Bio-Pulsed Stimulation Effectively Improves the Production of Avian Mesenchymal Stem Cell-Derived Extracellular Vesicles That Enhance the Bioactivity of Skin Fibroblasts and Hair Follicle Cells
Abstract
1. Introduction
2. Results
2.1. Characterization of AMSCs and Bioactivity of AMSCs Treated with Bio-Pulsed Reagent
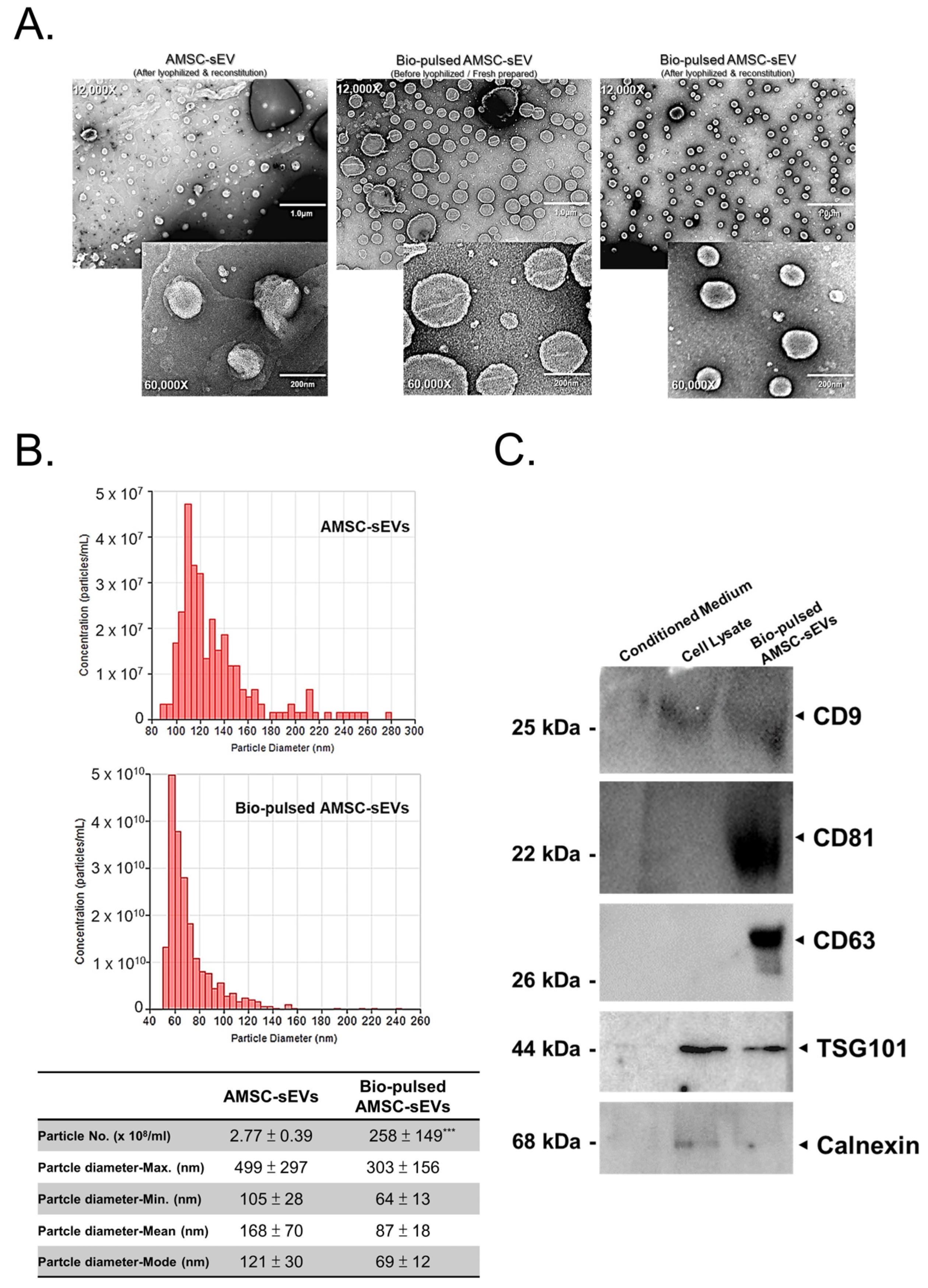
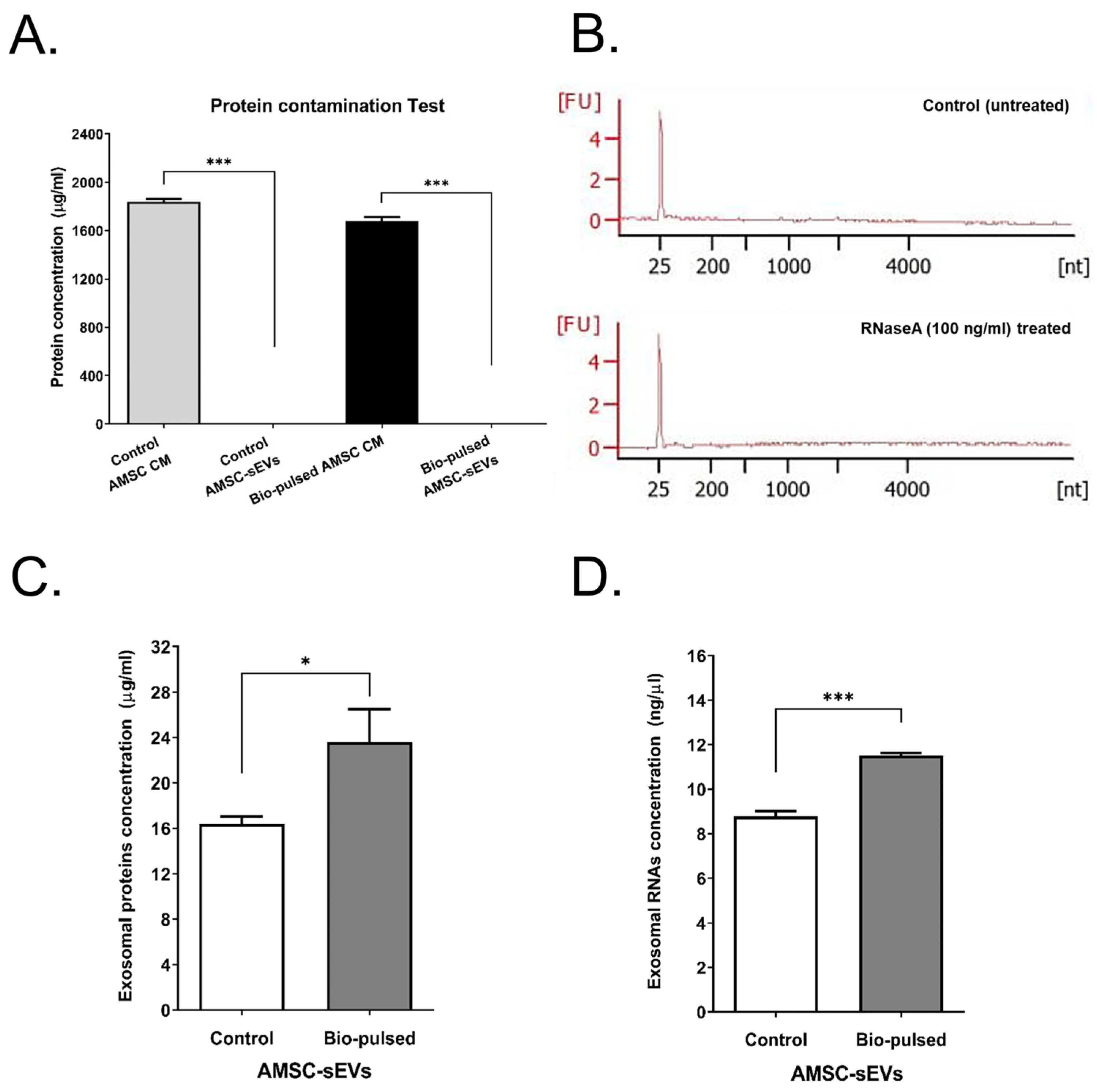
2.2. Quantity and Quality of AMSC-sEVs Were Enhanced by Bio-Pulsed Treatment
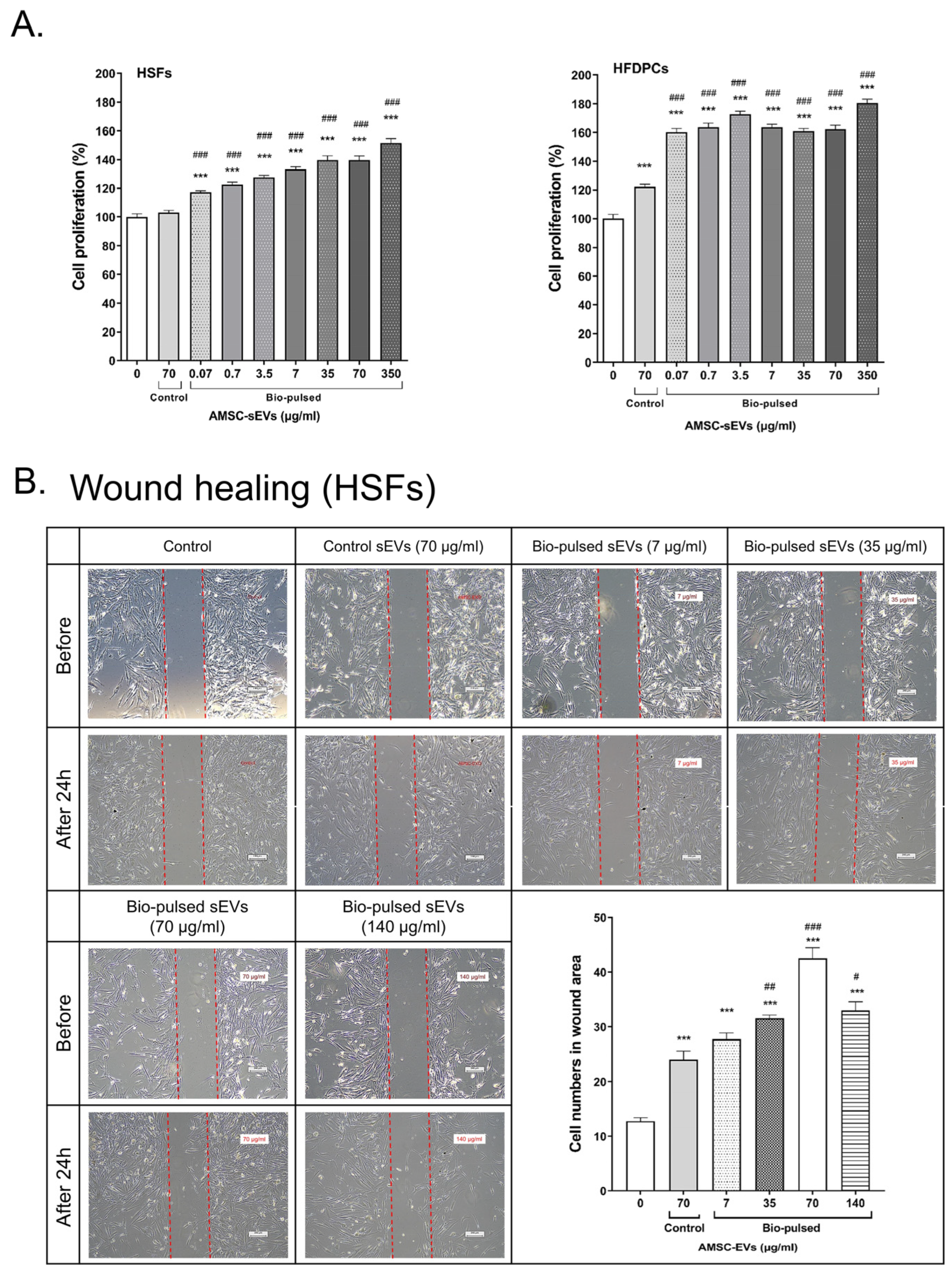
2.3. Bio-Pulsed AMSCs-sEVs Enhanced Cell Proliferation and Wound Healing
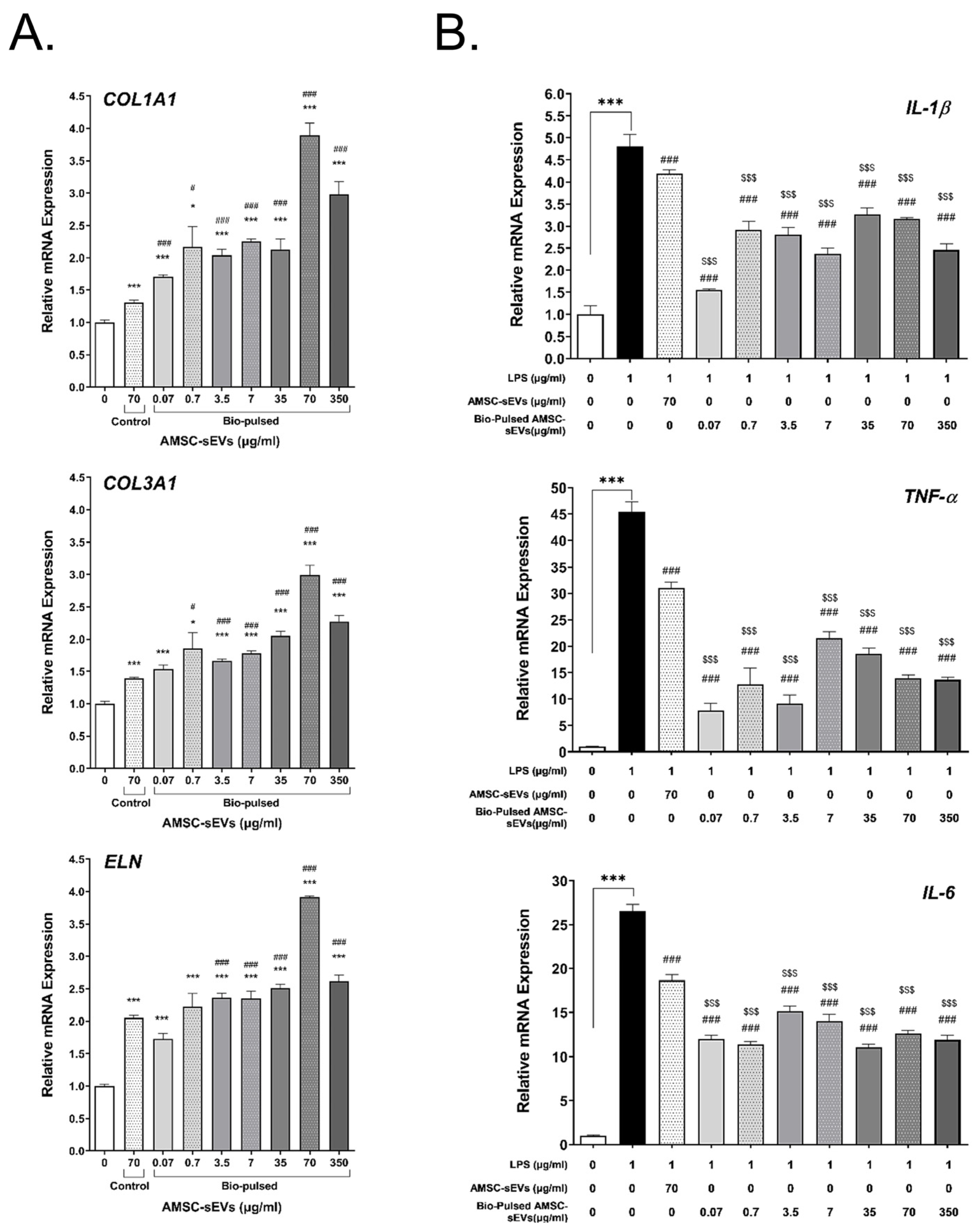
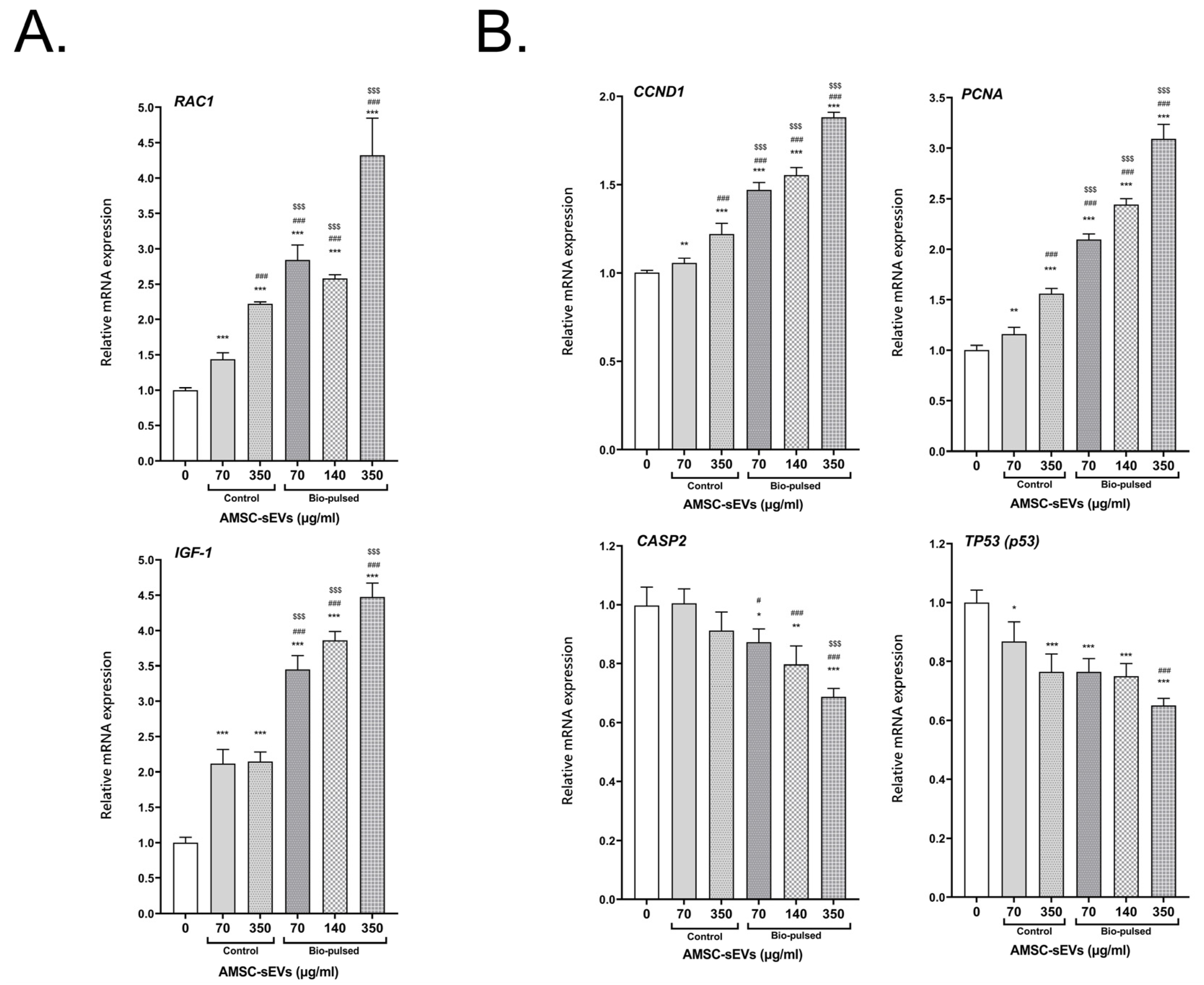
2.4. Bio-Pulsed AMSCs-sEVs Enhanced Collagen Expression and Anti-Inflammation
2.5. Bio-Pulsed AMSCs-sEVs Enhanced the Activity of Hair Follicle Cells
3. Discussion
4. Materials and Methods
4.1. Isolation and Culture of AMSCs
4.2. Generation of Bio-Pulsed AMSCs Conditioned Media
4.3. Isolation and Lyophilization of AMSC-sEVs
4.4. Transmission Electron Microscopy
4.5. Particle Size Distribution and Concentration Measurements
4.6. Western Blot Analysis
4.7. Protein Contamination Assay
4.8. RNAse Protection Assay
4.9. Exosomal Protein Concentration Examination
4.10. Exosomal RNA Concentration Examination
4.11. Cell Proliferation Assay (MTS Assay)
4.12. Wound Healing Assay
4.13. Quantitative Real-Time Polymerase Chain Reaction
4.14. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kavanagh, D.P.; Suresh, S.; Newsome, P.N.; Frampton, J.; Kalia, N. Pretreatment of Mesenchymal Stem Cells Manipulates Their Vasculoprotective Potential While Not Altering Their Homing Within the Injured Gut. Stem Cells 2015, 33, 2785–2797. [Google Scholar] [CrossRef]
- Gao, Y.; Bai, C.; Xiong, H.; Li, Q.; Shan, Z.; Huang, L.; Ma, Y.; Guan, W. Isolation and characterization of chicken dermis-derived mesenchymal stem/progenitor cells. BioMed Res. Int. 2013, 2013, 626258. [Google Scholar] [CrossRef] [PubMed]
- Rahmani-Moghadam, E.; Zarrin, V.; Mahmoodzadeh, A.; Owrang, M.; Talaei-Khozani, T. Comparison of the Characteristics of Breast Milk-derived Stem Cells with the Stem Cells Derived from the Other Sources: A Comparative Review. Curr. Stem Cell Res. Ther. 2022, 17, 71–90. [Google Scholar] [CrossRef] [PubMed]
- Bayreuther, K.; Rodemann, H.P.; Hommel, R.; Dittmann, K.; Albiez, M.; Francz, P.I. Human skin fibroblasts in vitro differentiate along a terminal cell lineage. Proc. Natl. Acad. Sci. USA 1988, 85, 5112–5116. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.A.; Kinsey, S.E.; English, A.; Jones, R.A.; Straszynski, L.; Meredith, D.M.; Markham, A.F.; Jack, A.; Emery, P.; McGonagle, D. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum. 2002, 46, 3349–3360. [Google Scholar] [CrossRef]
- Young, H.E.; Mancini, M.L.; Wright, R.P.; Smith, J.C.; Black, A.C., Jr.; Reagan, C.R.; Lucas, P.A. Mesenchymal stem cells reside within the connective tissues of many organs. Dev. Dyn. 1995, 202, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Crigler, L.; Kazhanie, A.; Yoon, T.J.; Zakhari, J.; Anders, J.; Taylor, B.; Virador, V.M. Isolation of a mesenchymal cell population from murine dermis that contains progenitors of multiple cell lineages. FASEB J. 2007, 21, 2050–2063. [Google Scholar] [CrossRef]
- Bissell, M.J.; Radisky, D. Putting tumours in context. Nat. Rev. Cancer 2001, 1, 46–54. [Google Scholar] [CrossRef]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef]
- Yeo, R.W.; Lai, R.C.; Zhang, B.; Tan, S.S.; Yin, Y.; Teh, B.J.; Lim, S.K. Mesenchymal stem cell: An efficient mass producer of exosomes for drug delivery. Adv. Drug Deliv. Rev. 2013, 65, 336–341. [Google Scholar] [CrossRef]
- Record, M.; Carayon, K.; Poirot, M.; Silvente-Poirot, S. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim. Biophys. Acta 2014, 1841, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Razeghian, E.; Margiana, R.; Chupradit, S.; Bokov, D.O.; Abdelbasset, W.K.; Marofi, F.; Shariatzadeh, S.; Tosan, F.; Jarahian, M. Mesenchymal Stem/Stromal Cells as a Vehicle for Cytokine Delivery: An Emerging Approach for Tumor Immunotherapy. Front. Med. 2021, 8, 721174. [Google Scholar] [CrossRef] [PubMed]
- Jadli, A.S.; Ballasy, N.; Edalat, P.; Patel, V.B. Inside(sight) of tiny communicator: Exosome biogenesis, secretion, and uptake. Mol. Cell. Biochem. 2020, 467, 77–94. [Google Scholar] [CrossRef]
- Hassanzadeh, A.; Rahman, H.S.; Markov, A.; Endjun, J.J.; Zekiy, A.O.; Chartrand, M.S.; Beheshtkhoo, N.; Kouhbanani, M.A.J.; Marofi, F.; Nikoo, M.; et al. Mesenchymal stem/stromal cell-derived exosomes in regenerative medicine and cancer; overview of development, challenges, and opportunities. Stem Cell Res. Ther. 2021, 12, 297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yang, P. A novel cell-cell communication mechanism in the nervous system: Exosomes. J. Neurosci. Res. 2018, 96, 45–52. [Google Scholar] [CrossRef]
- Ferreira, J.R.; Teixeira, G.Q.; Santos, S.G.; Barbosa, M.A.; Almeida-Porada, G.; Goncalves, R.M. Mesenchymal Stromal Cell Secretome: Influencing Therapeutic Potential by Cellular Pre-conditioning. Front. Immunol. 2018, 9, 2837. [Google Scholar] [CrossRef]
- Cunningham, C.J.; Redondo-Castro, E.; Allan, S.M. The therapeutic potential of the mesenchymal stem cell secretome in ischaemic stroke. J. Cereb. Blood Flow Metab. 2018, 38, 1276–1292. [Google Scholar] [CrossRef]
- Guo, J.A.; Yu, P.J.; Yang, D.Q.; Chen, W. The Antisenescence Effect of Exosomes from Human Adipose-Derived Stem Cells on Skin Fibroblasts. BioMed Res. Int. 2022, 2022, 1034316. [Google Scholar] [CrossRef]
- Tutuianu, R.; Rosca, A.M.; Iacomi, D.M.; Simionescu, M.; Titorencu, I. Human Mesenchymal Stromal Cell-Derived Exosomes Promote In Vitro Wound Healing by Modulating the Biological Properties of Skin Keratinocytes and Fibroblasts and Stimulating Angiogenesis. Int. J. Mol. Sci. 2021, 22, 6239. [Google Scholar] [CrossRef]
- Matsuoka, T.; Takanashi, K.; Dan, K.; Yamamoto, K.; Tomobe, K.; Shinozuka, T. Effects of mesenchymal stem cell-derived exosomes on oxidative stress responses in skin cells. Mol. Biol. Rep. 2021, 48, 4527–4535. [Google Scholar] [CrossRef]
- Liu, J.; Li, F.; Liu, B.; Yao, Z.; Li, L.; Liu, G.; Peng, L.; Wang, Y.; Huang, J. Adipose-derived mesenchymal stem cell exosomes inhibit transforming growth factor-beta1-induced collagen synthesis in oral mucosal fibroblasts. Exp. Ther. Med. 2021, 22, 1419. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Chen, B.; Zhang, X.; Chen, H. Human Adipose-Derived Mesenchymal Stem Cells-Derived Exosomal microRNA-19b Promotes the Healing of Skin Wounds Through Modulation of the CCL1/TGF-beta Signaling Axis. Clin. Cosmet. Investig. Dermatol. 2020, 13, 957–971. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Li, L. Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. J. Cell. Mol. Med. 2018, 22, 1428–1442. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tsai, T.L.; Li, W.J. Strategies to retain properties of bone marrow-derived mesenchymal stem cells ex vivo. Ann. N. Y. Acad. Sci. 2017, 1409, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Pendse, S.; Kale, V.; Vaidya, A. Extracellular Vesicles Isolated from Mesenchymal Stromal Cells Primed with Hypoxia: Novel Strategy in Regenerative Medicine. Curr. Stem Cell Res. Ther. 2021, 16, 243–261. [Google Scholar] [CrossRef]
- Qian, W.; Huang, L.; Xu, Y.; Lu, W.; Wen, W.; Guo, Z.; Zhu, W.; Li, Y. Hypoxic ASCs-derived Exosomes Attenuate Colitis by Regulating Macrophage Polarization via miR-216a-5p/HMGB1 Axis. Inflamm. Bowel Dis. 2022. [Google Scholar] [CrossRef]
- Kim, M.; Shin, D.I.; Choi, B.H.; Min, B.H. Exosomes from IL-1beta-Primed Mesenchymal Stem Cells Inhibited IL-1beta- and TNF-alpha-Mediated Inflammatory Responses in Osteoarthritic SW982 Cells. Tissue Eng. Regen. Med. 2021, 18, 525–536. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, J.; Wu, J.; Yu, Y.; Yao, W.; Zhou, M.; Tian, J.; Zhang, J.; Cui, L.; Zeng, X.; et al. Tetrahydroxystilbene glucoside isolated from Polygonum multiflorum Thunb. demonstrates osteoblast differentiation promoting activity. Exp. Ther. Med. 2017, 14, 2845–2852. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Q.; Zhou, Q. Assay of stilbene glucoside in Polygonum multiflorum Thunb and its processed products. Zhongguo Zhong Yao Za Zhi 1991, 16, 469–472, 511. [Google Scholar]
- Zhang, Y.Z.; Shen, J.F.; Xu, J.Y.; Xiao, J.H.; Wang, J.L. Inhibitory effects of 2,3,5,4’-tetrahydroxystilbene-2-O-beta-D-glucoside on experimental inflammation and cyclooxygenase 2 activity. J. Asian Nat. Prod. Res. 2007, 9, 355–363. [Google Scholar] [CrossRef]
- Jiang, Z.; Xu, J.; Long, M.; Tu, Z.; Yang, G.; He, G. 2, 3, 5, 4’-tetrahydroxystilbene-2-O-beta-D-glucoside (THSG) induces melanogenesis in B16 cells by MAP kinase activation and tyrosinase upregulation. Life Sci. 2009, 85, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Qin, R.; Li, X.; Li, G.; Tao, L.; Li, Y.; Sun, J.; Kang, X.; Chen, J. Protection by tetrahydroxystilbene glucoside against neurotoxicity induced by MPP+: The involvement of PI3K/Akt pathway activation. Toxicol. Lett. 2011, 202, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.H.; Wang, W.Q.; Wang, J.L. Protective effect of tetrahydroxystilbene glucoside on cardiotoxicity induced by doxorubicin in vitro and in vivo. Acta Pharmacol. Sin. 2009, 30, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ge, L.; Yang, Q.; Xie, Y.; Sun, J.; Cao, W.; Wang, S. Thinning of dermas with the increasing age may be against by tetrahydroxystilbene glucoside in mice. Int. J. Clin. Exp. Med. 2014, 7, 2017–2024. [Google Scholar]
- Shin, J.Y.; Choi, Y.H.; Kim, J.; Park, S.Y.; Nam, Y.J.; Lee, S.Y.; Jeon, J.H.; Jin, M.H.; Lee, S. Polygonum multiflorum extract support hair growth by elongating anagen phase and abrogating the effect of androgen in cultured human dermal papilla cells. BMC Complement. Med. Ther. 2020, 20, 144. [Google Scholar] [CrossRef]
- Chin, Y.T.; Liu, C.M.; Chen, T.Y.; Chung, Y.Y.; Lin, C.Y.; Hsiung, C.N.; Jan, Y.S.; Chiu, H.C.; Fu, E.; Lee, S.Y. 2,3,5,4’-tetrahydroxystilbene-2-O-beta-D-glucoside-stimulated dental pulp stem cells-derived conditioned medium enhances cell activity and anti-inflammation. J. Dent. Sci. 2021, 16, 586–598. [Google Scholar] [CrossRef]
- Ostrowski, M.; Carmo, N.B.; Krumeich, S.; Fanget, I.; Raposo, G.; Savina, A.; Moita, C.F.; Schauer, K.; Hume, A.N.; Freitas, R.P.; et al. Rab27a and Rab27b control. different steps of the exosome secretion pathway. Nat. Cell Biol. 2010, 12, 19–30. [Google Scholar] [CrossRef]
- Huang, H.; Hou, J.; Liu, K.; Liu, Q.; Shen, L.; Liu, B.; Lu, Q.; Zhang, N.; Che, L.; Li, J.; et al. RAB27A-dependent release of exosomes by liver cancer stem cells induces Nanog expression in their differentiated progenies and confers regorafenib resistance. J. Gastroenterol. Hepatol. 2021, 36, 3429–3437. [Google Scholar] [CrossRef]
- van Solinge, T.S.; Abels, E.R.; van de Haar, L.L.; Hanlon, K.S.; Maas, S.L.N.; Schnoor, R.; de Vrij, J.; Breakefield, X.O.; Broekman, M.L.D. Versatile Role of Rab27a in Glioma: Effects on Release of Extracellular Vesicles, Cell Viability, and Tumor Progression. Front. Mol. Biosci. 2020, 7, 554649. [Google Scholar] [CrossRef]
- Shin, K.O.; Ha, D.H.; Kim, J.O.; Crumrine, D.A.; Meyer, J.M.; Wakefield, J.S.; Lee, Y.; Kim, B.; Kim, S.; Kim, H.K.; et al. Exosomes from Human Adipose Tissue-Derived Mesenchymal Stem Cells Promote Epidermal Barrier Repair by Inducing de Novo Synthesis of Ceramides in Atopic Dermatitis. Cells 2020, 9, 680. [Google Scholar] [CrossRef]
- Ha, D.H.; Kim, H.K.; Lee, J.; Kwon, H.H.; Park, G.H.; Yang, S.H.; Jung, J.Y.; Choi, H.; Lee, J.H.; Sung, S.; et al. Mesenchymal Stem/Stromal Cell-Derived Exosomes for Immunomodulatory Therapeutics and Skin Regeneration. Cells 2020, 9, 1157. [Google Scholar] [CrossRef]
- Cho, B.S.; Kim, J.O.; Ha, D.H.; Yi, Y.W. Exosomes derived from human adipose tissue-derived mesenchymal stem cells alleviate atopic dermatitis. Stem Cell Res. Ther. 2018, 9, 187. [Google Scholar] [CrossRef]
- Wang, X.; C, F.H.; Wang, J.J.; Ji, H.; Guan, W.; Zhao, Y. Isolation, culture, and characterization of chicken lung-derived mesenchymal stem cells. Can. J. Vet. Res. 2018, 82, 225–235. [Google Scholar]
- Parekkadan, B.; Milwid, J.M. Mesenchymal stem cells as therapeutics. Annu. Rev. BioMed Eng. 2010, 12, 87–117. [Google Scholar] [CrossRef] [PubMed]
- Teli, P.; Kale, V.; Vaidya, A. Extracellular vesicles isolated from mesenchymal stromal cells primed with neurotrophic factors and signaling modifiers as potential therapeutics for neurodegenerative diseases. Curr. Res. Transl. Med. 2021, 69, 103286. [Google Scholar] [CrossRef]
- Huang, C.; Dai, J.; Zhang, X.A. Environmental physical cues determine the lineage specification of mesenchymal stem cells. Biochim. Biophys. Acta 2015, 1850, 1261–1266. [Google Scholar] [CrossRef]
- Nava, M.M.; Raimondi, M.T.; Pietrabissa, R. Controlling self-renewal and differentiation of stem cells via mechanical cues. J. BioMed Biotechnol. 2012, 2012, 797410. [Google Scholar] [CrossRef]
- Chen, S.Y.; Lin, M.C.; Tsai, J.S.; He, P.L.; Luo, W.T.; Herschman, H.; Li, H.J. EP4 Antagonist-Elicited Extracellular Vesicles from Mesenchymal Stem Cells Rescue Cognition/Learning Deficiencies by Restoring Brain Cellular Functions. Stem Cells Transl. Med. 2019, 8, 707–723. [Google Scholar] [CrossRef]
- Noronha, N.C.; Mizukami, A.; Caliari-Oliveira, C.; Cominal, J.G.; Rocha, J.L.M.; Covas, D.T.; Swiech, K.; Malmegrim, K.C.R. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res. Ther. 2019, 10, 131. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Li, Y.M.; Wang, Z. Preserving extracellular vesicles for biomedical applications: Consideration of storage stability before and after isolation. Drug Deliv. 2021, 28, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int. J. Nanomed. 2020, 15, 6917–6934. [Google Scholar] [CrossRef] [PubMed]
- Kwack, M.H.; Seo, C.H.; Gangadaran, P.; Ahn, B.C.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Exosomes derived from human dermal papilla cells promote hair growth in cultured human hair follicles and augment the hair-inductive capacity of cultured dermal papilla spheres. Exp. Dermatol. 2019, 28, 854–857. [Google Scholar] [CrossRef] [PubMed]
- Varani, J.; Dame, M.K.; Rittie, L.; Fligiel, S.E.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Decreased collagen production in chronologically aged skin: Roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am. J. Pathol. 2006, 168, 1861–1868. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Song, S.; Chen, N.; Liao, J.; Zeng, L. Stem cell-derived exosomes: A supernova in cosmetic dermatology. J. Cosmet. Dermatol. 2021, 20, 3812–3817. [Google Scholar] [CrossRef]
- Vu, D.M.; Nguyen, V.T.; Nguyen, T.H.; Do, P.T.X.; Dao, H.H.; Hai, D.X.; Le, N.T.; Nguyen, X.H.; Than, U.T.T. Effects of Extracellular Vesicles Secreted by TGFbeta-Stimulated Umbilical Cord Mesenchymal Stem Cells on Skin Fibroblasts by Promoting Fibroblast Migration and ECM Protein Production. Biomedicines 2022, 10, 1810. [Google Scholar] [CrossRef]
- Qiu, B.; Xu, X.; Yi, P.; Hao, Y. Curcumin reinforces MSC-derived exosomes in attenuating osteoarthritis via modulating the miR-124/NF-kB and miR-143/ROCK1/TLR9 signalling pathways. J. Cell. Mol. Med. 2020, 24, 10855–10865. [Google Scholar] [CrossRef]
- Semenova, E.; Koegel, H.; Hasse, S.; Klatte, J.E.; Slonimsky, E.; Bilbao, D.; Paus, R.; Werner, S.; Rosenthal, N. Overexpression of mIGF-1 in keratinocytes improves wound healing and accelerates hair follicle formation and cycling in mice. Am. J. Pathol. 2008, 173, 1295–1310. [Google Scholar] [CrossRef]
- Staecker, H.; Van De Water, T.R. Factors controlling hair-cell regeneration/repair in the inner ear. Curr. Opin. Neurobiol. 1998, 8, 480–487. [Google Scholar] [CrossRef]
- Castilho, R.M.; Squarize, C.H.; Leelahavanichkul, K.; Zheng, Y.; Bugge, T.; Gutkind, J.S. Rac1 is required for epithelial stem cell function during dermal and oral mucosal wound healing but not for tissue homeostasis in mice. PLoS ONE 2010, 5, e10503. [Google Scholar] [CrossRef]
- Castilho, R.M.; Squarize, C.H.; Patel, V.; Millar, S.E.; Zheng, Y.; Molinolo, A.; Gutkind, J.S. Requirement of Rac1 distinguishes follicular from interfollicular epithelial stem cells. Oncogene 2007, 26, 5078–5085. [Google Scholar] [CrossRef]
- Tsai, P.W.; Lee, Y.H.; Chen, L.G.; Lee, C.J.; Wang, C.C. In Vitro and In Vivo Anti-Osteoarthritis Effects of 2,3,5,4′-Tetrahydroxystilbene-2-O-beta-d-Glucoside from Polygonum Multiflorum. Molecules 2018, 23, 571. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.Y.; Chagnaadorj, A.; Huang, S.J.; Wang, C.C.; Chiang, Y.H.; Cheng, C.W. Hepatoprotective Activity of the Ethanolic Extract of Polygonum multiflorum Thunb. against Oxidative Stress-Induced Liver Injury. Evid. Based Complement. Altern. Med. 2018, 2018, 4130307. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.Y.; Bayarsengee, U.; Wang, C.C.; Chiang, Y.H.; Cheng, C.W. The natural compound 2,3,5,4′-tetrahydroxystilbene-2-O-beta-d glucoside protects against adriamycin-induced nephropathy through activating the Nrf2-Keap1 antioxidant pathway. Environ. Toxicol. 2018, 33, 72–82. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. In Current Protocols in Cell Biology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; Chapter 3, Unit 3 22. [Google Scholar] [CrossRef]
- Tracy, S.A.; Ahmed, A.; Tigges, J.C.; Ericsson, M.; Pal, A.K.; Zurakowski, D.; Fauza, D.O. A comparison of clinically relevant sources of mesenchymal stem cell-derived exosomes: Bone marrow and amniotic fluid. J. Pediatr. Surg. 2019, 54, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Vogel, R.; Savage, J.; Muzard, J.; Camera, G.D.; Vella, G.; Law, A.; Marchioni, M.; Mehn, D.; Geiss, O.; Peacock, B.; et al. Measuring particle concentration of multimodal synthetic reference materials and extracellular vesicles with orthogonal techniques: Who is up to the challenge? J. Extracell. Vesicles 2021, 10, e12052. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Sharples, R.A.; Scicluna, B.J.; Hill, A.F. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J. Extracell. Vesicles 2014, 3, 23743. [Google Scholar] [CrossRef] [PubMed]

| For Gallus gallus | |||
| Gene name | Forward | Backward | Accession No. |
| CD44 | CCGGGCTTTTCTTCCTTCTG | AGTTGGCCATTGTTTCCTCAG | NM_204860.5 |
| CD73 (NT5E) | CTCCCGTTTCAAGGGTCAGG | TTCATGGTTGCCCAAAGCCA | XM_040669143.1 |
| CD71 (TFRC) | TTGGAGACTCCTGATGCTATCG | TCACATAGACAGGTTTGCCAGA | NM_205256.2 |
| CD34 | TGGGAGTAAGAGTGGGGTCG | CCTGGAGCAGAAAAGGGACTT | XM_040691711.1 |
| MKI67 (Ki-67) | AAACGGAATGGGACTGACGG | TCACATTCTGTTCTCCTTCCAAA | XM_040674390.2 |
| PCNA | CGGATACGTTGGCTCTAGTGT | GGAATTCCAAGCTGCTCCAC | NM_204170.3 |
| RAB27A | AGAAAAAGGCAAATGTGGCTGC | GAGAGTTCACTAAGGCTGCATGA | FJ449551.1 |
| GAPDH | GTCAAGGCTGAGAACGGGAA | GCCCATTTGATGTTGCTGGG | NM_204305.2 |
| For Homo sapiens | |||
| Gene name | Forward | Backward | Accession No. |
| COL1A1 | GTCAGATGGGCCCCCG | CACCATCATTTCCACGAGCA | NM_000088.4 |
| COL3A1 | GAGGATGGTTGCACGAAACAC | CAGCCTTGCGTGTTCGATATT | NM_000090 |
| ELN | CAGGTGCGGTGGTTCCTC | CTGGGTATACACCTGGCAGC | M36860 |
| IL-1β | GCAGCCATGGCAGAAGTACC | AGTCATCCTCATTGCCACTGTAAT | NM_000576.2 |
| TNF-α | TAGCCCATGTTGTAGCAAACCC | TTATCTCTCAGCTCCACGCCA | NM_000594.3 |
| IL-6 | ACCCCCAGGAGAAGATTCCA | GATGCCGTCGAGGATGTACC | M54894.1 |
| RAC1 | TGGCTAAGGAGATTGGTGCTG | CGGATCGCTTCGTCAAACAC | AF498964.1 |
| IGF-1 | ATCAGCAGTCTTCCAACCCAAT | GCCAGGTAGAAGAGATGCGA | M29644.1 |
| CCND1 | ATCAAGTGTGACCCGGACTG | CTTGGGGTCCATGTTCTGCT | NM_053056.3 |
| PCNA | TCTGAGGGCTTCGACACCTA | TCATTGCCGGCGCATTTTAG | BC062439.1 |
| CASP2 | GCATGTACTCCCACCGTTGA | GACAGGCGGAGCTTCTTGTA | NM_032982.3 |
| TP53 | AAGTCTAGAGCCACCGTCCA | CAGTCTGGCTGCCAATCCA | NM_000546.5 |
| 18s | GTAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGCG | NR_003286 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shieh, J.-S.; Chin, Y.-T.; Chiu, H.-C.; Hsieh, Y.-Y.; Cheng, H.-R.; Gu, H.; Chang, F.-W. Bio-Pulsed Stimulation Effectively Improves the Production of Avian Mesenchymal Stem Cell-Derived Extracellular Vesicles That Enhance the Bioactivity of Skin Fibroblasts and Hair Follicle Cells. Int. J. Mol. Sci. 2022, 23, 15010. https://doi.org/10.3390/ijms232315010
Shieh J-S, Chin Y-T, Chiu H-C, Hsieh Y-Y, Cheng H-R, Gu H, Chang F-W. Bio-Pulsed Stimulation Effectively Improves the Production of Avian Mesenchymal Stem Cell-Derived Extracellular Vesicles That Enhance the Bioactivity of Skin Fibroblasts and Hair Follicle Cells. International Journal of Molecular Sciences. 2022; 23(23):15010. https://doi.org/10.3390/ijms232315010
Chicago/Turabian StyleShieh, Ju-Sheng, Yu-Tang Chin, Hsien-Chung Chiu, Ya-Yu Hsieh, Hui-Rong Cheng, Hai Gu, and Fung-Wei Chang. 2022. "Bio-Pulsed Stimulation Effectively Improves the Production of Avian Mesenchymal Stem Cell-Derived Extracellular Vesicles That Enhance the Bioactivity of Skin Fibroblasts and Hair Follicle Cells" International Journal of Molecular Sciences 23, no. 23: 15010. https://doi.org/10.3390/ijms232315010
APA StyleShieh, J.-S., Chin, Y.-T., Chiu, H.-C., Hsieh, Y.-Y., Cheng, H.-R., Gu, H., & Chang, F.-W. (2022). Bio-Pulsed Stimulation Effectively Improves the Production of Avian Mesenchymal Stem Cell-Derived Extracellular Vesicles That Enhance the Bioactivity of Skin Fibroblasts and Hair Follicle Cells. International Journal of Molecular Sciences, 23(23), 15010. https://doi.org/10.3390/ijms232315010









