Identification of Risk Genes Associated with Myocardial Infarction—Big Data Analysis and Literature Review
Abstract
:1. Introduction
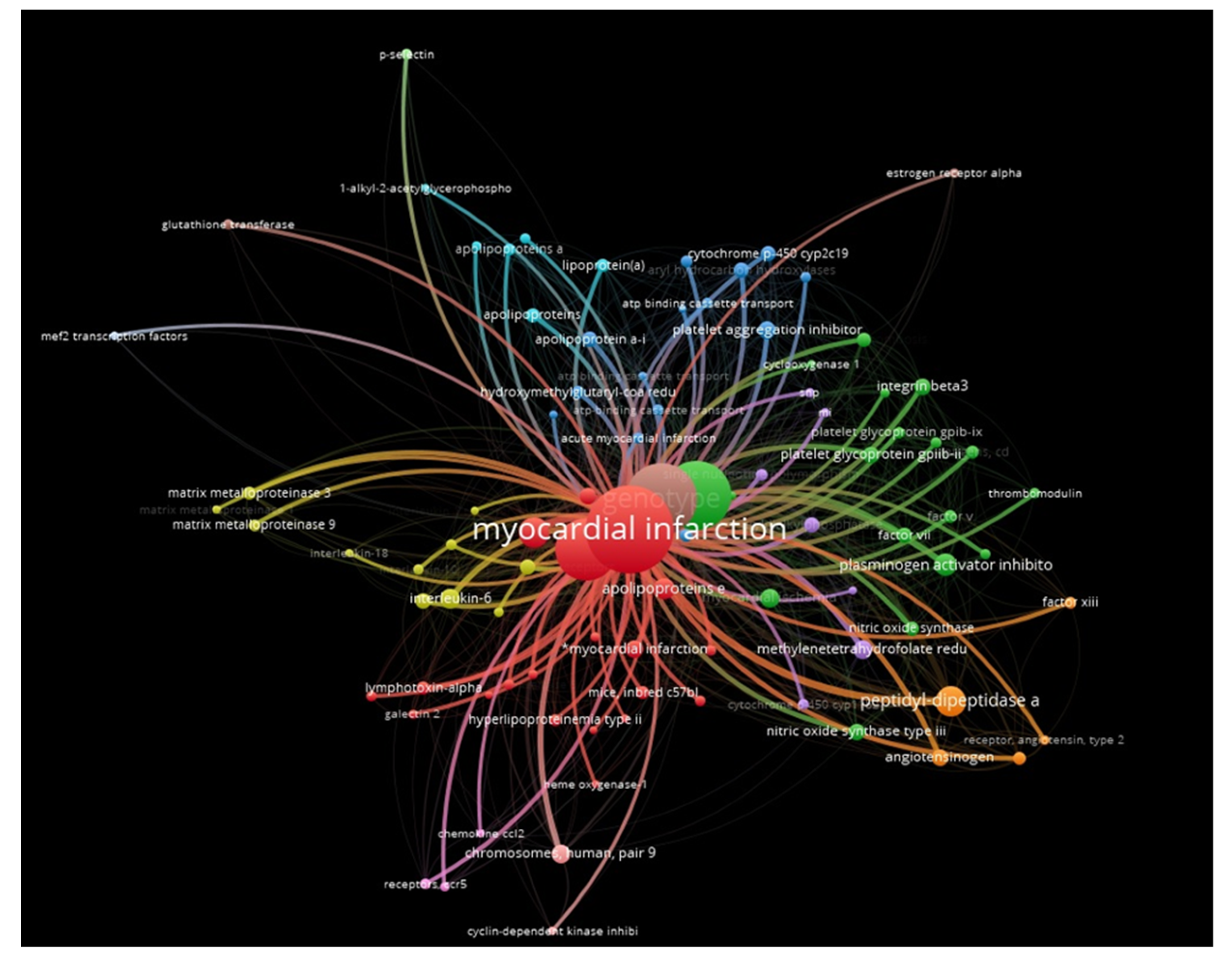
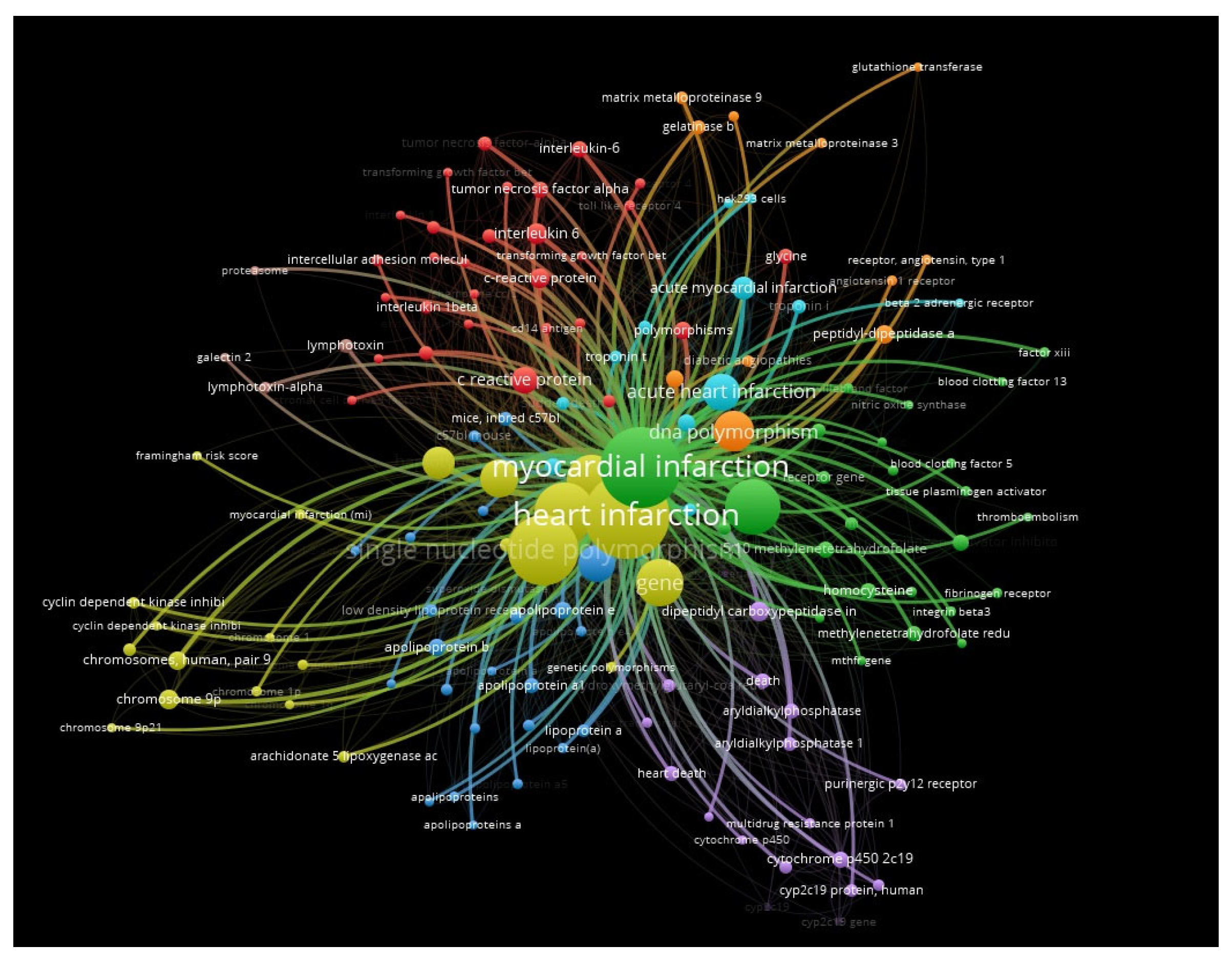
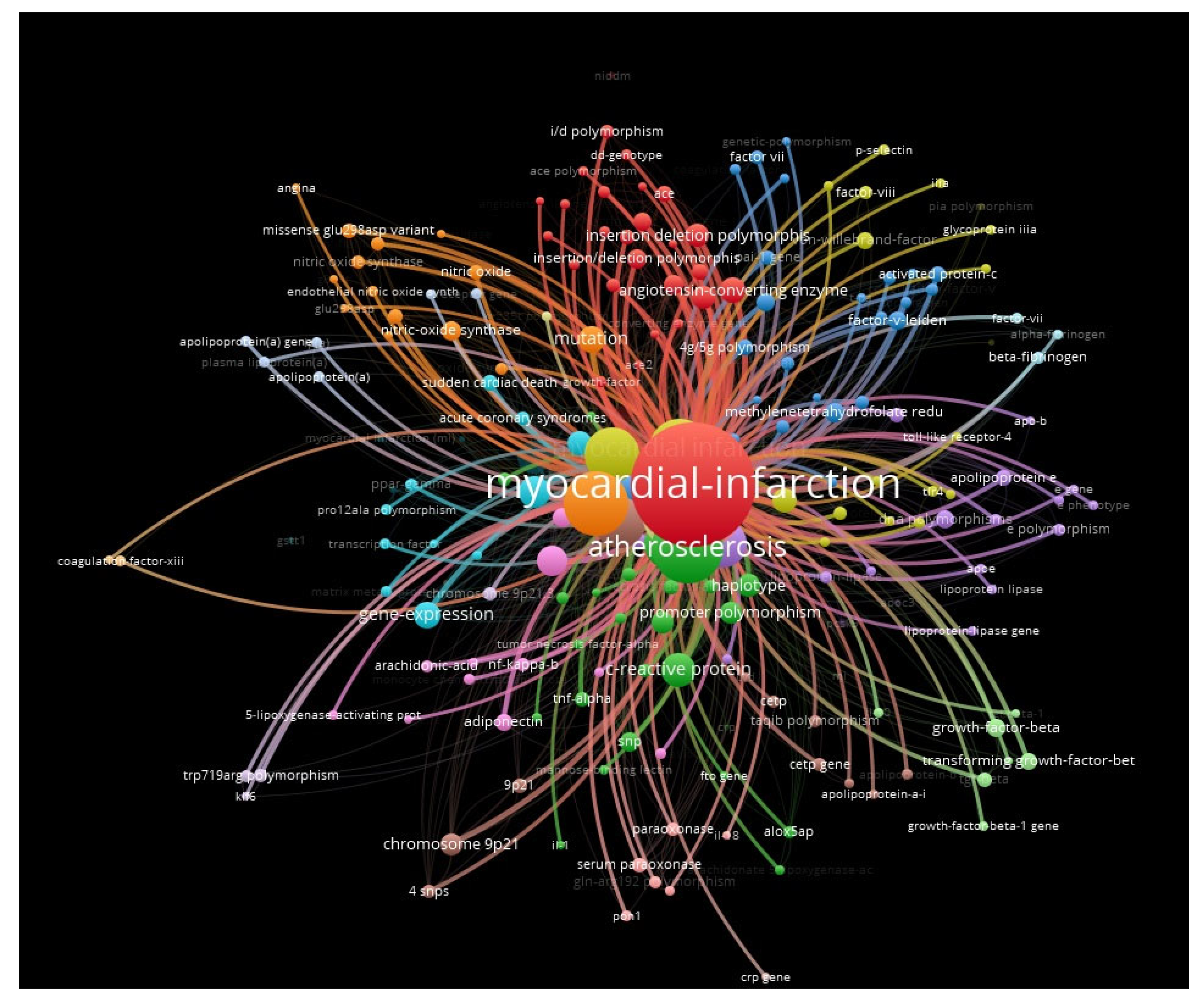
2. Results
3. Discussion
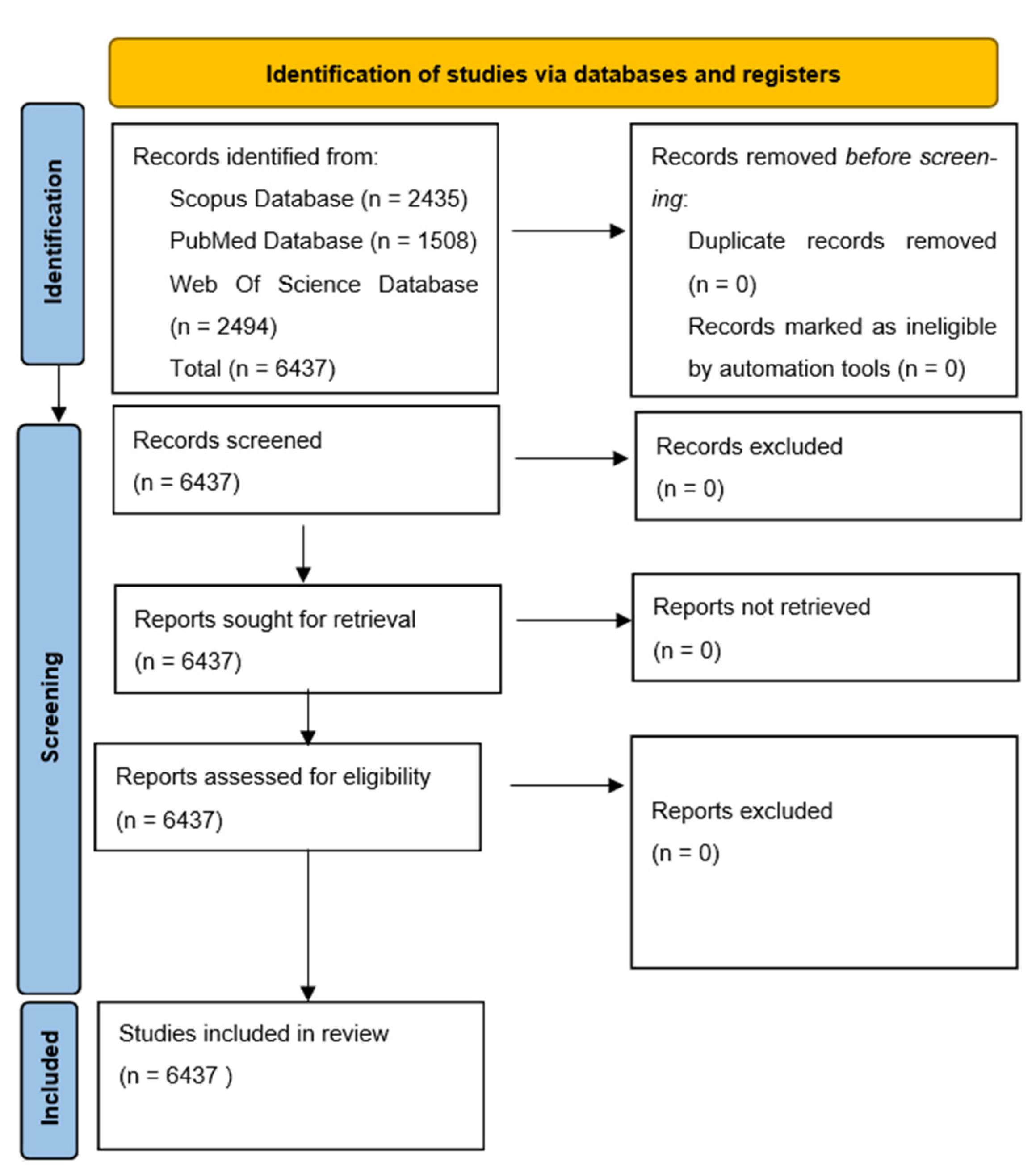
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Yang, X. Identification of risk genes associated with myocardial infarction based on the recursive feature elimination algorithm and support vector machine classifier. Mol. Med. Rep. 2018, 17, 1555–1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, H.; Hu, D.; Li, Q.; Tu, S. Risk gene identification and support vector machine learning to construct an early diagnosis model of myocardial infarction. Mol. Med. Rep. 2020, 22, 1775–1782. [Google Scholar] [CrossRef] [PubMed]
- Boateng, S.; Sanborn, T. Acute myocardial infarction. Dis. A Mon. 2013, 59, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.; Berry, J.D.; Borden, W.B.; Bravata, D.M.; Dai, S.; Ford, E.S.; Fox, C.S.; et al. Heart disease and stroke statistics-2013 update: A Report from the American Heart Association. Circulation 2013, 127, e6–e245. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, L.; Kaufman, D.W.; Helmrich, S.P.; Miller, D.R.; Stolley, P.D.; Shapiro, S. Myocardial Infarction and Cigarette Smoking in Women Younger Than 50 Years of Age. JAMA J. Am. Med. Assoc. 1985, 253, 2965–2969. [Google Scholar] [CrossRef]
- Leong, D.P.; Smyth, A.; Teo, K.K.; McKee, M.; Rangarajan, S.; Pais, P.; Liu, L.; Anand, S.S.; Yusuf, S. Patterns of alcohol consumption and myocardial infarction risk: Observations from 52 countries in the INTERHEART case-control study. Circulation 2014, 130, 390–398. [Google Scholar] [CrossRef] [Green Version]
- Poulter, N.R.; Chang, C.L.; Farley, T.M.M.; Kelaghan, J.; Meirlk, O.; Marmot, M.G.; Debert-Ribeiro, M.; Medina, E.; Artigas, J.; He, S.; et al. Acute myocardial infarction and combined oral contraceptives: Results of an international multicentre case-control study. Lancet 1997, 349, 1202–1209. Available online: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed4&NEWS=N&AN=1997133337 (accessed on 3 October 2022).
- Cassidy, A.; Mukamal, K.J.; Liu, L.; Franz, M.; Eliassen, A.H.; Rimm, E.B. High Anthocyanin Intake Is Associated with a Reduced Risk of Myocardial Infarction in Young and Middle-Aged Women. Circulation 2013, 127, 188–196. [Google Scholar] [CrossRef] [Green Version]
- Freiberg, M.S.; Chang, C.C.H.; Kuller, L.H.; Skanderson, M.; Lowy, E.; Kraemer, K.L.; Butt, A.A.; Goetz, M.B.; Leaf, D.; Oursler, K.A.; et al. HIV Infection and the Risk of Acute Myocardial Infarction. JAMA Intern. Med. 2013, 173, 614–622. [Google Scholar] [CrossRef]
- Helgadottir, A.; Manolescu, A.; Thorleifsson, G.; Gretarsdottir, S.; Jonsdottir, H.; Thorsteinsdottir, U.; Samani, N.J.; Gudmundsson, G.H.; Grant, S.; Thorgeirsson, G.; et al. The gene encoding 5-lipoxygenase activating protein confers risk of myocardial infarction and stroke. Nat. Genet. 2004, 36, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Do, R.; Project, N.E.S.; Stitziel, N.O.; Won, H.-H.; Jørgensen, A.B.; Duga, S.; Merlini, P.A.; Kiezun, A.; Farrall, M.; Goel, A.; et al. Exome sequencing identifies rare LDLR and APOA5 alleles conferring risk for myocardial infarction. Nature 2015, 518, 102–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, H.; Wang, H.; Yin, Z.; Jiang, H.; Fang, M.; Han, J. Association of genetic polymorphisms in ADH and ALDH2 with risk of coronary artery disease and myocardial infarction: A meta-analysis. Gene 2013, 526, 134–141. [Google Scholar] [CrossRef]
- Anand, S.S.; Xie, C.; Paré, G.; Montpetit, A.; Rangarajan, S.; McQueen, M.J.; Cordell, H.J.; Keavney, B.; Yusuf, S.; Hudson, T.J.; et al. Genetic variants associated with myocardial infarction risk factors in over 8000 individuals from five ethnic groups the INTERHEART genetics study. Circ. Cardiovasc. Genet. 2009, 2, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, P.; Kallin, B.; Hooft, F.M.V.; Båvenholm, P.; Hamsten, A. Allele-specific increase in basal transcription of the plasminogen-activator inhibitor 1 gene is associated with myocardial infarction. Proc. Natl. Acad. Sci. USA 1995, 92, 1851–1855. [Google Scholar] [CrossRef] [Green Version]
- Dawson, S.; Wiman, B.; Hamsten, A.; Green, F.; Humphries, S.; Henney, A. The two allele sequences of a common polymorphism in the promoter of the plasminogen activator inhibitor-1 (PAI-1) gene respond differently to interleukin-1 in HepG2 cells. J. Biol. Chem. 1993, 268, 10739–10745. [Google Scholar] [CrossRef]
- Jung, R.G.; Motazedian, P.; Ramirez, F.D.; Simard, T.; Di Santo, P.; Visintini, S.; Faraz, M.A.; Labinaz, A.; Jung, Y.; Hibbert, B. Association between plasminogen activator inhibitor-1 and cardiovascular events: A systematic review and meta-analysis. Thromb. J. 2018, 16, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Senno, S.L.; Pechet, L. Clinical Implications of Elevated PAI-1 Revisited: Multiple Arterial Thrombosis in a Patient with Essential Thrombocythemia and Elevated Plasminogen Activator Inhibitor-1 (PAI-1) Levels with a Review of the Literature. J. Thromb. Thrombolysis 1999, 8, 105–112. [Google Scholar] [CrossRef]
- Weiss, E.J.; Bray, P.F.; Tayback, M.; Schulman, S.P.; Kickler, T.S.; Becker, L.C.; Weiss, J.L.; Gerstenblith, G.; Goldschmidt-Clermont, P.J. A Polymorphism of A Platelet Glycoprotein Receptor as An Inherited Risk Factor for Coronary Thrombosis. N. Engl. J. Med. 1996, 334, 1090–1094. [Google Scholar] [CrossRef] [Green Version]
- Ridker, P.M.; Hennekens, C.H.; Schmitz, C.; Stampfer, M.J.; Lindpaintner, K. PIA1/A2 polymorphism of platelet glycoprotein IIIa and risks of myocardial infarction, stroke, and venous thrombosis. Lancet 1997, 349, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Iacoviello, L.; Di Castelnuovo, A.; De Knijff, P.; D’Orazio, A.; Amore, C.; Arboretti, R.; Kluft, C.; Donati, M.B. Polymorphisms in the Coagulation Factor Vii Gene and the Risk of Myocardial Infarction. N. Engl. J. Med. 1998, 338, 79–85. [Google Scholar] [CrossRef]
- Lindman, A.S.; Pedersen, J.I.; Arnesen, H.; Hjerkinn, E.M.; Veierød, M.B.; Prydz, H.; Seljeflot, I. Coagulation factor VII, R353Q polymorphism, and serum choline-containing phospholipids in males at high risk for coronary heart disease. Thromb. Res. 2004, 113, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Inoue, N.; Kawashima, S.; Kanazawa, K.; Yamada, S.; Akita, H.; Yokoyama, M. Polymorphism of the NADH/NADPH Oxidase p22 phox Gene in Patients with Coronary Artery Disease. Circulation 1998, 97, 135–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boerma, M.; Forsberg, L.; Van Zeijl, L.; Morgenstern, R.; De Faire, U.; Lemne, C.; Erlinge, D.; Thulin, T.; Hong, Y.; Cotgreave, I.A. A genetic polymorphism in connexin 37 as a prognostic marker for atherosclerotic plaque development. J. Intern. Med. 1999, 246, 211–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, Y.; Izawa, H.; Ichihara, S.; Takatsu, F.; Ishihara, H.; Hirayama, H.; Sone, T.; Tanaka, M.; Yokota, M. Prediction of the Risk of Myocardial Infarction from Polymorphisms in Candidate Genes. N. Engl. J. Med. 2002, 347, 1916–1923. [Google Scholar] [CrossRef]
- Lanfear, D.E.; Marsh, S.; Cresci, S.; Shannon, W.D.; Spertus, J.A.; McLeod, H.L. Genotypes associated with myocardial infarction risk are more common in African Americans than in European Americans. J. Am. Coll. Cardiol. 2004, 44, 165–167. [Google Scholar] [CrossRef] [Green Version]
- Tiret, L.; Godefroy, T.; Lubos, E.; Nicaud, V.; Tregouet, D.-A.; Barbaux, S.; Schnabel, R.; Bickel, C.; Espinola-Klein, C.; Poirier, O.; et al. Genetic Analysis of the Interleukin-18 System Highlights the Role of the Interleukin-18 Gene in Cardiovascular Disease. Circulation 2005, 112, 643–650. [Google Scholar] [CrossRef] [Green Version]
- Lian, Z.; Li, S.-F.; Cui, Y.-X.; Wu, M.-Y.; Su, L.-N.; Hu, D.; Xiong, W.-J.; Chen, H. Association between polymorphisms in interleukin-18 promoter and risk of coronary artery disease: A meta-analysis. Biosci. Rep. 2019, 39, BSR20192721. [Google Scholar] [CrossRef]
- Shiffman, D.; Ellis, S.G.; Rowland, C.M.; Malloy, M.J.; Luke, M.M.; Iakoubova, O.A.; Pullinger, C.R.; Cassano, J.; Aouizerat, B.E.; Fenwick, R.G.; et al. Identification of Four Gene Variants Associated with Myocardial Infarction. Am. J. Hum. Genet. 2005, 77, 596–605. [Google Scholar] [CrossRef] [Green Version]
- Ozaki, K.; Tanaka, T. Genome-wide association study to identify SNPs conferring risk of myocardial infarction and their functional analyses. Cell. Mol. Life Sci. 2005, 62, 1804–1813. [Google Scholar] [CrossRef] [PubMed]
- Helgadottir, A.; Thorleifsson, G.; Manolescu, A.; Gretarsdottir, S.; Blondal, T.; Jonasdottir, A.; Jonasdottir, A.; Sigurdsson, A.; Baker, A.; Palsson, A.; et al. A Common Variant on Chromosome 9p21 Affects the Risk of Myocardial Infarction. Science 2007, 316, 1491–1493. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Zhang, W.; Ruan, Z.-B.; Zhu, L.; Liu, Y.; Mi, Y.-Y.; Zhang, L.-F. New findings in the roles of Cyclin-dependent Kinase inhibitors 2B Antisense RNA 1 (CDKN2B-AS1) rs1333049 G/C and rs4977574 A/G variants on the risk to coronary heart disease. Bioengineered 2020, 11, 1084–1098. [Google Scholar] [CrossRef] [PubMed]
- Alfakih, K.; Brown, B.; Lawrance, R.; Warburton, P.; Maqbool, A.; Walters, K.; Samani, N.; Ball, S.; Balmforth, A.; Hall, A. Effect of a common X-linked angiotensin II type 2-receptor gene polymorphism (−1332 G/A) on the occurrence of premature myocardial infarction and stenotic atherosclerosis requiring revascularization. Atherosclerosis 2007, 195, e32–e38. [Google Scholar] [CrossRef] [PubMed]
| Genes/Alleles | Description | Possible Implication in MI |
|---|---|---|
| PAI-1 | Encodes a member of the serine proteinase inhibitor (serpin) superfamily | Reduced fibrinolytic capacity |
| Membrane receptor for fibrinogen and von Willebrand factor (platelet glycoprotein IIIa gene) | Platelet aggregation at the site of a ruptured coronary atherosclerotic plaque | |
| Alleles: R353Q H7H5, H7H6 | Coagulation Factor VII regulator gene | Factor VII clotting activity |
| Stromelysin-1 | Cleaves a number of extracellular matrix and non-extracellular matrix proteins | Progression of coronary atherosclerosis |
| IL Genes | Proinflammatory cytokine involved in both innate and acquired immune responses | Atherosclerosis |
| LTA LGALS2 | Lymphotoxin-alpha (LT-α) or tumor necrosis factor-beta (TNF-β) | |
| AT2 (-1332 G/A) | Angiotensin II type 2 (AT2) receptor | Stenotic atherosclerosis |
| RS671 | Coronary artery disease | |
| LDLR | Low-density lipoprotein receptor | Atherosclerosis |
| AKAP12 | Scaffolding proteins that regulate the cellular cyclic AMP response | Influencing cardiac contractility |
| OR8D2 | G-protein-coupled receptors | Atherosclerosis |
| GLRA2 | Glycine receptor | Influencing cardiac contractility |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tirdea, C.; Hostiuc, S.; Moldovan, H.; Scafa-Udriste, A. Identification of Risk Genes Associated with Myocardial Infarction—Big Data Analysis and Literature Review. Int. J. Mol. Sci. 2022, 23, 15008. https://doi.org/10.3390/ijms232315008
Tirdea C, Hostiuc S, Moldovan H, Scafa-Udriste A. Identification of Risk Genes Associated with Myocardial Infarction—Big Data Analysis and Literature Review. International Journal of Molecular Sciences. 2022; 23(23):15008. https://doi.org/10.3390/ijms232315008
Chicago/Turabian StyleTirdea, Cosmin, Sorin Hostiuc, Horatiu Moldovan, and Alexandru Scafa-Udriste. 2022. "Identification of Risk Genes Associated with Myocardial Infarction—Big Data Analysis and Literature Review" International Journal of Molecular Sciences 23, no. 23: 15008. https://doi.org/10.3390/ijms232315008
APA StyleTirdea, C., Hostiuc, S., Moldovan, H., & Scafa-Udriste, A. (2022). Identification of Risk Genes Associated with Myocardial Infarction—Big Data Analysis and Literature Review. International Journal of Molecular Sciences, 23(23), 15008. https://doi.org/10.3390/ijms232315008







