B4GALT1 as a New Biomarker of Idiopathic Pulmonary Fibrosis
Abstract
:1. Introduction
2. Results
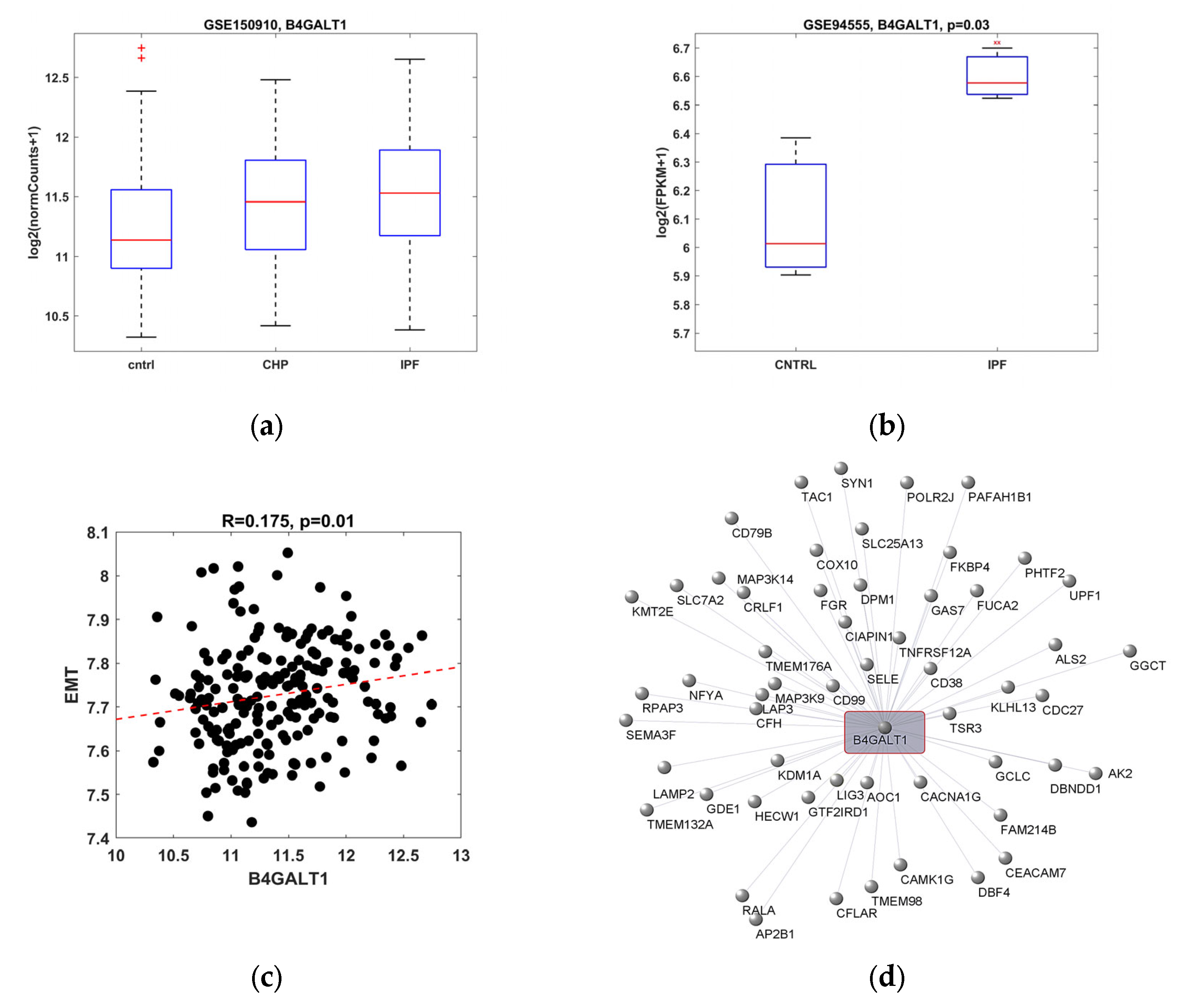
2.1. B4GALT1 Gene Expression Is Increased in IPF and It Correlates with EMT Gene Pathway
2.2. Protein and Gene Expression Analysis in Primary Fibroblast Cell Cultures from IPF Patient
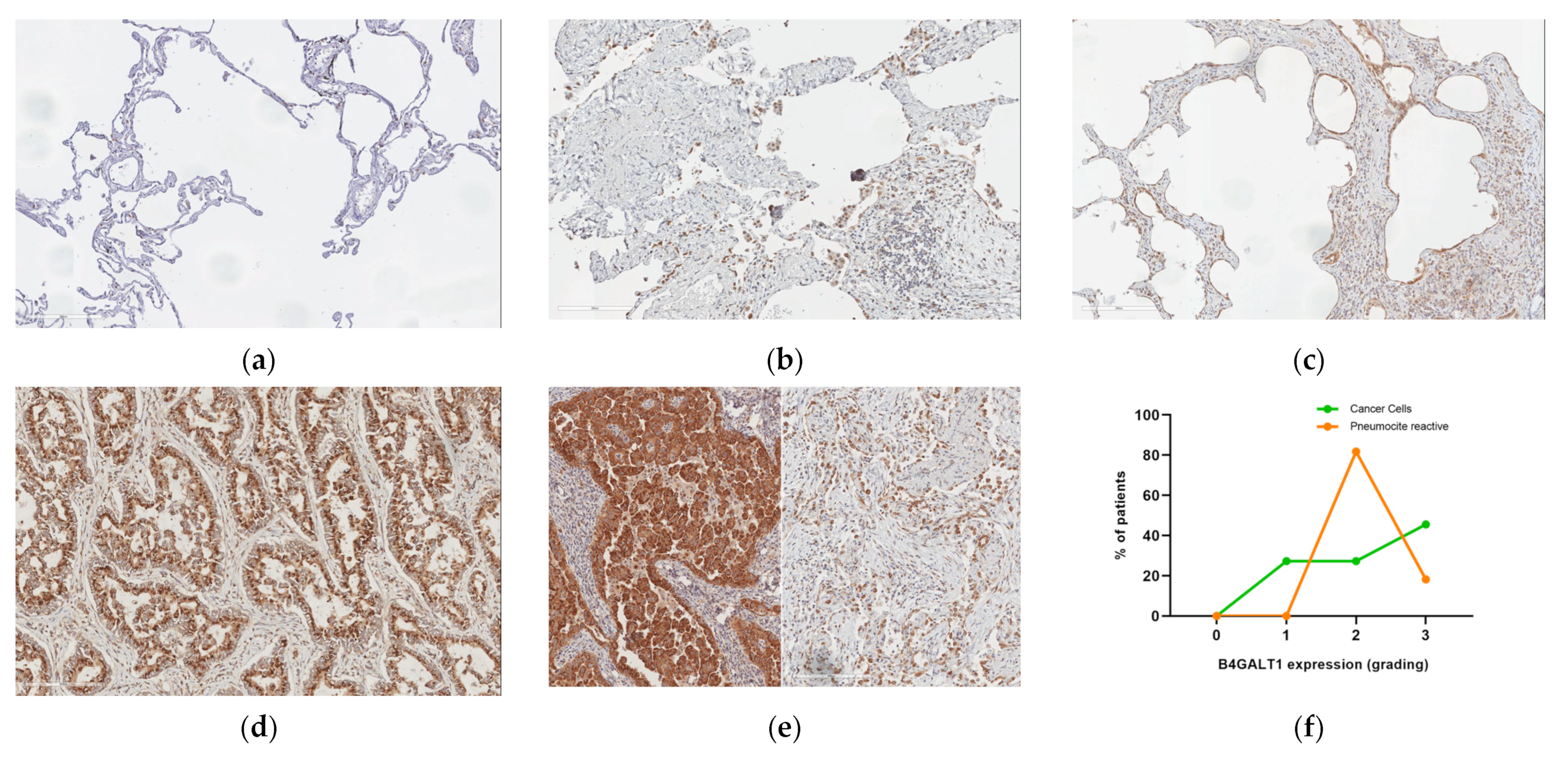
2.3. Protein Expression Analysis in Lung Speciemens from IPFpatients
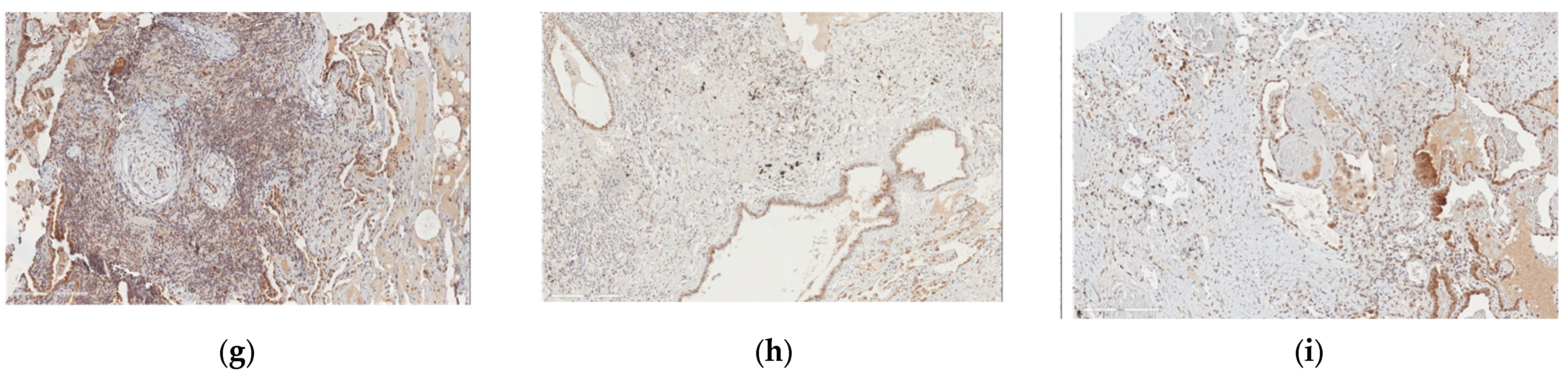
2.4. Protein and Gene Expression Analysis in Patients with Fibrosis in Other Non-Lung Tissues
2.5. B4GALT1 Is Overexpressed in UIP/IPF Associated with Lung Cancer
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Bioinformatic Analysis
4.3. Primary Cell Cultures
4.4. RNA Extraction and Real Time RT-PCR
4.5. Immunofluorescence
4.6. Immunohistochemistry
4.7. Optical Microscopy
4.8. Statistical Analysis
4.9. Ethics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, H.; Wu, X.; Li, Y.; Xia, Y. Research Progress in the Molecular Mechanisms, Therapeutic Targets, and Drug Development of Idiopathic Pulmonary Fibrosis. Front. Pharmacol. 2022, 13, 963054. [Google Scholar] [CrossRef] [PubMed]
- Ruaro, B.; Matucci Cerinic, M.; Salton, F.; Baratella, E.; Confalonieri, M.; Hughes, M. Editorial: Pulmonary fibrosis: One manifestation, various diseases. Front. Pharmacol. 2022, 13, 1027332. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, F.; Lunardi, F.; Tauro, V.; Pezzuto, F.; Fortarezza, F.; Vedovelli, L.; Faccioli, E.; Balestro, E.; Schiavon, M.; Esposito, G.; et al. RNA Sequencing of Epithelial Cell/Fibroblastic Foci Sandwich in Idiopathic Pulmonary Fibrosis: New Insights on the Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 3323. [Google Scholar] [CrossRef] [PubMed]
- Confalonieri, P.; Volpe, M.C.; Jacob, J.; Maiocchi, S.; Salton, F.; Ruaro, B.; Confalonieri, M.; Braga, L. Regeneration or Repair? The Role of Alveolar Epithelial Cells in the Pathogenesis of Idiopathic Pulmonary Fibrosis (IPF). Cells 2022, 11, 2095. [Google Scholar] [CrossRef] [PubMed]
- De Vitis, C.; Corleone, G.; Salvati, V.; Ascenzi, F.; Pallocca, M.; De Nicola, F.; Fanciulli, M.; di Martino, S.; Bruschini, S.; Napoli, C.; et al. B4GALT1 Is a New Candidate to Maintain the Stemness of Lung Cancer Stem Cells. J. Clin. Med. 2019, 8, 1928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Z.; Bailey, A.; Kuleshov, M.V.; Clarke, D.J.B.; Evangelista, J.E.; Jenkins, S.L.; Lachmann, A.; Wojciechowicz, M.L.; Kropiwnicki, E.; Jagodnik, K.M.; et al. Gene Set Knowledge Discovery with Enrichr. Curr. Protoc. 2021, 1, e90. [Google Scholar] [CrossRef]
- Kamp, J.C.; Neubert, L.; Stark, H.; Hinrichs, J.B.; Boekhoff, C.; Seidel, A.D.; Ius, F.; Haverich, A.; Gottlieb, J.; Welte, T.; et al. Comparative Analysis of Gene Expression in Fibroblastic Foci in Patients with Idiopathic Pulmonary Fibrosis and Pulmonary Sarcoidosis. Cells 2022, 11, 664. [Google Scholar] [CrossRef]
- Karampitsakos, T.; Spagnolo, P.; Mogulkoc, N.; Wuyts, W.A.; Tomassetti, S.; Bendstrup, E.; Molina-Molina, M.; Manali, E.D.; Unat, Ö.S.; Bonella, F.; et al. Lung cancer in patients with idiopathic pulmonary fibrosis: A retrospective multicentre study in Europe. Respirol. Carlton. Vic. 2022. [Google Scholar] [CrossRef]
- Xie, H.; Zhu, Y.; Zhang, J.; Liu, Z.; Fu, H.; Cao, Y.; Li, G.; Shen, Y.; Dai, B.; Xu, J.; et al. B4GALT1 expression predicts prognosis and adjuvant chemotherapy benefits in muscle-invasive bladder cancer patients. BMC Cancer 2018, 18, 590. [Google Scholar] [CrossRef]
- Xie, H.; Zhu, Y.; An, H.; Wang, H.; Zhu, Y.; Fu, H.; Wang, Z.; Fu, Q.; Xu, J.; Ye, D. Increased B4GALT1 expression associates with adverse outcome in patients with non-metastatic clear cell renal cell carcinoma. Oncotarget 2016, 7, 32723–32730. [Google Scholar] [CrossRef]
- Al-Obaide, M.A.I.; Alobydi, H.; Abdelsalam, A.G.; Zhang, R.; Srivenugopal, K.S. Multifaceted roles of 5’-regulatory region of the cancer associated gene B4GALT1 and its comparison with the gene family. Int. J. Oncol. 2015, 47, 1393–1404. [Google Scholar] [CrossRef] [Green Version]
- Nilius, V.; Killer, M.C.; Timmesfeld, N.; Schmitt, M.; Moll, R.; Lorch, A.; Beyer, J.; Mack, E.; Lohoff, M.; Burchert, A.; et al. High β-1,4-Galactosyltransferase-I expression in peripheral T-lymphocytes is associated with a low risk of relapse in germ-cell cancer patients receiving high-dose chemotherapy with autologous stem cell reinfusion. Oncoimmunology 2018, 7, e1423169. [Google Scholar] [CrossRef] [Green Version]
- B4GalT1 Regulates Apoptosis and Autophagy of Glioblastoma In Vitro and In Vivo—Pu Wang, Xiaolong Li, Yuan Xie. 2020. Available online: https://journals.sagepub.com/doi/full/10.1177/1533033820980104 (accessed on 24 October 2022).
- Chen, Y.; Su, L.; Huang, C.; Wu, S.; Qiu, X.; Zhao, X.; Meng, Q.; Meng, Y.-M.; Kong, X.; Wang, M.; et al. Galactosyltransferase B4GALT1 confers chemoresistance in pancreatic ductal adenocarcinomas by upregulating N-linked glycosylation of CDK11p110. Cancer Lett. 2021, 500, 228–243. [Google Scholar] [CrossRef]
- Shauchuk, A.; Szulc, B.; Maszczak-Seneczko, D.; Wiertelak, W.; Skurska, E.; Olczak, M. N-glycosylation of the human β1,4-galactosyltransferase 4 is crucial for its activity and Golgi localization. Glycoconj. J. 2020, 37, 577–588. [Google Scholar] [CrossRef]
- B4GALT1 Beta-1,4-galactosyltransferase 1 [Homo sapiens (human)]—Gene—NCBI. Available online: https://www.ncbi.nlm.nih.gov/gene/2683 (accessed on 24 October 2022).
- Connelly, M.A.; Gruppen, E.G.; Otvos, J.D.; Dullaart, R.P.F. Inflammatory glycoproteins in cardiometabolic disorders, autoimmune diseases and cancer. Clin. Chim. Acta Int. J. Clin. Chem. 2016, 459, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Nicastri, A.; Gaspari, M.; Sacco, R.; Elia, L.; Gabriele, C.; Romano, R.; Rizzuto, A.; Cuda, G. N-glycoprotein analysis discovers new up-regulated glycoproteins in colorectal cancer tissue. J. Proteome Res. 2014, 13, 4932–4941. [Google Scholar] [CrossRef]
- Thakkar, V.; Patel, P.; Prajapati, N.; Kaur, R.; Nandave, M. Serum Levels of Glycoproteins are Elevated in Patients with Ovarian Cancer. Indian J. Clin. Biochem. IJCB 2014, 29, 345–350. [Google Scholar] [CrossRef] [Green Version]
- Muramatsu, T. Glycoproteins associated with metastatic potential of cancer. Gan Kagaku Ryoho 1989, 16, 3354–3358. [Google Scholar]
- Bhavanandan, V.P. Cancer-associated mucins and mucin-type glycoproteins. Glycobiology 1991, 1, 493–503. [Google Scholar] [CrossRef]
- Miller, M.C.; Zheng, Y.; Zhou, Y.; Tai, G.; Mayo, K.H. Galectin-3 binds selectively to the terminal, non-reducing end of β(1→4)-galactans, with overall affinity increasing with chain length. Glycobiology 2019, 29, 74–84. [Google Scholar] [CrossRef]
- Qasba, P.K.; Ramakrishnan, B.; Boeggeman, E. Structure and Function of β-1,4-Galactosyltransferase. Curr. Drug Targets 2008, 9, 292–309. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, J.; Suzuki, R. On the origin of galectin and galactose—Part 1. Glycoforum 2022, 25, A6. [Google Scholar]
- Sciacchitano, S.; Lavra, L.; Morgante, A.; Ulivieri, A.; Magi, F.; De Francesco, G.P.; Bellotti, C.; Salehi, L.B.; Ricci, A. Galectin-3: One Molecule for an Alphabet of Diseases, from A to Z. Int. J. Mol. Sci. 2018, 19, 379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Li, J.; Gao, J. Functions of galectin-3 and its role in fibrotic diseases. J. Pharmacol. Exp. Ther. 2014, 351, 336–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishi, Y.; Sano, H.; Kawashima, T.; Okada, T.; Kuroda, T.; Kikkawa, K.; Kawashima, S.; Tanabe, M.; Goto, T.; Matsuzawa, Y.; et al. Role of galectin-3 in human pulmonary fibrosis. Allergol. Int. Off. J. Jpn. Soc. Allergol. 2007, 56, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Ho, J.E.; Gao, W.; Levy, D.; Santhanakrishnan, R.; Araki, T.; Rosas, I.O.; Hatabu, H.; Latourelle, J.C.; Nishino, M.; Dupuis, J.; et al. Galectin-3 Is Associated with Restrictive Lung Disease and Interstitial Lung Abnormalities. Am. J. Respir. Crit. Care Med. 2016, 194, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Slack, R.J.; Mills, R.; Mackinnon, A.C. The therapeutic potential of galectin-3 inhibition in fibrotic disease. Int. J. Biochem. Cell Biol. 2021, 130, 105881. [Google Scholar] [CrossRef]
- Hirani, N.; MacKinnon, A.C.; Nicol, L.; Ford, P.; Schambye, H.; Pedersen, A.; Nilsson, U.J.; Leffler, H.; Sethi, T.; Tantawi, S.; et al. Target inhibition of galectin-3 by inhaled TD139 in patients with idiopathic pulmonary fibrosis. Eur. Respir. J. 2021, 57, 2002559. [Google Scholar] [CrossRef]
- Raghu, G.; Collard, H.R.; Egan, J.J.; Martinez, F.J.; Behr, J.; Brown, K.K.; Colby, T.V.; Cordier, J.-F.; Flaherty, K.R.; Lasky, J.A.; et al. An Official ATS/ERS/JRS/ALAT Statement: Idiopathic Pulmonary Fibrosis: Evidence-based Guidelines for Diagnosis and Management. Am. J. Respir. Crit. Care Med. 2011, 183, 788–824. [Google Scholar] [CrossRef] [Green Version]
- Diagnosis of IPF: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline Implementation Tools. Available online: https://www.thoracic.org/statements/guideline-implementation-tools/diagnosis-of-ipf.php (accessed on 24 October 2022).
- Mino-Kenudson, M. Significance of tumor spread through air spaces (STAS) in lung cancer from the pathologist perspective. Transl. Lung Cancer Res. 2020, 9, 847–859. [Google Scholar] [CrossRef]
- Transcriptional Network Analysis for the Regulation of Left Ventricular Hypertrophy and Microvascular Remodeling|SpringerLink. Available online: https://link.springer.com/article/10.1007/s12265-013-9504-x (accessed on 14 November 2022).

| Pathways | p-Value | q-Value | Genes |
|---|---|---|---|
| Focal adhesion (WP306) | 3.54 × 10−28 | 1.04 × 10−25 | ITGB1, ITGB5, LAMA2, LAMA1, ITGB3, LAMA3, TNC, LAMC2, LAMC1, THBS2, THBS1, MYLK, COMP, SPP1, FLNA, ITGAV, PDGFRB, JUN, ITGA2, FN1, VEGFC, VEGFA, COL1A1, COL1A2, COL4A2, COL4A1, COL5A3, COL6A2, COL5A2, ITGA5, MYL9 |
| miRNA targets in ECM and membrane receptors (WP2911) | 1.87 × 10−23 | 2.77 × 10−21 | ITGB5, FN1, LAMC1, THBS2, THBS1, COL3A1, COL1A2, COL5A1, COL4A2, COL4A1, COL5A3, COL6A2, COL5A2, COL6A3 |
| Focal adhesion-PI3K-Akt-mTOR-signaling pathway (WP3932) | 1.93 × 10−22 | 1.89 × 10−20 | ITGB1, ITGB5, LAMA2, LAMA1, COL11A1, ITGB3, LAMA3, TNC, LAMC2, LAMC1, THBS2, FGF2, THBS1, COMP, SPP1, ITGAV, PDGFRB, ITGA2, FN1, VEGFC, VEGFA, COL1A1, COL3A1, COL1A2, COL4A2, COL5A1, COL4A1, COL5A3, COL6A2, COL5A2, ITGA5 |
| PI3K-Akt signaling pathway (WP4172) | 7.67 × 10−19 | 5.65 × 10−17 | ITGB1, ITGB5, LAMA2, LAMA1, ITGB3, LAMA3, TNC, LAMC2, LAMC1, THBS2, FGF2, THBS1, COMP, SPP1, ITGAV, PDGFRB, BDNF, ITGA2, FN1, VEGFC, VEGFA, COL1A1, IL6, COL1A2, COL4A2, COL4A1, COL6A2, COL6A3, ITGA5 |
| IL-18 signaling pathway (WP4754) | 4.42 × 10−16 | 2.60 × 10−14 | JUN, CXCL8, SDC4, MMP1, MMP2, MMP3, FN1, PLOD3, TNFAIP3, TNFRSF11B, VEGFA, COL1A1, ACTA2, MMP14, COL3A1, IL6, COL1A2, SPP1, TIMP3, FAS, PTX3, TIMP1, SNTB1, TGM2 |
| Type I collagen synthesis in the context of osteogenesis imperfecta (WP4786) | 1.21 × 10−14 | 5.95 × 10−13 | COL1A1, COL1A2, BMP1, LOX, SERPINH1, P3H1, PLOD2, TNFRSF11B, PLOD1, PPIB, COLGALT1 |
| Senescence and autophagy in cancer (WP615) | 1.48 × 10−13 | 6.27 × 10−12 | JUN, SPARC, TGFB1, CXCL8, IGFBP3, SERPINE1, FN1, CXCL1, INHBA, THBS1, COL1A1, IL6, MMP14, COL3A1, CD44 |
| Arrhythmogenic right ventricular cardiomyopathy (WP2118) | 9.15 × 10−12 | 3.37 × 10−10 | ITGB1, GJA1, SGCD, ITGB5, CDH2, LAMA2, SGCB, ITGB3, ITGA2, ITGAV, ITGA5, SGCG |
| Small cell lung cancer (WP4658) | 1.31 × 10−11 | 4.32 × 10−10 | ITGB1, LAMA2, GADD45B, LAMA1, GADD45A, ITGA2, LAMA3, FN1, LAMC2, LAMC1, COL4A2, COL4A1, ITGAV |
| Lung fibrosis (WP3624) | 6.43 × 10−10 | 1.48 × 10−8 | GREM1, IL6, CXCL8, TGFB1, ELN, MMP2, SPP1, PTX3, TIMP1, FGF2 |
| Information | Histology | Spirometry | Radiology | |||||||||||
| ID | Age | Sex | Smoke | Pack Year | Diagnosis | Gap Index and Stadiation | FEV1 (%) | FVC (%) | Ct Score | Ground Glass | Honey Combing | Consolidations | Cross Linking | Enphysema |
| UIP1 | 72 | M | - | - | Possible UIP | II | 90.5 | 77.8 | 30 | No | No | No | Yes | No |
| UIP2 | 72 | M | Yes | 37.5 | UIP | I | 110 | 98 | 45 | No | Yes | No | Yes | No |
| UIP3 | 78 | F | Yes | 3.75 | Possible UIP | I | 113.9 | 122.30 | 20 | No | Yes | No | Yes | No |
| UIP4 | 49 | M | Yes | 15 | UIP | I | 56 | 54 | 50 | Yes | Yes | No | Yes | No |
| UIP + LC1 | 75 | M | - | 40 | Lung AdK + UIP | IA2 | 129.30 | 154.70 | 15 | No | No | Yes | Yes | Yes |
| UIP + LC2 | 64 | M | Yes | 30 | Lung squamous K + possible UIP | IB | 71 | 68 | 80 | Yes | Yes | No | Yes | Yes |
| UIP + LC3 | 76 | M | - | 60 | Lung AdK + possible UIP | IB | 76.7 | 70.4 | 40 | Yes | Yes | Yes | Yes | Yes |
| UIP + LC4 | 63 | M | Yes | 34 | Lung squamous K+ possible UIP | IIB | 77.60 | 79.60 | 30 | No | Yes | No | Yes | No |
| UIP + LC5 | 74 | M | - | 30 | Lung AdK + UIP | IIB | 92 | 85 | 80 | Yes | Yes | Yes | Yes | No |
| UIP + LC6 | 68 | M | - | 5 | Lung AdK+ UIP | IB | 108 | 100 | 20 | Yes | No | Yes | Yes | No |
| UIP + LC7 | 79 | M | Yes | 20 | Lung AdK + UIP | IA2 | 129.1 | 119.1 | 15 | Yes | Yes | Yes | Yes | No |
| UIP + LC8 | 80 | M | - | - | Lung squamous K+ Possible UIP | IB | 88.4 | 81.1 | - | - | - | - | - | - |
| UIP + LC9 | 81 | M | No | - | Lung AdK + possible UIP | IA2 | 100.6 | 89.9 | 5 | Yes | No | Yes | No | No |
| UIP + LC10 | 67 | M | - | 80 | Lung AdK + possible UIP | IA3 | 70 | 112 | 20 | No | No | Yes | Yes | Yes |
| UIP + LC11 | 78 | M | - | - | Lung squamous K + possible UIP | IIB | 63.10 | 81.20 | 50 | No | Yes | Yes | Yes | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Vitis, C.; D’Ascanio, M.; Sacconi, A.; Pizzirusso, D.; Salvati, V.; Mancini, M.; Scafetta, G.; Cirombella, R.; Ascenzi, F.; Bruschini, S.; et al. B4GALT1 as a New Biomarker of Idiopathic Pulmonary Fibrosis. Int. J. Mol. Sci. 2022, 23, 15040. https://doi.org/10.3390/ijms232315040
De Vitis C, D’Ascanio M, Sacconi A, Pizzirusso D, Salvati V, Mancini M, Scafetta G, Cirombella R, Ascenzi F, Bruschini S, et al. B4GALT1 as a New Biomarker of Idiopathic Pulmonary Fibrosis. International Journal of Molecular Sciences. 2022; 23(23):15040. https://doi.org/10.3390/ijms232315040
Chicago/Turabian StyleDe Vitis, Claudia, Michela D’Ascanio, Andrea Sacconi, Dario Pizzirusso, Valentina Salvati, Massimiliano Mancini, Giorgia Scafetta, Roberto Cirombella, Francesca Ascenzi, Sara Bruschini, and et al. 2022. "B4GALT1 as a New Biomarker of Idiopathic Pulmonary Fibrosis" International Journal of Molecular Sciences 23, no. 23: 15040. https://doi.org/10.3390/ijms232315040
APA StyleDe Vitis, C., D’Ascanio, M., Sacconi, A., Pizzirusso, D., Salvati, V., Mancini, M., Scafetta, G., Cirombella, R., Ascenzi, F., Bruschini, S., Esposito, A., Castelli, S., Salvucci, C., Teodonio, L., Sposato, B., Catizone, A., Di Napoli, A., Vecchione, A., Ciliberto, G., ... Mancini, R. (2022). B4GALT1 as a New Biomarker of Idiopathic Pulmonary Fibrosis. International Journal of Molecular Sciences, 23(23), 15040. https://doi.org/10.3390/ijms232315040










