Barrier Membranes for Guided Bone Regeneration (GBR): A Focus on Recent Advances in Collagen Membranes
Abstract
1. Introduction
2. Nonabsorbable Barrier Membranes
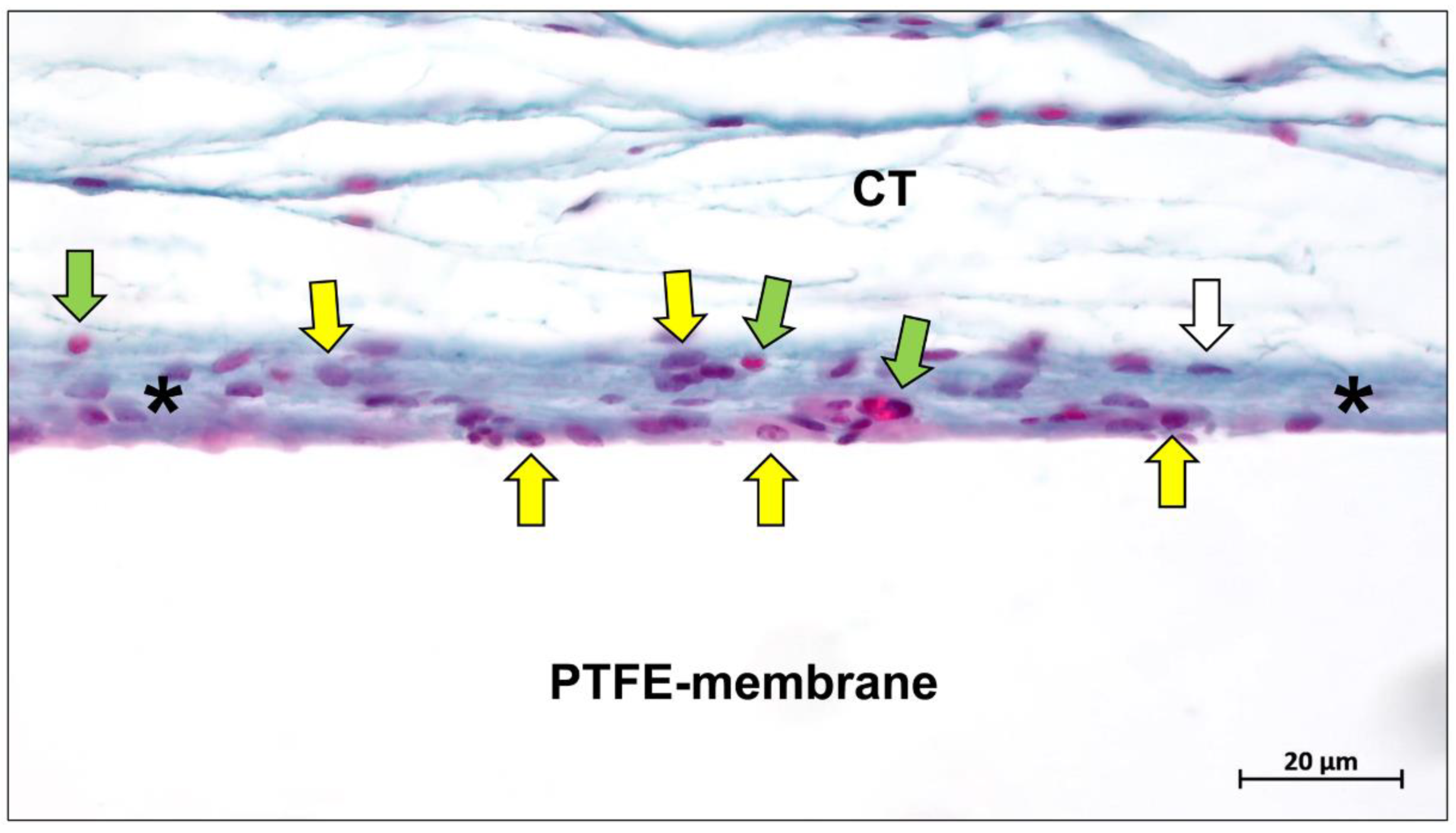
2.1. Polytetrafluoroethylene (PTFE)
2.1.1. e-PTFE and d-PTFE
- expanded polytetrafluoroethylene (e-PTFE);
- high-density polytetrafluoroethylene (d-PTFE).
2.1.2. Titanium-Reinforced PTFE-Membranes
2.2. Titanium Meshes and Cages
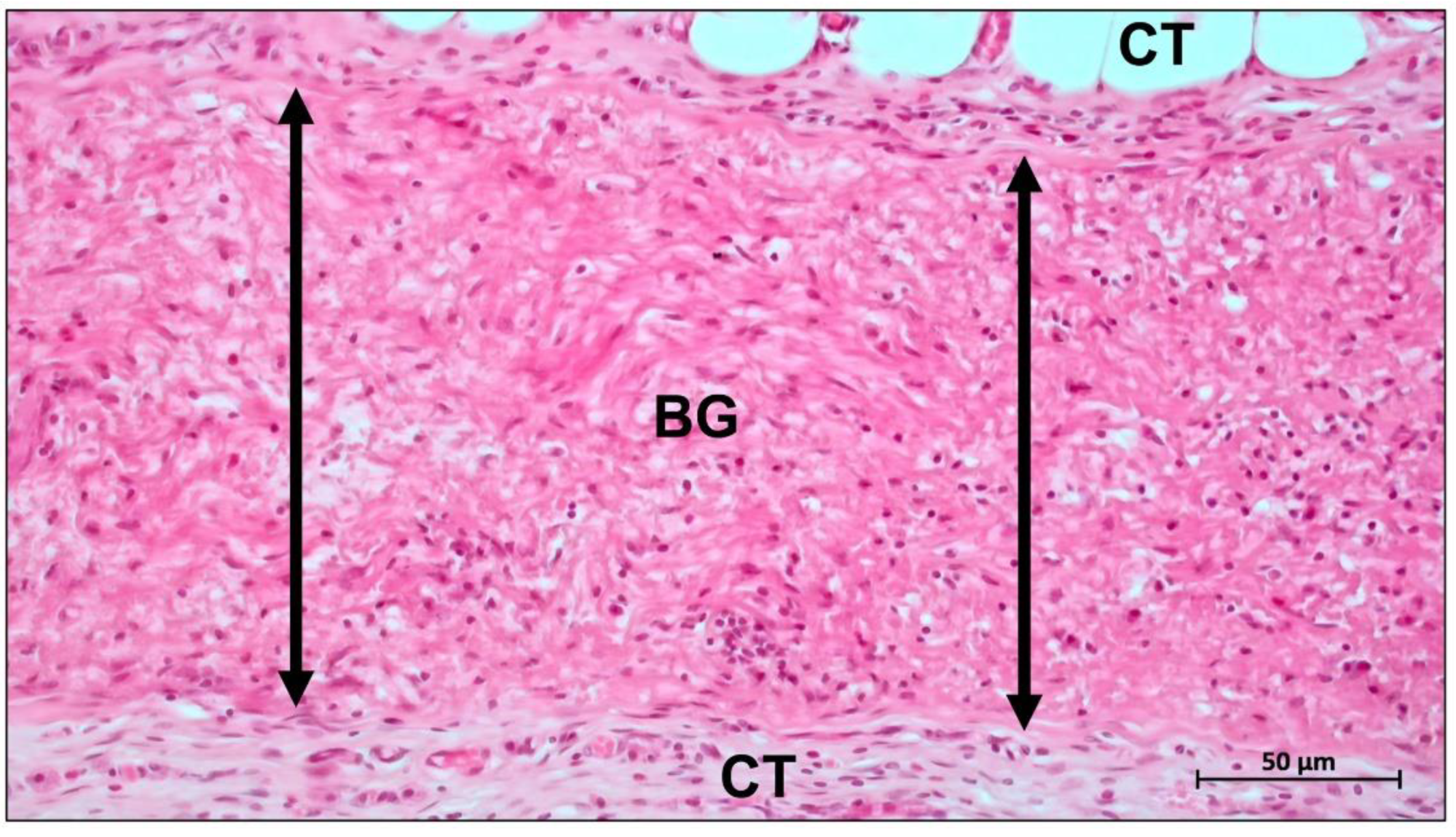
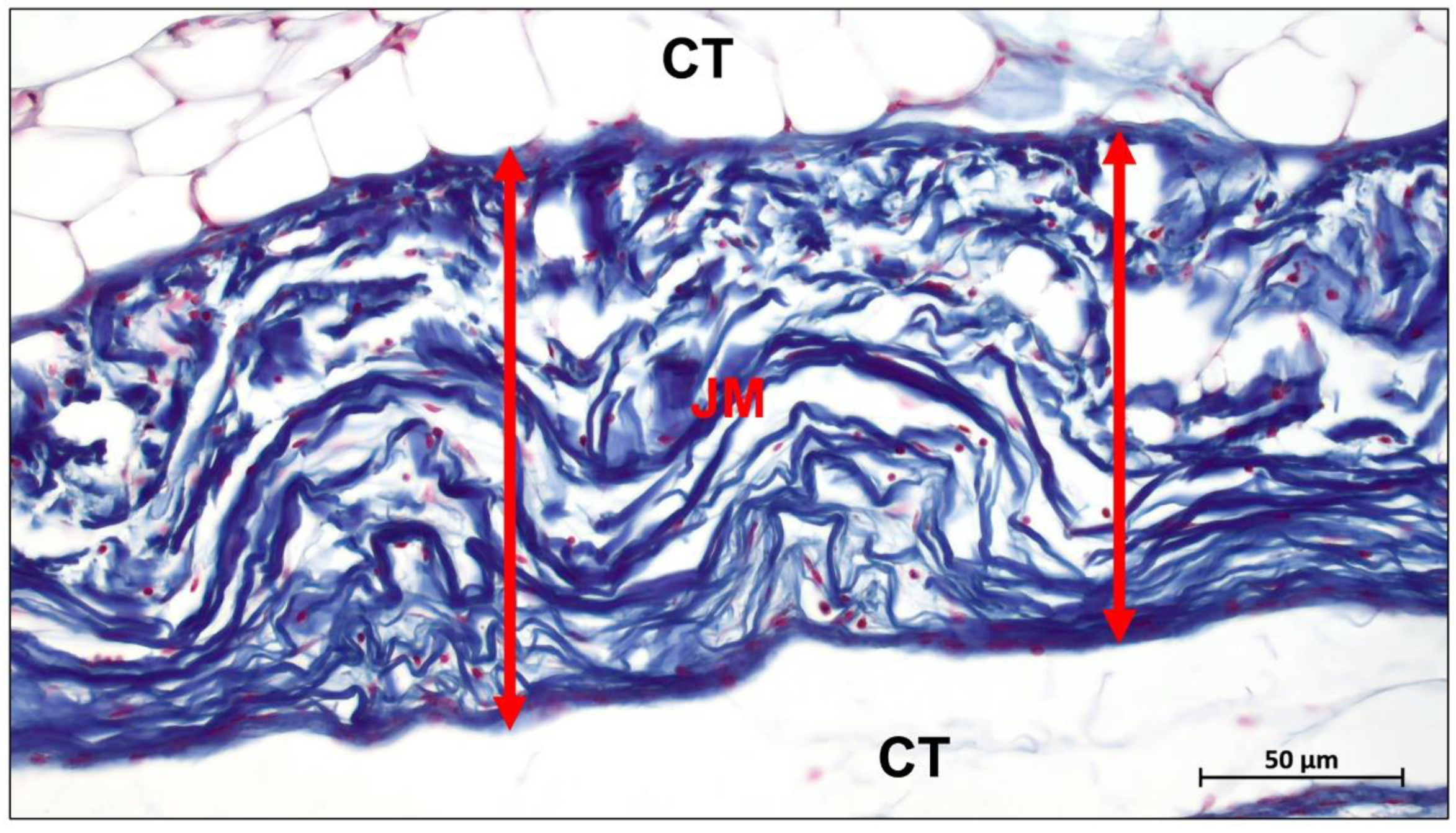
3. Absorbable Barrier Membrane
3.1. Modification of Collagen Membranes
3.1.1. Tissue Sources of Collagen Membranes
3.1.2. Collagen Crosslinking Strategies
Physical Strategies
Chemical Strategies
Enzymatic Strategies
| Author | Membranes Tested | Crosslinking Agents | Study Design | Mechanical Properties | Enzyme Resistance | Cell Cultivation | Osteogenesis and Organizational Integration Properties |
|---|---|---|---|---|---|---|---|
| Wang et al., 2022 [146] | Collagen/polycaprolactone methacryloyl/magnesium (Col/PCLMA/Mg) composite membrane | UV irradiation | In vivo and vitro | Increased elastic modulus, reduced swelling rate | Increased | Enhanced osteogenic capability | |
| Wu et al., 2022 [147] | Chemical crosslinking collagen membrane combined with zinc-doped nanohydroxyapatite (nZnHA) | Glutaraldehyde-alendronate | In vivo and vitro | Increased tensile modulus and extreme tensile strength | Increased | Noncytotoxic | Improved tissue integration and vascularization |
| He et al., 2022 [75] | Chemical Crosslinking collagen bilayer membrane | Oxidized sodium alginate (OSA) | In vivo and vitro | Improved structural stability, compressive strength, swelling behavior | Increased | Osteogenic differentiation was most promoted on the membrane with a large pore size (240–310 μm) | |
| Yang et al., 2021 [134] | Chemical Crosslinking collagen membrane | Oligomeric proanthocyanidins (OPCs) | In vitro | Promoted osteoblast proliferation and differentiation | |||
| Liang et al., 2021 [148] | Sequentially functionalized atelocollagen membrane | UV irradiation | In vivo and vitro | Improved compressive strength, swelling behavior | Increased | Increased metabolic activity | Enough safety, occlusivity, and soft tissue barrier functionality |
| Hong et al., 2021 [149] | Chemical and physical crosslinking collagen membrane | Carbodiimide, biphasic calcium phosphate (BCP)-supplemented UV irradiation | In vivo | Increased | Chemical crosslinking increased inflammatory response, both chemical and physical crosslinking distinctively enhanced new bone formation in the early phase of healing. | ||
| Rung et al., 2021 [138] | Chemical crosslinking collagen membrane | EDC/NHS and EGCG | In vivo and vitro | Moderately enhanced stiffness, slightly weakened elasticity. | Promoted cell viability, adhesion, and vessel formation | Upregulated angiogenesis-related factor VEGF, downregulated microphages markers F4/80. | |
| Zhao et al., 2020 [150] | Chemical crosslinking collagen bilayer membrane | Dialdehyde carboxymethyl cellulose | In vitro | Improved tensile strength, reduced swelling behavior | Increased | Good blood compatibility and cytocompatibility | Enhanced alkaline phosphatase (ALP) activity, promoted the differentiation of MC3T3-E1 cells. |
| Zhang et al., 2020 [108] | Amniotic membrane | UVA/riboflavin | In vivo and vitro | Increased brittleness and hardness | Increased | Enhanced resistance to tissue dissolution | |
| Ahn et al., 2020 [121] | Chemical crosslinking collagen membrane | EDC | In vivo and vitro | Improved tensile strength | Increased | Noncytotoxic | Similar bone regeneration compared with noncrosslinking membrane. |
| Li et al., 2019 [135] | Chemical crosslinking collagen membrane | Oligomeric proanthocyanidins (OPCs) | In vivo and vitro | Improved thermal stability and tensile modulus | Increased | Noncytotoxic but even promote L929 cells growth | Good tissue integration |
| Guo et al., 2019 [151] | Chemical crosslinking small intestinal submucosa membrane | Epigallocatechin-3-gallate (EGCG) | In vivo and vitro | Improved ultimate stress (US), elastic modulus (EM) | Enhanced the adhesion of fibroblasts and pre-osteoblasts, and promoted the osteogenic differentiation of MC3T3-E1 cells | Accelerated bone regeneration | |
| Russo et al., 2019 [152] | Porcine pericardium membrane | Polyphenol-rich pomace extract (PRPE) | In vitro | Improved stiffness and Young’s modulus | Increased | ||
| Muñoz-González et al., 2018 [153] | Chemical crosslinking collagen membrane | Trifunctional oligourethane | In vitro | Increased relaxation time | Increased | Imparted capacity to modulate macrophages | |
| An et al., 2018 [119] | Physical and chemical crosslinking collagen membrane | Dehydrothermally (DHT) and DHT/EDC | In vivo and vitro | Increased tensile strength | Increased | Promoted Angiogenesis and tissue integration | |
| Wei et al., 2018 [154] | Chemical crosslinking collagen membrane loading β-TCP | Oligomeric proanthocyanidins (OPCs) | In vitro | Increased compression modulus | Increased | Promoted the proliferation of MG-63 cells |
3.1.3. Incorporation of Bioactive Molecules
Cytokines and Growth Factors
Metal Ions
Antimicrobials and Antibiotics
| Characteristics | Modification | Author | Experimental Groups | Main Funding |
|---|---|---|---|---|
| Loading of growth factors or cytokines | PDGF | Nevins 2003 [157] | rhPDGF-BB incorporated in bone allograft | Purified rhPDGF-BB mixed with bone allograft results in robust periodontal regeneration in both Class II furcations and interproximal intrabony defects. |
| Yamano 2011 [158] | rhPDGF-BB incorporated in CM | PDGF significantly increased gene expression of osteoblast differentiation markers and ALP and cell proliferation activities with little cytotoxicity in MC3T3-E1 cells. | ||
| Joshi 2019 [159] | Platelet-Rich-Fibrin (PRF) membrane or CM incorporated with rhPDGF-BB | Both PRF membrane and CM incorporated with rhPDGF-BB showed comparable gingival crevicular fluid (GCF) levels of PDGF-BB initially, with PRF showing more sustained levels throughout the study period. | ||
| Elangovan 2014 [172] | pDNA encoding PDGF-B on a collagen scaffold | A significant increase in new bone volume/total volume (BV/TV) % (14-fold and 44-fold higher) compared to empty defects or empty scaffolds, respectively. | ||
| BMP | Chao 2021 [164] | rhBMP-2 loaded in the HAp/TCP/Col complex | HAp/TCP/Col with 0.2 mg/mL rhBMP-2 manifested strong osteogenic potential with more and faster new bone formation and better implant stability in Beagle dog model. | |
| Saulacic 2017 [167], Fujioka-Kobayashi 2017 [77] | BMP-9 loaded on CM. BMP-9 loading on deproteinized bovine bone mineral | BMP-9 loaded on collagen membranes induced better horizontal bone defect closure than loading on deproteinized bovine bone mineral, and both combinations positively induced bone regeneration. | ||
| Khorsand 2017 [173] | PEI-(pBMP-2+pFGF-2) embedded in collagen scaffolds. PEI-pBMP-2 embedded in collagen scaffolds | Synergistic delivery of pDNA encoding FGF-2 and BMP-2 also shows significant improvement in bone regeneration in diaphyseal long bone radial defects. | ||
| Elangovan 2015 [172] | PEI-pPDGF-B complex-loaded collagen scaffold | The PEI-cmRNA (encoding BMP-2) complex promoted significantly enhanced bone regeneration compared to PEI-DNA (encoding BMP-2) | ||
| Khorsand 2017 [194] | cmRNA (BMP-9) loaded collagen sponges. cmRNA (BMP-2) loaded collagen sponges. | cmRNA (BMP-9) had stronger bone regeneration efficacy than cmRNA (BMP-2), with a two-fold higher junctional density of regenerated bone. | ||
| Khorsand 2019 [23] | pDNA (BMP-9) loaded CM. cmRNA (BMP-9) loaded CM. | Calvarial bone defects treated with CM-cmRNA(BMP-9) trended toward being higher than defects treated with CM-pDNA(BMP-9) and CM alone. | ||
| SDF-1α | Yu 2020 [195] | Physical adsorption group with Bio-Oss+SDF-1αphysically adsorbed on the CM. Chemical crosslinking group with Bio-Oss+SDF-1α chemically crosslinked to the CM | Collagen membranes chemically conjugated to SDF-1α significantly promoted the formation of new bone and microvessels compared with SDF-1α physisorption and showed a similar effect of new bone formation to the BMSC seeding method. | |
| Loading of metal ions | Sr | Ehret 2017 [178] | Strontium-doped hydroxyapatite polysaccharide materials | Sr-doped matrices are not cytotoxic in vitro, regardless of the amount of Sr added. In vivo, subcutaneous implantation of these Sr-doped matrices induced a transformation of bone tissue and blood vessels. |
| Zn | Wu 2022 [147] | nZnHA-doped collagen membranes | 1% and 2% nZnHA-doped collagen membranes exhibited superior biocompatibility and stronger promotion of multinucleated giant cells (MNGC) formation in vitro and in vivo. | |
| Chou 2016 [181] | zinc hydroxyapatite loaded gelatin membrane | Both transforming growth factor-β (TGF-β) and osteoprotegerin (OPG) are upregulated when osteoblasts are exposed to zinc ions. | ||
| Xue 2021 [24] | PCL/Col/ZIF-8 Composite Membrane | PCL/Col/ZIF-8 composite membrane achieved PH-responsive release of zinc ions, showing enhanced osteoinductivity and angiogenesis both in vitro and in vivo. | ||
| Loading of antimicrobials and antibiotics | Silver nanoparticle | Chen 2018 [196] | Silver nanoparticle-coated collagen membrane | The AgNP-coated membrane also has effective anti-inflammatory effects by inhibiting the expression and release of anti-inflammatory cytokines such as IL-6 and TNF-α. In addition, the resultant membrane was able to induce osteogenic differentiation of mesenchymal stem cells. |
| Amoxicillin | Ho 2021 [189] | Amoxicillin loaded poly (D, L-lactic acid) membrane | Early reduction in inflammation and accelerate periodontal repair in vivo. | |
| Gentamicin | Cibor 2017 [190] | Gentamicin loaded Polysaccharide membrane | Resultant membrane support osteoblast growth and show favorable pharmacokinetics, bactericidal activity, cytocompatibility and good mechanical properties. | |
| Curcumin Aspirin | Ghavimi 2020 [191] | Nanofibrous asymmetric collagen/curcumin membrane containing aspirin loaded PLGA nanoparticles | The asymmetric membrane achieved complete bone regeneration after 28 days in animal test. | |
| Vancomycin | Xie 2022 [192] | Vancomycin loaded PMMA membranes | PMMA membranes loaded with relatively low concentrations of vancomycin (1–4 g/cement dose) can slightly promote osteoblast viability and angiogenesis. | |
| Antimicrobial peptide | Zhou 2022 [193] | AgNPs@AMP functionally coated peptide rods | AgNPs@AMP functional coating promote osteogenic gene expression (ALP, COL 1, β-Actin, OCN and Runx-2) and osseointegration in vivo. |
4. Biological Mechanisms of Collagen Membrane
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caballé-Serrano, J.; Abdeslam-Mohamed, Y.; Munar-Frau, A.; Fujioka-Kobayashi, M.; Hernández-Alfaro, F.; Miron, R. Adsorption and Release Kinetics of Growth Factors on Barrier Membranes for Guided Tissue/Bone Regeneration: A Systematic Review. Arch. Oral Biol. 2019, 100, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, J.-I.; Abe, G.L.; Li, A.; Thongthai, P.; Tsuboi, R.; Kohno, T.; Imazato, S. Barrier Membranes for Tissue Regeneration in Dentistry. Biomater. Investig. Dent. 2021, 8, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Aprile, P.; Letourneur, D.; Simon-Yarza, T. Membranes for Guided Bone Regeneration: A Road from Bench to Bedside. Adv. Healthc. Mater. 2020, 9, e2000707. [Google Scholar] [CrossRef] [PubMed]
- Ottenbacher, N.; Alkildani, S.; Korzinskas, T.; Pissarek, J.; Ulm, C.; Jung, O.; Sundag, B.; Bellmann, O.; Stojanovic, S.; Najman, S.; et al. Novel Histomorphometrical Approach to Evaluate the Integration Pattern and Functionality of Barrier Membranes. Dent. J. 2021, 9, 127. [Google Scholar] [CrossRef] [PubMed]
- Naenni, N.; Lim, H.C.; Strauss, F.J.; Jung, R.E.; Hämmerle, C.H.F.; Thoma, D.S. Local Tissue Effects of Various Barrier Membranes in a Rat Subcutaneous Model. J. Periodontal. Implant Sci. 2020, 50, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Spinell, T.; Saliter, J.; Hackl, B.; Unger, K.; Hickel, R.; Folwaczny, M. In-Vitro Cytocompatibility and Growth Factor Content of GBR/GTR Membranes. Dent. Mater. 2019, 35, 963–969. [Google Scholar] [CrossRef]
- Nyman, S.; Lindhe, J.; Karring, T.; Rylander, H. New Attachment Following Surgical Treatment of Human Periodontal Disease. J. Clin. Periodontol. 1982, 9, 290–296. [Google Scholar] [CrossRef]
- Nyman, S.; Gottlow, J.; Karring, T.; Lindhe, J. The Regenerative Potential of the Periodontal Ligament. An Experimental Study in the Monkey. J. Clin. Periodontol. 1982, 9, 257–265. [Google Scholar] [CrossRef]
- Gottlow, J.; Nyman, S.; Lindhe, J.; Karring, T.; Wennström, J. New Attachment Formation in the Human Periodontium by Guided Tissue Regeneration. Case Reports. J. Clin. Periodontol. 1986, 13, 604–616. [Google Scholar] [CrossRef]
- Blanco, J.; Alonso, A.; Sanz, M. Long-Term Results and Survival Rate of Implants Treated with Guided Bone Regeneration: A 5-Year Case Series Prospective Study. Clin. Oral Implants Res. 2005, 16, 294–301. [Google Scholar] [CrossRef]
- Carbonell, J.M.; Martín, I.S.; Santos, A.; Pujol, A.; Sanz-Moliner, J.D.; Nart, J. High-Density Polytetrafluoroethylene Membranes in Guided Bone and Tissue Regeneration Procedures: A Literature Review. Int. J. Oral Maxillofac. Surg. 2014, 43, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Dodge, A.; Luepke, P.; Wang, H.-L.; Kapila, Y.; Lin, G.-H. Effect of Membrane Exposure on Guided Bone Regeneration: A Systematic Review and Meta-Analysis. Clin. Oral Implants Res. 2018, 29, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhou, Z.; Yun, J.; Liu, R.; Li, J.; Chen, Y.; Cai, H.; Jiang, H.B.; Lee, E.-S.; Han, J.; et al. Effect of Different Membranes on Vertical Bone Regeneration: A Systematic Review and Network Meta-Analysis. Biomed Res. Int. 2022, 2022, 7742687. [Google Scholar] [CrossRef] [PubMed]
- Ghensi, P.; Stablum, W.; Bettio, E.; Soldini, M.C.; Tripi, T.R.; Soldini, C. Management of the Exposure of a Dense PTFE (d-PTFE) Membrane in Guided Bone Regeneration (GBR): A Case Report. Oral Implantol. 2017, 10, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Soldatos, N.K.; Stylianou, P.; Koidou, V.P.; Angelov, N.; Yukna, R.; Romanos, G.E. Limitations and Options Using Resorbable versus Nonresorbable Membranes for Successful Guided Bone Regeneration. Quintessence Int. 2017, 48, 131–147. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef]
- Sheikh, Z.; Qureshi, J.; Alshahrani, A.M.; Nassar, H.; Ikeda, Y.; Glogauer, M.; Ganss, B. Collagen Based Barrier Membranes for Periodontal Guided Bone Regeneration Applications. Odontology 2017, 105, 1–12. [Google Scholar] [CrossRef]
- Naung, N.Y.; Shehata, E.; Van Sickels, J.E. Resorbable Versus Nonresorbable Membranes: When and Why? Dent. Clin. N. Am. 2019, 63, 419–431. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, S.; Shi, B.; Wang, Y.; Chen, Y.; Wang, X.; Lee, E.-S.; Jiang, H.-B. Advances in Modification Methods Based on Biodegradable Membranes in Guided Bone/Tissue Regeneration: A Review. Polymers 2022, 14, 871. [Google Scholar] [CrossRef]
- Jiang, Y.-H.; Lou, Y.-Y.; Li, T.-H.; Liu, B.-Z.; Chen, K.; Zhang, D.; Li, T. Cross-Linking Methods of Type I Collagen-Based Scaffolds for Cartilage Tissue Engineering. Am. J. Transl. Res. 2022, 14, 1146–1159. [Google Scholar]
- Venkatesan, N.; Lavu, V.; Balaji, S.K. Clinical Efficacy of Amniotic Membrane with Biphasic Calcium Phosphate in Guided Tissue Regeneration of Intrabony Defects- a Randomized Controlled Clinical Trial. Biomater. Res. 2021, 25, 15. [Google Scholar] [CrossRef]
- Friedmann, A.; Fickl, S.; Fischer, K.R.; Dalloul, M.; Goetz, W.; Kauffmann, F. Horizontal Augmentation of Chronic Mandibular Defects by the Guided Bone Regeneration Approach: A Randomized Study in Dogs. Materials 2021, 15, 238. [Google Scholar] [CrossRef]
- Khorsand, B.; Elangovan, S.; Hong, L.; Kormann, M.S.D.; Salem, A.K. A Bioactive Collagen Membrane That Enhances Bone Regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 1824–1832. [Google Scholar] [CrossRef]
- Xue, Y.; Zhu, Z.; Zhang, X.; Chen, J.; Yang, X.; Gao, X.; Zhang, S.; Luo, F.; Wang, J.; Zhao, W.; et al. Accelerated Bone Regeneration by MOF Modified Multifunctional Membranes through Enhancement of Osteogenic and Angiogenic Performance. Adv. Healthc. Mater. 2021, 10, e2001369. [Google Scholar] [CrossRef]
- Fujioka-Kobayashi, M.; Marjanowski, S.D.; Kono, M.; Hino, S.; Saulacic, N.; Schaller, B. Osteoinductive Potential of Recombinant BMP-9 in Bone Defects of Mice Treated with Antiresorptive Agents. Int. J. Oral Maxillofac. Surg. 2022, 51, 566–575. [Google Scholar] [CrossRef]
- Elgali, I.; Turri, A.; Xia, W.; Norlindh, B.; Johansson, A.; Dahlin, C.; Thomsen, P.; Omar, O. Guided Bone Regeneration Using Resorbable Membrane and Different Bone Substitutes: Early Histological and Molecular Events. Acta Biomater. 2016, 29, 409–423. [Google Scholar] [CrossRef]
- Wu, L.; Morrow, B.R.; Jefferson, M.M.; Li, F.; Hong, L. Antibacterial Collagen Composite Membranes Containing Minocycline. J. Pharm. Sci. 2021, 110, 2177–2184. [Google Scholar] [CrossRef] [PubMed]
- Ronda, M.; Rebaudi, A.; Torelli, L.; Stacchi, C. Expanded vs. Dense Polytetrafluoroethylene Membranes in Vertical Ridge Augmentation around Dental Implants: A Prospective Randomized Controlled Clinical Trial. Clin. Oral Implants Res. 2014, 25, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Caballé-Serrano, J.; Munar-Frau, A.; Ortiz-Puigpelat, O.; Soto-Penaloza, D.; Peñarrocha, M.; Hernández-Alfaro, F. On the Search of the Ideal Barrier Membrane for Guided Bone Regeneration. J. Clin. Exp. Dent. 2018, 10, e477–e483. [Google Scholar] [CrossRef]
- Cohen, E.S. Guided Tissue Regeneration in Combination with Demineralized Freeze-Dried Bone Allograft Using a Nonresorbable Membrane. Compend. Contin. Educ. Dent. 1995, 16, 846, 848, 851–852 passim; quiz 864. [Google Scholar] [PubMed]
- Feng, S.; Zhong, Z.; Wang, Y.; Xing, W.; Drioli, E. Progress and Perspectives in PTFE Membrane: Preparation, Modification, and Applications. J. Membr. Sci. 2018, 549, 332–349. [Google Scholar] [CrossRef]
- Dhanumalayan, E.; Joshi, G.M. Performance Properties and Applications of Polytetrafluoroethylene (PTFE)—A Review. Adv. Compos. Mater. 2018, 1, 247–268. [Google Scholar] [CrossRef]
- Al-Kattan, R. An Update on the Utilization of High-Density Polytetrafluoroethylene (d-PTFE) Membranes for Guided Bone Regeneration. Int. J. Oral Care Res. 2020, 8, 91. [Google Scholar] [CrossRef]
- Sattar, M.M.; Patel, M.; Alani, A. Clinical Applications of Polytetrafluoroethylene (PTFE) Tape in Restorative Dentistry. Br. Dent. J. 2017, 222, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, L.; Zhou, Z.; Lai, H.; Xu, P.; Liao, L.; Wei, J. Biodegradable Polymer Membranes Applied in Guided Bone/Tissue Regeneration: A Review. Polymers 2016, 8, 115. [Google Scholar] [CrossRef]
- Teng, H. Overview of the Development of the Fluoropolymer Industry. Appl. Sci. 2012, 2, 496–512. [Google Scholar] [CrossRef]
- Hou, X.; Deem, P.T.; Choy, K.L. Hydrophobicity Study of Polytetrafluoroethylene Nanocomposite Films. Thin Solid Films 2012, 520, 4916–4920. [Google Scholar] [CrossRef]
- Turri, A.; Čirgić, E.; Shah, F.A.; Hoffman, M.; Omar, O.; Dahlin, C.; Trobos, M. Early Plaque Formation on PTFE Membranes with Expanded or Dense Surface Structures Applied in the Oral Cavity of Human Volunteers. Clin. Exp. Dent. Res. 2021, 7, 137. [Google Scholar] [CrossRef]
- Trobos, M.; Juhlin, A.; Shah, F.A.; Hoffman, M.; Sahlin, H.; Dahlin, C. In Vitro Evaluation of Barrier Function against Oral Bacteria of Dense and Expanded Polytetrafluoroethylene (PTFE) Membranes for Guided Bone Regeneration. Clin. Implant Dent. Relat. Res. 2018, 20, 738–748. [Google Scholar] [CrossRef]
- Tan, X.M.; Rodrigue, D. A Review on Porous Polymeric Membrane Preparation. Part II: Production Techniques with Polyethylene, Polydimethylsiloxane, Polypropylene, Polyimide, and Polytetrafluoroethylene. Polymers 2019, 11, 1310. [Google Scholar] [CrossRef]
- Vroom, M.; Gründemann, L.; Gallo, P. Clinical Classification of Healing Complications and Management in Guided Bone Regeneration Procedures with a Nonresorbable D-PTFE Membrane. Int. J. Periodontics Restor. Dent. 2022, 42, 419–427. [Google Scholar] [CrossRef]
- Chiapasco, M.; Zaniboni, M. Clinical Outcomes of GBR Procedures to Correct Peri-Implant Dehiscences and Fenestrations: A Systematic Review. Clin. Oral Implants Res. 2009, 20 (Suppl. 4), 113–123. [Google Scholar] [CrossRef] [PubMed]
- Korzinskas, T.; Jung, O.; Smeets, R.; Stojanovic, S.; Najman, S.; Glenske, K.; Hahn, M.; Wenisch, S.; Schnettler, R.; Barbeck, M. In Vivo Analysis of the Biocompatibility and Macrophage Response of a Non-Resorbable PTFE Membrane for Guided Bone Regeneration. Int. J. Mol. Sci. 2018, 19, 2952. [Google Scholar] [CrossRef] [PubMed]
- Cucchi, A.; Ghensi, P. Vertical Guided Bone Regeneration Using Titanium-Reinforced d-PTFE Membrane and Prehydrated Corticocancellous Bone Graft. Open Dent. J. 2014, 8, 194. [Google Scholar] [CrossRef] [PubMed]
- Cucchi, A.; Vignudelli, E.; Napolitano, A.; Marchetti, C.; Corinaldesi, G. Evaluation of Complication Rates and Vertical Bone Gain after Guided Bone Regeneration with Non-resorbable Membranes versus Titanium Meshes and Resorbable Membranes. A Randomized Clinical Trial. Clin. Implant Dent. Relat. Res. 2017, 19, 821. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Tonda-Turo, C.; Ferreira, A.M.; Ciardelli, G. Polymeric Membranes for Guided Bone Regeneration. Biotechnol. J. 2011, 6, 1187–1197. [Google Scholar] [CrossRef]
- Xie, Y.; Li, S.; Zhang, T.; Wang, C.; Cai, X. Titanium Mesh for Bone Augmentation in Oral Implantology: Current Application and Progress. Oral Sci. Int. 2020, 12, 37. [Google Scholar] [CrossRef]
- Hasegawa, H.; Kaneko, T.; Endo, M.; Kanno, C.; Yamazaki, M.; Yaginuma, S.; Igarashi, H.; Honma, H.; Masui, S.; Suto, M.; et al. Comparing the Efficacy of a Microperforated Titanium Membrane for Guided Bone Regeneration with an Existing Mesh Retainer in Dog Mandibles. Materials 2021, 14, 3358. [Google Scholar] [CrossRef] [PubMed]
- Majewski, P. The Ti-Mesh Technique: Guided Bone Regeneration for Three-Dimensional Augmentations. Clinical Aspects: A Case Series. Int. J. Periodontics Restor. Dent. 2022, 42, 145–153. [Google Scholar] [CrossRef]
- Uehara, S.; Kurita, H.; Shimane, T.; Sakai, H.; Kamata, T.; Teramoto, Y.; Yamada, S. Predictability of Staged Localized Alveolar Ridge Augmentation Using a Micro Titanium Mesh. J. Oral Maxillofac. Surg. 2015, 19, 411–416. [Google Scholar] [CrossRef]
- Sagheb, K.; Schiegnitz, E.; Moergel, M.; Walter, C.; Al-Nawas, B.; Wagner, W. Clinical Outcome of Alveolar Ridge Augmentation with Individualized CAD-CAM-Produced Titanium Mesh. Int. J. Dent. 2017, 3, 36. [Google Scholar] [CrossRef]
- Rasia dal Polo, M.; Poli, P.P.; Rancitelli, D.; Beretta, M.; Maiorana, C. Alveolar Ridge Reconstruction with Titanium Meshes: A Systematic Review of the Literature. Med. Oral Patol. Oral Cir. Bucal 2014, 19, e639. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Ji, P.; Li, X.; Gao, H.; Li, L.; Wang, C. Mechanical Characterization of 3D-Printed Individualized Ti-Mesh (Membrane) for Alveolar Bone Defects. J. Healthc. Eng. 2019, 2019, 4231872. [Google Scholar] [CrossRef]
- Cucchi, A.; Vignudelli, E.; Franceschi, D.; Randellini, E.; Lizio, G.; Fiorino, A.; Corinaldesi, G. Vertical and Horizontal Ridge Augmentation Using Customized CAD/CAM Titanium Mesh with versus without Resorbable Membranes. A Randomized Clinical Trial. Clin. Oral Implants Res. 2021, 32, 1411. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhao, J.; Xie, Y.; Tian, T.; Zhang, T.; Cai, X. Hard Tissue Stability after Guided Bone Regeneration: A Comparison between Digital Titanium Mesh and Resorbable Membrane. Int. J. Oral Sci. 2021, 13, 37. [Google Scholar] [CrossRef]
- Li, H.P.; Zheng, J.S.; Zhang, S.; Yang, C.; Kwon, Y.D.; Kim, Y.J. Experiment of GBR for Repair of Peri-Implant Alveolar Defects in Beagle Dogs. Sci. Rep. 2018, 8, 16532. [Google Scholar] [CrossRef]
- Cucchi, A.; Sartori, M.; Parrilli, A.; Aldini, N.N.; Vignudelli, E.; Corinaldesi, G. Histological and Histomorphometric Analysis of Bone Tissue after Guided Bone Regeneration with Non-Resorbable Membranes vs Resorbable Membranes and Titanium Mesh. Clin. Implant Dent. Relat. Res. 2019, 21, 693–701. [Google Scholar] [CrossRef]
- Cucchi, A.; Vignudelli, E.; Fiorino, A.; Pellegrino, G.; Corinaldesi, G. Vertical Ridge Augmentation (VRA) with Ti-Reinforced d-PTFE Membranes or Ti Meshes and Collagen Membranes: 1-Year Results of a Randomized Clinical Trial. Clin. Oral Implants Res. 2021, 32, 1–14. [Google Scholar] [CrossRef]
- Tanaskovic, N.; Trajkovski, B.; Kačarević, Ž.P.; Rider, P.M.; Houshmand, A.; Xiong, X.; Jung, O.; Barbeck, M. Periorbital Reconstruction by “Periorbital Patch” Technique Using a Pericardium-Based Collagen Membrane and Titanium Mesh. Materials 2019, 12, 2343. [Google Scholar] [CrossRef]
- Bottino, M.C.; Thomas, V.; Schmidt, G.; Vohra, Y.K.; Chu, T.M.G.; Kowolik, M.J.; Janowski, G.M. Recent Advances in the Development of GTR/GBR Membranes for Periodontal Regeneration—A Materials Perspective. Dent. Mater. 2012, 28, 703–721. [Google Scholar] [CrossRef]
- Dubey, N.; Ferreira, J.A.; Daghrery, A.; Aytac, Z.; Malda, J.; Bhaduri, S.B.; Bottino, M.C. Highly Tunable Bioactive Fiber-Reinforced Hydrogel for Guided Bone Regeneration. Acta Biomater. 2020, 113, 164. [Google Scholar] [CrossRef] [PubMed]
- Ovcharenko, N.; Greenwell, H.; Katwal, D.; Patel, A.; Hill, M.; Shumway, B.; Cockerham, B. A Comparison of the Effect of Barrier Membranes on Clinical and Histologic Hard and Soft Tissue Healing with Ridge Preservation. Int. J. Periodontics Restor. Dent. 2020, 40, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Xu, L.; Guo, G.; Wang, Y. Visible Light–Induced Cross-Linking of Porcine Pericardium for the Improvement of Endothelialization, Anti-Tearing, and Anticalcification Properties. J. Biomed. Mater. Res. A 2022, 110, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Kumari, C.B.N.; Ramakrishnan, T.; Devadoss, P.; Vijayalakshmi, R.; Alzahrani, K.J.; Almasri, M.A.; Al-Ahmari, M.M.; Dira, H.S.A.; Suhluli, M.; Bhati, A.K.; et al. Use of Collagen Membrane in the Treatment of Periodontal Defects Distal to Mandibular Second Molars Following Surgical Removal of Impacted Mandibular Third Molars: A Comparative Clinical Study. Biology 2021, 10, 1348. [Google Scholar] [CrossRef] [PubMed]
- Gueldenpfennig, T.; Houshmand, A.; Najman, S.; Stojanovic, S.; Korzinskas, T.; Smeets, R.; Gosau, M.; Pissarek, J.; Emmert, S.; Jung, O.; et al. The Condensation of Collagen Leads to an Extended Standing Time and a Decreased Pro-Inflammatory Tissue Response to a Newly Developed Pericardium-Based Barrier Membrane for Guided Bone Regeneration. In Vivo 2020, 34, 985. [Google Scholar] [CrossRef]
- Von Arx, T.; Buser, D. Horizontal Ridge Augmentation Using Autogenous Block Grafts and the Guided Bone Regeneration Technique with Collagen Membranes: A Clinical Study with 42 Patients. Clin. Oral Implants Res. 2006, 17, 359–366. [Google Scholar] [CrossRef]
- Wessing, B.; Lettner, S.; Zechner, W. Guided Bone Regeneration with Collagen Membranes and Particulate Graft Materials: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Implants 2018, 33, 87–100. [Google Scholar] [CrossRef]
- Sbricoli, L.; Guazzo, R.; Annunziata, M.; Gobbato, L.; Bressan, E.; Nastri, L. Selection of Collagen Membranes for Bone Regeneration: A Literature Review. Materials 2020, 13, 786. [Google Scholar] [CrossRef]
- Vajra Madhuri, S. Membranes for Periodontal Regeneration. Online. 2016, Volume 5, pp. 19–24. Available online: http://www.ijpsi.org/Papers/Vol5(6)/E0506019024.pdf (accessed on 24 November 2022).
- Turri, A.; Elgali, I.; Vazirisani, F.; Johansson, A.; Emanuelsson, L.; Dahlin, C.; Thomsen, P.; Omar, O. Guided Bone Regeneration Is Promoted by the Molecular Events in the Membrane Compartment. Biomaterials 2016, 84, 167–183. [Google Scholar] [CrossRef]
- Caballé-Serrano, J.; Sawada, K.; Miron, R.J.; Bosshardt, D.D.; Buser, D.; Gruber, R. Collagen Barrier Membranes Adsorb Growth Factors Liberated from Autogenous Bone Chips. Clin. Oral Implants Res. 2017, 28, 236–241. [Google Scholar] [CrossRef]
- Kuchler, U.; Rybaczek, T.; Dobask, T.; Heimel, P.; Tangl, S.; Klehm, J.; Menzel, M.; Gruber, R. Bone-Conditioned Medium Modulates the Osteoconductive Properties of Collagen Membranes in a Rat Calvaria Defect Model. Clin. Oral Implants Res. 2018, 29, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.K.; Möhler, H.; Busch, F.; Mehl, A. Preclinical and Clinical Studies of a Collagen Membrane (Bio-Gide®). Biomaterials 1997, 18, 535–538. [Google Scholar] [CrossRef]
- Radenković, M.; Alkildani, S.; Stoewe, I.; Bielenstein, J.; Sundag, B.; Bellmann, O.; Jung, O.; Najman, S.; Stojanović, S.; Barbeck, M. Comparative In Vivo Analysis of the Integration Behavior and Immune Response of Collagen-Based Dental Barrier Membranes for Guided Bone Regeneration (GBR). Membranes 2021, 11, 712. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Tian, Y.; Zhang, W.; Wang, X.; Yang, X.; Li, B.; Ge, L.; Bai, D.; Li, D. Fabrication of Oxidized Sodium Alginate-Collagen Heterogeneous Bilayer Barrier Membrane with Osteogenesis-Promoting Ability. Int. J. Biol. Macromol. 2022, 202, 55–67. [Google Scholar] [CrossRef]
- Zhou, T.; Chen, S.; Ding, X.; Hu, Z.; Cen, L.; Zhang, X. Fabrication and Characterization of Collagen/PVA Dual-Layer Membranes for Periodontal Bone Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 437. [Google Scholar] [CrossRef] [PubMed]
- Fujioka-Kobayashi, M.; Kobayashi, E.; Schaller, B.; Mottini, M.; Miron, R.J.; Saulacic, N. Effect of Recombinant Human Bone Morphogenic Protein 9 (RhBMP9) Loaded onto Bone Grafts versus Barrier Membranes on New Bone Formation in a Rabbit Calvarial Defect Model. J. Biomed. Mater. Res. A 2017, 105, 2655–2661. [Google Scholar] [CrossRef]
- Ratiu, C.; Brocks, M.; Costea, T.; Moldovan, L.; Cavalu, S. PRGF-Modified Collagen Membranes for Guided Bone Regeneration: Spectroscopic, Microscopic and Nano-Mechanical Investigations. Appl. Sci. 2019, 9, 1035. [Google Scholar] [CrossRef]
- Vahabi, S.; Yadegary, Z.; Karamshahi, M. Evaluating the Adhesion of Human Gingival Fibroblasts and MG-63 Osteoblast-like Cells to Activated PRP-Coated Membranes. Cell Tissue Bank 2019, 20, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Gielkens, P.F.M.; Schortinghuis, J.; De Jong, J.R.; Raghoebar, G.M.; Stegenga, B.; Bos, R.R.M. Vivosorb, Bio-Gide, and Gore-Tex as Barrier Membranes in Rat Mandibular Defects: An Evaluation by Microradiography and Micro-CT. Clin. Oral Implants Res. 2008, 19, 516–521. [Google Scholar] [CrossRef]
- Sela, M.N.; Babitski, E.; Steinberg, D.; Kohavi, D.; Rosen, G. Degradation of Collagen-Guided Tissue Regeneration Membranes by Proteolytic Enzymes of Porphyromonas Gingivalis and Its Inhibition by Antibacterial Agents. Clin. Oral Implants Res. 2009, 20, 496–502. [Google Scholar] [CrossRef]
- Meyer, M. Processing of Collagen Based Biomaterials and the Resulting Materials Properties. Biomed. Eng. Online 2019, 18, 24. [Google Scholar] [CrossRef] [PubMed]
- Terzi, A.; Gallo, N.; Bettini, S.; Sibillano, T.; Altamura, D.; Madaghiele, M.; De Caro, L.; Valli, L.; Salvatore, L.; Sannino, A.; et al. Sub- and Supramolecular X-Ray Characterization of Engineered Tissues from Equine Tendon, Bovine Dermis, and Fish Skin Type-I Collagen. Macromol. Biosci. 2020, 20, 2000017. [Google Scholar] [CrossRef] [PubMed]
- Terzi, A.; Storelli, E.; Bettini, S.; Sibillano, T.; Altamura, D.; Salvatore, L.; Madaghiele, M.; Romano, A.; Siliqi, D.; Ladisa, M.; et al. Effects of Processing on Structural, Mechanical and Biological Properties of Collagen-Based Substrates for Regenerative Medicine. Sci. Rep. 2018, 8, 1429. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Raines, R.T. Collagen-Based Biomaterials for Wound Healing. Biopolymers 2014, 101, 821. [Google Scholar] [CrossRef] [PubMed]
- Gorlov, I.F.; Titov, E.I.; Semenov, G.V.; Slozhenkina, M.I.; Sokolov, A.Y.; Omarov, R.S.; Goncharov, A.I.; Zlobina, E.Y.; Litvinova, E.V.; Karpenko, E.V. Collagen from Porcine Skin: A Method of Extraction and Structural Properties. Int. J. Food Prop. 2018, 21, 1031–1042. [Google Scholar] [CrossRef]
- Barbeck, M.; Lorenz, J.; Kubesch, A.; Bohm, N.; Booms, P.; Choukroun, J.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Porcine Dermis-Derived Collagen Membranes Induce Implantation Bed Vascularization Via Multinucleated Giant Cells: A Physiological Reaction? J. Oral Implantol. 2015, 41, e238–e251. [Google Scholar] [CrossRef]
- Franchi, M.; Trirè, A.; Quaranta, M.; Orsini, E.; Ottani, V. Collagen Structure of Tendon Relates to Function. Sci. World J. 2007, 7, 404–420. [Google Scholar] [CrossRef]
- Ghodbane, S.A.; Dunn, M.G. Physical and Mechanical Properties of Cross-Linked Type I Collagen Scaffolds Derived from Bovine, Porcine, and Ovine Tendons. J. Biomed. Mater. Res. A 2016, 104, 2685. [Google Scholar] [CrossRef]
- Terzi, A.; Gallo, N.; Bettini, S.; Sibillano, T.; Altamura, D.; Campa, L.; Natali, M.L.; Salvatore, L.; Madaghiele, M.; De Caro, L.; et al. Investigations of Processing–Induced Structural Changes in Horse Type-i Collagen at Sub and Supramolecular Levels. Front. Bioeng. Biotechnol. 2019, 7, 203. [Google Scholar] [CrossRef]
- Vallecillo-Rivas, M.; Toledano-Osorio, M.; Vallecillo, C.; Osorio, R.; Toledano, M. The Collagen Origin Influences the Degradation Kinetics of Guided Bone Regeneration Membranes. Polymers 2021, 13, 3007. [Google Scholar] [CrossRef]
- Blatt, S.; Burkhardt, V.; Kämmerer, P.W.; Pabst, A.M.; Sagheb, K.; Heller, M.; Al-Nawas, B.; Schiegnitz, E. Biofunctionalization of Porcine-Derived Collagen Matrices with Platelet Rich Fibrin: Influence on Angiogenesis in Vitro and in Vivo. Clin. Oral Investig. 2020, 24, 3425–3436. [Google Scholar] [CrossRef]
- Noble, C.; Morse, D.; Lerman, A.; Young, M. Evaluation of Pericardial Tissues from Assorted Species as a Tissue-Engineered Heart Valve Material. Med. Biol. Eng. Comput. 2022, 60, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Gallo, N.; Natali, M.L.; Sannino, A.; Salvatore, L. An Overview of the Use of Equine Collagen as Emerging Material for Biomedical Applications. J. Funct. Biomater. 2020, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Silvipriya, K.S.; Krishna Kumar, K.; Bhat, A.R.; Dinesh Kumar, B.; John, A.; Lakshmanan, P. Collagen: Animal Sources and Biomedical Application. J. Appl. Pharm. Sci. 2015, 5, 123–127. [Google Scholar] [CrossRef]
- Bianchini, P.; Parma, B. Immunological Safety Evaluation of a Horse Collagen Haemostatic Pad. Arzneim.-Forsch. 2001, 51, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Angele, P.; Abke, J.; Kujat, R.; Faltermeier, H.; Schumann, D.; Nerlich, M.; Kinner, B.; Englert, C.; Ruszczak, Z.; Mehrl, R.; et al. Influence of Different Collagen Species on Physico-Chemical Properties of Crosslinked Collagen Matrices. Biomaterials 2004, 25, 2831–2841. [Google Scholar] [CrossRef]
- Toledano, M.; Asady, S.; Toledano-Osorio, M.; García-Godoy, F.; Serrera-Figallo, M.A.; Benítez-García, J.A.; Osorio, R. Differential Biodegradation Kinetics of Collagen Membranes for Bone Regeneration. Polymers 2020, 12, 1290. [Google Scholar] [CrossRef]
- Liu, S.; Lau, C.S.; Liang, K.; Wen, F.; Teoh, S.H. Marine Collagen Scaffolds in Tissue Engineering. Curr. Opin. Biotechnol. 2022, 74, 92–103. [Google Scholar] [CrossRef]
- Ahmed, Z.; Powell, L.C.; Matin, N.; Mearns-Spragg, A.; Thornton, C.A.; Khan, I.M.; Francis, L.W. Jellyfish Collagen: A Biocompatible Collagen Source for 3d Scaffold Fabrication and Enhanced Chondrogenicity. Mar. Drugs 2021, 19, 405. [Google Scholar] [CrossRef]
- Addad, S.; Exposito, J.Y.; Faye, C.; Ricard-Blum, S.; Lethias, C. Isolation, Characterization and Biological Evaluation of Jellyfish Collagen for Use in Biomedical Applications. Mar. Drugs 2011, 9, 967. [Google Scholar] [CrossRef]
- Alkildani, S.; Jung, O.; Barbeck, M. In Vitro Investigation of Jellyfish Collagen as a Tool in Cell Culture and (Bone) Tissue Engineering. Anticancer. Res. 2021, 41, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Flaig, I.; Radenković, M.; Najman, S.; Pröhl, A.; Jung, O.; Barbeck, M. In Vivo Analysis of the Biocompatibility and Immune Response of Jellyfish Collagen Scaffolds and Its Suitability for Bone Regeneration. Int. J. Mol. Sci. 2020, 21, 4518. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Kamali, A.; Moshiri, A.; Baharvand, H.; Daemi, H. Chemical Crosslinking of Biopolymeric Scaffolds: Current Knowledge and Future Directions of Crosslinked Engineered Bone Scaffolds. Int. J. Biol. Macromol. 2018, 107, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Shan, T.; Ma, Y.X.; Tay, F.R.; Niu, L. Novel Biomedical Applications of Crosslinked Collagen. Trends Biotechnol. 2019, 37, 464–491. [Google Scholar] [CrossRef]
- Adamiak, K.; Sionkowska, A. Current Methods of Collagen Cross-Linking: Review. Int. J. Biol. Macromol. 2020, 161, 550–560. [Google Scholar] [CrossRef]
- Bailey, A.J. Effect of Ionizing Radiation on Connective Tissue Components. Int. Rev. Connect. Tissue Res. 1968, 4, 233–281. [Google Scholar] [CrossRef]
- Zhang, C.; Du, T.; Mu, G.; Wang, J.; Gao, X.; Long, F.; Wang, Q. Evaluation and Ultrastructural Changes of Amniotic Membrane Fragility after UVA/Riboflavin Cross-Linking and Its Effects on Biodegradation. Medicine 2020, 99, e20091. [Google Scholar] [CrossRef]
- Gaudet, I.D.; Shreiber, D.I. Characterization of Methacrylated Type-I Collagen as a Dynamic, Photoactive Hydrogel. Biointerphases 2012, 7, 25. [Google Scholar] [CrossRef]
- Tronci, G.; Grant, C.A.; Thomson, N.H.; Russell, S.J.; Wood, D.J. Influence of 4-Vinylbenzylation on the Rheological and Swelling Properties of Photo-Activated Collagen Hydrogels. MRS Adv. 2016, 1, 533–538. [Google Scholar] [CrossRef]
- Tronci, G.; Yin, J.; Holmes, R.A.; Liang, H.; Russell, S.J.; Wood, D.J. Protease-Sensitive Atelocollagen Hydrogels Promote Healing in a Diabetic Wound Model. J. Mater. Chem. B 2016, 4, 7249–7258. [Google Scholar] [CrossRef]
- Yannas, I.V.; Tobolsky, A.V. Cross-Linking of Gelatine by Dehydration. Nature 1967, 215, 509–510. [Google Scholar] [CrossRef] [PubMed]
- Weadock, K.S.; Miller, E.J.; Keuffel, E.L.; Dunnl, M.G. Effect of Physical Crosslinking Methods on Collagen-Fiber Durability in Proteolytic Solutions. J. Biomed. Mater. Res. A 1996, 32, 221–226. [Google Scholar] [CrossRef]
- Gorham, S.D.; Light, N.D.; Diamond, A.M.; Willins, M.J.; Bailey, A.J.; Wess, T.J.; Leslie, N.J. Effect of Chemical Modifications on the Susceptibility of Collagen to Proteolysis. II. Dehydrothermal Crosslinking. Int. J. Biol. Macromol. 1992, 14, 129–138. [Google Scholar] [CrossRef]
- Wess, T.J.; Orgel, J.P. Changes in Collagen Structure: Drying, Dehydrothermal Treatment and Relation to Long Term Deterioration. Thermochim. Acta 2000, 365, 119–128. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, L.; Xu, H.; Yamamoto, M.; Shinoda, M.; Kishimoto, M.; Tanaka, T.; Yamane, H. Effect of the Application of a Dehydrothermal Treatment on the Structure and the Mechanical Properties of Collagen Film. Materials 2020, 13, 377. [Google Scholar] [CrossRef] [PubMed]
- Nakada, A.; Shigeno, K.; Sato, T.; Kobayashi, T.; Wakatsuki, M.; Uji, M.; Nakamura, T. Manufacture of a Weakly Denatured Collagen Fiber Scaffold with Excellent Biocompatibility and Space Maintenance Ability. Biomed. Mater. 2013, 8, 045010. [Google Scholar] [CrossRef] [PubMed]
- Nakada, A.; Shigeno, K.; Sato, T.; Hatayama, T.; Wakatsuki, M.; Nakamura, T. Optimal Dehydrothermal Processing Conditions to Improve Biocompatibility and Durability of a Weakly Denatured Collagen Scaffold. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 2301–2307. [Google Scholar] [CrossRef] [PubMed]
- An, Y.Z.; Kim, Y.K.; Lim, S.M.; Heo, Y.K.; Kwon, M.K.; Cha, J.K.; Lee, J.S.; Jung, U.W.; Choi, S.H. Physiochemical Properties and Resorption Progress of Porcine Skin-Derived Collagen Membranes: In Vitro and in Vivo Analysis. Dent. Mater. J. 2018, 37, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Olde Damink, L.H.; Dijkstra, P.J.; van Luyn, M.J.; van Wachem, P.B.; Nieuwenhuis, P.; Feijen, J. Cross-Linking of Dermal Sheep Collagen Using a Water-Soluble Carbodiimide. Biomaterials 1996, 17, 765–773. [Google Scholar] [CrossRef]
- Ahn, J.J.; Kim, H.J.; Bae, E.B.; Cho, W.T.; Choi, Y.; Hwang, S.H.; Jeong, C.M.; Huh, J.B. Evaluation of 1-Ethyl-3-(3-Dimethylaminopropyl) Carbodiimide Cross-Linked Collagen Membranes for Guided Bone Regeneration in Beagle Dogs. Materials 2020, 13, 4599. [Google Scholar] [CrossRef]
- Bax, D.V.; Davidenko, N.; Gullberg, D.; Hamaia, S.W.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Fundamental Insight into the Effect of Carbodiimide Crosslinking on Cellular Recognition of Collagen-Based Scaffolds. Acta Biomater. 2017, 49, 218–234. [Google Scholar] [CrossRef] [PubMed]
- Bryś, M.; Urbańska, K.; Olas, B. Novel Findings Regarding the Bioactivity of the Natural Blue Pigment Genipin in Human Diseases. Int. J. Mol. Sci. 2022, 23, 902. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ao, J.; Lu, H.; Zhao, Q.; Ma, Y.; Zhang, J.; Ren, H.; Zhang, Y. Osteoimmune Modulation and Guided Steogenesis Promoted by Barrier Membranes Incorporated with S-Nitrosoglutathione (Gsno) and Mesenchymal Stem Cell-Derived Exosomes. Int. J. Nanomed. 2020, 15, 3483. [Google Scholar] [CrossRef]
- Nair, M.; Johal, R.K.; Hamaia, S.W.; Best, S.M.; Cameron, R.E. Tunable Bioactivity and Mechanics of Collagen-Based Tissue Engineering Constructs: A Comparison of EDC-NHS, Genipin and TG2 Crosslinkers. Biomaterials 2020, 254, 120109. [Google Scholar] [CrossRef] [PubMed]
- Shavandi, A.; Bekhit, A.E.D.A.; Saeedi, P.; Izadifar, Z.; Bekhit, A.A.; Khademhosseini, A. Polyphenol Uses in Biomaterials Engineering. Biomaterials 2018, 167, 91. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Jaurequi, J.; Tang, B.W.; Nimni, M.E. Proanthocyanidin: A Natural Crosslinking Reagent for Stabilizing Collagen Matrices. J. Biomed. Mater. Res. A 2003, 65A, 118–124. [Google Scholar] [CrossRef]
- Green, B.; Yao, X.; Ganguly, A.; Xu, C.; Dusevich, V.; Walker, M.P.; Wang, Y. Grape Seed Proanthocyanidins Increase Collagen Biodegradation Resistance in the Dentin/Adhesive Interface When Included in an Adhesive. J. Dent. 2010, 38, 908. [Google Scholar] [CrossRef]
- Yang, F.; Xue, F.; Guan, J.; Zhang, Z.; Yin, J.; Kang, Q. Stromal-Cell-Derived Factor (SDF) 1-Alpha Overexpression Promotes Bone Regeneration by Osteogenesis and Angiogenesis in Osteonecrosis of the Femoral Head. Cell. Physiol. Biochem. 2018, 46, 2561–2575. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Pang, Y.; Zhu, Z.; Zhang, Y.; Liu, Q.; Zhang, X.; Liu, Y. Modification of Collagen with Proanthocyanidins by Mimicking the Bridging Role of Glycosaminoglycans for Dentine Remineralization. Mater. Des. 2021, 210, 110067. [Google Scholar] [CrossRef]
- Bunte, K.; Hensel, A.; Beikler, T. Polyphenols in the Prevention and Treatment of Periodontal Disease: A Systematic Review of in Vivo, Ex Vivo and in Vitro Studies. Fitoterapia 2019, 132, 30–39. [Google Scholar] [CrossRef]
- Savickiene, N.; Jekabsone, A.; Raudone, L.; Abdelgeliel, A.S.; Cochis, A.; Rimondini, L.; Makarova, E.; Grinberga, S.; Pugovics, O.; Dambrova, M.; et al. Efficacy of Proanthocyanidins from Pelargonium Sidoides Root Extract in Reducing P. Gingivalis Viability While Preserving Oral Commensal S. Salivarius. Materials 2018, 11, 1499. [Google Scholar] [CrossRef] [PubMed]
- Kulakowski, D.; Leme-Kraus, A.A.; Nam, J.W.; McAlpine, J.; Chen, S.N.; Pauli, G.F.; Ravindran, S.; Bedran-Russo, A.K. Oligomeric Proanthocyanidins Released from Dentin Induce Regenerative Dental Pulp Cell Response. Acta Biomater. 2017, 55, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, W.C.; Liu, X.; Li, Y.; Lin, C.; Lin, Y.M.; Wang, A.N.; Nguyen, T.T.; Lin, Y.C.; Chung, R.J. Study on Proanthocyanidins Crosslinked Collagen Membrane for Guided Bone Tissue Regeneration. J. Appl. Biomater. Funct. Mater. 2021, 19, 22808000211005379. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, W.C.; Chang, Y.H.; Liu, X.; Chang, C.L.; Lin, C.; Chung, R.J. Preparation and in Vivo Investigation of Oligomeric Proanthocyanidins Cross-Linked Collagen Serving as Synthesized Tissue Regeneration Membrane. Mater. Sci. Eng. C 2019, 101, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Wang, Y.; Wang, Y.; Yang, R.; Liu, L.; Rung, S.; Xiang, L.; Wu, Y.; Du, S.; Man, Y.; et al. Evaluation of Epigallocatechin-3-Gallate (EGCG) Modified Collagen in Guided Bone Regeneration (GBR) Surgery and Modulation of Macrophage Phenotype. Mater. Sci. Eng. C 2019, 99, 73–82. [Google Scholar] [CrossRef]
- Chu, C.; Deng, J.; Sun, X.; Qu, Y.; Man, Y. Collagen Membrane and Immune Response in Guided Bone Regeneration: Recent Progress and Perspectives. Tissue Eng. Part B Rev. 2017, 23, 421–435. [Google Scholar] [CrossRef]
- Rung, S.; Zhao, X.; Chu, C.; Yang, R.; Qu, Y.; Man, Y. Application of Epigallocatechin-3-Gallate (EGCG) Modified 1-Ethyl-3-(3-Dimethylaminopropylcarbodiimide Hydrochloride/N-Hydroxy-Succinimide (EDC/NHS) Cross-Linked Collagen Membrane to Promote Macrophage Adhesion. Materials 2021, 14, 4660. [Google Scholar] [CrossRef]
- Kaartinen, M.T.; El-Maadawy, S.; Räsänen, N.H.; McKee, M.D. Tissue Transglutaminase and Its Substrates in Bone. J. Bone Miner. Res. 2002, 17, 2161–2173. [Google Scholar] [CrossRef]
- Chau, D.Y.S.; Collighan, R.J.; Verderio, E.A.M.; Addy, V.L.; Griffin, M. The Cellular Response to Transglutaminase-Cross-Linked Collagen. Biomaterials 2005, 26, 6518–6529. [Google Scholar] [CrossRef]
- Fortunati, D.; Chau, D.Y.S.; Wang, Z.; Collighan, R.J.; Griffin, M. Cross-Linking of Collagen I by Tissue Transglutaminase Provides a Promising Biomaterial for Promoting Bone Healing. Amino Acids 2014, 46, 1751–1761. [Google Scholar] [CrossRef]
- Ciardelli, G.; Gentile, P.; Chiono, V.; Mattioli-Belmonte, M.; Vozzi, G.; Barbani, N.; Giusti, P. Enzymatically Crosslinked Porous Composite Matrices for Bone Tissue Regeneration. J. Biomed. Mater. Res. A 2010, 92, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.H.; Uemura, T.; Yamaguchi, I.; Ikoma, T.; Tanaka, J. Effect of Enzymatically Cross-Linked Tilapia Scale Collagen for Osteoblastic Differentiation of Human Mesenchymal Stem Cells. J. Bioact. Compat. Pol. 2016, 31, 31–41. [Google Scholar] [CrossRef]
- Shanmugasundaram, S.; Logan-Mauney, S.; Burgos, K.; Nurminskaya, M. Tissue Transglutaminase Regulates Chondrogenesis in Mesenchymal Stem Cells on Collagen Type XI Matrices. Amino Acids 2012, 42, 1045. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Xiao, Z.; Long, H.; Ma, K.; Zhang, J.; Ren, X.; Zhang, J. Assessment of the Characteristics and Biocompatibility of Gelatin Sponge Scaffolds Prepared by Various Crosslinking Methods. Sci. Rep. 2018, 8, 1616. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xia, D.; Wang, S.; Gu, R.; Yang, F.; Zhao, X.; Liu, X.; Zhu, Y.; Liu, H.; Xu, Y.; et al. Photocrosslinkable Col/PCL/Mg Composite Membrane Providing Spatiotemporal Maintenance and Positive Osteogenetic Effects during Guided Bone Regeneration. Bioact. Mater. 2022, 13, 53–63. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, S.; Luo, P.; Deng, S.; Shan, Z.; Fang, J.; Liu, X.; Xie, J.; Liu, R.; Wu, S.; et al. Optimizing the Bio-Degradability and Biocompatibility of a Biogenic Collagen Membrane through Cross-Linking and Zinc-Doped Hydroxyapatite. Acta Biomater. 2022, 143, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Yin, J.; Man, K.; Yang, X.B.; Calciolari, E.; Donos, N.; Russell, S.J.; Wood, D.J.; Tronci, G. A Long-Lasting Guided Bone Regeneration Membrane from Sequentially Functionalised Photoactive Atelocollagen. Acta Biomater. 2022, 140, 190–205. [Google Scholar] [CrossRef] [PubMed]
- Hong, I.; Khalid, A.W.; Pae, H.C.; Song, Y.W.; Cha, J.K.; Lee, J.S.; Paik, J.W.; Choi, S.H. Diverse Patterns of Bone Regeneration in Rabbit Calvarial Defects Depending on the Type of Collagen Membrane. J. Periodontal. Implant Sci. 2021, 51, 40. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, X.; Xie, X.; Lei, J.; Ge, L.; Yuan, L.; Li, D.; Mu, C. Controlling the Pore Structure of Collagen Sponge by Adjusting the Cross-Linking Degree for Construction of Heterogeneous Double-Layer Bone Barrier Membranes. ACS Biomater. Sci. Eng. 2020, 3, 2058–2067. [Google Scholar] [CrossRef]
- Gou, M.; Huang, Y.Z.; Hu, J.G.; Jiang, Y.L.; Zhang, X.Z.; Su, N.C.; Lei, Y.; Zhang, H.; Wang, H.; Xie, H.Q. Epigallocatechin-3-Gallate Cross-Linked Small Intestinal Submucosa for Guided Bone Regeneration. ACS Biomater. Sci. Eng. 2019, 5, 5024–5035. [Google Scholar] [CrossRef]
- Russo, N.; Cassinelli, C.; Torre, E.; Morra, M.; Iviglia, G. Improvement of the Physical Properties of Guided Bone Regeneration Membrane from Porcine Pericardium by Polyphenols-Rich Pomace Extract. Materials 2019, 12, 2564. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-González, P.U.; Rivera-Debernardi, O.; Mendoza-Novelo, B.; Claudio-Rizo, J.A.; Mata-Mata, J.L.; Delgadillo-Holtfort, I.; Carriles, R.; Flores-Moreno, M.; González-García, G.; Cauich-Rodríguez, J.V.; et al. Design of Silica-Oligourethane-Collagen Membranes for Inflammatory Response Modulation: Characterization and Polarization of a Macrophage Cell Line. Macromol Biosci. 2018, 18, 1800099. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Chang, Y.H.; Liu, C.J.; Chung, R.J. Integrated Oxidized-Hyaluronic Acid/Collagen Hydrogel with β-TCP Using Proanthocyanidins as a Crosslinker for Drug Delivery. Pharmaceutics 2018, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Chen, D.; Xu, C.; Harris, S.E.; Mundy, G.R.; Yoneda, T. Patterns of Gene Expression Associated with BMP-2-Induced Osteoblast and Adipocyte Differentiation of Mesenchymal Progenitor Cell 3T3-F442A. J. Bone Miner. Metab. 2000, 18, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Boyan, L.A.; Bhargava, G.; Nishimura, F.; Orman, R.; Price, R.; Terranova, V.P. Mitogenic and Chemotactic Responses of Human Periodontal Ligament Cells to the Different Isoforms of Platelet-Derived Growth Factor. J. Dent. Res. 1994, 73, 1593–1600. [Google Scholar] [CrossRef]
- Nevins, M.; Camelo, M.; Nevins, M.L.; Schenk, R.K.; Lynch, S.E. Periodontal Regeneration in Humans Using Recombinant Human Platelet-Derived Growth Factor-BB (RhPDGF-BB) and Allogenic Bone. J. Periodontol. 2003, 74, 1282–1292. [Google Scholar] [CrossRef]
- Yamano, S.; TY, L.; Dai, J. Bioactive Collagen Membrane as a Carrier for Sustained Release of PDGF. J. Tissue Eng. 2011, 2, 6. [Google Scholar] [CrossRef]
- Joshi, A.A.; Padhye, A.M.; Gupta, H.S. Platelet Derived Growth Factor-BB Levels in Gingival Crevicular Fluid of Localized Intrabony Defect Sites Treated with Platelet Rich Fibrin Membrane or Collagen Membrane Containing Recombinant Human Platelet Derived Growth Factor-BB: A Randomized Clinical and Biochemical Study. J. Periodontol. 2019, 90, 701–708. [Google Scholar] [CrossRef]
- Bessa, P.C.; Casal, M.; Reis, R.L. Bone Morphogenetic Proteins in Tissue Engineering: The Road from Laboratory to Clinic, Part II (BMP Delivery). J Tissue Eng. Regen. Med. 2008, 2, 81–96. [Google Scholar] [CrossRef]
- Wu, W.; Li, B.; Liu, Y.; Wang, X.; Tang, L. Effect of Multilaminate Small Intestinal Submucosa as a Barrier Membrane on Bone Formation in a Rabbit Mandible Defect Model. BioMed Res. Int. 2018, 2018, 3270293. [Google Scholar] [CrossRef]
- Sun, Y.K.; Cha, J.K.; Thoma, D.S.; Yoon, S.R.; Lee, J.S.; Choi, S.H.; Jung, U.W. Bone Regeneration of Peri-Implant Defects Using a Collagen Membrane as a Carrier for Recombinant Human Bone Morphogenetic Protein-2. BioMed Res. Int. 2018, 2018, 5437361. [Google Scholar] [CrossRef] [PubMed]
- Onji, K.; Kabir, M.A.; Zhu, B.; Yokozeki, K.; Saito, T.; Akazawa, T.; Murata, M. Human Fresh Fibrin Membrane with Bone Morphogenetic Protein-2 (BMP-2) Induces Bone Formation in the Subcutaneous Tissues of Nude Mice. Materials 2021, 14, 150. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.L.; Wang, T.M.; Chang, H.H.; Lin, L.D. Effects of Low-Dose RhBMP-2 on Peri-Implant Ridge Augmentation in a Canine Model. J. Clin. Periodontol. 2021, 48, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Fujioka-Kobayashi, M.; Abd El Raouf, M.; Saulacic, N.; Kobayashi, E.; Zhang, Y.; Schaller, B.; Miron, R.J. Superior Bone-Inducing Potential of RhBMP9 Compared to RhBMP2. J. Biomed. Mater. Res. A 2018, 106, 1561–1574. [Google Scholar] [CrossRef]
- Saulacic, N.; Schaller, B.; Muñoz, F.; Fujioka-Kobayashi, M.; Kobayashi, E.; Lang, N.P.; Miron, R.J. Recombinant Human BMP9 (RhBMP9) in Comparison with RhBMP2 for Ridge Augmentation Following Tooth Extraction: An Experimental Study in the Beagle Dog. Clin. Oral Implants Res. 2018, 29, 1050–1059. [Google Scholar] [CrossRef]
- Saulacic, N.; Fujioka-Kobayashi, M.; Kobayashi, E.; Schaller, B.; Miron, R.J. Guided Bone Regeneration with Recombinant Human Bone Morphogenetic Protein 9 Loaded on Either Deproteinized Bovine Bone Mineral or a Collagen Barrier Membrane. Clin. Implant Dent. Relat. Res. 2017, 19, 600–607. [Google Scholar] [CrossRef]
- Ying, J.W.; Wen, T.Y.; Pei, S.S.; Su, L.H.; Ruan, K.K. Stromal Cell-Derived Factor-1α Promotes Recruitment and Differentiation of Nucleus Pulposus-Derived Stem Cells. World J. Stem Cells 2019, 11, 196. [Google Scholar] [CrossRef]
- Kramer, P.R.; Nares, S.; Kramer, S.F.; Grogan, D.; Kaiser, M. Mesenchymal Stem Cells Acquire Characteristics of Cells in the Periodontal Ligament in Vitro. J. Dent. Res. 2004, 83, 27–34. [Google Scholar] [CrossRef]
- Xu, M.; Wei, X.; Fang, J.; Xiao, L. Combination of SDF-1 and BFGF Promotes Bone Marrow Stem Cell-Mediated Periodontal Ligament Regeneration. Biosci. Rep. 2019, 39, 20190785. [Google Scholar] [CrossRef]
- Sam, G.; Madhavan Pillai, B.R. Evolution of Barrier Membranes in Periodontal Regeneration-“Are the Third Generation Membranes Really Here?”. J. Clin. Diagn. Res. 2014, 8, ZE14. [Google Scholar] [CrossRef]
- Elangovan, S.; D’Mello, S.R.; Hong, L.; Ross, R.D.; Allamargot, C.; Dawson, D.V.; Stanford, C.M.; Johnson, G.K.; Sumner, D.R.; Salem, A.K. The Enhancement of Bone Regeneration by Gene Activated Matrix Encoding for Platelet Derived Growth Factor. Biomaterials 2014, 35, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Khorsand, B.; Nicholson, N.; Do, A.V.; Femino, J.E.; Martin, J.A.; Petersen, E.; Guetschow, B.; Fredericks, D.C.; Salem, A.K. Regeneration of Bone Using Nanoplex Delivery of FGF-2 and BMP-2 Genes in Diaphyseal Long Bone Radial Defects in a Diabetic Rabbit Model. J. Control. Release 2017, 248, 53. [Google Scholar] [CrossRef] [PubMed]
- Ponsaerts, P.; Van Tendeloo, V.F.I.; Berneman, Z.N. Cancer Immunotherapy Using RNA-Loaded Dendritic Cells. Clin. Exp. Immunol. 2003, 134, 378. [Google Scholar] [CrossRef] [PubMed]
- Querido, W.; Rossi, A.L.; Farina, M. The Effects of Strontium on Bone Mineral: A Review on Current Knowledge and Microanalytical Approaches. Micron 2016, 80, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Marie, P.J. Strontium Ranelate: A Dual Mode of Action Rebalancing Bone Turnover in Favour of Bone Formation. Curr. Opin. Rheumatol. 2006, 18 (Suppl. 1), S11–S15. [Google Scholar] [CrossRef]
- Glenske, K.; Donkiewicz, P.; Köwitsch, A.; Milosevic-Oljaca, N.; Rider, P.; Rofall, S.; Franke, J.; Jung, O.; Smeets, R.; Schnettler, R.; et al. Applications of Metals for Bone Regeneration. Int. J. Mol. Sci. 2018, 19, 826. [Google Scholar] [CrossRef]
- Ehret, C.; Aid-Launais, R.; Sagardoy, T.; Siadous, R.; Bareille, R.; Rey, S.; Pechev, S.; Etienne, L.; Kalisky, J.; De Mones, E.; et al. Strontium-Doped Hydroxyapatite Polysaccharide Materials Effect on Ectopic Bone Formation. PLoS ONE 2017, 12, e0184663. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Cheng, M.; Wang, Q.; Yeung, K.W.K.; Chu, P.K.; Zhang, X. Zinc-Modified Sulfonated Polyetheretherketone Surface with Immunomodulatory Function for Guiding Cell Fate and Bone Regeneration. Adv. Sci. 2018, 5, 1800749. [Google Scholar] [CrossRef]
- Osorio, R.; Yamauti, M.; Osorio, E.; Ruiz-Requena, M.E.; Pashley, D.H.; Tay, F.R.; Toledano, M. Zinc Reduces Collagen Degradation in Demineralized Human Dentin Explants. J. Dent. 2011, 39, 148–153. [Google Scholar] [CrossRef]
- Chou, J.; Komuro, M.; Hao, J.; Kuroda, S.; Hattori, Y.; Ben-Nissan, B.; Milthorpe, B.; Otsuka, M. Bioresorbable Zinc Hydroxyapatite Guided Bone Regeneration Membrane for Bone Regeneration. Clin. Oral Implants Res. 2016, 27, 354–360. [Google Scholar] [CrossRef]
- Balalaie, A.; Rezvani, M.B.; Mohammadi Basir, M. Dual Function of Proanthocyanidins as Both MMP Inhibitor and Crosslinker in Dentin Biomodification: A Literature Review. Dent. Mater. J. 2018, 37, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Jahnen-Dechent, W.; Ketteler, M. Magnesium Basics. Clin. Kidney J. 2012, 5, i3. [Google Scholar] [CrossRef]
- Jung, O.; Hesse, B.; Stojanovic, S.; Seim, C.; Weitkamp, T.; Batinic, M.; Goerke, O.; Kačarević, Ž.P.; Rider, P.; Najman, S.; et al. Biocompatibility Analyses of Hf-Passivated Magnesium Screws for Guided Bone Regeneration (Gbr). Int. J. Mol. Sci. 2021, 22, 12567. [Google Scholar] [CrossRef]
- Lu, J.; Wei, J.; Yan, Y.; Li, H.; Jia, J.; Wei, S.; Guo, H.; Xiao, T.; Liu, C. Preparation and Preliminary Cytocompatibility of Magnesium Doped Apatite Cement with Degradability for Bone Regeneration. J. Mater. Sci. Mater. Med. 2011, 22, 607–615. [Google Scholar] [CrossRef]
- Leem, Y.H.; Lee, K.S.; Kim, J.H.; Seok, H.K.; Chang, J.S.; Lee, D.H. Magnesium Ions Facilitate Integrin Alpha 2- and Alpha 3-Mediated Proliferation and Enhance Alkaline Phosphatase Expression and Activity in HBMSCs. J. Tissue Eng. Regen. Med. 2016, 10, E527–E536. [Google Scholar] [CrossRef]
- Xue, J.; He, M.; Niu, Y.; Liu, H.; Crawford, A.; Coates, P.; Chen, D.; Shi, R.; Zhang, L. Preparation and in Vivo Efficient Anti-Infection Property of GTR/GBR Implant Made by Metronidazole Loaded Electrospun Polycaprolactone Nanofiber Membrane. Int. J. Pharm. 2014, 475, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wu, Z.; Leung, A.; Chen, X.; Landao-Bassonga, E.; Gao, J.; Chen, L.; Zheng, M.; Yao, F.; Yang, H.; et al. Fabrication of a Silver Nanoparticle-Coated Collagen Membrane with Anti-Bacterial and Anti-Inflammatory Activities for Guided Bone Regeneration. Biomed. Mater. 2018, 13, 065014. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.H.; Claudia, J.C.; Tai, W.C.; Huang, K.Y.; Lai, C.H.; Chang, C.H.; Chang, Y.C.; Wu, Y.C.; Kuo, M.Y.P.; Chang, P.C. The Treatment Response of Barrier Membrane with Amoxicillin-Loaded Nanofibers in Experimental Periodontitis. J. Periodontol. 2021, 92, 886–895. [Google Scholar] [CrossRef]
- Cibor, U.; Krok-Borkowicz, M.; Brzychczy-Włoch, M.; Rumian, Ł.; Pietryga, K.; Kulig, D.; Chrzanowski, W.; Pamuła, E. Gentamicin-Loaded Polysaccharide Membranes for Prevention and Treatment of Post-Operative Wound Infections in the Skeletal System. Pharm. Res. 2017, 34, 2075–2083. [Google Scholar] [CrossRef]
- Ghavimi, M.A.; Bani Shahabadi, A.; Jarolmasjed, S.; Memar, M.Y.; Maleki Dizaj, S.; Sharifi, S. Nanofibrous Asymmetric Collagen/Curcumin Membrane Containing Aspirin-Loaded PLGA Nanoparticles for Guided Bone Regeneration. Sci. Rep. 2020, 10, 18200. [Google Scholar] [CrossRef]
- Xie, J.; Wang, W.; Fan, X.; Li, H.; Wang, H.; Liao, R.; Hu, Y.; Zeng, M. Masquelet Technique: Effects of Vancomycin Concentration on Quality of the Induced Membrane. Injury 2022, 53, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Bai, T.; Wang, L.; Cheng, Y.; Xia, D.; Yu, S.; Zheng, Y. Biomimetic AgNPs@antimicrobial Peptide/Silk Fibroin Coating for Infection-Trigger Antibacterial Capability and Enhanced Osseointegration. Bioact. Mater. 2022, 20, 64–80. [Google Scholar] [CrossRef] [PubMed]
- Khorsand, B.; Elangovan, S.; Hong, L.; Dewerth, A.; Kormann, M.S.D.; Salem, A.K. A Comparative Study of the Bone Regenerative Effect of Chemically Modified RNA Encoding BMP-2 or BMP-9. AAPS J. 2017, 19, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Sun, H.; Yang, J.; Liu, Y.; Zhang, Z.; Wang, J.; Deng, F. Evaluation of Bone-Regeneration Effects and Ectopic Osteogenesis of Collagen Membrane Chemically Conjugated with Stromal Cell-Derived Factor-1 in Vivo. Biomed. Mater. 2020, 15, 015009. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, L.; Liu, R.; Lin, Y.; Chen, S.; Lu, S.; Lin, Z.; Chen, Z.; Wu, C.; Xiao, Y. The Osteoimmunomodulatory Property of a Barrier Collagen Membrane and Its Manipulation: Via Coating Nanometer-Sized Bioactive Glass to Improve Guided Bone Regeneration. Biomater. Sci. 2018, 6, 1007–1019. [Google Scholar] [CrossRef]
- Omar, O.; Elgali, I.; Dahlin, C.; Thomsen, P. Barrier Membranes: More than the Barrier Effect? J. Clin. Periodontol. 2019, 46, 103–123. [Google Scholar] [CrossRef]
- Lima, L.L.; Gonçalves, P.F.; Sallum, E.A.; Casati, M.Z.; Nociti, F.H. Guided Tissue Regeneration May Modulate Gene Expression in Periodontal Intrabony Defects: A Human Study. J. Periodont. Res. 2008, 43, 459–464. [Google Scholar] [CrossRef]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided Bone Regeneration: Materials and Biological Mechanisms Revisited. Eur. J. Oral Sci. 2017, 125, 315. [Google Scholar] [CrossRef]
- Kitaori, T.; Ito, H.; Schwarz, E.M.; Tsutsumi, R.; Yoshitomi, H.; Oishi, S.; Nakano, M.; Fujii, N.; Nagasawa, T.; Nakamura, T. Stromal Cell-Derived Factor 1/CXCR4 Signaling Is Critical for the Recruitment of Mesenchymal Stem Cells to the Fracture Site during Skeletal Repair in a Mouse Model. Arthritis Rheumatol. 2009, 60, 813–823. [Google Scholar] [CrossRef]
- Xing, Z.; Lu, C.; Hu, D.; Yu, Y.Y.; Wang, X.; Colnot, C.; Nakamura, M.; Wu, Y.; Miclau, T.; Marcucio, R.S. Multiple Roles for CCR2 during Fracture Healing. Dis. Models Mech. 2010, 3, 451–458. [Google Scholar] [CrossRef]
- Bottino, M.C.; Thomas, V. Membranes for Periodontal Regeneration-A Materials Perspective. Front. Oral Biol. 2015, 17, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.J.; Eaton, J.W.; Tang, L. Molecular Basis of Biomaterial-Mediated Foreign Body Reactions. Blood 2001, 98, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Mountziaris, P.M.; Mikos, A.G. Modulation of the Inflammatory Response for Enhanced Bone Tissue Regeneration. Tissue Eng. Part B Rev. 2008, 14, 179. [Google Scholar] [CrossRef] [PubMed]
- Rolvien, T.; Barbeck, M.; Wenisch, S.; Amling, M.; Krause, M. Cellular Mechanisms Responsible for Success and Failure of Bone Substitute Materials. Int. J. Mol. Sci. 2018, 19, 2893. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, Q.; Wang, X.; Yao, X.; Zhang, B.; Wu, J.; Sun, C. Exosomal NcRNAs Facilitate Interactive “dialogue” between Tumor Cells and Tumor-Associated Macrophages. Cancer Lett. 2022, 552, 215975. [Google Scholar] [CrossRef]
- Lee, J.; Byun, H.; Madhurakkat Perikamana, S.K.; Lee, S.; Shin, H. Current Advances in Immunomodulatory Biomaterials for Bone Regeneration. Adv. Healthc. Mater. 2019, 8, 1801106. [Google Scholar] [CrossRef]
- Maruyama, M.; Rhee, C.; Utsunomiya, T.; Zhang, N.; Ueno, M.; Yao, Z.; Goodman, S.B. Modulation of the Inflammatory Response and Bone Healing. Front. Endocrinol. 2020, 11, 386. [Google Scholar] [CrossRef]
- Brown, B.N.; Valentin, J.E.; Stewart-Akers, A.M.; McCabe, G.P.; Badylak, S.F. Macrophage Phenotype and Remodeling Outcomes in Response to Biologic Scaffolds with and without a Cellular Component. Biomaterials 2009, 30, 1482. [Google Scholar] [CrossRef]
- Barbeck, M.; Motta, A.; Migliaresi, C.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Heterogeneity of Biomaterial-Induced Multinucleated Giant Cells: Possible Importance for the Regeneration Process? J. Biomed. Mater. Res. A 2016, 104, 413–418. [Google Scholar] [CrossRef]
- Barbeck, M.; Booms, P.; Unger, R.; Hoffmann, V.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Multinucleated Giant Cells in the Implant Bed of Bone Substitutes Are Foreign Body Giant Cells—New Insights into the Material-Mediated Healing Process. J. Biomed. Mater. Res. A 2017, 105, 1105–1111. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. FOREIGN BODY REACTION TO BIOMATERIALS. Semin. Immunol. 2008, 20, 86. [Google Scholar] [CrossRef]
- Miron, R.J.; Zohdi, H.; Fujioka-Kobayashi, M.; Bosshardt, D.D. Giant Cells around Bone Biomaterials: Osteoclasts or Multi-Nucleated Giant Cells? Acta Biomater. 2016, 46, 15–28. [Google Scholar] [CrossRef]
- Miron, R.J.; Bosshardt, D.D. Multinucleated Giant Cells: Good Guys or Bad Guys? Tissue Eng. Part B Rev. 2018, 24, 53–65. [Google Scholar] [CrossRef]
- Ghanaati, S.; Barbeck, M.; Detsch, R.; Deisinger, U.; Hilbig, U.; Rausch, V.; Sader, R.; Unger, R.E.; Ziegler, G.; Kirkpatrick, C.J. The Chemical Composition of Synthetic Bone Substitutes Influences Tissue Reactions in Vivo: Histological and Histomorphometrical Analysis of the Cellular Inflammatory Response to Hydroxyapatite, Beta-Tricalcium Phosphate and Biphasic Calcium Phosphate Ceramics. Biomed. Mater. 2012, 7, 015005. [Google Scholar] [CrossRef]
- Barbeck, M.; Dard, M.; Kokkinopoulou, M.; Markl, J.; Booms, P.; Sader, R.A.; Kirkpatrick, C.J.; Ghanaati, S. Small-Sized Granules of Biphasic Bone Substitutes Support Fast Implant Bed Vascularization. Biomatter 2015, 5, e1056943. [Google Scholar] [CrossRef]
- Herten, M.; Jung, R.E.; Ferrari, D.; Rothamel, D.; Golubovic, V.; Molenberg, A.; Hämmerle, C.H.F.; Becker, J.; Schwarz, F. Biodegradation of Different Synthetic Hydrogels Made of Polyethylene Glycol Hydrogel/RGD-Peptide Modifications: An Immunohistochemical Study in Rats. Clin. Oral Implants Res. 2009, 20, 116–125. [Google Scholar] [CrossRef]
- Ghanaati, S.; Schlee, M.; Webber, M.J.; Willershausen, I.; Barbeck, M.; Balic, E.; Görlach, C.; Stupp, S.I.; Sader, R.A.; Kirkpatrick, C.J. Evaluation of the Tissue Reaction to a New Bilayered Collagen Matrix in Vivo and Its Translation to the Clinic. Biomed. Mater. 2011, 6, 015010. [Google Scholar] [CrossRef]
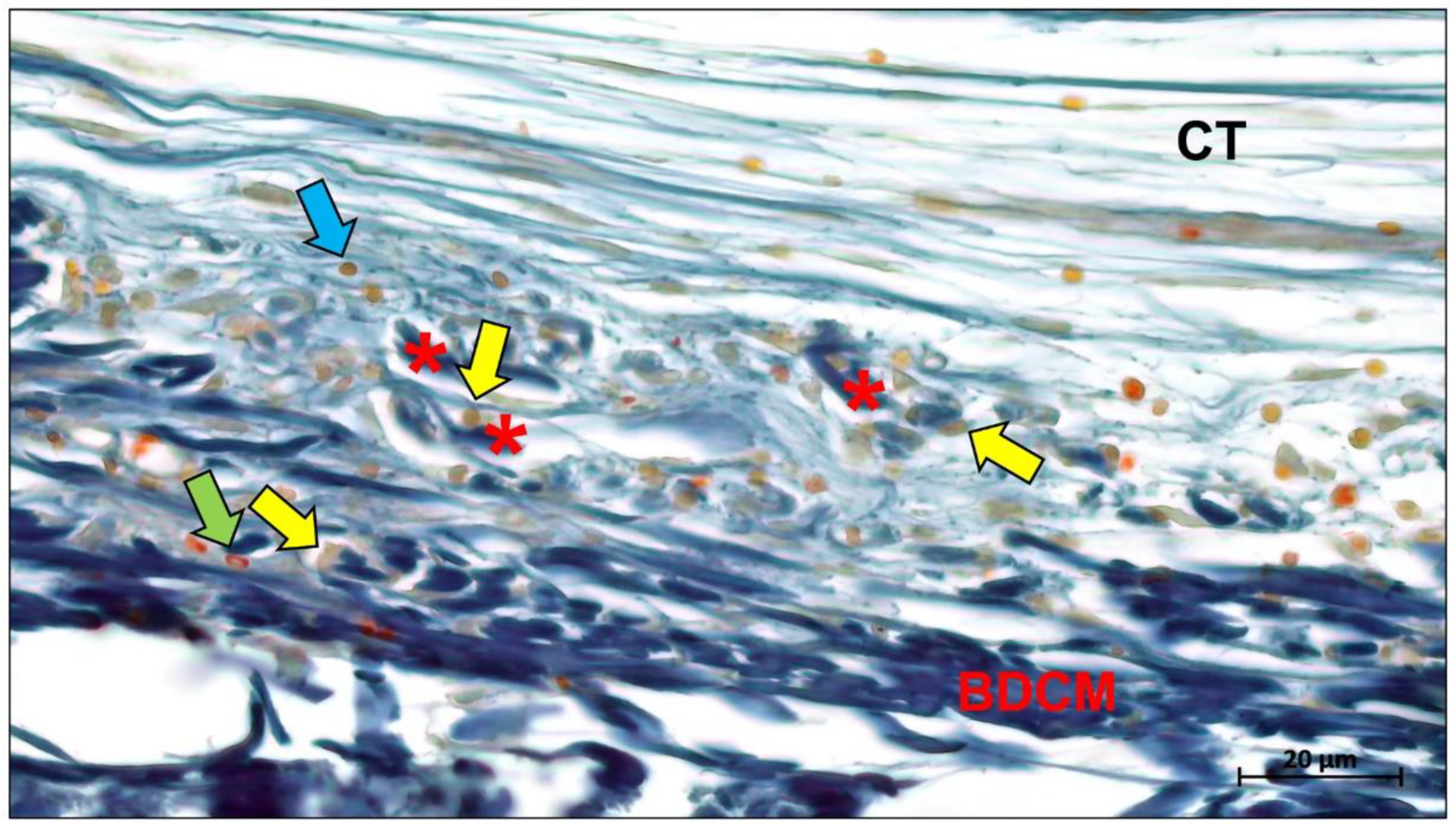
- Kapogianni, E.; Alkildani, S.; Radenkovic, M.; Xiong, X.; Krastev, R.; Stöwe, I.; Bielenstein, J.; Jung, O.; Najman, S.; Barbeck, M.; et al. The Early Fragmentation of a Bovine Dermis-Derived Collagen Barrier Membrane Contributes to Transmembraneous Vascularization—A Possible Paradigm Shift for Guided Bone Regeneration. Membranes 2021, 11, 185. [Google Scholar] [CrossRef]
- Lindner, C.; Alkildani, S.; Stojanovic, S.; Najman, S.; Jung, O.; Barbeck, M. In Vivo Biocompatibility Analysis of a Novel Barrier Membrane Based on Bovine Dermis-Derived Collagen for Guided Bone Regeneration (GBR). Membranes 2022, 12, 378. [Google Scholar] [CrossRef]
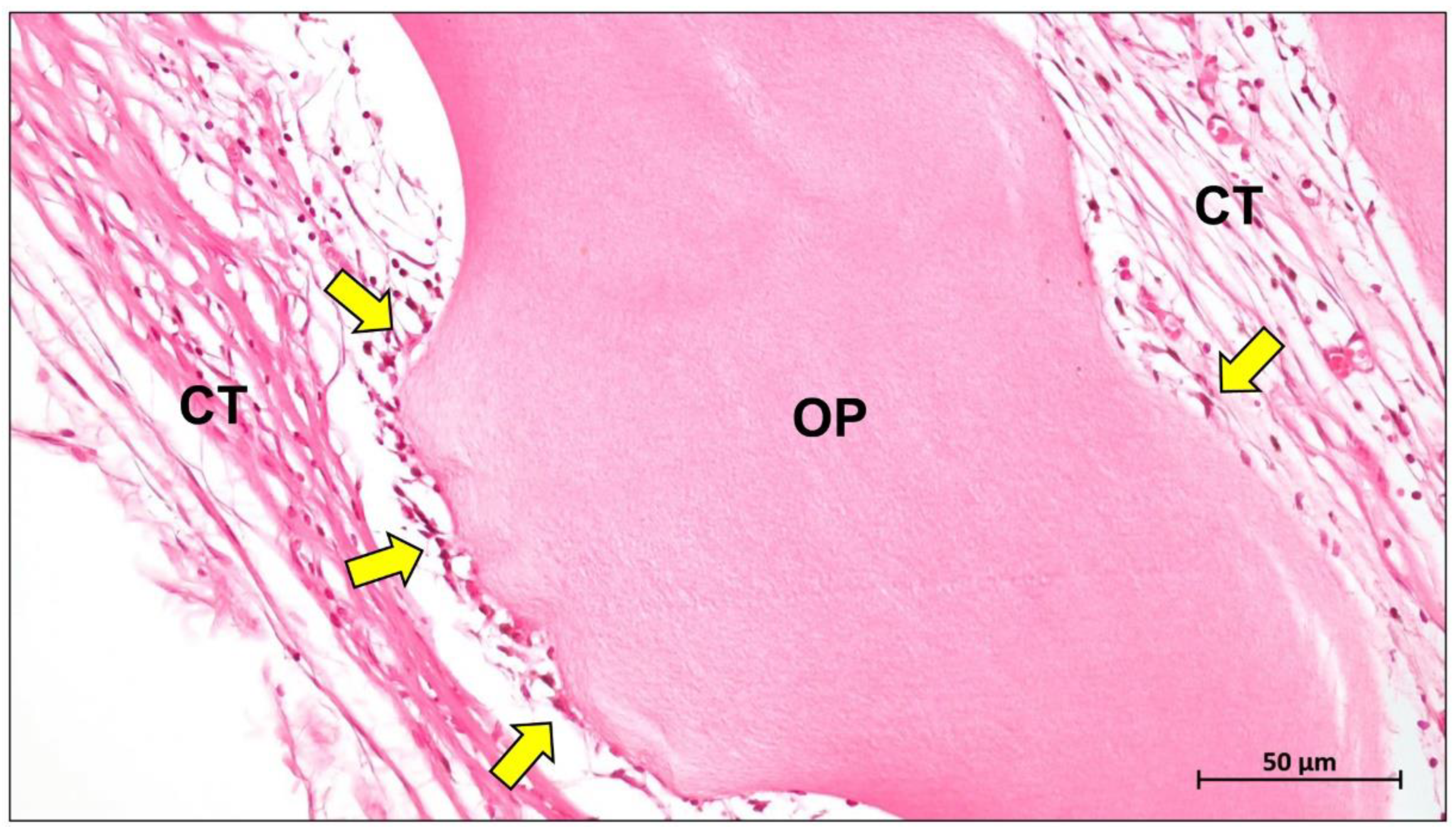
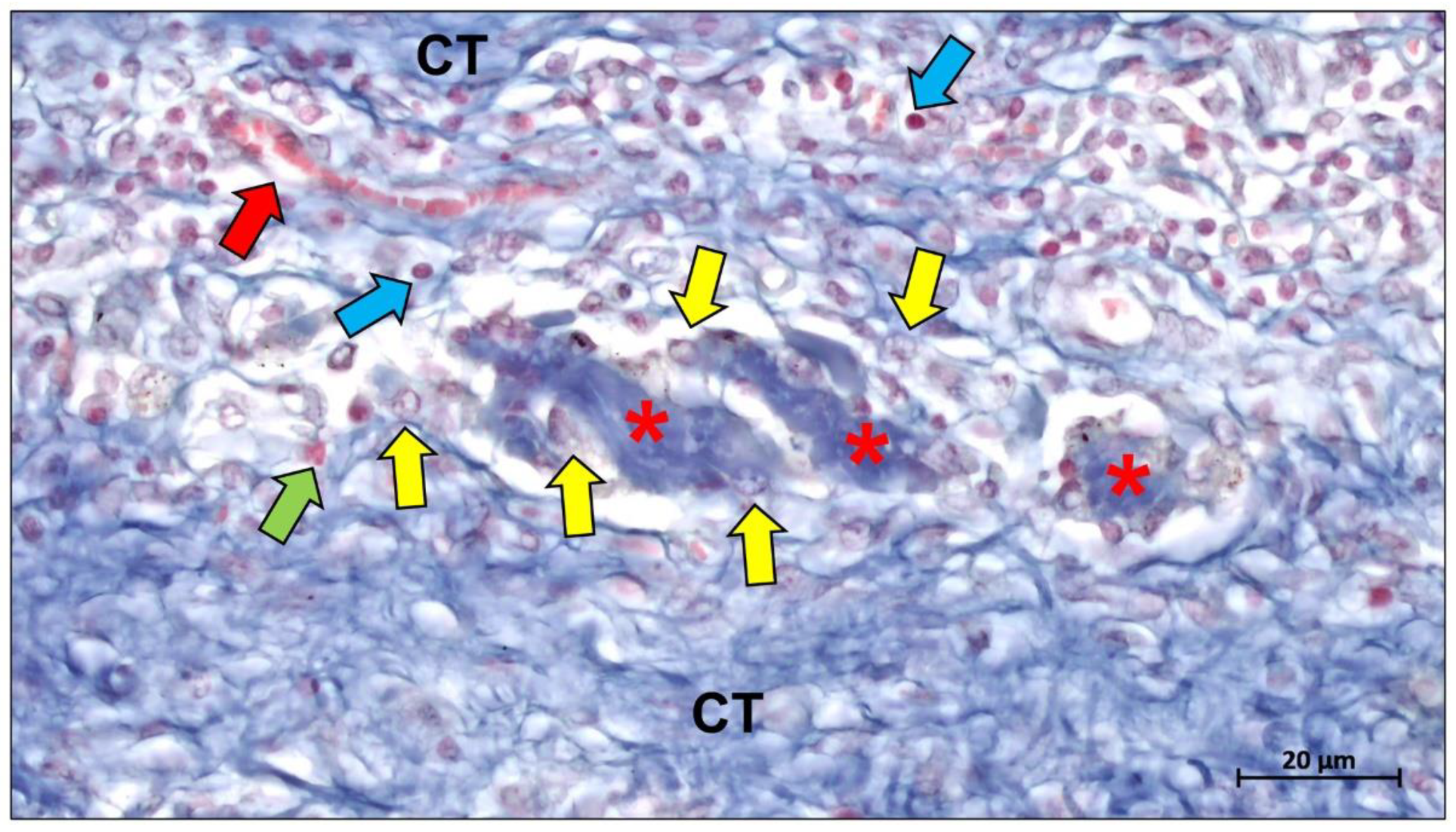
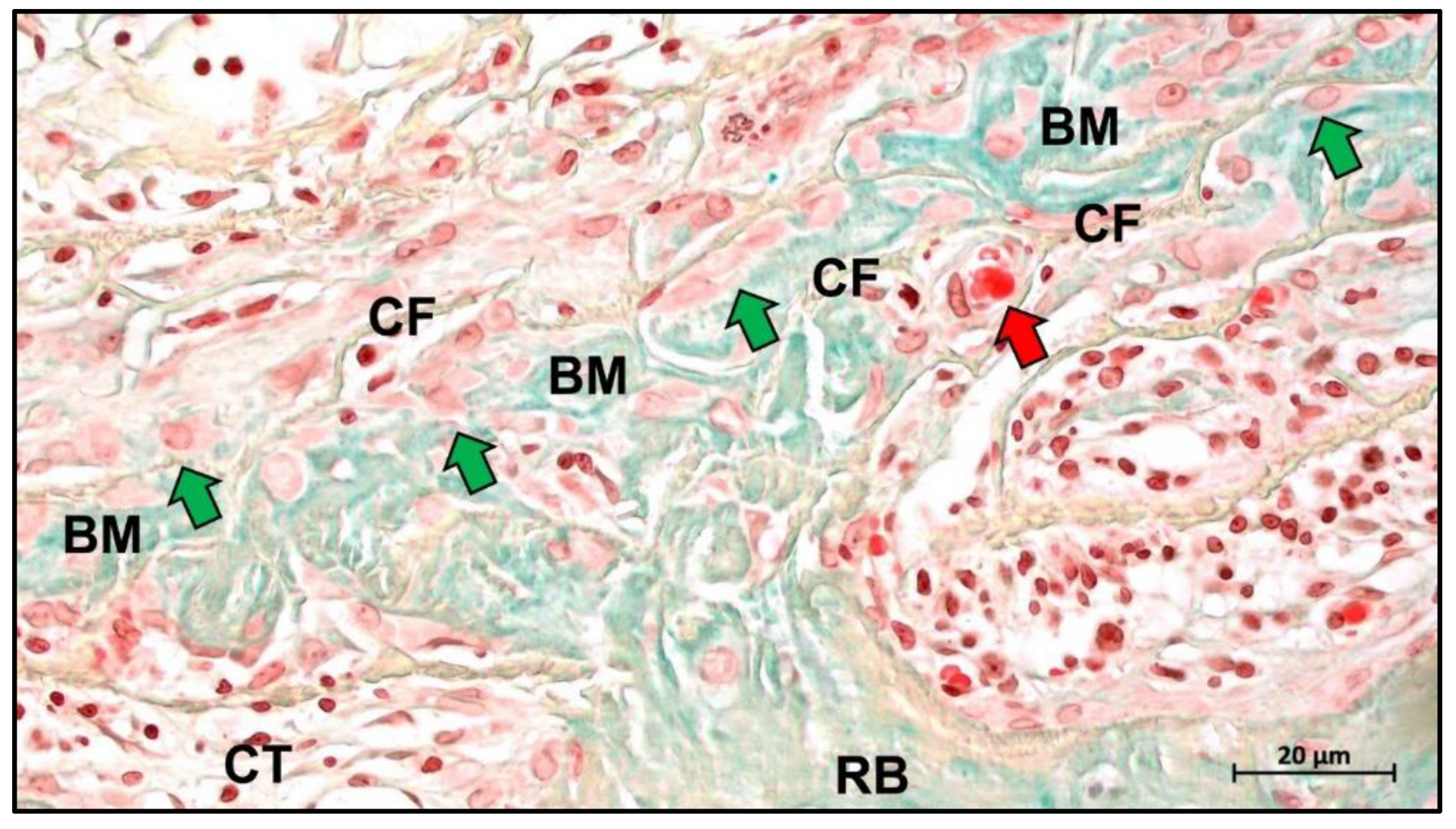

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Fan, L.; Alkildani, S.; Liu, L.; Emmert, S.; Najman, S.; Rimashevskiy, D.; Schnettler, R.; Jung, O.; Xiong, X.; et al. Barrier Membranes for Guided Bone Regeneration (GBR): A Focus on Recent Advances in Collagen Membranes. Int. J. Mol. Sci. 2022, 23, 14987. https://doi.org/10.3390/ijms232314987
Ren Y, Fan L, Alkildani S, Liu L, Emmert S, Najman S, Rimashevskiy D, Schnettler R, Jung O, Xiong X, et al. Barrier Membranes for Guided Bone Regeneration (GBR): A Focus on Recent Advances in Collagen Membranes. International Journal of Molecular Sciences. 2022; 23(23):14987. https://doi.org/10.3390/ijms232314987
Chicago/Turabian StyleRen, Yanru, Lu Fan, Said Alkildani, Luo Liu, Steffen Emmert, Stevo Najman, Denis Rimashevskiy, Reinhard Schnettler, Ole Jung, Xin Xiong, and et al. 2022. "Barrier Membranes for Guided Bone Regeneration (GBR): A Focus on Recent Advances in Collagen Membranes" International Journal of Molecular Sciences 23, no. 23: 14987. https://doi.org/10.3390/ijms232314987
APA StyleRen, Y., Fan, L., Alkildani, S., Liu, L., Emmert, S., Najman, S., Rimashevskiy, D., Schnettler, R., Jung, O., Xiong, X., & Barbeck, M. (2022). Barrier Membranes for Guided Bone Regeneration (GBR): A Focus on Recent Advances in Collagen Membranes. International Journal of Molecular Sciences, 23(23), 14987. https://doi.org/10.3390/ijms232314987












