Towards the Search for Potential Biomarkers in Osteosarcoma: State-of-the-Art and Translational Expectations
Abstract
:1. Introduction
| Marker | Translational Applications | Ref. |
|---|---|---|
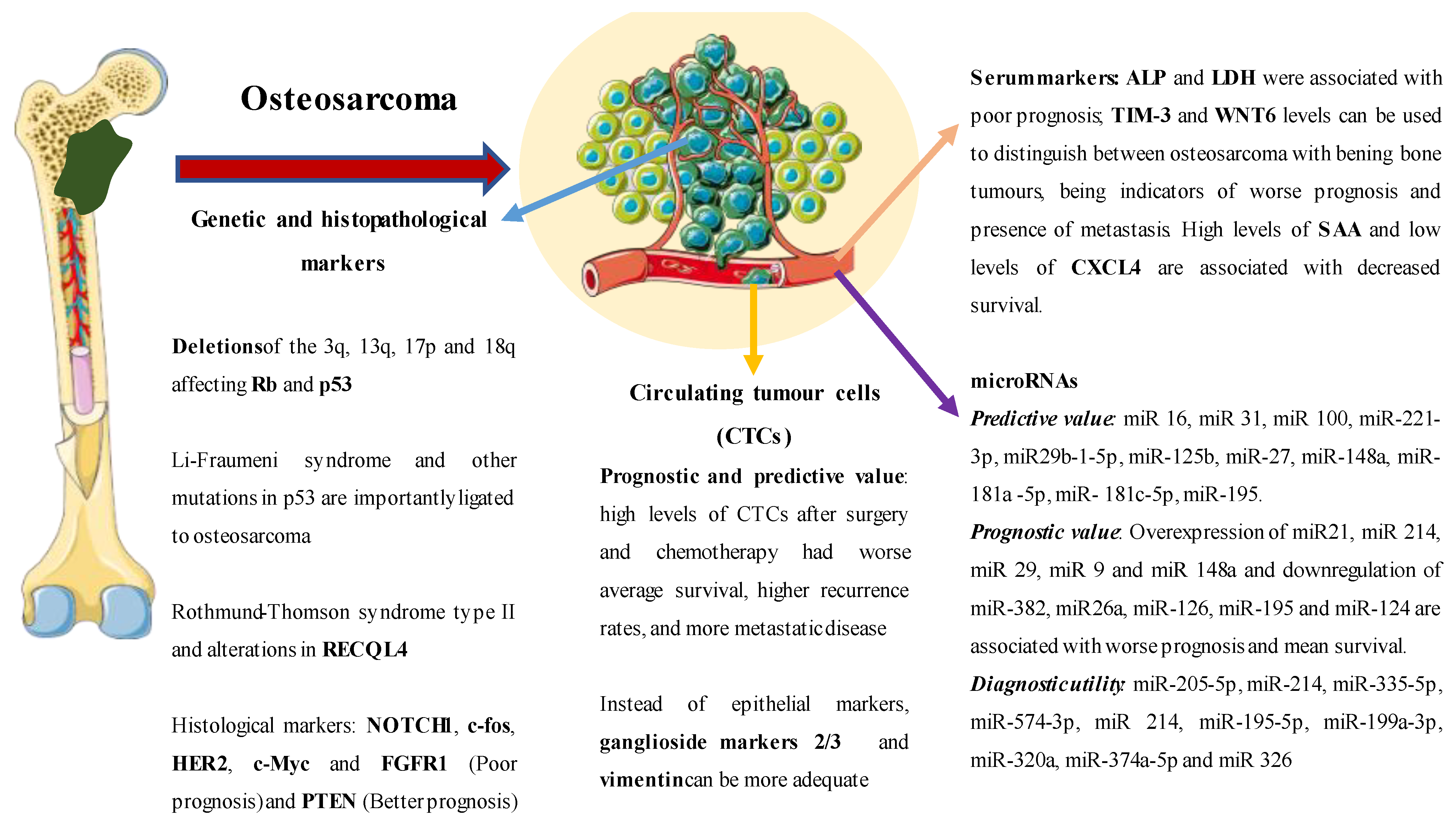
| Lactate dehydrogenase (serological) | Elevated serological levels are associated with a worse prognosis. | [28] |
| Alkaline phosphatase (serological) | Elevated serological levels are associated with a worse prognosis. | [29] |
| TIM-3 (serological) | Elevated serological levels are associated with a worse prognosis and allow differentiation between benign bone lesions and osteosarcoma. | [30] |
| WNT6 (serological) | Elevated serological levels are associated with a worse prognosis and allow differentiation between benign bone lesions and osteosarcoma (AUC 0.854) | [31] |
| SAA and CXCL4 (serological) | Elevated serological levels are associated with a worse prognosis. | [32] |
| P53 (genetic) | Mutations in P53 are associated with more aggressive tumours and Li–Fraumeni syndrome. | [33] |
| Tb1 (genetic) | Mutations in Rb1 are associated with more aggressive tumours. | [34] |
| NOTCH1 (genetic) | Worse prognosis and higher rate of metastatic disease. | [35] |
| C-Fos (genetic) | Greater histological aggressiveness and invasion. | [36] |
| HER2 (genetic) | Limited prognostic utility. | [37] |
| C-Myc (genetic) | Worse prognosis and more aggressive lesions. | [38] |
| FGFR1 (genetic) | Worse response to chemotherapy and worse prognosis. | [39] |
| PTEN (genetic) | It is associated with a better prognosis. | [40] |
| miR16 upregulation | Less histological invasion and greater response to cisplatin. | [41] |
| miR21, miR 214, miR 29, miR 9 and miR 148a upregulation | Worse prognosis and worse average survival. | [42] |
| miR-382, miR26a, miR-126, miR-195 and miR-124 downregulation | Worse prognosis and worse average survival. | [42] |
| MiR- 205-5p, MiR-214, MiR-335-5p y MiR-574-3p | Diagnostic utility with AUC of 0.70, 0.8 and 0.88, respectively. | [43] |
| miR 214 | Low plasma levels were associated with better median survival. | [43] |
| Combination of miR-195-5p, miR-199a-3p, miR-320a and miR-374a-5p | AUC of 0.96 in differentiating patients with osteosarcoma versus healthy controls. | [44] |
| miR-152 downregulation | AUC 0.956—sensitivity of 92.5% and specificity of 96.2% in differentiating osteosarcoma from periostitis and healthy controls. | [45] |
| miR 326 | AUC of 0.817 diagnosing osteosarcoma compared to healthy controls and decreased levels of miR 326 tend to present a worse prognosis and a higher probability of metastatic disease. | [46] |
| Circulating tumour cells | Worse prognosis and predictive sensitivity to different chemotherapies. | [47,48,49] |
| Genetic risk factor ~30% osteosarcomas | Acquired risk factor ~70% osteosarcomas |
| Alterations in Rb1 Li–Fraumeni Syndrome Rothmund–Thomson syndrome Bloom syndrome and Werner syndrome | Previous use of alkylating agents, such as nitrogen mustards or platinum derivatives Prior use of radiotherapy Paget disease |
2. Serological Markers
3. Genetic Markers
4. MicroRNA
5. Circulating Tumour Cells
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burningham, Z.; Hashibe, M.; Spector, L.; Schiffman, J.D. The Epidemiology of Sarcoma. Clin. Sarcoma Res. 2012, 2, 14. [Google Scholar] [CrossRef] [Green Version]
- Hui, J.Y.C. Epidemiology and Etiology of Sarcomas. Surg. Clin. N. Am. 2016, 96, 901–914. [Google Scholar] [CrossRef] [PubMed]
- SEOM. Las Cifras del Cancer en España 2021. 2021. Available online: https://seom.org/images/Cifras_del_cancer_en_Espnaha_2021.pdf (accessed on 22 October 2022).
- Ottaviani, G.; Jaffe, N. The Epidemiology of Osteosarcoma. In Cancer Treatment and Research; Springer: Boston, MA, USA, 2009; pp. 3–13. [Google Scholar] [CrossRef]
- Le Vu, B.; de Vathaire, F.; Shamsaldin, A.; Hawkins, M.M.; Grimaud, E.; Hardiman, C.; Diallo, I.; Vassal, G.; Bessa, E.; Campbell, S.; et al. Radiation dose, chemotherapy and risk of osteosarcoma after solid tumours during childhood. Int. J. Cancer 1998, 77, 370–377. [Google Scholar] [CrossRef]
- Tucker, M.A.; D’Angio, G.J.; Boice, J.D., Jr.; Strong, L.C.; Li, F.P.; Stovall, M.; Stone, B.J.; Green, D.M.; Lombardi, F.; Newton, W.; et al. Bone Sarcomas Linked to Radiotherapy and Chemotherapy in Children. N. Engl. J. Med. 1987, 317, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.W.; Squire, J.A.; Zielenska, M. The Genetics of Osteosarcoma. Sarcoma 2012, 2012, 627254. [Google Scholar] [CrossRef] [Green Version]
- Imbert-Bouteille, M.; Gauthier-Villars, M.; Leroux, D.; Meunier, I.; Aerts, I.; Lumbroso-Le Rouic, L.; Lejeune, S.; Delnatte, C.; Abadie, C.; Pujol, P.; et al. Osteosarcoma without prior retinoblastoma related to RB1 low-penetrance germline pathogenic variants: A novel type of RB1-related hereditary predisposition syndrome? Mol. Genet. Genom. Med. 2019, 7, e913. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, G.J.; Fuchimoto, Y.; Osumi, T.; Shimada, H.; Hosaka, S.; Morioka, H.; Mukai, M.; Masugi, Y.; Sakamoto, M.; Kuroda, T. Li-Fraumeni syndrome with simultaneous osteosarcoma and liver cancer: Increased expression of a CD44 variant isoform after chemotherapy. BMC Cancer 2012, 12, 444. [Google Scholar] [CrossRef] [Green Version]
- Insuasty-Enríquez, J.S.; Ortega Apraez, V.; Arias-Quiroz, E.J.; Alarcón-Tarazona, M.L.; Calderón-Cortés, C.A. Síndrome de Li-Fraumeni. Acta Méd. Colomb. 2021, 47, 1–3. [Google Scholar] [CrossRef]
- Salih, A.; Inoue, S.; Onwuzurike, N. Rothmund-Thomson syndrome (RTS) with osteosarcoma due to RECQL4 mutation. BMJ Case Rep. 2018, 2018, bcr-2017. [Google Scholar] [CrossRef]
- Hameed, M.; Mandelker, D. Tumor Syndromes Predisposing to Osteosarcoma. Adv. Anat. Pathol. 2018, 25, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Seton, M.; Hansen, M.F. Osteosarcoma in Paget’s Disease of Bone. In Advances in Pathobiology and Management of Paget’s Disease of Bone; Elsevier: Amsterdam, The Netherlands, 2016; pp. 89–104. [Google Scholar] [CrossRef]
- Hansen, M.F.; Nellissery, M.J.; Bhatia, P. Common mechanisms of osteosarcoma and paget’s disease. J. Bone Miner. Res. 1999, 14, 39–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahlawat, S.; Fayad, L.M. Revisiting the WHO classification system of bone tumours: Emphasis on advanced magnetic resonance imaging sequences. Part 2. Pol. J. Radiol. 2020, 85, 409–419. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, Q.; Gong, X.; Liu, J.; Ma, Y. Osteosarcoma: A review of current and future therapeutic approaches. Biomed. Eng. Online 2021, 20, 24. [Google Scholar] [CrossRef]
- Bacci, G.; Fabbri, N.; Balladelli, A.; Forni, C.; Palmerini, E.; Picci, P. Treatment and prognosis for synchronous multifocal osteosarcoma in 42 patients. J. Bone Jt. Surg. Br. Vol. 2006, 88, 1071–1075. [Google Scholar] [CrossRef] [Green Version]
- Klein, M.J.; Siegal, G.P. Osteosarcoma. Am. J. Clin. Pathol. 2006, 125, 555–581. [Google Scholar] [CrossRef]
- Weaver, R.; O’Connor, M.; Carey Smith, R.; Halkett, G.K. The complexity of diagnosing sarcoma in a timely manner: Perspectives of health professionals, patients, and carers in Australia. BMC Health Serv. Res. 2020, 20, 711. [Google Scholar] [CrossRef]
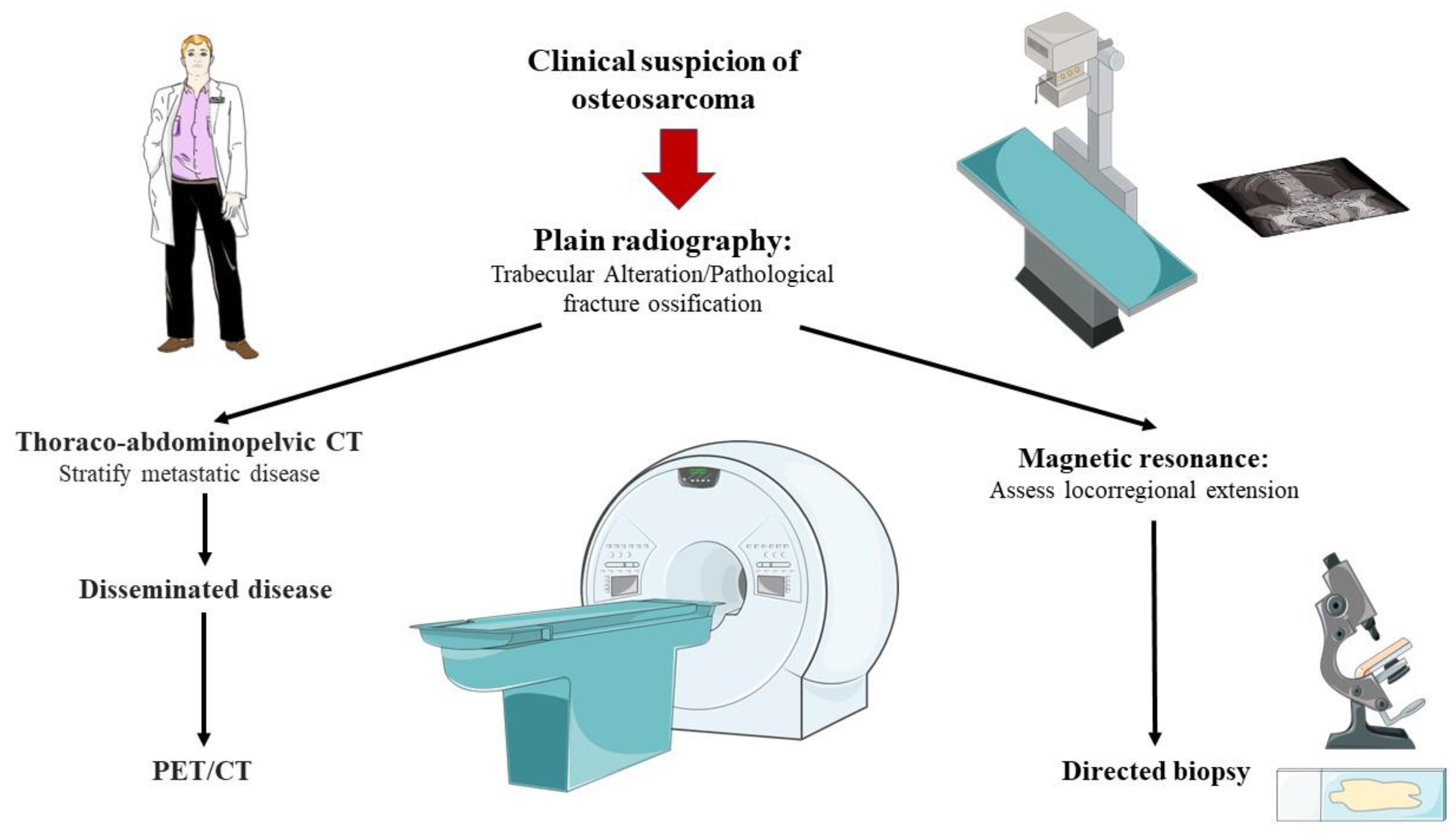
- Bajpai, J.; Gamnagatti, S.; Kumar, R.; Sreenivas, V.; Sharma, M.C.; Alam Khan, S.; Rastogi, S.; Malhotra, A.; Safaya, R.; Bakhshi, S. Role of MRI in osteosarcoma for evaluation and prediction of chemotherapy response: Correlation with histological necrosis. Pediatr. Radiol. 2010, 41, 441–450. [Google Scholar] [CrossRef]
- Wadhwa, N. Osteosarcoma: Diagnostic dilemmas in histopathology and prognostic factors. Indian J. Orthop. 2014, 48, 247–254. [Google Scholar] [CrossRef]
- Guan, Z. PET CT in the diagnosis and prognosis of osteosarcoma. Front. Biosci. 2018, 23, 2157–2165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powers, J.M.; Cost, C.; Cederberg, K.; Leonard, D.; Leavey, P.J. Bone Scintigraphy in Osteosarcoma. J. Pediatr. Hematol. Oncol. 2014, 36, e543–e545. [Google Scholar] [CrossRef] [PubMed]
- Smeland, S.; Bielack, S.S.; Whelan, J.; Bernstein, M.; Hogendoorn, P.; Krailo, M.D.; Gorlick, R.; Janeway, K.A.; Ingleby, F.C.; Anninga, J.; et al. Survival and prognosis with osteosarcoma: Outcomes in more than 2000 patients in the EURAMOS-1 (European and American Osteosarcoma Study) cohort. Eur. J. Cancer 2019, 109, 36–50. [Google Scholar] [CrossRef] [Green Version]
- Federman, N.; Bernthal, N.; Eilber, F.C.; Tap, W.D. The Multidisciplinary Management of Osteosarcoma. Curr. Treat. Options Oncol. 2009, 10, 82–93. [Google Scholar] [CrossRef] [Green Version]
- Whelan, J.S.; Bielack, S.S.; Marina, N.; Smeland, S.; Jovic, G.; Hook, J.M.; Krailo, M.; Anninga, J.; Butterfass-Bahloul, T.; Böhling, T.; et al. EURAMOS-1, an international randomised study for osteosarcoma: Results from pre-randomisation treatment. Ann. Oncol. 2015, 26, 407–414. [Google Scholar] [CrossRef]
- Rathore, R.; Van Tine, B.A. Pathogenesis and Current Treatment of Osteosarcoma: Perspectives for Future Therapies. J. Clin. Med. 2021, 10, 1182. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Lan, T.; Cai, H.; Lu, A.; Yu, W. Meta-analysis of serum lactate dehydrogenase and prognosis for osteosarcoma. Medicine 2018, 97, e0741. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Chen, L.; Huang, D.; Ge, J.; Qiu, Y.; Hao, L. Meta-analysis of alkaline phosphatase and prognosis for osteosarcoma. Eur. J. Cancer Care 2016, 26, e12536. [Google Scholar] [CrossRef]
- Ge, W.; Li, J.; Fan, W.; Xu, D.; Sun, S. Tim-3 as a diagnostic and prognostic biomarker of osteosarcoma. Tumor Biol. 2017, 39, 101042831771564. [Google Scholar] [CrossRef] [Green Version]
- Jiang, K.; Li, S.; Li, L.; Wang, X.; Gu, Y.; Jin, Z. WNT6 is an effective marker for osteosarcoma diagnosis and prognosis. Medicine 2018, 97, e13011. [Google Scholar] [CrossRef]
- Flores, R.J. Abstract 459: The use of circulating SAA and CXCL4 to predict outcome of osteosarcoma at diagnosis. Cancer Res. 2016, 76, 459. [Google Scholar] [CrossRef]
- Chen, Z.; Guo, J.; Zhang, K.; Guo, Y. TP53 Mutations and Survival in Osteosarcoma Patients: A Meta-Analysis of Published Data. Dis. Mark. 2016, 2016, 4639575. [Google Scholar] [CrossRef] [Green Version]
- Ren, W.; Gu, G. Prognostic implications of RB1 tumour suppressor gene alterations in the clinical outcome of human osteosarcoma: A meta-analysis. Eur. J. Cancer Care 2015, 26, e12401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Na Li, N.; Lu, S.; Chen, Y.; Shan, L.; Zhao, X.; Xu, Y. The role of Notch ligand Jagged1 in osteosarcoma proliferation, metastasis, and recurrence. J. Orthop. Surg. Res. 2021, 16, 226. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, H.; Wang, Q.; Zhou, F.; Liu, Y.; Zhang, Y.; Ding, H.; Yuan, M.; Li, F.; Chen, Y. Involvement of c-Fos in cell proliferation, migration, and invasion in osteosarcoma cells accompanied by altered expression of Wnt2 and Fzd9. PLoS ONE 2017, 12, e0180558. [Google Scholar] [CrossRef] [Green Version]
- Gorlick, S.; Barkauskas, D.A.; Krailo, M.; Piperdi, S.; Sowers, R.; Gill, J.; Geller, D.; Randall, R.L.; Janeway, K.; Schwartz, C.; et al. HER-2 expression is not prognostic in osteosarcoma; a Children’s Oncology Group prospective biology study. Pediatr. Blood Cancer 2014, 61, 1558–1564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.G.; Geng, X. A meta-analysis on the association of HER-2 overexpression with prognosis in human osteosarcoma. Eur. J. Cancer Care 2010, 19, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Amary, M.F.; Ye, H.; Berisha, F.; Khatri, B.; Forbes, G.; Lehovsky, K.; Frezza, A.M.; Behjati, S.; Tarpey, P.; Pillay, N.; et al. Fibroblastic growth factor receptor 1 amplification in osteosarcoma is associated with poor response to neo-adjuvant chemotherapy. Cancer Med. 2014, 3, 980–987. [Google Scholar] [CrossRef]
- Zhou, J.; Xiao, X.; Wang, W.; Luo, Y. Association between PTEN and clinical-pathological features of osteosarcoma. Biosci. Rep. 2019, 339, BSR20190954. [Google Scholar] [CrossRef] [Green Version]
- Gu, Z.; Li, Z.; Xu, R.; Zhu, X.; Hu, R.; Xue, Y.; Xu, W. miR-16-5p Suppresses Progression and Invasion of Osteosarcoma via Targeting at Smad3. Front. Pharmacol. 2020, 11, 1324. [Google Scholar] [CrossRef]
- Cheng, D.; Qiu, X.; Zhuang, M.; Zhu, C.; Zou, H.; Liu, Z. MicroRNAs with prognostic significance in osteosarcoma: A systemic review and meta-analysis. Oncotarget 2017, 8, 81062–81074. [Google Scholar] [CrossRef] [Green Version]
- Allen-Rhoades, W.; Kurenbekova, L.; Satterfield, L.; Parikh, N.; Fuja, D.; Shuck, R.L.; Rainusso, N.; Trucco, M.; Barkauskas, D.A.; Jo, E.; et al. Cross-species identification of a plasma microRNA signature for detection, therapeutic monitoring, and prognosis in osteosarcoma. Cancer Med. 2015, 4, 977–988. [Google Scholar] [CrossRef]
- Lian, F.; Cui, Y.; Zhou, C.; Gao, K.; Wu, L. Identification of a Plasma Four-microRNA Panel as Potential Noninvasive Biomarker for Osteosarcoma. PLoS ONE 2015, 10, e0121499. [Google Scholar] [CrossRef]
- Wang, N.G.; Wang, D.C.; Tan, B.Y.; Wang, F.; Yuan, Z.N. Down-regulation of microRNA152 is associated with the diagnosis and prognosis of patients with osteosarcoma. Int. J. Clin. Exp. Pathol. 2015, 8, 9314–9319. [Google Scholar]
- Cao, L.; Wang, J.; Wang, Q. MiR-326 is a diagnostic biomarker and regulates cell survival and apoptosis by targeting Bcl-2 in osteosarcoma. Biomed. Pharmacother. 2016, 84, 828–835. [Google Scholar] [CrossRef]
- Wu, Z.-J.; Tan, J.-C.; Qin, X.; Liu, B.; Yuan, Z.-C. Significance of circulating tumor cells in osteosarcoma patients treated by neoadjuvant chemotherapy and surgery. Cancer Manag. Res. 2018, 2018, 3333–3339. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Lu, Y.; Long, Z.; Li, M.; Kong, J.; Chen, G.; Wang, Z. Prognostic and clinicopathological significance of circulating tumor cells in osteosarcoma. J. Bone Oncol. 2019, 16, 100236. [Google Scholar] [CrossRef]
- Han, X.; Yang, S.; Mo, H.; Wang, M.; Zhou, F.; Li, H.; Qiao, H.; Mei, J.; Wang, Y.; Cheng, Y.; et al. Targeting of CXCR1 on Osteosarcoma Circulating Tumor Cells and Precise Treatment via Cisplatin Nanodelivery. Adv. Funct. Mater. 2019, 29, 1902246. [Google Scholar] [CrossRef]
- Chander, Y.; Subramanya, H. Serological tumor markers—Their role. Med. J. Armed Forces India 2000, 56, 279–281. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.-C.; Hsu, C.-W.; Chen, C.-D.; Yu, C.-J.; Chang, K.-P.; Tai, D.-I.; Liu, H.-P.; Su, W.-H.; Chang, Y.-S.; Yu, J.-S. Candidate Serological Biomarkers for Cancer Identified from the Secretomes of 23 Cancer Cell Lines and the Human Protein Atlas. Mol. Cell. Proteom. 2010, 9, 1100–1117. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, T.; Asanuma, K.; Hagi, T.; Sudo, A. Is Serum Lactate Dehydrogenase Useful for Predicting Oncological Outcome in Patients with Soft Tissue Sarcoma? Anticancer Res. 2019, 39, 6871–6875. [Google Scholar] [CrossRef]
- Yahaya, S.; Sofian, A.M.; Saad, A.Z.M.; Zulmi, W.; Azman, M.N.; Faisham, W. Pre-treatment serum lactate dehydrogenase (LDH) and serum alkaline phosphatase (ALP) as prognostic factors in patients with osteosarcoma. J. Cancer Prev. Curr. Res. 2018, 9, 1. [Google Scholar] [CrossRef]
- Czarnecka, A.M.; Synoradzki, K.; Firlej, W.; Bartnik, E.; Sobczuk, P.; Fiedorowicz, M.; Grieb, P.; Rutkowski, P. Molecular Biology of Osteosarcoma. Cancers 2020, 12, 2130. [Google Scholar] [CrossRef] [PubMed]
- Mirabello, L.; Yeager, M.; Mai, P.L.; Gastier-Foster, J.M.; Gorlick, R.; Khanna, C.; Patiño-García, A.; Sierrasesúmaga, L.; Lecanda, F.; Andrulis, I.L.; et al. Germline TP53 Variants and Susceptibility to Osteosarcoma. JNCI J. Natl. Cancer Inst. 2015, 107, djv101. [Google Scholar] [CrossRef]
- Gonzalez, K.D.; Noltner, K.A.; Buzin, C.H.; Gu, D.; Wen-Fong, C.Y.; Nguyen, V.Q.; Han, J.H.; Lowstuter, K.; Longmate, J.; Sommer, S.S.; et al. Beyond Li Fraumeni Syndrome: Clinical Characteristics of Families With p53 Germline Mutations. J. Clin. Oncol. 2009, 27, 1250–1256. [Google Scholar] [CrossRef]
- Gianferante, D.M.; Mirabello, L.; Savage, S.A. Germline and somatic genetics of osteosarcoma—Connecting aetiology, biology and therapy. Nat. Rev. Endocrinol. 2017, 13, 480–491. [Google Scholar] [CrossRef]
- Calvert, G.T.; Randall, R.L.; Jones, K.B.; Cannon-Albright, L.; Lessnick, S.; Schiffman, J.D. At-Risk Populations for Osteosarcoma: The Syndromes and Beyond. Sarcoma 2012, 2012, 152382. [Google Scholar] [CrossRef]
- Wagh, A.; Kokane, G.; Jendi, S.; Khatib, S.; Mistry, J.; Vaidya, K. Early Diagnosis: A Seeming Misfortune for Osteosarcoma of Mandible—Rare Case Report. Indian J. Otolaryngol. Head Neck Surg. 2018, 71, 748–751. [Google Scholar] [CrossRef]
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.S.; Dutta, A. MicroRNAs in Cancer. Annu. Rev. Pathol. Mech. Dis. 2009, 4, 199–227. [Google Scholar] [CrossRef]
- Sasaki, R.; Osaki, M.; Okada, F. MicroRNA-Based Diagnosis and Treatment of Metastatic Human Osteosarcoma. Cancers 2019, 11, 553. [Google Scholar] [CrossRef]
- Gally, T.B.; Aleluia, M.M.; Borges, G.F.; Kaneto, C.M. Circulating MicroRNAs as Novel Potential Diagnostic Biomarkers for Osteosarcoma: A Systematic Review. Biomolecules 2021, 11, 1432. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, X.; Huang, Y.; Wang, M.; Cen, C.; Tang, S.; Dique, M.R.; Cai, L.; Luis, M.A.; Smollar, J.; et al. Detection Methods and Clinical Applications of Circulating Tumor Cells in Breast Cancer. Front. Oncol. 2021, 11, 652253. [Google Scholar] [CrossRef]
- Lin, D.; Shen, L.; Luo, M.; Zhang, K.; Li, J.; Yang, Q.; Zhu, F.; Zhou, D.; Zheng, S.; Chen, Y.; et al. Circulating tumor cells: Biology and clinical significance. Signal Transduct. Target. Ther. 2021, 6, 404. [Google Scholar] [CrossRef]
- Zhong, X.; Zhang, H.; Zhu, Y.; Liang, Y.; Yuan, Z.; Li, J.; Li, J.; Li, X.; Jia, Y.; He, T.; et al. Circulating tumor cells in cancer patients: Developments and clinical applications for immunotherapy. Mol. Cancer 2020, 19, 15. [Google Scholar] [CrossRef] [Green Version]
- Millner, L.M.; Linder, M.W.; Valdes, R., Jr. Circulating tumor cells: A review of present methods and the need to identify heterogeneous phenotypes. Ann. Clin. Lab. Sci. 2013, 43, 295–304. [Google Scholar] [PubMed]
- Rushton, A.J.; Nteliopoulos, G.; Shaw, J.A.; Coombes, R.C. A Review of Circulating Tumour Cell Enrichment Technologies. Cancers 2021, 13, 970. [Google Scholar] [CrossRef] [PubMed]
- Kowalik, A.; Kowalewska, M.; Góźdź, S. Current approaches for avoiding the limitations of circulating tumor cells detection methods—Implications for diagnosis and treatment of patients with solid tumors. Transl. Res. 2017, 185, 58–84.e15. [Google Scholar] [CrossRef] [Green Version]
- Fasanya, H.O.; Dopico, P.J.; Yeager, Z.; Fan, Z.H.; Siemann, D.W. Using a combination of gangliosides and cell surface vimentin as surface biomarkers for isolating osteosarcoma cells in microfluidic devices. J. Bone Oncol. 2021, 28, 100357. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pekarek, L.; De la Torre-Escuredo, B.; Fraile-Martinez, O.; García-Montero, C.; Saez, M.A.; Cobo-Prieto, D.; Guijarro, L.G.; Saz, J.V.; De Castro-Martinez, P.; Torres-Carranza, D.; et al. Towards the Search for Potential Biomarkers in Osteosarcoma: State-of-the-Art and Translational Expectations. Int. J. Mol. Sci. 2022, 23, 14939. https://doi.org/10.3390/ijms232314939
Pekarek L, De la Torre-Escuredo B, Fraile-Martinez O, García-Montero C, Saez MA, Cobo-Prieto D, Guijarro LG, Saz JV, De Castro-Martinez P, Torres-Carranza D, et al. Towards the Search for Potential Biomarkers in Osteosarcoma: State-of-the-Art and Translational Expectations. International Journal of Molecular Sciences. 2022; 23(23):14939. https://doi.org/10.3390/ijms232314939
Chicago/Turabian StylePekarek, Leonel, Basilio De la Torre-Escuredo, Oscar Fraile-Martinez, Cielo García-Montero, Miguel A. Saez, David Cobo-Prieto, Luis G. Guijarro, Jose V. Saz, Patricia De Castro-Martinez, Diego Torres-Carranza, and et al. 2022. "Towards the Search for Potential Biomarkers in Osteosarcoma: State-of-the-Art and Translational Expectations" International Journal of Molecular Sciences 23, no. 23: 14939. https://doi.org/10.3390/ijms232314939
APA StylePekarek, L., De la Torre-Escuredo, B., Fraile-Martinez, O., García-Montero, C., Saez, M. A., Cobo-Prieto, D., Guijarro, L. G., Saz, J. V., De Castro-Martinez, P., Torres-Carranza, D., Pekarek, T., Carrera, A. C., Alvarez-Mon, M., & Ortega, M. A. (2022). Towards the Search for Potential Biomarkers in Osteosarcoma: State-of-the-Art and Translational Expectations. International Journal of Molecular Sciences, 23(23), 14939. https://doi.org/10.3390/ijms232314939









