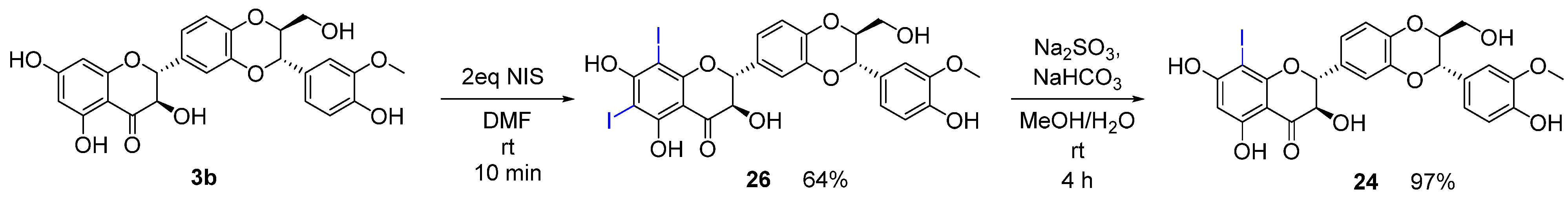
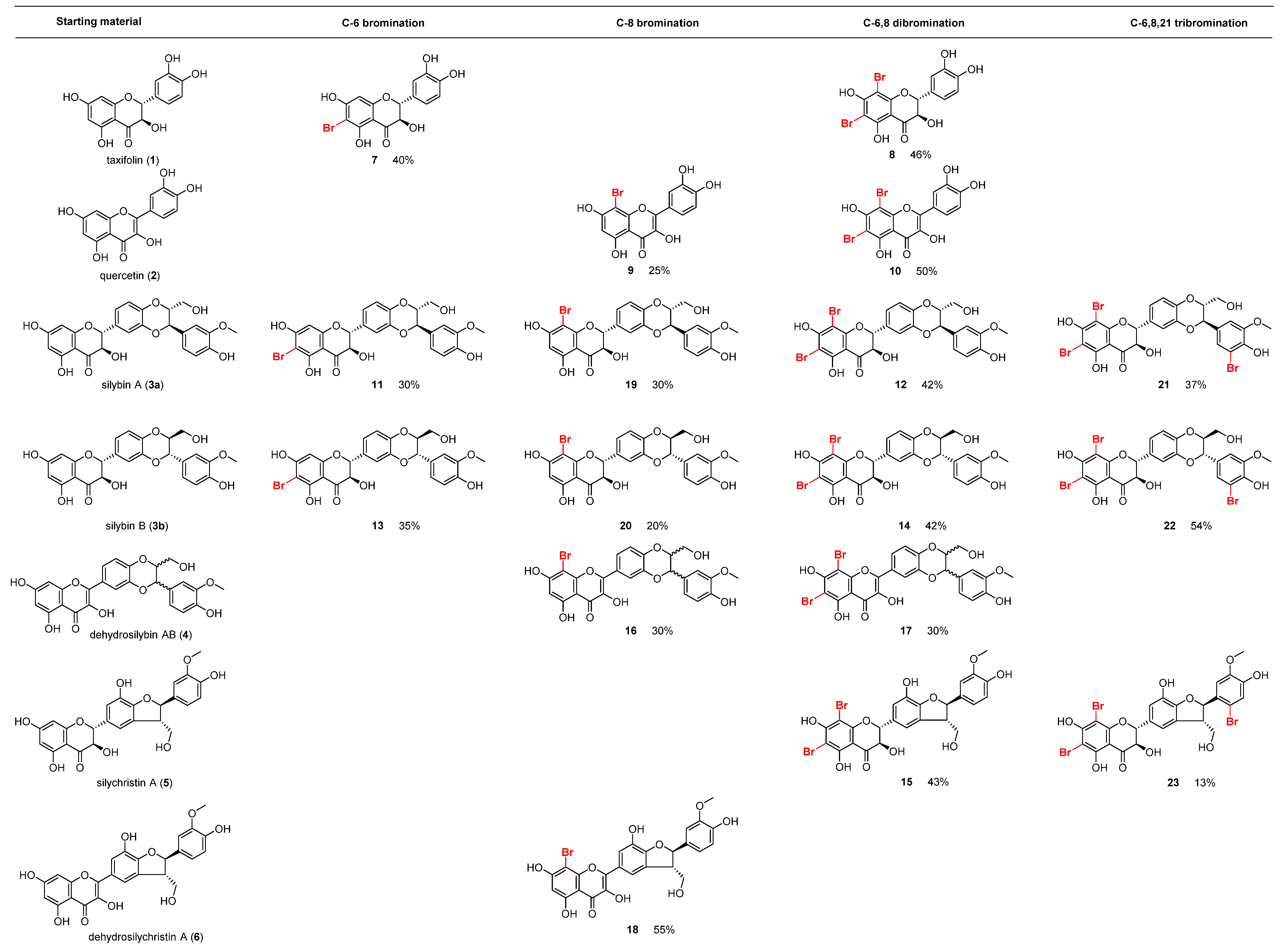
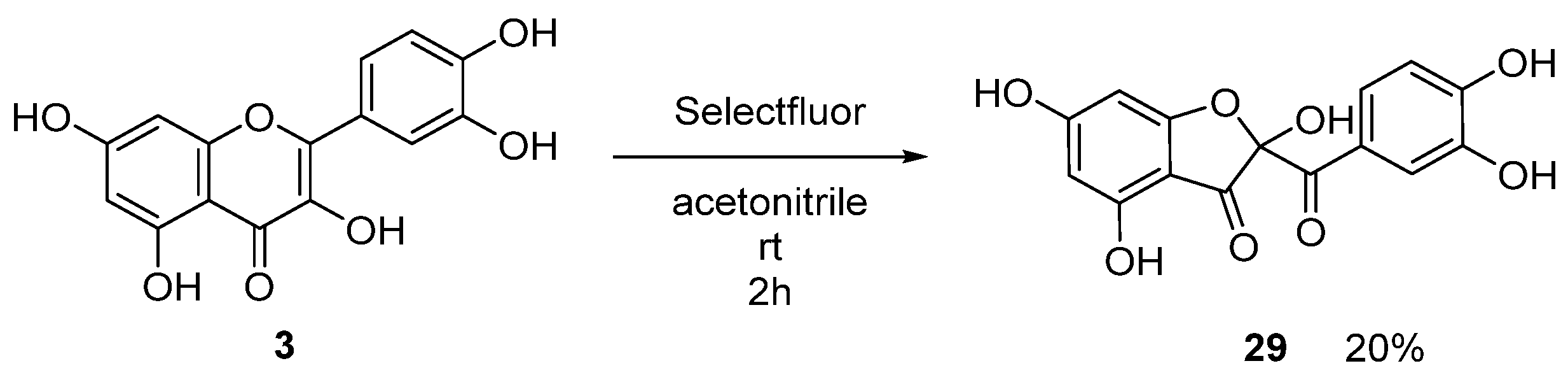
2.1. Chemistry
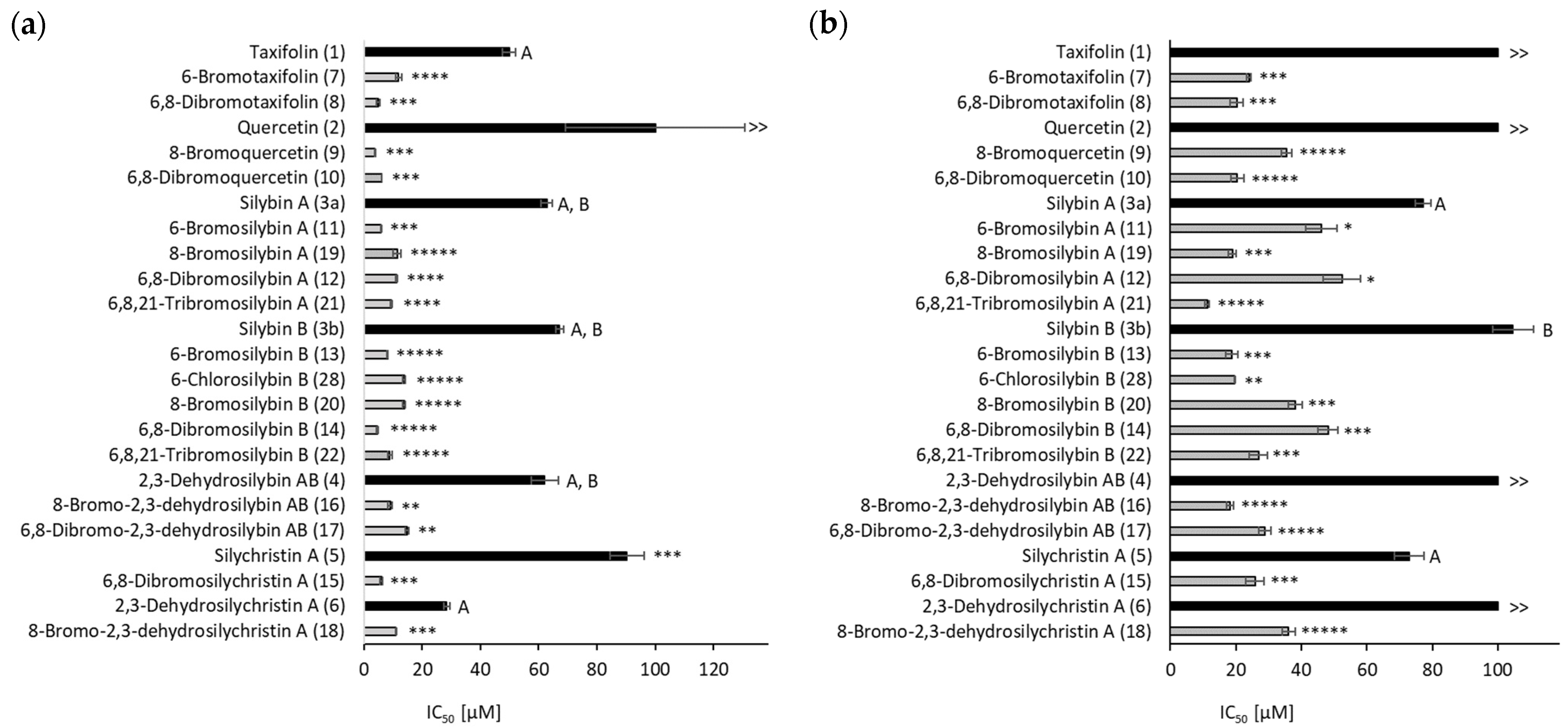
2.2. Biological Activity
2.2.1. Inhibition of Bacterial Communication
2.2.2. Effect on Biofilm Formation
2.2.3. Modulation of Antibiotic-Resistant Phenotype in Resistant Bacteria
2.2.4. Antioxidant Capacity, Reducing Potential and Lipoperoxidation Inhibition
2.2.5. Cytotoxicity and Anti-Inflammatory Activity
3. Materials and Methods
3.1. General Experimental Procedures
3.2. General Procedure A for Tribromination
3.3. General Procedure B for Diiodination
3.4. General Procedure C for Chlorination
3.5. General Procedure D for Selective Dehalogenation
3.6. Inhibition of Quorum Sensing
3.7. Sensitization of Antibiotic-Resistant Bacteria
3.8. Inhibition of Biofilm Formation and Disruption of Maturated Biofilm
3.9. Antioxidant Activity, Reducing Potential, and Lipid Peroxidation Inhibition
3.10. Cytotoxicity
3.11. Inhibition of Nitric Oxide Production
3.12. Sensitization of Doxorubicin-Resistant Human Ovarian Carcinoma Cells
3.13. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, C.; La, M.P.; Tang, H.; Pan, W.H.; Sun, P.; Krohn, K.; Yi, Y.H.; Li, L.; Zhang, W. Bioactive briarane diterpenoids from the South China Sea gorgonian Dichotella gemmacea. Bioorg. Med. Chem. Lett. 2012, 22, 4368–4372. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.J.; Dattelbaum, J.D.; Field, J.J.; Smart, Z.; Woolly, E.F.; Barber, J.M.; Heathcott, R.; Miller, J.H.; Northcote, P.T. Structurally diverse hamigerans from the New Zealand marine sponge Hamigera tarangaensis: NMR-directed isolation, structure elucidation and antifungal activity. Org. Biomol. Chem. 2013, 11, 8041–8051. [Google Scholar] [CrossRef] [PubMed]
- Smitha, D.; Kumar, M.M.K.; Ramana, H.; Rao, D.V. Rubrolide R: A new furanone metabolite from the ascidian Synoicum of the Indian Ocean. Nat. Prod. Res. 2014, 28, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Parker, W.B.; Cheng, Y.C. Metabolism and mechanism of action of 5-fluorouracil. Pharmacol. Ther. 1990, 48, 381–395. [Google Scholar] [CrossRef]
- Senderowicz, A.M. Flavopiridol: The first cyclin-dependent kinase inhibitor in human clinical trials. Investig. New Drugs 1999, 17, 313–320. [Google Scholar] [CrossRef]
- Deep, A.; Marwaha, R.K.; Marwaha, M.G.; Jyoti; Nandal, R.; Sharma, A.K. Flavopiridol as cyclin dependent kinase (CDK) inhibitor: A review. New J. Chem. 2018, 42, 18500–18507. [Google Scholar] [CrossRef]
- Naderi, G.A.; Asgary, S.; Sarraf-Zadegan, N.; Shirvany, H. Anti-oxidant effect of flavonoids on the susceptibility of LDL oxidation. Mol. Cell Biochem. 2003, 246, 193–196. [Google Scholar] [CrossRef]
- Chambers, C.S.; Holečková, V.; Petrásková, L.; Biedermann, D.; Valentová, K.; Buchta, M.; Křen, V. The silymarin composition… and why does it matter? Food Res. Int. 2017, 100, 339–353. [Google Scholar] [CrossRef]
- Křen, V.; Valentová, K. Silybin and its congeners: From traditional medicine to molecular effects. Nat. Prod. Rep. 2022, 39, 1264–1281. [Google Scholar] [CrossRef]
- Biedermann, D.; Vavříková, E.; Cvak, L.; Křen, V. Chemistry of silybin. Nat. Prod. Rep. 2014, 31, 1138–1157. [Google Scholar] [CrossRef]
- Valentová, K.; Biedermann, D.; Křen, V. 2,3-Dehydroderivatives of silymarin flavonolignans: Prospective natural compounds for the prevention of chronic diseases. Proceedings 2019, 11, 21. [Google Scholar] [CrossRef]
- Holasová, K.; Křížkovská, B.; Hoang, L.; Dobiasová, S.; Lipov, J.; Macek, T.; Křen, V.; Valentová, K.; Ruml, T.; Viktorová, J. Flavonolignans from silymarin modulate antibiotic resistance and virulence in Staphylococcus aureus. Biomed. Pharmacother. 2022, 149, 112806. [Google Scholar] [CrossRef] [PubMed]
- Karimova, E.; Baltina, L.; Spirikhin, L.; Gabbasov, T.; Orshanskaya, Y.; Zarubaev, V. Synthesis and antiviral activity of quercetin brominated derivatives. Nat. Prod. Commun. 2015, 10, 1565–1568. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Yang, K.; Ma, Y.; Zhao, X.; Lu, K.; Yu, P. Synthesis of 6- or 8-bromo flavonoids by regioselective mono-bromination and deprotection protocol from flavonoid alkyl ethers. Bull. Korean Chem. Soc. 2015, 36, 1460–1466. [Google Scholar] [CrossRef]
- Kiehlmann, E.; Szczepina, M.G. Epimerization, transacylation and bromination of dihydroquercetin acetates; synthesis of 8-bromodihydroquercetin. Cent. Eur. J. Chem. 2011, 9, 492–498. [Google Scholar] [CrossRef]
- Hurtová, M.; Biedermann, D.; Kuzma, M.; Křen, V. Mild and selective method of bromination of flavonoids. J. Nat. Prod. 2020, 83, 3324–3331. [Google Scholar] [CrossRef]
- Binsack, R.; Boersma, B.J.; Patel, R.P.; Kirk, M.; White, C.R.; Darley-Usmar, V.; Barnes, S.; Zhou, F.; Parks, D.A. Enhanced antioxidant activity after chlorination of quercetin by hypochlorous acid. Alcohol. Clin. Exp. Res. 2001, 25, 434–443. [Google Scholar] [CrossRef]
- Freitas, M.; Ribeiro, D.; Tome, S.M.; Silva, A.M.; Fernandes, E. Synthesis of chlorinated flavonoids with anti-inflammatory and pro-apoptotic activities in human neutrophils. Eur. J. Med. Chem. 2014, 86, 153–164. [Google Scholar] [CrossRef]
- Lu, K.; Chu, J.; Wang, H.; Fu, X.; Quan, D.; Ding, H.; Yao, Q.; Yu, P. Regioselective iodination of flavonoids by N-iodosuccinimide under neutral conditions. Tetrahedron Lett. 2013, 54, 6345–6348. [Google Scholar] [CrossRef]
- Pérez, M.; Ruiz, D.; Autino, J.; Sathicq, A.; Romanelli, G. A very simple solvent-free method for the synthesis of 2-arylchromones using KHSO4 as a recyclable catalyst. Comptes Rendus Chim. 2016, 19, 551–555. [Google Scholar] [CrossRef]
- Zhang, J.W.; Yang, W.W.; Chen, L.L.; Chen, P.; Wang, Y.B.; Chen, D.Y. An efficient tandem synthesis of chromones from o-bromoaryl ynones and benzaldehyde oxime. Org. Biomol. Chem. 2019, 17, 7461–7467. [Google Scholar] [CrossRef] [PubMed]
- Tao, T.; He, C.; Deng, J.; Huang, Y.; Su, Q.; Peng, M.; Yi, M.; Darko, K.O.; Zou, H.; Yang, X. A novel synthetic derivative of quercetin, 8-trifluoromethyl-3,5,7,3′,4′-O-pentamethyl-quercetin, inhibits bladder cancer growth by targeting the AMPK/mTOR signaling pathway. Oncotarget 2017, 8, 71657–71671. [Google Scholar] [CrossRef] [PubMed]
- Proenca, C.; Ribeiro, D.; Soares, T.; Tome, S.M.; Silva, A.M.S.; Lima, J.; Fernandes, E.; Freitas, M. Chlorinated flavonoids modulate the inflammatory process in human blood. Inflammation 2017, 40, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Justino, G.C.; Rodrigues, M.; Florencio, M.H.; Mira, L. Structure and antioxidant activity of brominated flavonols and flavanones. J. Mass Spectrom. 2009, 44, 1459–1468. [Google Scholar] [CrossRef] [PubMed]
- Ahmed-Belkacem, A.; Pozza, A.; Muñoz-Martínez, F.; Bates, S.E.; Castanys, S.; Gamarro, F.; Di Pietro, A.; Pérez-Victoria, J.M. Flavonoid structure-activity studies identify 6-prenylchrysin and tectochrysin as potent and specific inhibitors of breast cancer resistance protein ABCG2. Cancer Res. 2005, 65, 4852–4860. [Google Scholar] [CrossRef]
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef]
- Girard, L. Quorum sensing in Vibrio spp.: The complexity of multiple signalling molecules in marine and aquatic environments. Crit. Rev. Microbiol. 2019, 45, 451–471. [Google Scholar] [CrossRef]
- Paczkowski, J.E.; Mukherjee, S.; McCready, A.R.; Cong, J.P.; Aquino, C.J.; Kim, H.; Henke, B.R.; Smith, C.D.; Bassler, B.L. Flavonoids suppress Pseudomonas aeruginosa virulence through allosteric inhibition of quorum-sensing receptors. J. Biol. Chem. 2017, 292, 4064–4076. [Google Scholar] [CrossRef]
- Manefield, M.; de Nys, R.; Naresh, K.; Roger, R.; Givskov, M.; Peter, S.; Kjelleberg, S. Evidence that halogenated furanones from Delisea pulchra inhibit acylated homoserine lactone (AHL)-mediated gene expression by displacing the AHL signal from its receptor protein. Microbiology 1999, 145, 283–291. [Google Scholar] [CrossRef]
- Soukarieh, F.; Liu, R.; Romero, M.; Roberston, S.N.; Richardson, W.; Lucanto, S.; Oton, E.V.; Qudus, N.R.; Mashabi, A.; Grossman, S.; et al. Hit identification of new potent PqsR antagonists as inhibitors of quorum sensing in planktonic and biofilm grown Pseudomonas aeruginosa. Front. Chem. 2020, 8, 204. [Google Scholar] [CrossRef]
- Lopes, L.A.A.; Dos Santos Rodrigues, J.B.; Magnani, M.; de Souza, E.L.; de Siqueira-Júnior, J.P. Inhibitory effects of flavonoids on biofilm formation by Staphylococcus aureus that overexpresses efflux protein genes. Microb. Pathog. 2017, 107, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Topal, F.; Nar, M.; Gocer, H.; Kalin, P.; Kocyigit, U.M.; Gülçin, İ.; Alwasel, S.H. Antioxidant activity of taxifolin: An activity–structure relationship. J. Enzym. Inhib. Med. Chem. 2016, 31, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Trouillas, P.; Marsal, P.; Svobodová, A.; Vostálová, J.; Gažák, R.; Hrbáč, J.; Sedmera, P.; Křen, V.; Lazzaroni, R.; Duroux, J.-L.; et al. Mechanism of the antioxidant action of silybin and 2,3-dehydrosilybin flavonolignans: A joint experimental and theoretical study. J. Phys. Chem. A 2008, 112, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Viktorová, J.; Dobiasová, S.; Řehořová, K.; Biedermann, D.; Káňová, K.; Šeborová, K.; Václavíková, R.; Valentová, K.; Ruml, T.; Křen, V.; et al. Antioxidant, anti-inflammatory, and multidrug resistance modulation activity of silychristin derivatives. Antioxidants 2019, 8, 303. [Google Scholar] [CrossRef]
- Biedermann, D.; Buchta, M.; Holečková, V.; Sedlák, D.; Valentová, K.; Cvačka, J.; Bednárová, L.; Křenková, A.; Kuzma, M.; Škuta, C.; et al. Silychristin: Skeletal alterations and biological activities. J. Nat. Prod. 2016, 79, 3086–3092. [Google Scholar] [CrossRef]
- Pyszková, M.; Biler, M.; Biedermann, D.; Valentová, K.; Kuzma, M.; Vrba, J.; Ulrichová, J.; Sokolová, R.; Mojović, M.; Popović-Bijelić, A.; et al. Flavonolignan 2,3-dehydroderivatives: Preparation, antiradical and cytoprotective activity. Free Radic. Biol. Med. 2016, 90, 114–125. [Google Scholar] [CrossRef]
- Valentová, K.; Káňová, K.; Di Meo, F.; Pelantová, H.; Chambers, C.; Rydlová, L.; Petrásková, L.; Křenková, A.; Cvačka, J.; Trouillas, P.; et al. Chemoenzymatic preparation and biophysical properties of sulfated quercetin metabolites. Int. J. Mol. Sci. 2017, 18, 2231. [Google Scholar] [CrossRef]
- Dobiasová, S.; Řehořová, K.; Kučerová, D.; Biedermann, D.; Káňová, K.; Petrásková, L.; Koucká, K.; Václavíková, R.; Valentová, K.; Ruml, T.; et al. Multidrug resistance modulation activity of silybin derivatives and their anti-inflammatory potential. Antioxidants 2020, 9, 455. [Google Scholar] [CrossRef]
- Waheed Janabi, A.H.; Kamboh, A.A.; Saeed, M.; Xiaoyu, L.; BiBi, J.; Majeed, F.; Naveed, M.; Mughal, M.J.; Korejo, N.A.; Kamboh, R.; et al. Flavonoid-rich foods (FRF): A promising nutraceutical approach. Iran J. Basic Med. Sci. 2020, 23, 140–153. [Google Scholar]
- Gažák, R.; Marhol, P.; Purchartová, K.; Monti, D.; Biedermann, D.; Riva, S.; Cvak, L.; Křen, V. Large-scale separation of silybin diastereoisomers using lipases. Process Biochem. 2010, 45, 1657–1663. [Google Scholar] [CrossRef]
- Gažák, R.; Trouillas, P.; Biedermann, D.; Fuksová, K.; Marhol, P.; Kuzma, M.; Křen, V. Base-catalyzed oxidation of silybin and isosilybin into 2,3-dehydro derivatives. Tetrahedron Lett. 2013, 54, 315–317. [Google Scholar] [CrossRef]
- Szemerédi, N.; Kincses, A.; Řehořová, K.; Hoang, L.; Salardón-Jiménez, N.; Sevilla-Hernández, C.; Viktorová, J.; Domínguez-Álvarez, E.; Spengler, G. Ketone- and cyano-selenoesters to overcome efflux pump, quorum-sensing, and biofilm-mediated resistance. Antibiotics 2020, 9, 896. [Google Scholar] [CrossRef] [PubMed]
- Hoang, L.; Beneš, F.; Fenclová, M.; Kronusová, O.; Švarcová, V.; Řehořová, K.; Baldassarre Švecová, E.; Vosátka, M.; Hajšlová, J.; Kaštánek, P.; et al. Phytochemical composition and in vitro biological activity of iris spp. (iridaceae): A new source of bioactive constituents for the inhibition of oral bacterial biofilms. Antibiotics 2020, 9, 403. [Google Scholar] [CrossRef]
- Joyeux, M.; Mortier, F.; Fleurentin, J. Screening of antiradical, antilipoperoxidant and hepatoprotective effects of nine plant extracts used in Caribbean folk medicine. Phytother. Res. 1995, 9, 228–230. [Google Scholar] [CrossRef]
- Roubalová, L.; Purchartová, K.; Papoušková, B.; Vacek, J.; Křen, V.; Ulrichová, J.; Vrba, J. Sulfation modulates the cell uptake, antiradical activity and biological effects of flavonoids in vitro: An examination of quercetin, isoquercitrin and taxifolin. Bioorg. Med. Chem. 2015, 23, 5402–5409. [Google Scholar] [CrossRef] [PubMed]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Vavříková, E.; Vacek, J.; Valentová, K.; Marhol, P.; Ulrichová, J.; Kuzma, M.; Křen, V. Chemo-enzymatic synthesis of silybin and 2,3-dehydrosilybin dimers. Molecules 2014, 19, 4115–4134. [Google Scholar] [CrossRef]
- Tran, V.N.; Viktorová, J.; Augustýnková, K.; Jelenová, N.; Dobiasová, S.; Řehořová, K.; Fenclová, M.; Stránská-Zachariášová, M.; Vítek, L.; Hajšlová, J.; et al. In silico and in vitro studies of mycotoxins and their cocktails; their toxicity and its mitigation by silibinin pre-treatment. Toxins 2020, 12, 148. [Google Scholar] [CrossRef]

| Vibrio campbellii BAA 1118 (AI-1) | Vibrio campbellii BAA 1119 (AI-2) | |||||||
|---|---|---|---|---|---|---|---|---|
| EC50 [µM] | IC50 [µM] | SI | SA $ | EC50 [µM] | IC50 [µM] | SI | SA $ | |
| Taxifolin (1) | 63.7 ± 2.7 | 71.0 ± 3.0 | 1.1 ± 0.1 | E | 7.3 ± 0.3 | 80.0 ± 1.7 | 11.0 ± 0.6 | A |
| 6-Bromotaxifolin (7) | 38.9 ± 1.6 | 59.0 ± 0.1 | 1.5 ± 0.1 | * | 11.9 ± 0.5 | 19.7 ± 1.0 | 1.7 ± 0.2 | *** |
| 6,8-Dibromotaxifolin (8) | 38.1 ± 2.0 | 55.5 ± 1.0 | 1.5 ± 0.1 | * | 10.1 ± 0.4 | 109.3 ± 0.8 | 10.8 ± 0.6 | |
| Quercetin (2) | 15.9 ± 0.5 | 24.5 ± 0.8 | 1.5 ± 0.1 | C, D | 4.8 ± 0.2 | 10.6 ± 0.5 | 2.2 ± 0.2 | C |
| 8-Bromoquercetin (9) | 25.6 ± 0.5 | 27.8 ± 0.5 | 1.08 ± 0.04 | ***** | 0.4 ± 0.02 | 1.1 ± 0.1 | 2.9 ± 0.3 | ***** |
| 6,8-Dibromoquercetin (10) | 7.3 ± 0.4 | 13.8 ± 0.5 | 1.9 ± 0.2 | ** | 0.7 ± 0.02 | 3.5 ± 0.1 | 5.0 ± 0.3 | ** |
| Silybin A (3a) | 97.1 ± 1.0 | 131.0 ± 3.7 | 1.3 ± 0.1 | D | 17.1 ± 0.5 | 119.6 ± 3.1 | 7.0 ± 0.4 | B |
| 6-Bromosilybin A (11) | 24.4 ± 0.7 | 51.7 ± 0.7 | 2.1 ± 0.1 | ***** | 9.4 ± 0.5 | 51.5 ± 0.6 | 5.5 ± 0.3 | * |
| 8-Bromosilybin A (19) | 33.9 ± 1.8 | 114.9 ± 5.4 | 3.4 ± 0.3 | *** | 4.6 ± 0.3 | >200 | ≥43.1 ± 2.6 | *** |
| 6,8-Dibromosilybin A (12) | 12.2 ± 0.4 | 23.5 ± 1.0 | 1.9 ± 0.2 | *** | 13.2 ± 0.3 | 80.5 ± 4.8 | 6.1 ± 0.5 | |
| 6,8,21-Tribromosilybin A (21) | >200 | >200 | 30.7 ± 1.6 | >200 | ≥6.5 ± 0.3 | |||
| Silybin B (3b) | 121.5 ± 0.6 | 116.6 ± 0.9 | 0.96 ± 0.01 | E | 22.3 ± 0.5 | 152.4 ± 2.4 | 6.8 ± 0.2 | B |
| 6-Bromosilybin B (13) | 14.5 ± 0.5 | 16.1 ± 0.1 | 1.11 ± 0.04 | * | 26.1 ± 1.3 | 52.7 ± 0.6 | 2.0 ± 0.1 | ***** |
| 6-Chlorosilybin B (28) | 114.2 ± 5.0 | 130.1 ± 5.9 | 1.1 ± 0.1 | 3.2 ± 0.2 | >200 | ≥62.6 ± 3.6 | *** | |
| 8-Bromosilybin B (20) | 44.2 ± 0.7 | 126.6 ± 4.0 | 2.9 ± 0.1 | *** | 4.7 ± 0.2 | 200 | ≥42.9 ± 2.1 | *** |
| 6,8-Dibromosilybin B (14) | 14.7 ± 0.7 | 16.6 ± 0.8 | 1.1 ± 0.1 | 19.3 ± 0.5 | 60.2 ± 3.4 | 3.1 ± 0.3 | ***** | |
| 6,8,21-Tribromosilybin B (22) | 4.3 ± 0.3 | 7.3 ± 0.1 | 1.7 ± 0.1 | * | 12.0 ± 0.5 | 34.4 ± 1.8 | 2.9 ± 0.3 | **** |
| 2,3-Dehydrosilybin AB (4) | 59.9 ± 2.0 | 163.2 ± 3.8 | 2.7 ± 0.2 | A | 10.2 ± 0.4 | 25.3 ± 1.0 | 2.5 ± 0.2 | C |
| 8-Bromo-2,3-dehydrosilybin AB (16) | >200 | >200 | 6.9 ± 0.3 | 87.0 ± 2.5 | 12.7 ± 1.0 | ***** | ||
| 6,8-Dibromo-2,3-dehydrosilybin AB (17) | 55.5 ± 1.6 | >200 | ≥3.6 ± 0.1 | *** | 4.1 ± 0.2 | 126.8 ± 7.4 | 31.2 ± 3.5 | **** |
| Silychristin A (5) | >200 | >200 | >200 | >200 | ||||
| 6,8-Dibromosilychristin A (15) | >200 | >200 | >200 | >200 | ||||
| 2,3-dehydrosilychristin A (6) | 22.3 ± 1.0 | 43.0 ± 1.5 | 1.9 ± 0.2 | B | 11.1 ± 0.1 | 11.0 ± 0.4 | 0.99 ± 0.05 | D |
| 8-Bromo-2,3-dehydrosilychristin A (18) | 29.4 ± 0.7 | 80.1 ± 1.7 | 2.7 ± 0.1 | ** | 10.5 ± 0.2 | 23.0 ± 0.2 | 2.2 ± 0.1 | ***** |
| 5-Fluorouracil (PC) | (0.39 ± 0.05) × 10−3 | (0.68 ± 0.01) × 10−3 | 1.73 ± 0.05 | B, C | (0.44 ± 0.03) × 10−3 | (3.41 ± 0.03) × 10−3 | 7.8 ± 0.5 | B |
| Pseudomonas aeruginosa | Staphylococcus aureus | |||
|---|---|---|---|---|
| Imipenem | Colistin | Chloramphenicol | Gentamicin | |
| 4 mg/L | 2 mg/L | 8 mg/L | 1 mg/L | |
| Taxifolin (1) | - | - | - | 37 ± 1% |
| 6-Bromotaxifolin (7) | - | - | - | 46 ± 4% |
| 6,8-Dibromotaxifolin (8) | - | - | - | 54 ± 5% |
| Quercetin (2) | - | - | - | - |
| 8-Bromoquercetin (9) | - | - | - | - |
| 6,8-Dibromoquercetin (10) | - | - | - | - |
| Silybin A (3a) | - | - | - | - |
| 6-Bromosilybin A (11) | - | - | - | 37 ± 1% |
| 8-Bromosilybin A (19) | - | - | - | 41 ± 3% |
| 6,8-Dibromosilybin A (12) | - | - | - | 40 ± 1% |
| 6,8,21-Tribromosilybin A (21) | - | - | - | reversion of resistance |
| Silybin B (3b) | - | - | - | - |
| 6-Bromosilybin B (13) | - | - | - | 67 ± 4% |
| 6-Chlorosilybin B (28) | - | - | - | 34 ± 2% |
| 8-Bromosilybin B (20) | - | - | - | 48 ± 9% |
| 6,8-Dibromosilybin B (14) | - | - | - | 41 ± 1% |
| 6,8,21-Tribromosilybin B (22) | - | - | - | reversion of resistance |
| 2,3-Dehydrosilybin AB (4) | - | - | - | reversion of resistance [5 µM] |
| 8-Bromo-2,3-dehydrosilybin AB (16) | - | reversion of resistance | - | - |
| 6,8-Dibromo-2,3-dehydrosilybin AB (17) | - | 47 ± 3% | - | - |
| Silychristin A (5) | - | - | - | - |
| 6,8-Dibromosilychristin A (15) | - | - | - | viability 63 ± 3% |
| 2,3-Dehydrosilychristin A (6) | - | - | 69 ± 4% [10 µM] | - |
| 8-Bromo-2,3-dehydrosilychristin A (18) | - | - | - | - |
| Sulbactam (PC) | - | - | reversion of resistance | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hurtová, M.; Káňová, K.; Dobiasová, S.; Holasová, K.; Čáková, D.; Hoang, L.; Biedermann, D.; Kuzma, M.; Cvačka, J.; Křen, V.; et al. Selectively Halogenated Flavonolignans—Preparation and Antibacterial Activity. Int. J. Mol. Sci. 2022, 23, 15121. https://doi.org/10.3390/ijms232315121
Hurtová M, Káňová K, Dobiasová S, Holasová K, Čáková D, Hoang L, Biedermann D, Kuzma M, Cvačka J, Křen V, et al. Selectively Halogenated Flavonolignans—Preparation and Antibacterial Activity. International Journal of Molecular Sciences. 2022; 23(23):15121. https://doi.org/10.3390/ijms232315121
Chicago/Turabian StyleHurtová, Martina, Kristýna Káňová, Simona Dobiasová, Kateřina Holasová, Denisa Čáková, Lan Hoang, David Biedermann, Marek Kuzma, Josef Cvačka, Vladimír Křen, and et al. 2022. "Selectively Halogenated Flavonolignans—Preparation and Antibacterial Activity" International Journal of Molecular Sciences 23, no. 23: 15121. https://doi.org/10.3390/ijms232315121
APA StyleHurtová, M., Káňová, K., Dobiasová, S., Holasová, K., Čáková, D., Hoang, L., Biedermann, D., Kuzma, M., Cvačka, J., Křen, V., Viktorová, J., & Valentová, K. (2022). Selectively Halogenated Flavonolignans—Preparation and Antibacterial Activity. International Journal of Molecular Sciences, 23(23), 15121. https://doi.org/10.3390/ijms232315121