Turner Syndrome Mosaicism 45,X/46,XY with Genital Ambiguity and Duchenne Muscular Dystrophy: Translational Approach of a Rare Italian Case
Abstract
1. Introduction
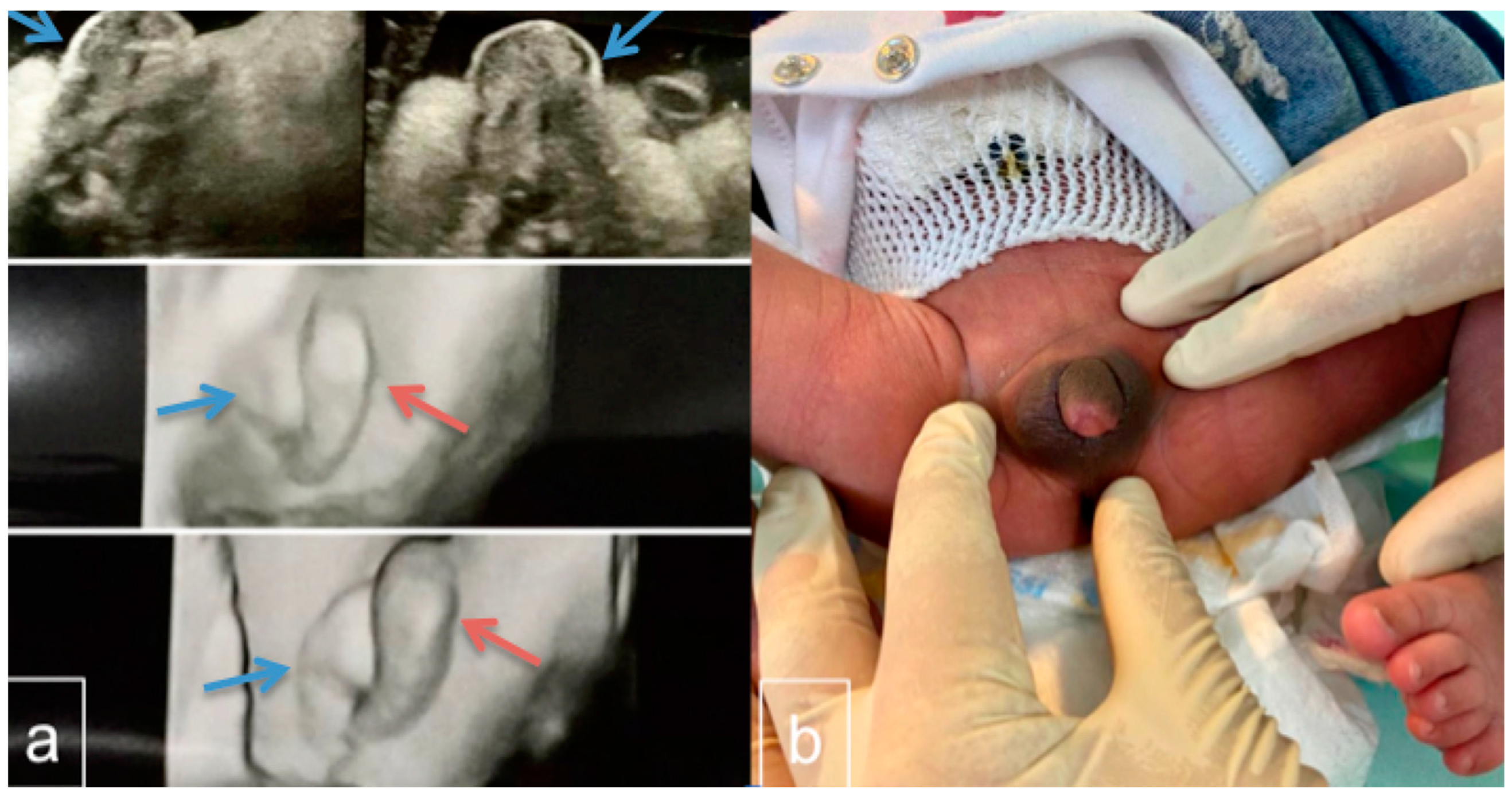
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eggers, S.; Ohnesorg, T.; Sinclair, A. Genetic regulation of mammalian gonad development. Nat. Rev. Endocrinol. 2014, 10, 673–683. [Google Scholar] [CrossRef]
- Brennan, J.; Capel, B. One tissue, two fates: Molecular genetic events that underlie testis versus ovary development. Nat. Rev. Genet. 2004, 5, 509–521. [Google Scholar] [CrossRef]
- Wilhelm, D.; Palmer, S.; Koopman, P. Sex determination and gonadal development in mammals. Physiol. Rev. 2007, 87, 1–28. [Google Scholar] [CrossRef]
- Tanaka, S.S.; Nishinakamura, R. Regulation of male sex determination: Genital ridge formation and Sry activation in mice. Cell Mol. Life Sci. 2014, 71, 4781–4802. [Google Scholar] [CrossRef]
- Vorona, E.; Zitzmann, M.; Gromoll, J.; Schuring, A.N.; Nieschlag, E. Clinical, endocrinological, and epigenetic features of the 46,XX male syndrome, compared with 47,XXY Klinefelter patients. J. Clin. Endocrinol. Metab. 2007, 92, 3458–3465. [Google Scholar] [CrossRef]
- Gravholt, C.H.; Viuff, M.H.; Brun, S.; Stochholm, K.; Andersen, N.H. Turner syndrome: Mechanisms and management. Nat. Rev. Endocrinol. 2019, 15, 601–614. [Google Scholar] [CrossRef]
- Bonomi, M.; Rochira, V.; Pasquali, D.; Balercia, G.; Jannini, E.A.; Ferlin, A.; Klinefelter Italia, N.G. Klinefelter syndrome (KS): Genetics, clinical phenotype and hypogonadism. J. Endocrinol. Investig. 2017, 40, 123–134. [Google Scholar] [CrossRef]
- Sybert, V.P.; McCauley, E. Turner’s syndrome. N. Engl. J. Med. 2004, 351, 1227–1238. [Google Scholar] [CrossRef]
- Gravholt, C.H. Epidemiological, endocrine and metabolic features in Turner syndrome. Eur. J. Endocrinol. 2004, 151, 657–687. [Google Scholar] [CrossRef]
- Donaldson, M.D.; Gault, E.J.; Tan, K.W.; Dunger, D.B. Optimising management in Turner syndrome: From infancy to adult transfer. Arch. Dis. Child 2006, 91, 513–520. [Google Scholar] [CrossRef]
- Jones, K.L.; McNamara, E.A.; Longoni, M.; Miller, D.E.; Rohanizadegan, M.; Newman, L.A.; Hayes, F.; Levitsky, L.L.; Herrington, B.L.; Lin, A.E. Dual diagnoses in 152 patients with Turner syndrome: Knowledge of the second condition may lead to modification of treatment and/or surveillance. Am. J. Med. Genet. A 2018, 176, 2435–2445. [Google Scholar] [CrossRef]
- Shvetsova, E.; Sofronova, A.; Monajemi, R.; Gagalova, K.; Draisma, H.H.M.; White, S.J.; Santen, G.W.E.; Chuva de Sousa Lopes, S.M.; Heijmans, B.T.; van Meurs, J.; et al. Skewed X-inactivation is common in the general female population. Eur. J. Hum. Genet. 2019, 27, 455–465. [Google Scholar] [CrossRef]
- Fortunato, F.; Rossi, R.; Falzarano, M.S.; Ferlini, A. Innovative Therapeutic Approaches for Duchenne Muscular Dystrophy. J. Clin. Med. 2021, 10, 820. [Google Scholar] [CrossRef]
- Nowak, K.J.; Davies, K.E. Duchenne muscular dystrophy and dystrophin: Pathogenesis and opportunities for treatment. EMBO Rep. 2004, 5, 872–876. [Google Scholar] [CrossRef]
- de Martinville, B.; Kunkel, L.M.; Bruns, G.; Morle, F.; Koenig, M.; Mandel, J.L.; Horwich, A.; Latt, S.A.; Gusella, J.F.; Housman, D.; et al. Localization of DNA sequences in region Xp21 of the human X chromosome: Search for molecular markers close to the Duchenne muscular dystrophy locus. Am. J. Hum. Genet. 1985, 37, 235–249. [Google Scholar]
- Kaczorowska, E.; Zimowski, J.; Cichon-Kotek, M.; Mrozinska, A.; Purzycka, J.; Wierzba, J.; Limon, J.; Lipska-Zietkiewicz, B.S. Co-incidence of Turner syndrome and Duchenne muscular dystrophy—An important problem for the clinician. Dev. Period. Med. 2016, 20, 273–278. [Google Scholar]
- Rink, R.C.; Adams, M.C.; Misseri, R. A new classification for genital ambiguity and urogenital sinus anomalies. BJU Int. 2005, 95, 638–642. [Google Scholar] [CrossRef]
- Murugan, S.; Chandramohan, A.; Lakshmi, B.R. Use of multiplex ligation-dependent probe amplification (MLPA) for Duchenne muscular dystrophy (DMD) gene mutation analysis. Indian J. Med. Res. 2010, 132, 303–311. [Google Scholar]
- Birnkrant, D.J.; Bushby, K.; Bann, C.M.; Apkon, S.D.; Blackwell, A.; Brumbaugh, D.; Case, L.E.; Clemens, P.R.; Hadjiyannakis, S.; Pandya, S.; et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: Diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol. 2018, 17, 251–267. [Google Scholar] [CrossRef]
- Gole, L.A.; Lim, J.; Crolla, J.A.; Loke, K.Y. Gonadal mosaicism 45,X/46,X,psu dic(Y)(q11.2) resulting in a Turner phenotype with mixed gonadal dysgenesis. Singapore Med. J. 2008, 49, 349–351. [Google Scholar]
- Baer, T.G.; Freeman, C.E.; Cujar, C.; Mansukhani, M.; Singh, B.; Chen, X.; Abellar, R.; Oberfield, S.E.; Levy, B. Prevalence and Physical Distribution of SRY in the Gonads of a Woman with Turner Syndrome: Phenotypic Presentation, Tubal Formation, and Malignancy Risk. Horm. Res. Paediatr. 2017, 88, 291–297. [Google Scholar] [CrossRef]
- Bjerglund Nielsen, L.; Nielsen, I.M. Turner’s syndrome and Duchenne muscular dystrophy in a girl with an X; autosome translocation. Ann. Genet. 1984, 27, 173–177. [Google Scholar]
- Dowlut-McElroy, T.; Vilchez, D.A.; Taboada, E.M.; Strickland, J.L. Dysgerminoma in a 10-Year Old with 45X/46XY Turner Syndrome Mosaicism. J. Pediatr. Adolesc. Gynecol. 2019, 32, 555–557. [Google Scholar] [CrossRef]
- Canto, P.; Kofman-Alfaro, S.; Jimenez, A.L.; Soderlund, D.; Barron, C.; Reyes, E.; Mendez, J.P.; Zenteno, J.C. Gonadoblastoma in Turner syndrome patients with nonmosaic 45,X karyotype and Y chromosome sequences. Cancer Genet. Cytogenet. 2004, 150, 70–72. [Google Scholar] [CrossRef]
- Panarello, C.; Acquila, M.; Caprino, D.; Gimelli, G.; Pecorara, M.; Mori, P.G. Concomitant Turner syndrome and hemophilia A in a female with an idic(X)(p11) heterozygous at locus DXS52. Cytogenet. Cell Genet. 1992, 59, 241–242. [Google Scholar] [CrossRef]
- Ferrier, P.; Bamatter, F.; Klein, D. Muscular Dystrophy (Duchenne) in a Girl with Turner’s Syndrome. J. Med. Genet. 1965, 2, 38–46. [Google Scholar] [CrossRef][Green Version]
- Wu, Q.; Ma, X.; Kong, X.; Shi, H.; Chen, Z.; Jiao, Z.; Liu, L.; Jiang, M. [Two cases of rare diseases with abnormalities of X chromosome]. Chin. J. Med. Genet. 2019, 36, 151–153. [Google Scholar] [CrossRef]
- Ou, Z.; Li, S.; Li, Q.; Chen, X.; Liu, W.; Sun, X. Duchenne muscular dystrophy in a female patient with a karyotype of 46,X,i(X)(q10). Tohoku J. Exp. Med. 2010, 222, 149–153. [Google Scholar] [CrossRef]
- Wulfsberg, E.A.; Skoglund, R.R. Duchenne muscular dystrophy in a 46 XY female. Clin. Pediatr. 1986, 25, 276–278. [Google Scholar] [CrossRef]
- Chen, J.J.; Cao, B.Y.; Su, C.; Liu, M.; Wu, D.; Li, W.J.; Gong, C.X. A Chinese girl with Turner syndrome and Duchenne muscular dystrophy: Diagnosis and management of this “dual diagnosis”. Chin. Med. J. 2020, 134, 743–745. [Google Scholar] [CrossRef]
- Verma, S.; Goyal, P.; Beam, C.; Shah, D. Turner syndrome and Duchenne muscular dystrophy. Muscle Nerve 2017, 56, E12–E15. [Google Scholar] [CrossRef]
- Chelly, J.; Marlhens, F.; Le Marec, B.; Jeanpierre, M.; Lambert, M.; Hamard, G.; Dutrillaux, B.; Kaplan, J.C. De novo DNA microdeletion in a girl with Turner syndrome and Duchenne muscular dystrophy. Hum. Genet. 1986, 74, 193–196. [Google Scholar] [CrossRef]
- Kinoshita, M.; Ikeda, K.; Yoshimura, M.; Saku, A.; Watanabe, K. [Duchenne muscular dystrophy carrier presenting with mosaic X chromosome constitution and muscular symptoms--with analysis of the barr bodies in the muscle]. Rinsho Shinkeigaku 1990, 30, 643–646. [Google Scholar]
- Sano, M.; Saito, F.; Yamamoto, K.; Tonomura, A.; Tsukagoshi, H. Duchenne muscular dystrophy in a female with 45,X/46,XX chromosome constitution. Jpn. J. Hum. Genet. 1987, 32, 257–262. [Google Scholar] [CrossRef]
- Satre, V.; Monnier, N.; Devillard, F.; Amblard, F.; Lunardi, J. Prenatal diagnosis of DMD in a female foetus affected by Turner syndrome. Prenat. Diagn. 2004, 24, 913–917. [Google Scholar] [CrossRef]
- Bortolini, E.R.; da Silva, D.M.; Chequer, R.S.; Vianna-Morgante, A.M.; Zatz, M. Duchenne muscular dystrophy in a girl with a 45,X/46,XX/47,XXX chromosome constitution. Am. J. Med. Genet. 1986, 25, 239–243. [Google Scholar] [CrossRef]
- Garcia, S.; de Haro, T.; Zafra-Ceres, M.; Poyatos, A.; Gomez-Capilla, J.A.; Gomez-Llorente, C. Identification of de novo mutations of Duchenne/Becker muscular dystrophies in southern Spain. Int. J. Med. Sci. 2014, 11, 988–993. [Google Scholar] [CrossRef][Green Version]
- Mercuri, E.; Bönnemann, C.G.; Muntoni, F. Muscular dystrophies. Lancet 2019, 394, 2025–2038. [Google Scholar] [CrossRef]
- Rosenfeld, R.G.; Attie, K.M.; Frane, J.; Brasel, J.A.; Burstein, S.; Cara, J.F.; Chernausek, S.; Gotlin, R.W.; Kuntze, J.; Lippe, B.M.; et al. Growth hormone therapy of Turner’s syndrome: Beneficial effect on adult height. J. Pediatr. 1998, 132, 319–324. [Google Scholar] [CrossRef]
| Paper ID | Year of Publication | Karyotype | Turner Phenotype | DMD Onset | DMD Symptoms | CPK at Onset (UI/L) | Origin | Mutation DMD Gene |
|---|---|---|---|---|---|---|---|---|
| Chen et al. [30] | 2020 | 45,X | yes | 9 yrs old | typical | 6.566 | China | Familiar |
| Verma et al. [31] | 2017 | 45,X | yes | 5 yrs old | typical | 4504 | USA | De novo |
| Kaczorowska et al. [16] | 2016 | 45,X | yes | 14 months old | atypical | 20.451 | Poland | - |
| Wu et al. [27] | 2019 | 45,X | yes | 8 yrs old | typical | - | China | - |
| Bjerglund et al. [22] | 1984 | 45,X | yes | - | typical | - | Denmark | De novo |
| Chelly et al. [32] | 1986 | 45,X | yes | 2 yrs old | typical | - | France | De novo |
| Ou et al. [28] | 2010 | 46,X,i(X)(q10) | no | 4 yrs old | typical | - | China | De novo |
| Kinoshita et al. [33] | 1990 | 45,X/46,XX/47,XXX | incomplete | 52 yrs old | atypical | - | Japan | - |
| Wulfsberg et al. [29] | 1986 | 46,XY | no | 2 yrs old | typical | 7.555 | USA | De novo |
| Sano et al. [34] | 1987 | 45,X/46,XX | no | 5 yrs old | typical | 4.130 | Japan | Familiar |
| Satre et al. [35] | 2004 | 45,X/46,XX | - | Prenatal diagnosis | - | - | France | Familiar |
| Ferrier et al. [26] | 1965 | 45,X/46,XX | yes | 6 yrs old | typical | R49.000 | Switzerland | De novo |
| Bartolini et al. [36] | 1986 | 45,X/46,XX/47,XXX | - | 4 yrs old | typical | Elevated CPK | Brazil | Familiar |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamanna, B.; Vinciguerra, M.; Dellino, M.; Cascella, G.; Cazzato, G.; Macorano, E.; Malvasi, A.; Scacco, S.; Cicinelli, E.; Loizzi, V.; et al. Turner Syndrome Mosaicism 45,X/46,XY with Genital Ambiguity and Duchenne Muscular Dystrophy: Translational Approach of a Rare Italian Case. Int. J. Mol. Sci. 2022, 23, 14408. https://doi.org/10.3390/ijms232214408
Lamanna B, Vinciguerra M, Dellino M, Cascella G, Cazzato G, Macorano E, Malvasi A, Scacco S, Cicinelli E, Loizzi V, et al. Turner Syndrome Mosaicism 45,X/46,XY with Genital Ambiguity and Duchenne Muscular Dystrophy: Translational Approach of a Rare Italian Case. International Journal of Molecular Sciences. 2022; 23(22):14408. https://doi.org/10.3390/ijms232214408
Chicago/Turabian StyleLamanna, Bruno, Marina Vinciguerra, Miriam Dellino, Gabriele Cascella, Gerardo Cazzato, Enrica Macorano, Antonio Malvasi, Salvatore Scacco, Ettore Cicinelli, Vera Loizzi, and et al. 2022. "Turner Syndrome Mosaicism 45,X/46,XY with Genital Ambiguity and Duchenne Muscular Dystrophy: Translational Approach of a Rare Italian Case" International Journal of Molecular Sciences 23, no. 22: 14408. https://doi.org/10.3390/ijms232214408
APA StyleLamanna, B., Vinciguerra, M., Dellino, M., Cascella, G., Cazzato, G., Macorano, E., Malvasi, A., Scacco, S., Cicinelli, E., Loizzi, V., Vimercati, A., Cormio, G., Paduano, F., Cascardi, E., & Tatullo, M. (2022). Turner Syndrome Mosaicism 45,X/46,XY with Genital Ambiguity and Duchenne Muscular Dystrophy: Translational Approach of a Rare Italian Case. International Journal of Molecular Sciences, 23(22), 14408. https://doi.org/10.3390/ijms232214408













