Golgi Complex form and Function: A Potential Hub Role Also in Skeletal Muscle Pathologies?
Abstract
:1. Introduction
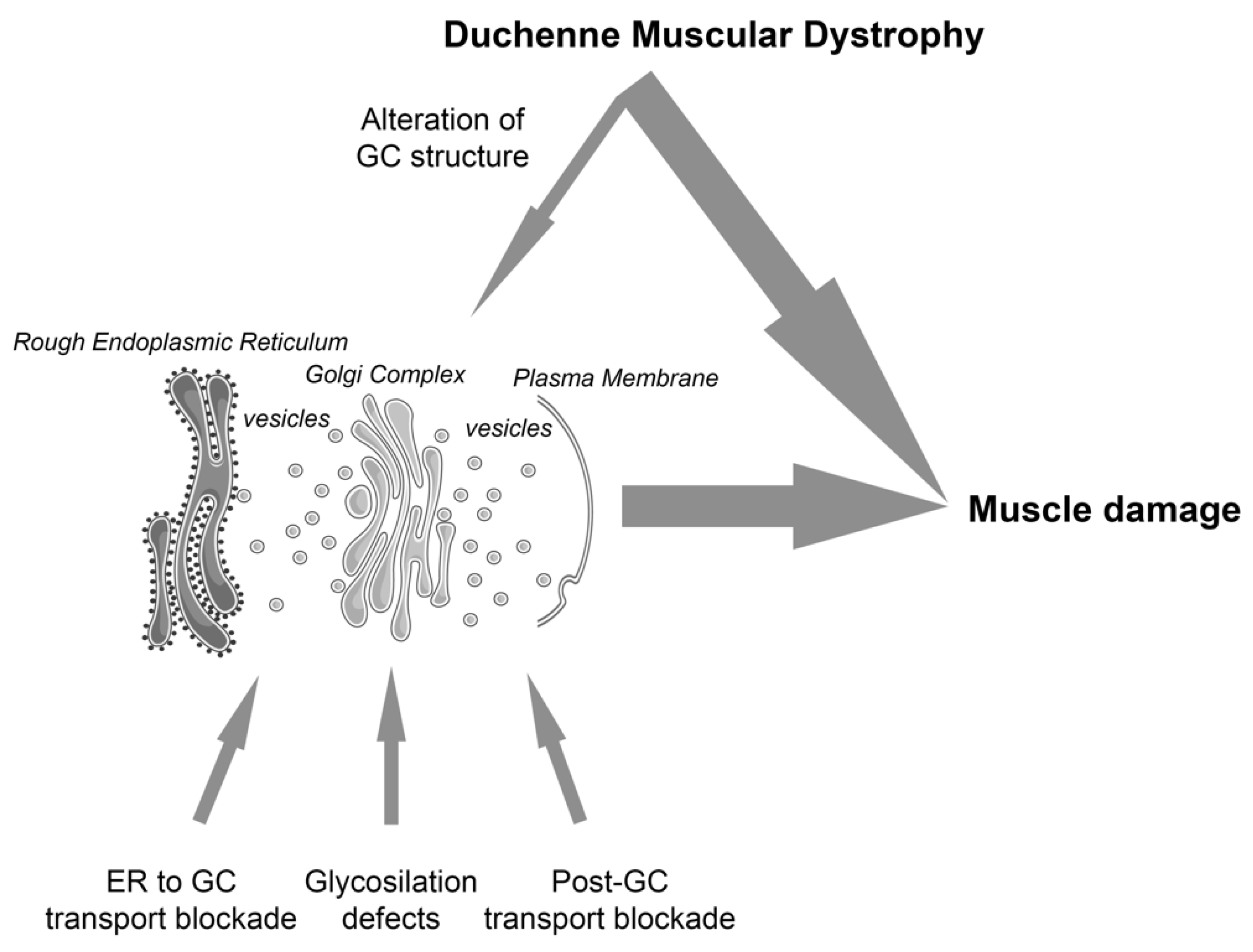
2. The Early Secretory Pathway and Skeletal Muscle Dystrophies
3. The Golgi Complex: A Hub Organelle in the Early Secretory Pathway
4. The Components of the Early Secretory Pathway as Therapeutic Targets
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dalkilic, I.; Kunkel, L.M. Muscular Dystrophies: Genes to Pathogenesis. Curr. Opin. Genet. Dev. 2003, 13, 231–238. [Google Scholar] [CrossRef]
- Gissen, P.; Maher, E.R. Cargos and Genes: Insights into Vesicular Transport from Inherited Human Disease. J. Med. Genet. 2007, 44, 545–555. [Google Scholar] [CrossRef] [Green Version]
- García-Cazorla, A.; Oyarzábal, A.; Saudubray, J.-M.; Martinelli, D.; Dionisi-Vici, C. Genetic Disorders of Cellular Trafficking. Trends Genet. 2022, 38, 724–751. [Google Scholar] [CrossRef]
- Anelli, T.; Sitia, R. Protein Quality Control in the Early Secretory Pathway. EMBO J. 2008, 27, 315–327. [Google Scholar] [CrossRef] [Green Version]
- Giacomello, E.; Ronchi, P.; Pepperkok, R. GM130 and P115 Play a Key Role in the Organisation of the Early Secretory Pathway during Skeletal Muscle Differentiation. J. Cell Sci. 2019, 132, jcs222083. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.; Joseph, D.; Bugnard, E.; Zaal, K.J.; Ralston, E. Golgi Complex Reorganization during Muscle Differentiation: Visualization in Living Cells and Mechanism. Mol. Biol. Cell 2001, 12, 795–808. [Google Scholar] [CrossRef] [Green Version]
- Ralston, E.; Lu, Z.; Ploug, T. The Organization of the Golgi Complex and Microtubules in Skeletal Muscle Is Fiber Type-Dependent. J. Neurosci. Off. J. Soc. Neurosci. 1999, 19, 10694–10705. [Google Scholar] [CrossRef] [Green Version]
- Ronchi, P.; Tischer, C.; Acehan, D.; Pepperkok, R. Positive Feedback between Golgi Membranes, Microtubules and ER Exit Sites Directs de Novo Biogenesis of the Golgi. J. Cell Sci. 2014, 127, 4620–4633. [Google Scholar] [CrossRef] [Green Version]
- Luini, A.; Parashuraman, S. Signaling at the Golgi: Sensing and Controlling the Membrane Fluxes. Curr. Opin. Cell Biol. 2016, 39, 37–42. [Google Scholar] [CrossRef]
- Bexiga, M.G.; Simpson, J.C. Human Diseases Associated with Form and Function of the Golgi Complex. Int. J. Mol. Sci. 2013, 14, 18670–18681. [Google Scholar] [CrossRef]
- Petrosyan, A. Unlocking Golgi: Why Does Morphology Matter? Biochem. Biokhimiia 2019, 84, 1490–1501. [Google Scholar] [CrossRef]
- Cohn, R.D.; Campbell, K.P. Molecular Basis of Muscular Dystrophies. Muscle Nerve 2000, 23, 1456–1471. [Google Scholar] [CrossRef]
- Falsaperla, R.; Praticò, A.D.; Ruggieri, M.; Parano, E.; Rizzo, R.; Corsello, G.; Vitaliti, G.; Pavone, P. Congenital Muscular Dystrophy: From Muscle to Brain. Ital. J. Pediatr. 2016, 42, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanagawa, M. Dystroglycanopathy: From Elucidation of Molecular and Pathological Mechanisms to Development of Treatment Methods. Int. J. Mol. Sci. 2021, 22, 13162. [Google Scholar] [CrossRef]
- Angelini Corrado, L.N. Heterogeneous Pathogenesis of LGMD2: Consequences for Therapy. Basic Appl. Myol. 2007, 17, 173–179. [Google Scholar]
- Brockington, M.; Yuva, Y.; Prandini, P.; Brown, S.C.; Torelli, S.; Benson, M.A.; Herrmann, R.; Anderson, L.V.; Bashir, R.; Burgunder, J.M.; et al. Mutations in the Fukutin-Related Protein Gene (FKRP) Identify Limb Girdle Muscular Dystrophy 2I as a Milder Allelic Variant of Congenital Muscular Dystrophy MDC1C. Hum. Mol. Genet. 2001, 10, 2851–2859. [Google Scholar] [CrossRef]
- Minetti, C.; Sotgia, F.; Bruno, C.; Scartezzini, P.; Broda, P.; Bado, M.; Masetti, E.; Mazzocco, M.; Egeo, A.; Donati, M.A.; et al. Mutations in the Caveolin-3 Gene Cause Autosomal Dominant Limb-Girdle Muscular Dystrophy. Nat. Genet. 1998, 18, 365–368. [Google Scholar] [CrossRef]
- Bogershausen, N.; Shahrzad, N.; Chong, J.X.; von Kleist-Retzow, J.C.; Stanga, D.; Li, Y.; Bernier, F.P.; Loucks, C.M.; Wirth, R.; Puffenberger, E.G.; et al. Recessive TRAPPC11 Mutations Cause a Disease Spectrum of Limb Girdle Muscular Dystrophy and Myopathy with Movement Disorder and Intellectual Disability. Am. J. Hum. Genet. 2013, 93, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Fee, D.B.; Harmelink, M.; Monrad, P.; Pyzik, E. Siblings with Mutations in TRAPPC11 Presenting with Limb-Girdle Muscular Dystrophy 2S. J. Clin. Neuromuscul. Dis. 2017, 19, 27–30. [Google Scholar] [CrossRef]
- Bashir, R.; Britton, S.; Strachan, T.; Keers, S.; Vafiadaki, E.; Lako, M.; Richard, I.; Marchand, S.; Bourg, N.; Argov, Z.; et al. A Gene Related to Caenorhabditis Elegans Spermatogenesis Factor Fer-1 Is Mutated in Limb-Girdle Muscular Dystrophy Type 2B. Nat. Genet. 1998, 20, 37–42. [Google Scholar] [CrossRef]
- Shamseldin, H.E.; Bennett, A.H.; Alfadhel, M.; Gupta, V.; Alkuraya, F.S. GOLGA2, Encoding a Master Regulator of Golgi Apparatus, Is Mutated in a Patient with a Neuromuscular Disorder. Hum. Genet. 2016, 135, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, U.; Mistri, M.; Shah, N.; Shah, P.S.; Gupta, V.A. Bi-Allelic Loss of Function Variants in GOLGA2 Are Associated with a Complex Neurological Phenotype: Report of a Second Family. Clin. Genet. 2021, 100, 748–751. [Google Scholar] [CrossRef] [PubMed]
- Donkervoort, S.; Krause, N.; Dergai, M.; Yun, P.; Koliwer, J.; Gorokhova, S.; Geist Hauserman, J.; Cummings, B.B.; Hu, Y.; Smith, R.; et al. BET1 Variants Establish Impaired Vesicular Transport as a Cause for Muscular Dystrophy with Epilepsy. EMBO Mol. Med. 2021, 13, e13787. [Google Scholar] [CrossRef] [PubMed]
- Linders, P.T.A.; Peters, E.; ter Beest, M.; Lefeber, D.J.; van den Bogaart, G. Sugary Logistics Gone Wrong: Membrane Trafficking and Congenital Disorders of Glycosylation. Int. J. Mol. Sci. 2020, 21, 4654. [Google Scholar] [CrossRef]
- Saraste, J.; Prydz, K. A New Look at the Functional Organization of the Golgi Ribbon. Front. Cell Dev. Biol. 2019, 7, 171. [Google Scholar] [CrossRef]
- Duden, R. ER-to-Golgi Transport: COP I and COP II Function (Review). Mol. Membr. Biol. 2003, 20, 197–207. [Google Scholar] [CrossRef]
- Hellicar, J.; Stevenson, N.L.; Stephens, D.J.; Lowe, M. Supply Chain Logistics—The Role of the Golgi Complex in Extracellular Matrix Production and Maintenance. J. Cell Sci. 2022, 135, jcs258879. [Google Scholar] [CrossRef]
- Donalies, M.; Cramer, M.; Ringwald, M.; Starzinski-Powitz, A. Expression of M-Cadherin, a Member of the Cadherin Multigene Family, Correlates with Differentiation of Skeletal Muscle Cells. Proc. Natl. Acad. Sci. USA 1991, 88, 8024–8028. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Mei, M.; Li, Q.; Roboti, P.; Pang, Q.; Ying, Z.; Gao, F.; Lowe, M.; Bao, S. Loss of the Golgin GM130 Causes Golgi Disruption, Purkinje Neuron Loss, and Ataxia in Mice. Proc. Natl. Acad. Sci. USA 2017, 114, 346–351. [Google Scholar] [CrossRef] [Green Version]
- Percival, J.M.; Froehner, S.C. Golgi Complex Organization in Skeletal Muscle: A Role for Golgi-Mediated Glycosylation in Muscular Dystrophies? Traffic 2007, 8, 184–194. [Google Scholar] [CrossRef]
- Percival, J.M.; Gregorevic, P.; Odom, G.L.; Banks, G.B.; Chamberlain, J.S.; Froehner, S.C. RAAV6-Microdystrophin Rescues Aberrant Golgi Complex Organization in Mdx Skeletal Muscles. Traffic 2007, 8, 1424–1439. [Google Scholar] [CrossRef] [PubMed]
- Oddoux, S.; Randazzo, D.; Kenea, A.; Alonso, B.; Zaal, K.J.M.; Ralston, E. Misplaced Golgi Elements Produce Randomly Oriented Microtubules and Aberrant Cortical Arrays of Microtubules in Dystrophic Skeletal Muscle Fibers. Front. Cell Dev. Biol. 2019, 7, 176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowe, M. The Physiological Functions of the Golgin Vesicle Tethering Proteins. Front. Cell Dev. Biol. 2019, 7, 94. [Google Scholar] [CrossRef]
- Sicari, D.; Igbaria, A.; Chevet, E. Control of Protein Homeostasis in the Early Secretory Pathway: Current Status and Challenges. Cells 2019, 8, 1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Gee, H.Y.; Lee, M.G. Unconventional Protein Secretion—New Insights into the Pathogenesis and Therapeutic Targets of Human Diseases. J. Cell Sci. 2018, 131, jcs213686. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toniolo, L.; Sirago, G.; Fiotti, N.; Giacomello, E. Golgi Complex form and Function: A Potential Hub Role Also in Skeletal Muscle Pathologies? Int. J. Mol. Sci. 2022, 23, 14989. https://doi.org/10.3390/ijms232314989
Toniolo L, Sirago G, Fiotti N, Giacomello E. Golgi Complex form and Function: A Potential Hub Role Also in Skeletal Muscle Pathologies? International Journal of Molecular Sciences. 2022; 23(23):14989. https://doi.org/10.3390/ijms232314989
Chicago/Turabian StyleToniolo, Luana, Giuseppe Sirago, Nicola Fiotti, and Emiliana Giacomello. 2022. "Golgi Complex form and Function: A Potential Hub Role Also in Skeletal Muscle Pathologies?" International Journal of Molecular Sciences 23, no. 23: 14989. https://doi.org/10.3390/ijms232314989






