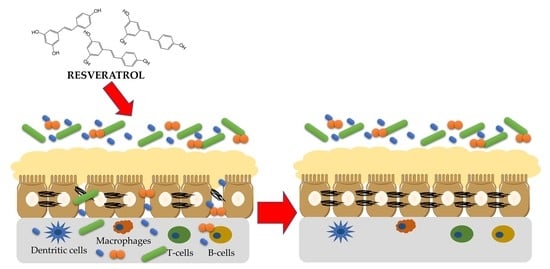
Crosstalk between Resveratrol and Gut Barrier: A Review
Abstract
:1. Introduction
2. Resveratrol—Overview
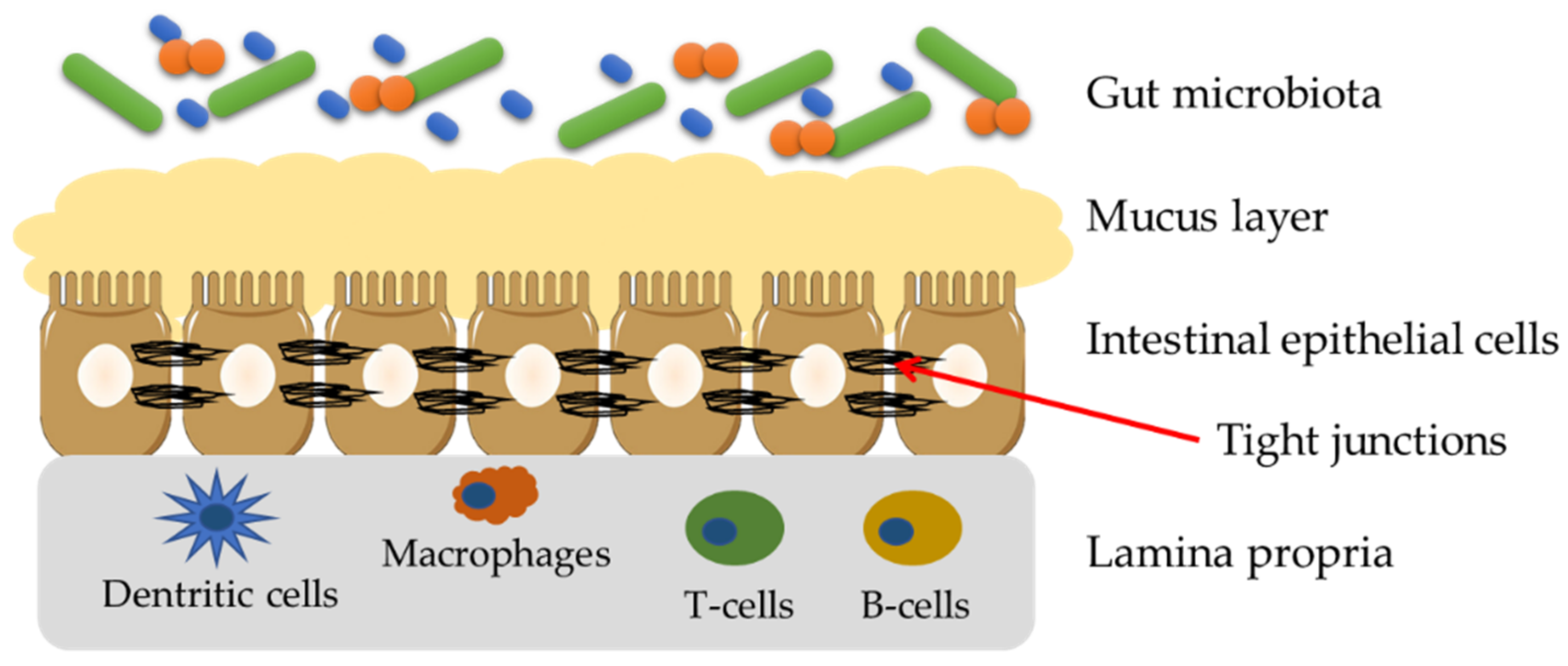
3. Gut Barrier—Structure and Importance
4. Non-Invasive Methods of Assessment of Gut Barrier in Clinical Trails
4.1. Absorption Methods for the Assessment of Gut Barrier Integrity
4.2. Methods for the Assessment of Gut Barrier Integrity Related to Bacterial Metabolism
4.3. Inflammation-Related Markers for the Assessment of Gut Barrier Integrity
4.4. Markers Associated with the Damage of the Mucosa
5. Principles of Polyphenols Action on the Gut Barrier
6. Effect of Resveratrol on the Gut Barrier
6.1. In Vitro Studies
6.2. Animal Studies
6.3. Human Studies
7. Summary and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cutrim, C.S.; Cortez, M.A.S. A Review on Polyphenols: Classification, Beneficial Effects and Their Application in Dairy Products. Int. J. Dairy Technol. 2018, 71, 564–578. [Google Scholar] [CrossRef]
- de Souza, E.L.; de Albuquerque, T.M.R.; dos Santos, A.S.; Massa, N.M.L.; de Brito Alves, J.L. Potential Interactions among Phenolic Compounds and Probiotics for Mutual Boosting of Their Health-Promoting Properties and Food Functionalities—A Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1645–1659. [Google Scholar] [CrossRef]
- Jiang, B.; Guo, L.; Li, B.-Y.; Zhen, J.-H.; Song, J.; Peng, T.; Yang, X.-D.; Hu, Z.; Gao, H.-Q. Resveratrol Attenuates Early Diabetic Nephropathy by Down-Regulating Glutathione S-Transferases Mu in Diabetic Rats. J. Med. Food 2013, 16, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Li, A.N.; Li, S.; Zhang, Y.J.; Xu, X.R.; Chen, Y.M.; Li, H.B. Resources and Biological Activities of Natural Polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef] [Green Version]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Teplova, V.; Isakova, E.P.; Klein, O.I.; Dergachova, D.I.; Gessler, N.N.; Deryabina, Y.I. Natural Polyphenols: Biological Activity, Pharmacological Potential, Means of Metabolic Engineering (Review). Appl. Biochem. Microbiol. 2018, 54, 221–237. [Google Scholar] [CrossRef]
- Kobayashi, S.; Shinohara, M.; Nagai, T.; Konishi, Y. Transport Mechanisms for Soy Isoflavones and Microbial Metabolites Dihydrogenistein and Dihydrodaidzein Across Monolayers and Membranes. Biosci. Biotechnol. Biochem. 2013, 77, 2210–2217. [Google Scholar] [CrossRef] [Green Version]
- Carbonell-Capella, J.M.; Buniowska, M.; Barba, F.J.; Esteve, M.J.; Frígola, A. Analytical Methods for Determining Bioavailability and Bioaccessibility of Bioactive Compounds from Fruits and Vegetables: A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 155–171. [Google Scholar] [CrossRef]
- Zhang, X.; Song, J.; Shi, X.; Miao, S.; Li, Y.; Wen, A. Absorption and Metabolism Characteristics of Rutin in Caco-2 Cells. Sci. World J. 2013, 2013, 382350. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Li, Z.; Wang, L.; Gao, W.; Zhang, Z.; Guo, C. Sodium-Dependent Glucose Transporter 1 and Glucose Transporter 2 Mediate Intestinal Transport of Quercetrin in Caco-2 Cells. Food Nutr. Res. 2020, 64, 3745. [Google Scholar] [CrossRef] [PubMed]
- Kamonpatana, K.; Giusti, M.M.; Chitchumroonchokchai, C.; MorenoCruz, M.; Riedl, K.M.; Kumar, P.; Failla, M.L. Susceptibility of Anthocyanins to Ex Vivo Degradation in Human Saliva. Food Chem. 2012, 135, 738–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Daza, M.C.; Pulido-Mateos, E.C.; Lupien-Meilleur, J.; Guyonnet, D.; Desjardins, Y.; Roy, D. Polyphenol-Mediated Gut Microbiota Modulation: Toward Prebiotics and Further. Front. Nutr. 2021, 8, 689456. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Peng, H. Bioaccessibility and Bioavailability of Phenolic Compounds. J. Food Bioact. 2018, 4, 11–68. [Google Scholar] [CrossRef] [Green Version]
- Cosme, P.; Rodríguez, A.B.; Espino, J.; Garrido, M. Plant Phenolics: Bioavailability as a Key Determinant of Their Potential Health-Promoting Applications. Antioxidants 2020, 9, 1263. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Corrêa, T.A.F.; Rogero, M.M.; Hassimotto, N.M.A.; Lajolo, F.M. The Two-Way Polyphenols-Microbiota Interactions and Their Effects on Obesity and Related Metabolic Diseases. Front. Nutr. 2019, 6, 188. [Google Scholar] [CrossRef] [Green Version]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The Therapeutic Potential of Resveratrol: A Review of Clinical Trials. NPJ Precis. Oncol. 2017, 1, 35. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef] [Green Version]
- Bavaresco, L.; Trevisan, M.; Cinerea, B.; Bavaresco, I.L.; Petegolli, I.D.; Cantu, I.E.; Fregoni, M.; Chiusaz, G.; Trevisan, M. Elicitation and Accumulation of Stilbene Phytoalexins in Grapevine Berries Infected by Botrytis Cinerea. Vitis 2017, 36, 77–83. [Google Scholar]
- Renaud, S.; de Lorgeril, M. Wine, Alcohol, Platelets, and the French Paradox for Coronary Heart Disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Chung, S.; Yao, H.; Caito, S.; Hwang, J.; Arunachalam, G.; Rahman, I. Regulation of SIRT1 in Cellular Functions: Role of Polyphenols. Arch. Biochem. Biophys. 2010, 501, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Vanamala, J.; Reddivari, L.; Radhakrishnan, S.; Tarver, C. Resveratrol Suppresses IGF-1 Induced Human Colon Cancer Cell Proliferation and Elevates Apoptosis via Suppression of IGF-1R/Wnt and Activation of P53 Signaling Pathways. BMC Cancer 2010, 10, 238. [Google Scholar] [CrossRef] [Green Version]
- Park, E.-S.; Lim, Y.; Hong, J.-T.; Yoo, H.-S.; Lee, C.-K.; Pyo, M.-Y.; Yun, Y.-P. Pterostilbene, a Natural Dimethylated Analog of Resveratrol, Inhibits Rat Aortic Vascular Smooth Muscle Cell Proliferation by Blocking Akt-Dependent Pathway. Vascul. Pharmacol. 2010, 53, 61–67. [Google Scholar] [CrossRef]
- Alayev, A.; Doubleday, P.F.; Berger, S.M.; Ballif, B.A.; Holz, M.K. Phosphoproteomics Reveals Resveratrol-Dependent Inhibition of Akt/MTORC1/S6K1 Signaling. J. Proteome Res. 2014, 13, 5734–5742. [Google Scholar] [CrossRef] [Green Version]
- Alayev, A.; Berger, S.M.; Holz, M.K. Resveratrol as a Novel Treatment for Diseases with MTOR Pathway Hyperactivation. Ann. N. Y. Acad. Sci. 2015, 1348, 116–123. [Google Scholar] [CrossRef]
- Walle, T. Bioavailability of Resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef]
- Pannu, N.; Bhatnagar, A. Resveratrol: From Enhanced Biosynthesis and Bioavailability to Multitargeting Chronic Diseases. Biomed. Pharmacother. 2019, 109, 2237–2251. [Google Scholar] [CrossRef]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E.; Walle, U.K. High Absorption but Very Low Bioavailability of Oral Resveratrol in Humans. Drug Metab. Dispos. 2004, 32, 1377. [Google Scholar] [CrossRef] [Green Version]
- Bode, L.M.; Bunzel, D.; Huch, M.; Cho, G.-S.; Ruhland, D.; Bunzel, M.; Bub, A.; Franz, C.M.A.P.; Kulling, S.E. In Vivo and in Vitro Metabolism of Trans-Resveratrol by Human Gut Microbiota. Am. J. Clin. Nutr. 2013, 97, 295–309. [Google Scholar] [CrossRef] [Green Version]
- Candeliere, F.; Raimondi, S.; Ranieri, R.; Musmeci, E.; Zambon, A.; Amaretti, A.; Rossi, M. β-Glucuronidase Pattern Predicted From Gut Metagenomes Indicates Potentially Diversified Pharmacomicrobiomics. Front. Microbiol. 2022, 13, 826994. [Google Scholar] [CrossRef] [PubMed]
- Kocot, A.M.; Jarocka-Cyrta, E.; Drabińska, N. Overview of the Importance of Biotics in Gut Barrier Integrity. Int. J. Mol. Sci. 2022, 23, 2896. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M. Human Intestinal Barrier: Effects of Stressors, Diet, Prebiotics, and Probiotics. Clin. Transl. Gastroenterol. 2021, 12, e00308. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-Y.; Osaka, T.; Moriyama, E.; Date, Y.; Kikuchi, J.; Tsuneda, S. Strengthening of the Intestinal Epithelial Tight Junction by Bifidobacterium Bifidum. Physiol. Rep. 2015, 3, e12327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assimakopoulos, S.F.; Triantos, C.; Maroulis, I.; Gogos, C. The Role of the Gut Barrier Function in Health and Disease. Gastroenterol. Res. 2018, 11, 261–263. [Google Scholar] [CrossRef] [Green Version]
- Rao, A.S.; Camilleri, M.; Eckert, D.J.; Busciglio, I.; Burton, D.D.; Ryks, M.; Wong, B.S.; Lamsam, J.; Singh, R.; Zinsmeister, A.R. Urine Sugars for in Vivo Gut Permeability: Validation and Comparisons in Irritable Bowel Syndrome-Diarrhea and Controls. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G919–G928. [Google Scholar] [CrossRef]
- Ordiz, M.I.; Davitt, C.; Stephenson, K.; Agapova, S.; Divala, O.; Shaikh, N.; Manary, M.J. EB 2017 Article: Interpretation of the Lactulose:Mannitol Test in Rural Malawian Children at Risk for Perturbations in Intestinal Permeability. Exp. Biol. Med. 2018, 243, 677–683. [Google Scholar] [CrossRef]
- Musa, M.A.; Kabir, M.; Hossain, M.I.; Ahmed, E.; Siddique, A.; Rashid, H.; Mahfuz, M.; Mondal, D.; Ahmed, T.; Petri, W.A.; et al. Measurement of Intestinal Permeability Using Lactulose and Mannitol with Conventional Five Hours and Shortened Two Hours Urine Collection by Two Different Methods: HPAE-PAD and LC-MSMS. PLoS ONE 2019, 14, e0220397. [Google Scholar] [CrossRef] [Green Version]
- Drabińska, N.; Krupa-kozak, U.; Jarocka-cyrta, E. Intestinal Permeability in Children with Celiac Disease after the Administration of Oligofructose-Enriched Inulin into a Gluten-Free Diet—Results of a Randomized, Placebo-Controlled, Pilot Trial. Nutrients 2020, 12, 1736. [Google Scholar] [CrossRef]
- Grootjans, J.; Thuijls, G.; Verdam, F.; Derikx, J.P.; Lenaerts, K.; Buurman, W.A. Non-Invasive Assessment of Barrier Integrity and Function of the Human Gut. World J. Gastrointest Surg. 2010, 2, 61–69. [Google Scholar] [CrossRef]
- van Wijck, K.; Verlinden, T.J.M.; van Eijk, H.M.H.; Dekker, J.; Buurman, W.A.; Dejong, C.H.C.; Lenaerts, K. Novel Multi-Sugar Assay for Site-Specific Gastrointestinal Permeability Analysis: A Randomized Controlled Crossover Trial. Clin. Nutr. 2013, 32, 245–251. [Google Scholar] [CrossRef]
- Wang, L.; Llorente, C.; Hartmann, P.; Yang, A.-M.; Chen, P.; Schnabl, B. Methods to Determine Intestinal Permeability and Bacterial Translocation during Liver Disease. J. Immunol. Methods 2015, 421, 44–53. [Google Scholar] [CrossRef] [Green Version]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal Permeability—A New Target for Disease Prevention and Therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.M.; Eng, R.H.K.; Buccini, F. Use of D-Lactic Acid Measurements in the Diagnosis of Bacterial Infections. J. Infect. Dis. 1986, 154, 658–664. [Google Scholar] [CrossRef]
- Herrera, D.J.; Morris, K.; Johnston, C.; Griffiths, P. Automated Assay for Plasma D-Lactate by Enzymatic Spectrophotometric Analysis with Sample Blank Correction. Ann. Clin. Biochem. 2008, 45, 177–183. [Google Scholar] [CrossRef]
- Fasano, A.; Not, T.; Wang, W.; Uzzau, S.; Berti, I.; Tommasini, A.; Goldblum, S.E. Zonulin, a Newly Discovered Modulator of Intestinal Permeability, and Its Expression in Coeliac Disease. Lancet 2000, 355, 1518–1519. [Google Scholar] [CrossRef]
- Tripathi, A.; Lammers, K.M.; Goldblum, S.; Shea-Donohue, T.; Netzel-Arnett, S.; Buzza, M.S.; Antalis, T.M.; Vogel, S.N.; Zhao, A.; Yang, S.; et al. Identification of Human Zonulin, a Physiological Modulator of Tight Junctions, as Prehaptoglobin-2. Proc. Natl. Acad. Sci. USA 2009, 106, 16799–16804. [Google Scholar] [CrossRef] [Green Version]
- Goldblum, S.E.; Rai, U.; Tripathi, A.; Thakar, M.; de Leo, L.; di Toro, N.; Not, T.; Ramachandran, R.; Puche, A.C.; Hollenberg, M.D.; et al. The Active Zot Domain (Aa 288–293) Increases ZO-1 and Myosin 1C Serine/Threonine Phosphorylation, Alters Interaction between ZO-1 and Its Binding Partners, and Induces Tight Junction Disassembly through Proteinase Activated Receptor 2 Activation. FASEB J. 2011, 25, 144–158. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Navarrete, J.M.; Sabater, M.; Ortega, F.; Ricart, W.; Fernández-Real, J.M. Circulating Zonulin, a Marker of Intestinal Permeability, Is Increased in Association with Obesity-Associated Insulin Resistance. PLoS ONE 2012, 7, e37160. [Google Scholar] [CrossRef] [Green Version]
- Rangachari, P.K. Histamine: Mercurial Messenger in the Gut. Am. J. Physiol.-Gastrointest. Liver Physiol. 1992, 262, G1–G13. [Google Scholar] [CrossRef]
- Luk, G.D.; Bayless, T.M.; Baylin, S.B. Diamine Oxidase (Histaminase). A Circulating Marker for Rat Intestinal Mucosal Maturation and Integrity. J. Clin. Investig. 1980, 66, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Honzawa, Y.; Nakase, H.; Matsuura, M.; Chiba, T. Clinical Significance of Serum Diamine Oxidase Activity in Inflammatory Bowel Disease: Importance of Evaluation of Small Intestinal Permeability. Inflamm. Bowel. Dis. 2011, 17, E23–E25. [Google Scholar] [CrossRef] [PubMed]
- Pathirana, W.G.W.; Chubb, S.P.; Gillett, M.J.; Vasikaran, S.D. Faecal Calprotectin. Clin. Biochem. Rev. 2018, 39, 77–90. [Google Scholar] [PubMed]
- Malcomson, F.C.; Willis, N.D.; McCallum, I.; Xie, L.; Ibero-Baraibar, I.; Leung, W.C.; Kelly, S.; Bradburn, D.M.; Belshaw, N.J.; Johnson, I.T.; et al. Effects of Supplementation with Nondigestible Carbohydrates on Fecal Calprotectin and on Epigenetic Regulation of SFRP1 Expression in the Large-Bowel Mucosa of Healthy Individuals. Am. J. Clin. Nutr. 2017, 105, 400–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casellas, F.; Borruel, N.; Torrejón, A.; Varela, E.; Antolin, M.; Guarner, F.; Malagelada, J.R. Oral Oligofructose-Enriched Inulin Supplementation in Acute Ulcerative Colitis Is Well Tolerated and Associated with Lowered Faecal Calprotectin. Aliment. Pharm. Ther. 2007, 25, 1061–1067. [Google Scholar] [CrossRef]
- Montalto, M.; Santoro, L.; Curigliano, V.; D’Onofrio, F.; Cammarota, G.; Panunzi, S.; Ricci, R.; Gallo, A.; Grieco, A.; Gasbarrini, A.; et al. Faecal Calprotectin Concentrations in Untreated Coeliac Patients. Scand. J. Gastroenterol. 2007, 42, 957–961. [Google Scholar] [CrossRef]
- D’Amico, F.; Nancey, S.; Danese, S.; Peyrin-Biroulet, L. A Practical Guide for Faecal Calprotectin Measurement: Myths and Realities. J. Crohns. Colitis. 2020, 15, 152–161. [Google Scholar] [CrossRef]
- Kosek, M.; Haque, R.; Lima, A.; Babji, S.; Shrestha, S.; Qureshi, S.; Amidou, S.; Mduma, E.; Lee, G.; Yori, P.P.; et al. Fecal Markers of Intestinal Inflammation and Permeability Associated with the Subsequent Acquisition of Linear Growth Deficits in Infants. Am. Soc. Trop. Med. Hyg. 2013, 88, 390–396. [Google Scholar] [CrossRef] [Green Version]
- Alam, A.N.; Sarker, S.A.; Wahed, M.A.; Khatun, M.; Rahaman, M.M. Enteric Protein Loss and Intestinal Permeability Changes in Children during Acute Shigellosis and after Recovery: Effect of Zinc Supplementation. Gut 1994, 35, 1707. [Google Scholar] [CrossRef] [Green Version]
- Sharpstone, D.; Rowbottom, A.; Nelson, M.; Gazzard, B. Faecal Alpha 1 Antitrypsin as a Marker of Gastrointestinal Disease in HIV Antibody Positive Individuals. Gut 1996, 38, 206. [Google Scholar] [CrossRef] [Green Version]
- Gondolesi, G.; Ghirardo, S.; Raymond, K.; Hoppenhauer, L.; Surillo, D.; Rumbo, C.; Fishbein, T.; Sansaricq, C.; Sauter, B. The Value of Plasma Citrulline to Predict Mucosal Injury in Intestinal Allografts. Am. J. Transplant. 2006, 6, 2786–2790. [Google Scholar] [CrossRef]
- Ceballos, I.; Chauveau, P.; Guerin, V.; Bardet, J.; Parvy, P.; Kamoun, P.; Jungers, P. Early Alterations of Plasma Free Amino Acids in Chronic Renal Failure. Clin. Chim. Acta 1990, 188, 101–108. [Google Scholar] [CrossRef]
- Wu, G.; Fang, Y.-Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione Metabolism and Its Implications for Health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef] [Green Version]
- McMonagle, M.P.; Halpenny, M.; McCarthy, A.; Mortell, A.; Manning, F.; Kilty, C.; Mannion, D.; Wood, A.E.; Corbally, M.T. Glutathione-S-Transferase: A Potential Marker of Ischemia-Reperfusion Injury of the Intestine after Cardiac Surgery? J. Pediatr. Surg. 2006, 41, 1526–1531. [Google Scholar] [CrossRef]
- Niewold, T.A.; Meinen, M.; van der Meulen, J. Plasma Intestinal Fatty Acid Binding Protein (I-FABP) Concentrations Increase Following Intestinal Ischemia in Pigs. Res. Vet. Sci. 2004, 77, 89–91. [Google Scholar] [CrossRef]
- van de Poll, M.C.G.; Derikx, J.P.M.; Buurman, W.A.; Peters, W.H.M.; Roelofs, H.M.J.; Wigmore, S.J.; Dejong, C.H.C. Liver Manipulation Causes Hepatocyte Injury and Precedes Systemic Inflammation in Patients Undergoing Liver Resection. World J. Surg. 2007, 31, 2033–2038. [Google Scholar] [CrossRef] [Green Version]
- Derikx, J.P.M.; Blijlevens, N.M.A.; Donnelly, J.P.; Fujii, H.; Kanda, T.; van Bijnen, A.A.; Heineman, E.; Buurman, W.A. Loss of Enterocyte Mass Is Accompanied by Diminished Turnover of Enterocytes after Myeloablative Therapy in Haematopoietic Stem-Cell Transplant Recipients. Ann. Oncol. 2009, 20, 337–342. [Google Scholar] [CrossRef]
- Adriaanse, M.P.M.; Tack, G.J.; Passos, V.L.; Damoiseaux, J.G.M.C.; Schreurs, M.W.J.; van Wijck, K.; Riedl, R.G.; Masclee, A.A.M.; Buurman, W.A.; Mulder, C.J.J.; et al. Serum I-FABP as Marker for Enterocyte Damage in Coeliac Disease and Its Relation to Villous Atrophy and Circulating Autoantibodies. Aliment. Pharmacol. Ther. 2013, 37, 482–490. [Google Scholar] [CrossRef]
- Guthmann, F.; Börchers, T.; Wolfrum, C.; Wustrack, T.; Bartholomäus, S.; Spener, F. Plasma Concentration of Intestinal- and Liver-FABP in Neonates Suffering from Necrotizing Enterocolitis and in Healthy Preterm Neonates. Mol. Cell Biochem. 2002, 239, 227–234. [Google Scholar] [CrossRef]
- Guerreiro, C.S.; Calado, Â.; Sousa, J.; Fonseca, J.E. Diet, Microbiota, and Gut Permeability—The Unknown Triad in Rheumatoid Arthritis. Front. Med. 2018, 5, 349. [Google Scholar] [CrossRef] [Green Version]
- de Filippo, C.; Cavalieri, D.; di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of Diet in Shaping Gut Microbiota Revealed by a Comparative Study in Children from Europe and Rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Luo, X.; Tang, J.; Mo, Q.; Zhong, H.; Zhang, H.; Feng, F. A Bridge for Short-Chain Fatty Acids to Affect Inflammatory Bowel Disease, Type 1 Diabetes, and Non-Alcoholic Fatty Liver Disease Positively: By Changing Gut Barrier. Eur. J. Nutr. 2021, 60, 2317–2330. [Google Scholar] [CrossRef] [PubMed]
- Mrduljaš, N.; Krešić, G.; Bilušić, T. Polyphenols: Food Sources and Health Benefits. In Functional Food; Hueda, M.C., Ed.; IntechOpen: Rijeka, Croatia, 2017; Chapter 2; ISBN 978-953-51-3440-4. [Google Scholar]
- Bernardi, S.; del Bo’, C.; Marino, M.; Gargari, G.; Cherubini, A.; Andrés-Lacueva, C.; Hidalgo-Liberona, N.; Peron, G.; González-Dominguez, R.; Kroon, P.; et al. Polyphenols and Intestinal Permeability: Rationale and Future Perspectives. J. Agric. Food Chem. 2020, 68, 1816–1829. [Google Scholar] [CrossRef] [PubMed]
- Kaulmann, A.; Bohn, T. Bioactivity of Polyphenols: Preventive and Adjuvant Strategies toward Reducing Inflammatory Bowel Diseases—Promises, Perspectives, and Pitfalls. Oxid. Med. Cell Longev. 2016, 2016, 9346470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luescher, S.; Urmann, C.; Butterweck, V. Effect of Hops Derived Prenylated Phenols on TNF-α Induced Barrier Dysfunction in Intestinal Epithelial Cells. J. Nat. Prod. 2017, 80, 925–931. [Google Scholar] [CrossRef]
- Das, J.; Ramani, R.; Suraju, M.O. Polyphenol Compounds and PKC Signaling. Biochim. Biophys. Acta Gen. Subj. 2016, 1860, 2107–2121. [Google Scholar] [CrossRef] [Green Version]
- Boto-Ordóñez, M.; Urpi-Sarda, M.; Queipo-Ortuño, M.I.; Tulipani, S.; Tinahones, F.J.; Andres-Lacueva, C. High Levels of Bifidobacteria Are Associated with Increased Levels of Anthocyanin Microbial Metabolites: A Randomized Clinical Trial. Food Funct. 2014, 5, 1932–1938. [Google Scholar] [CrossRef] [Green Version]
- Queipo-Ortuño, M.I.; Boto-Ordóñez, M.; Murri, M.; Gomez-Zumaquero, J.M.; Clemente-Postigo, M.; Estruch, R.; Cardona Diaz, F.; Andrés-Lacueva, C.; Tinahones, F.J. Influence of Red Wine Polyphenols and Ethanol on the Gut Microbiota Ecology and Biochemical Biomarkers. Am. J. Clin. Nutr. 2012, 95, 1323–1334. [Google Scholar] [CrossRef] [Green Version]
- Mosele, J.I.; Macià, A.; Motilva, M.J. Metabolic and Microbial Modulation of the Large Intestine Ecosystem by Non-Absorbed Diet Phenolic Compounds: A Review. Molecules 2015, 20, 17429–17468. [Google Scholar] [CrossRef] [Green Version]
- Edwards, C.A.; Havlik, J.; Cong, W.; Mullen, W.; Preston, T.; Morrison, D.J.; Combet, E. Polyphenols and Health: Interactions between Fibre, Plant Polyphenols and the Gut Microbiota. Nutr. Bull. 2017, 42, 356–360. [Google Scholar] [CrossRef]
- Carrasco-Pozo, C.; Morales, P.; Gotteland, M. Polyphenols Protect the Epithelial Barrier Function of Caco-2 Cells Exposed to Indomethacin through the Modulation of Occludin and Zonula Occludens-1 Expression. J. Agric. Food Chem. 2013, 61, 5291–5297. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Han, Q.; Wang, G.; Ma, W.P.; Wang, J.; Wu, W.X.; Guo, Y.; Liu, L.; Jiang, X.Y.; Xie, X.L.; et al. Resveratrol Protects Oxidative Stress-Induced Intestinal Epithelial Barrier Dysfunction by Upregulating Heme Oxygenase-1 Expression. Dig. Dis. Sci. 2016, 61, 2522–2534. [Google Scholar] [CrossRef]
- Jo, H.A.; Hwang, D.; Kim, J.K.; Lim, Y.H. Oxyresveratrol Improves Tight Junction Integrity through the PKC and MAPK Signaling Pathways in Caco-2 Cells. Food Chem. Toxicol. 2017, 108, 203–213. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Liu, X.; Yin, J.; Li, X.; Zhang, X.; Xing, X.; Wang, J.; Wang, S. Differential Protective Effect of Resveratrol and Its Microbial Metabolites on Intestinal Barrier Dysfunction Is Mediated by the AMPK Pathway. J. Agric. Food Chem. 2022, 70, 11301–11313. [Google Scholar] [CrossRef] [PubMed]
- Ling, K.H.; Wan, M.L.Y.; El-Nezami, H.; Wang, M. Protective Capacity of Resveratrol, a Natural Polyphenolic Compound, against Deoxynivalenol-Induced Intestinal Barrier Dysfunction and Bacterial Translocation. Chem. Res. Toxicol. 2016, 29, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Horn, N.; Ajuwon, K.M. Mechanisms of Deoxynivalenol-Induced Endocytosis and Degradation of Tight Junction Proteins in Jejunal IPEC-J2 Cells Involve Selective Activation of the MAPK Pathways. Arch. Toxicol. 2021, 95, 2065–2079. [Google Scholar] [CrossRef]
- Ergün, O.; Ergün, G.; Öktem, G.; Selvi, N.; Doǧan, H.; Tunçyürek, M.; Saydam, G.; Erdener, A. Enteral Resveratrol Supplementation Attenuates Intestinal Epithelial Inducible Nitric Oxide Synthase Activity and Mucosal Damage in Experimental Necrotizing Enterocolitis. J. Pediatr. Surg. 2007, 42, 1687–1694. [Google Scholar] [CrossRef]
- Etxeberria, U.; Arias, N.; Boqué, N.; Macarulla, M.T.; Portillo, M.P.; Martínez, J.A.; Milagro, F.I. Reshaping Faecal Gut Microbiota Composition by the Intake of Trans-Resveratrol and Quercetin in High-Fat Sucrose Diet-Fed Rats. J. Nutr. Biochem. 2015, 26, 651–660. [Google Scholar] [CrossRef]
- Siu, F.Y.K.; Ye, S.; Lin, H.; Li, S. Galactosylated PLGA Nanoparticles for the Oral Delivery of Resveratrol: Enhanced Bioavailability and in Vitro Anti-Inflammatory Activity. Int. J. Nanomed. 2018, 13, 4133–4144. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Hou, P.; Zhou, M.; Ren, Q.; Wang, X.; Huang, L.; Hui, S.; Yi, L.; Mi, M. Resveratrol Attenuates High-Fat Diet-Induced Non-Alcoholic Steatohepatitis by Maintaining Gut Barrier Integrity and Inhibiting Gut Inflammation through Regulation of the Endocannabinoid System. Clin. Nutr. 2020, 39, 1264–1275. [Google Scholar] [CrossRef]
- Bereswill, S.; Muñoz, M.; Fischer, A.; Plickert, R.; Haag, L.M.; Otto, B.; Kühl, A.A.; Loddenkemper, C.; Göbel, U.B.; Heimesaat, M.M. Anti-Inflammatory Effects of Resveratrol, Curcumin and Simvastatin in Acute Small Intestinal Inflammation. PLoS ONE 2010, 5, e15099. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.T.; Ye, X.L.; Li, R.R.; Chen, H.; Wang, Y.Y.; Yong, H.J.; Pan, M.L.; Lu, W.; Tang, Y.; Miao, H.; et al. Resveratrol Modulates the Gut Microbiota and Inflammation to Protect Against Diabetic Nephropathy in Mice. Front. Pharmacol. 2020, 11, 1249. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhao, H.; Shu, L.; Xing, H.; Wang, C.; Lu, C.; Song, G. Effect of Resveratrol on Intestinal Tight Junction Proteins and the Gut Microbiome in High-Fat Diet-Fed Insulin Resistant Mice. Int. J. Food Sci. Nutr. 2020, 71, 965–978. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, J.; Li, D.; Ke, W.; Chen, F.; Hu, X. Targeting the Gut Microbiota with Resveratrol: A Demonstration of Novel Evidence for the Management of Hepatic Steatosis. J. Nutr. Biochem. 2020, 81, 108363. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, Y.; Lv, H.; Pang, W.; Wang, J.; Ma, H.; Wang, S. Intestinal Pharmacokinetics of Resveratrol and Regulatory Effects of Resveratrol Metabolites on Gut Barrier and Gut Microbiota. Food Chem. 2021, 357, 129532. [Google Scholar] [CrossRef]
- Hao, W.; Zhu, X.; Liu, Z.; Song, Y.; Wu, S.; Lu, X.; Yang, J.; Jin, C. Resveratrol Alleviates Aluminum-Induced Intestinal Barrier Dysfunction in Mice. Environ. Toxicol. 2022, 37, 1373–1381. [Google Scholar] [CrossRef]
- Song, X.; Liu, L.; Peng, S.; Liu, T.; Chen, Y.; Jia, R.; Zou, Y.; Li, L.; Zhao, X.; Liang, X.; et al. Resveratrol Regulates Intestinal Barrier Function in Cyclophosphamide-Induced Immunosuppressed Mice. J. Sci. Food Agric. 2022, 102, 1205–1215. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Y.; Jin, S.; Pang, Q.; Shan, A.; Feng, X. Dietary Resveratrol Alleviated Lipopolysaccharide-Induced Ileitis through Nrf2 and NF-ΚB Signalling Pathways in Ducks (Anas Platyrhynchos). J. Anim. Physiol. Anim. Nutr. 2022, 106, 1306–1320. [Google Scholar] [CrossRef]
- Alharris, E.; Mohammed, A.; Alghetaa, H. The Ability of Resveratrol to Attenuate Ovalbumin-Mediated Allergic Asthma Is Associated With Changes in Microbiota Involving the Gut-Lung Axis, Enhanced Barrier Function and Decreased Inflammation in the Lungs. Front. Immunol. 2022, 13, 805770. [Google Scholar] [CrossRef]
- Guglielmetti, S.; Bernardi, S.; del Bo’, C.; Cherubini, A.; Porrini, M.; Gargari, G.; Hidalgo-Liberona, N.; Gonzalez-Dominguez, R.; Peron, G.; Zamora-Ros, R.; et al. Effect of a Polyphenol-Rich Dietary Pattern on Intestinal Permeability and Gut and Blood Microbiomics in Older Subjects: Study Protocol of the MaPLE Randomised Controlled Trial. BMC Geriatr. 2020, 20, 77. [Google Scholar] [CrossRef] [Green Version]
- del Bo’, C.; Bernardi, S.; Cherubini, A.; Porrini, M.; Gargari, G.; Hidalgo-Liberona, N.; González-Domínguez, R.; Zamora-Ros, R.; Peron, G.; Marino, M.; et al. A Polyphenol-Rich Dietary Pattern Improves Intestinal Permeability, Evaluated as Serum Zonulin Levels, in Older Subjects: The MaPLE Randomised Controlled Trial. Clin. Nutr. 2021, 40, 3006–3018. [Google Scholar] [CrossRef]
- Peron, G.; Gargari, G.; Meroño, T.; Miñarro, A.; Lozano, E.V.; Escuder, P.C.; González-Domínguez, R.; Hidalgo-Liberona, N.; del Bo, C.; Bernardi, S.; et al. Crosstalk among Intestinal Barrier, Gut Microbiota and Serum Metabolome after a Polyphenol-Rich Diet in Older Subjects with “Leaky Gut”: The MaPLE Trial. Clin. Nutr. 2021, 40, 5288–5297. [Google Scholar] [CrossRef]
- Walker, J.M.; Eckardt, P.; Aleman, J.O.; Correa da Rosa, J.; Liang, Y.; Iizumi, T.; Etheve, S.; Blaser, M.J.; Breslow, J.L.; Holt, P.R. The Effects of Trans-Resveratrol on Insulin Resistance, Inflammation, and Microbiota in Men with the Metabolic Syndrome: A Pilot Randomized, Placebo Controlled Clinical Trial. J. Clin. Transl. Res. 2018, 4, 122–135. [Google Scholar] [CrossRef]
- Tomé-Carneiro, J.; Larrosa, M.; Yáñez-Gascón, M.J.; Dávalos, A.; Gil-Zamorano, J.; Gonzálvez, M.; García-Almagro, F.J.; Ruiz Ros, J.A.; Tomás-Barberán, F.A.; Espín, J.C.; et al. One-Year Supplementation with a Grape Extract Containing Resveratrol Modulates Inflammatory-Related MicroRNAs and Cytokines Expression in Peripheral Blood Mononuclear Cells of Type 2 Diabetes and Hypertensive Patients with Coronary Artery Disease. Pharmacol. Res. 2013, 72, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, H.; Sia, C.L.; Korzeniewski, K.; Lohano, T.; Abuaysheh, S.; Marumganti, A.; Chaudhuri, A.; Dandona, P. A Resveratrol and Polyphenol Preparation Suppresses Oxidative and Inflammatory Stress Response to a High-Fat, High-Carbohydrate Meal. J. Clin. Endocrinol. Metab. 2011, 96, 1409–1414. [Google Scholar] [CrossRef] [Green Version]
- Apostolidou, C.; Adamopoulos, K.; Iliadis, S.; Kourtidou-Papadeli, C. Alterations of Antioxidant Status in Asymptomatic Hypercholesterolemic Individuals after Resveratrol Intake. Int. J. Food Sci. Nutr. 2016, 67, 541–552. [Google Scholar] [CrossRef]
- Most, J.; Penders, J.; Lucchesi, M.; Goossens, G.H.; Blaak, E.E. Gut Microbiota Composition in Relation to the Metabolic Response to 12-Week Combined Polyphenol Supplementation in Overweight Men and Women. Eur. J. Clin. Nutr. 2017, 71, 1040–1045. [Google Scholar] [CrossRef]


| Method | Analysed Marker | Localization | Type of Sample | Analytical Method | Disadvantages |
|---|---|---|---|---|---|
| Sugar absorption test (SAT) | Sugars of various molecular weights (lactulose, mannitol, sucralose, sucrose, raffinose) | Small intestine (can be extended to other parts of the intestinal tract by adding additional sugars to the mixture) | Urine | Chromatography | Time-consuming; requires chromatographical equipment for sugars analyses |
| PEG 400/4000 | Polyethylene glycols (PEG) | The whole intestinal tract | Urine | Chromatography | Time-consuming; requires chromatographical equipment which can analyse PEG; big individual variation in response |
| 51Cr-EDTA | Isotopically labelled EDTA | The whole intestinal tract | Urine | Chromatography | Radioactivity |
| FITC-dextran | Fluorescent-labelled dextran | The whole intestinal tract | Urine/serum | Chromatography/fluorimeter | Other substances (i.e., bilirubin) can give a fluorescence response |
| LAL | Endotoxin LPS | The whole intestinal tract | Plasma | ELISA | Low concentration—requires portal vein blood collection |
| EndoCAb | Antibodies anty-LPS | The whole intestinal tract | Serum | ELISA | Only in acute phase inflammations |
| D-lactate | Bacterial lactic acid | The whole intestinal tract | Plasma | ELISA/chromatography | Low specificity |
| Zonulin | Zonulin protein | - | Serum | ELISA | |
| DAO | Diamine oxidase | Small intestine | Plasma | ELISA | Requires the administration of heparin |
| Calprotectin | Calprotectin | Colon | Faeces | ELISA | Low specificity |
| AAT | Alfa-1-antitrypsin | Small intestine | Faeces/serum | ELISA | Unknown specificity |
| Citrulline | Citrulline | Small intestine | Plasma | ELISA/chromatography | Time-consuming; requires chromatographical equipment for amino acids analyses |
| GST | Glutathione S-transferases | - | Plasma/urine | ELISA | Low specificity |
| FABP | Fatty acid-binding proteins | Depending on the type of FABP | Plasma/urine | ELISA | Only in acute phase inflammations |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drabińska, N.; Jarocka-Cyrta, E. Crosstalk between Resveratrol and Gut Barrier: A Review. Int. J. Mol. Sci. 2022, 23, 15279. https://doi.org/10.3390/ijms232315279
Drabińska N, Jarocka-Cyrta E. Crosstalk between Resveratrol and Gut Barrier: A Review. International Journal of Molecular Sciences. 2022; 23(23):15279. https://doi.org/10.3390/ijms232315279
Chicago/Turabian StyleDrabińska, Natalia, and Elżbieta Jarocka-Cyrta. 2022. "Crosstalk between Resveratrol and Gut Barrier: A Review" International Journal of Molecular Sciences 23, no. 23: 15279. https://doi.org/10.3390/ijms232315279
APA StyleDrabińska, N., & Jarocka-Cyrta, E. (2022). Crosstalk between Resveratrol and Gut Barrier: A Review. International Journal of Molecular Sciences, 23(23), 15279. https://doi.org/10.3390/ijms232315279