Therapeutic Effects of Acer palmatum Thumb. Leaf Extract (KIOM-2015E) on Benzalkonium Chloride-Induced Dry Eye in a Mouse Model
Abstract
:1. Introduction
2. Results
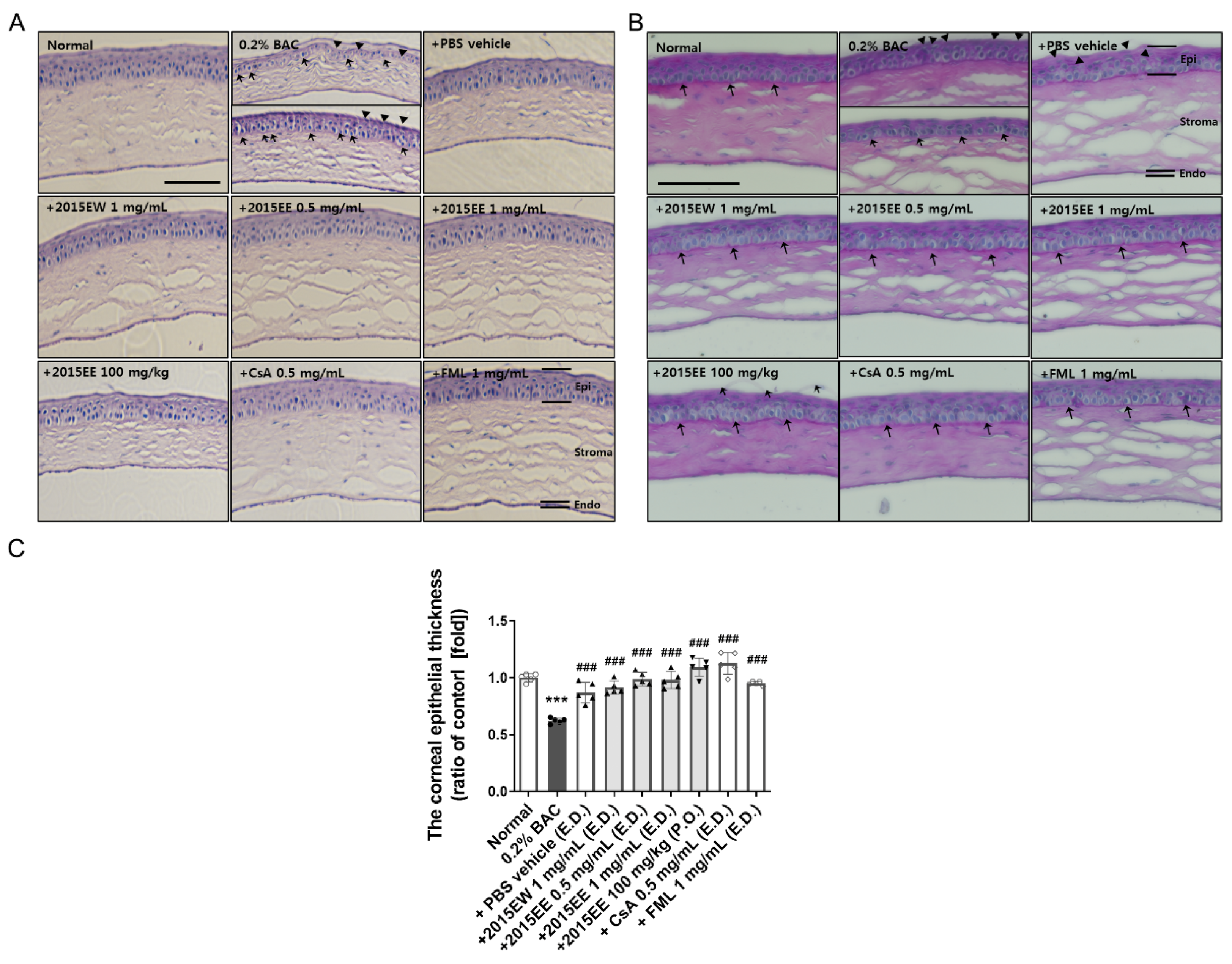
2.1. KIOM-2015E Ameliorates Corneal Dysfunction in a BAC-Induced Dry Eye Mouse Model
2.2. KIOM-2015E Application Reduces Inflammatory Cytokine Expression on the Ocular Surface in a BAC-Induced Dry Eye Mouse Model
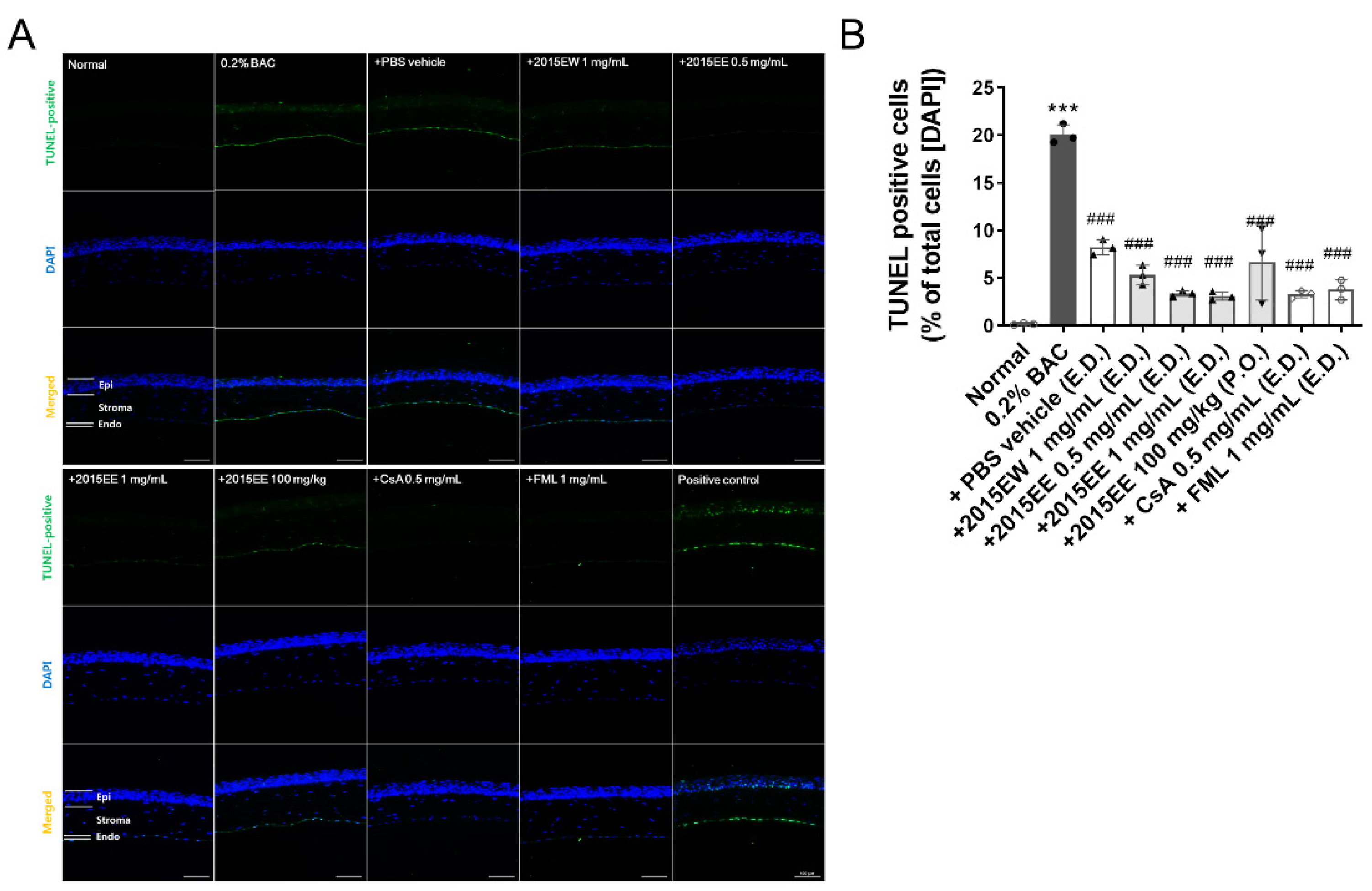
2.3. KIOM-2015E Application Reduces Damage to the Cornea on the Ocular Surface in a BAC-Induced Dry Eye Mouse Model
2.4. Histological Analysis of the Ocular Surface Following Treatment with Topical Eye Drops or Oral Administration of KIOM-2015E
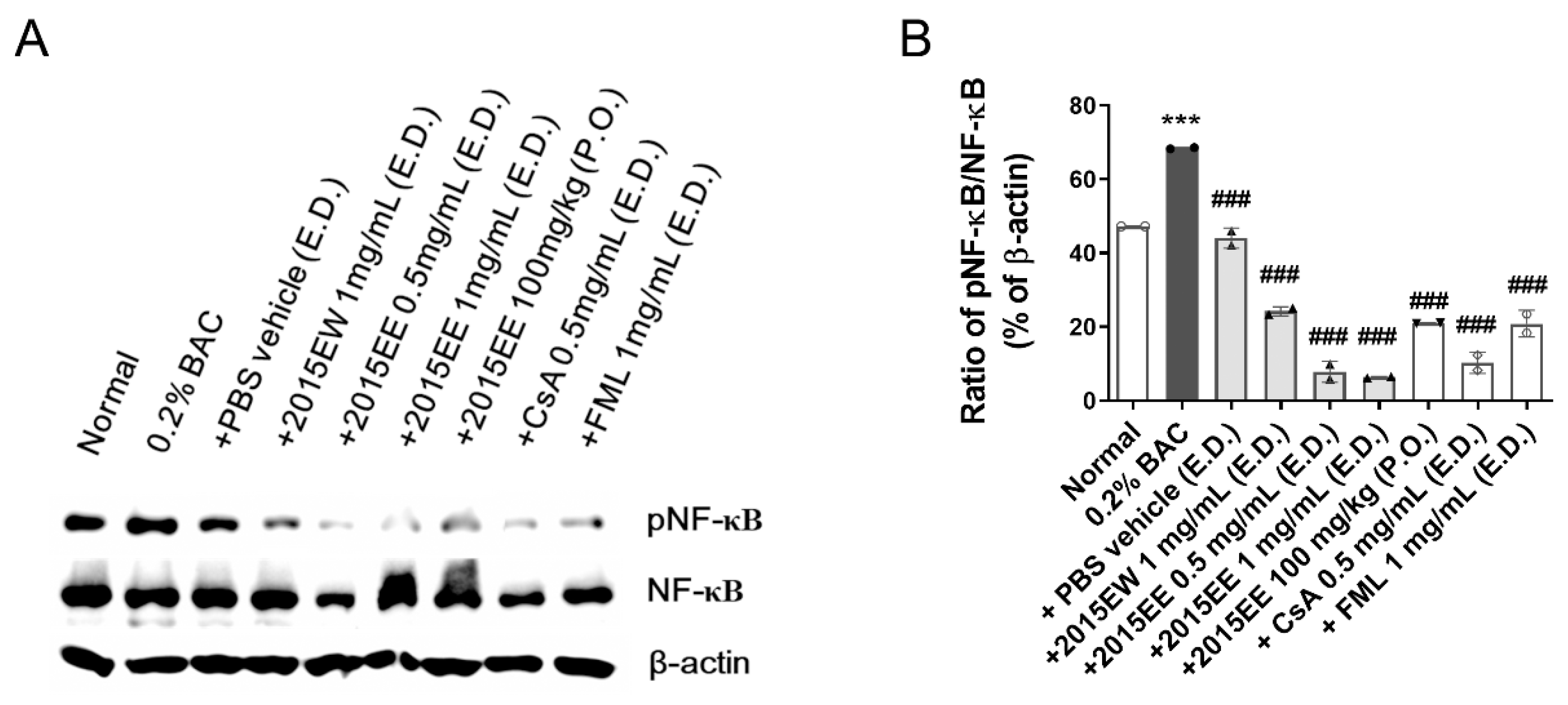
2.5. KIOM-2015E Suppresses BAC-Induced NF-κB Activation through p65 Nuclear Translocation Blockage in a Dry Eye Mouse Model
2.6. HPLC Analysis of the Marker Constituents in KIOM-2015E Extracts
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals and Procedures
4.3. Lissamine Green B Staining and Quantification
4.4. Paraffin Embedding and Histological Examination
4.5. Measurement of Tear Volume
4.6. PAS Staining
4.7. TUNEL Assay
4.8. Immunofluorescence Staining
4.9. RNA Isolation and Real-Time Reverse Transcription-Polymerase Chain Reaction Analysis
4.10. Preparation of Cellular Protein Extraction and Western Blot Analysis
4.11. Preparation of Standard Compound Solutions and Samples for HPLC Analysis
4.12. Chromatographic Conditions
4.13. Statistical analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schaumberg, D.A.; Sullivan, D.A.; Buring, J.E.; Dana, M.R. Prevalence of dry eye syndrome among US women. Am. J. Ophthalmol. 2003, 136, 318–326. [Google Scholar] [CrossRef]
- Luo, L.; Li, D.Q.; Pflugfelder, S.C. Hyperosmolarity-induced apoptosis in human corneal epithelial cells is mediated by cytochrome c and MAPK pathways. Cornea 2007, 26, 452–460. [Google Scholar] [CrossRef]
- Luo, L.; Li, D.Q.; Corrales, R.M.; Pflugfelder, S.C. Hyperosmolar saline is a proinflammatory stress on the mouse ocular surface. Eye Contact Lens 2005, 31, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Okahara, A.; Tanioka, H.; Takada, K.; Kawazu, K. Ocular toxicity of benzalkonium chloride homologs compared with their mixtures. J. Toxicol. Pathol. 2013, 26, 343–349. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.; He, H.; Zhou, T.; Liu, X.; Wang, Y.; He, H.; Wu, H.; Liu, Z. A mouse model of limbal stem cell deficiency induced by topical medication with the preservative benzalkonium chloride. Investig. Opthalmol. Vis. Sci. 2013, 54, 6314–6325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becquet, F.; Goldschild, M.; Moldovan, M.S.; Ettaiche, M.; Gastaud, P.; Baudouin, C. Histopathological effects of topical ophthalmic preservatives on rat corneoconjunctival surface. Curr. Eye Res. 1998, 17, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Burstein, N.L. Preservative cytotoxic threshold for benzalkonium chloride and chlorhexidine digluconate in cat and rabbit corneas. Investig. Ophthalmol. Vis. Sci. 1980, 19, 308–313. [Google Scholar]
- Baudouin, C.; Labbe, A.; Liang, H.; Pauly, A.; Brignole-Baudouin, F. Preservatives in eyedrops: The good, the bad and the ugly. Prog. Retin. Eye Res. 2010, 29, 312–334. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, X.; Zhou, T.; Wang, Y.; Bai, L.; He, H.; Liu, Z. A mouse dry eye model induced by topical administration of benzalkonium chloride. Mol. Vis. 2011, 17, 257–264. [Google Scholar]
- The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul. Surf. 2007, 5, 75–92. [CrossRef]
- Niederkorn, J.Y.; Stern, M.E.; Pflugfelder, S.C.; De Paiva, C.S.; Corrales, R.M.; Gao, J.; Siemasko, K. Desiccating stress induces T cell-mediated Sjogren’s Syndrome-like lacrimal keratoconjunctivitis. J. Immunol. 2006, 176, 3950–3957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Topouzis, S.; Liang, L.F.; Stotish, R.L. Myostatin signaling through Smad2, Smad3 and Smad4 is regulated by the inhibitory Smad7 by a negative feedback mechanism. Cytokine 2004, 26, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Lopez Bernal, D.; Ubels, J.L. Artificial tear composition and promotion of recovery of the damaged corneal epithelium. Cornea 1993, 12, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.C.; Jeong, I.Y.; Park, Y.G.; Yang, S.Y. Interleukin-6 and tumor necrosis factor-alpha levels in tears of patients with dry eye syndrome. Cornea 2007, 26, 431–437. [Google Scholar] [CrossRef]
- Solomon, A.; Dursun, D.; Liu, Z.; Xie, Y.; Macri, A.; Pflugfelder, S.C. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2283–2292. [Google Scholar]
- Pflugfelder, S.C.; Jones, D.; Ji, Z.; Afonso, A.; Monroy, D. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjogren’s syndrome keratoconjunctivitis sicca. Curr. Eye Res. 1999, 19, 201–211. [Google Scholar] [CrossRef]
- Corrales, R.M.; Villarreal, A.; Farley, W.; Stern, M.E.; Li, D.Q.; Pflugfelder, S.C. Strain-related cytokine profiles on the murine ocular surface in response to desiccating stress. Cornea 2007, 26, 579–584. [Google Scholar] [CrossRef]
- Hernandez-Illas, M.; Tozman, E.; Fulcher, S.F.; Jundt, J.W.; Davis, J.; Pflugfelder, S.C. Recombinant human tumor necrosis factor receptor Fc fusion protein (Etanercept): Experience as a therapy for sight-threatening scleritis and sterile corneal ulceration. Eye Contact Lens 2004, 30, 2–5. [Google Scholar] [CrossRef]
- Marsh, P.; Pflugfelder, S.C. Topical nonpreserved methylprednisolone therapy for keratoconjunctivitis sicca in Sjogren syndrome. Ophthalmology 1999, 106, 811–816. [Google Scholar] [CrossRef] [PubMed]
- McCabe, E.; Narayanan, S. Advancements in anti-inflammatory therapy for dry eye syndrome. Optometry 2009, 80, 555–566. [Google Scholar] [CrossRef]
- Bi, W.; Gao, Y.; Shen, J.; He, C.; Liu, H.; Peng, Y.; Zhang, C.; Xiao, P. Traditional uses, phytochemistry, and pharmacology of the genus Acer (maple): A review. J. Ethnopharmacol. 2016, 189, 31–60. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Oh, T.W.; Park, E.; Yim, N.H.; Park, K.I.; Cho, W.K.; Ma, J.Y. Anti-Inflammatory and Anti-Apoptotic Effects of Acer Palmatum Thumb. Extract, KIOM-2015EW, in a Hyperosmolar-Stress-Induced In Vitro Dry Eye Model. Nutrients 2018, 10, 282–300. [Google Scholar] [CrossRef] [Green Version]
- Nyunt, A.K.; Ishida, Y.; Yu, Y.; Shimada, S. Topical apolipoprotein A-1 may have a beneficial effect on the corneal epithelium in a mouse model of dry eye: A pilot study. Eye Contact Lens 2008, 34, 287–292. [Google Scholar] [CrossRef]
- Xiong, C.; Chen, D.; Liu, J.; Liu, B.; Li, N.; Zhou, Y.; Liang, X.; Ma, P.; Ye, C.; Ge, J.; et al. A rabbit dry eye model induced by topical medication of a preservative benzalkonium chloride. Investig. Opthalmol. Vis. Sci. 2008, 49, 1850–1856. [Google Scholar] [CrossRef] [Green Version]
- Calonge, M.; Enriquez-de-Salamanca, A.; Diebold, Y.; Gonzalez-Garcia, M.J.; Reinoso, R.; Herreras, J.M.; Corell, A. Dry eye disease as an inflammatory disorder. Ocul. Immunol. Inflamm. 2010, 18, 244–253. [Google Scholar] [CrossRef]
- Pflugfelder, S.C. Antiinflammatory therapy for dry eye. Am. J. Ophthalmol. 2004, 137, 337–342. [Google Scholar] [CrossRef]
- Spaeth, G.L.; Monteiro de Barros, D.S.; Fudemberg, S.J. Visual loss caused by corticosteroid-induced glaucoma: How to avoid it. Retina 2009, 29, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.F.; Tan, J.J.; Tripodis, Y. Risk factors for steroid response among cataract patients. J. Cataract Refract. Surg. 2011, 37, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Abengozar-Vela, A.; Calonge, M.; Stern, M.E.; Gonzalez-Garcia, M.J.; Enriquez-De-Salamanca, A. Quercetin and Resveratrol Decrease the Inflammatory and Oxidative Responses in Human Ocular Surface Epithelial Cells. Investig. Opthalmol. Vis. Sci. 2015, 56, 2709–2719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, C.S.; Lee, M.Y.; Shin, I.S.; Lee, J.A.; Ha, H.; Shin, H.K. Spirodela polyrhiza (L.) Sch. ethanolic extract inhibits LPS-induced inflammation in RAW264.7 cells. Immunopharmacol. Immunotoxicol. 2012, 34, 794–802. [Google Scholar] [CrossRef]
- Nam, T.G.; Lim, T.G.; Lee, B.H.; Lim, S.; Kang, H.; Eom, S.H.; Yoo, M.; Jang, H.W.; Kim, D.O. Comparison of Anti-Inflammatory Effects of Flavonoid-Rich Common and Tartary Buckwheat Sprout Extracts in Lipopolysaccharide-Stimulated RAW 264.7 and Peritoneal Macrophages. Oxidative Med. Cell. Longev. 2017, 2017, 9658030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gou, K.J.; Zeng, R.; Dong, Y.; Hu, Q.Q.; Hu, H.W.; Maffucci, K.G.; Dou, Q.L.; Yang, Q.B.; Qin, X.H.; Qu, Y. Anti-inflammatory and Analgesic Effects of Polygonum orientale L. Extracts. Front. Pharmacol. 2017, 8, 562. [Google Scholar] [CrossRef] [Green Version]
- Pflugfelder, S.C.; Solomon, A.; Dursun, D.; Li, D.Q. Dry eye and delayed tear clearance: “A call to arms”. Adv. Exp. Med. Biol. 2002, 506 Pt B, 739–743. [Google Scholar] [PubMed]
- Tomlinson, A.; Khanal, S.; Ramaesh, K.; Diaper, C.; McFadyen, A. Tear film osmolarity: Determination of a referent for dry eye diagnosis. Investig. Opthalmol. Vis. Sci. 2006, 47, 4309–4315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, L.; Li, D.Q.; Doshi, A.; Farley, W.; Corrales, R.M.; Pflugfelder, S.C. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Investig. Opthalmol. Vis. Sci. 2004, 45, 4293–4301. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Q.; Luo, L.; Chen, Z.; Kim, H.S.; Song, X.J.; Pflugfelder, S.C. JNK and ERK MAP kinases mediate induction of IL-1beta, TNF-alpha and IL-8 following hyperosmolar stress in human limbal epithelial cells. Exp. Eye Res. 2006, 82, 588–596. [Google Scholar] [CrossRef] [Green Version]
- Yeh, S.; Song, X.J.; Farley, W.; Li, D.Q.; Stern, M.E.; Pflugfelder, S.C. Apoptosis of ocular surface cells in experimentally induced dry eye. Investig. Opthalmol. Vis. Sci. 2003, 44, 124–129. [Google Scholar] [CrossRef] [Green Version]
- Vijmasi, T.; Chen, F.Y.; Balasubbu, S.; Gallup, M.; McKown, R.L.; Laurie, G.W.; McNamara, N.A. Topical administration of lacritin is a novel therapy for aqueous-deficient dry eye disease. Investig. Opthalmol. Vis. Sci. 2014, 55, 5401–5409. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.H.; Jung, J.C.; Jung, S.Y.; Yu, S.; Lee, K.W.; Park, Y.J. Comparison of the Efficacy of Fluorometholone With and Without Benzalkonium Chloride in Ocular Surface Disease. Cornea 2016, 35, 234–242. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, R.; Bennett, E.S.; Henry, V.A.; Paragina, S.; Narumi, T.; Izumi, Y.; Kamei, Y.; Nagatomi, E.; Miyanaga, Y.; Hamano, H.; et al. The phenol red thread tear test: A cross-cultural study. Investig. Opthalmol. Vis. Sci. 1993, 34, 3510–3514. [Google Scholar]
- Kim, Y.H.; Jung, J.C.; Jung, S.Y.; Kim, Y.I.; Lee, K.W.; Park, Y.J. Cyclosporine A Downregulates MMP-3 and MMP-13 Expression in Cultured Pterygium Fibroblasts. Cornea 2015, 34, 1137–1143. [Google Scholar] [CrossRef] [PubMed]



| Gene Name | NCBI Accession No. | Primer Sequences | |
|---|---|---|---|
| TNF-α | M13049.1 | F | 5′-GGTTCTGTCCCTTTCACTCA-3′ |
| R | 5′-CCTCTTCTGCCAGTTCCA-3′ | ||
| IL-1β | M15131.1 | F | 5′-CCTCACAAGCAGAGCACAA-3′ |
| R | 5′-AGAAACAGTCCAGCCCATAC-3′ | ||
| IL-6 | DQ788722.1 | F | 5′-CTCTGGGAAATCGTGGAAAT-3′ |
| R | 5′-CCAGTTTGGTAGCATCCATC-3′ | ||
| GAPDH | GU214026.1 | F | 5′-CTGCTCCTCCCTGTTCCA-3′ |
| R | 5′-CACACCGACCTTCACCAT-3′ | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yim, N.-H.; Park, E.; Cho, W.-K.; Kim, Y.-H.; Ma, J.Y. Therapeutic Effects of Acer palmatum Thumb. Leaf Extract (KIOM-2015E) on Benzalkonium Chloride-Induced Dry Eye in a Mouse Model. Int. J. Mol. Sci. 2022, 23, 14964. https://doi.org/10.3390/ijms232314964
Yim N-H, Park E, Cho W-K, Kim Y-H, Ma JY. Therapeutic Effects of Acer palmatum Thumb. Leaf Extract (KIOM-2015E) on Benzalkonium Chloride-Induced Dry Eye in a Mouse Model. International Journal of Molecular Sciences. 2022; 23(23):14964. https://doi.org/10.3390/ijms232314964
Chicago/Turabian StyleYim, Nam-Hui, Eunhee Park, Won-Kyung Cho, Yeoun-Hee Kim, and Jin Yeul Ma. 2022. "Therapeutic Effects of Acer palmatum Thumb. Leaf Extract (KIOM-2015E) on Benzalkonium Chloride-Induced Dry Eye in a Mouse Model" International Journal of Molecular Sciences 23, no. 23: 14964. https://doi.org/10.3390/ijms232314964
APA StyleYim, N.-H., Park, E., Cho, W.-K., Kim, Y.-H., & Ma, J. Y. (2022). Therapeutic Effects of Acer palmatum Thumb. Leaf Extract (KIOM-2015E) on Benzalkonium Chloride-Induced Dry Eye in a Mouse Model. International Journal of Molecular Sciences, 23(23), 14964. https://doi.org/10.3390/ijms232314964






