The Role of Skeletal Muscle Mitochondria in Colorectal Cancer Related Cachexia: Friends or Foes?
Abstract
:1. Introduction
2. Underlying Mechanisms of Mitochondrial Dysfunction
2.1. The impact of Systemic Inflammation on Skeletal Muscle Mitochondria in CRC Cachexia
2.2. Mitochondrial Biogenesis, Fusion and Fission in CRC Cachexia
2.3. The role of Proteolytic Systems in Skeletal Muscle Mitochondrial Dysfunction in CRC Cachexia
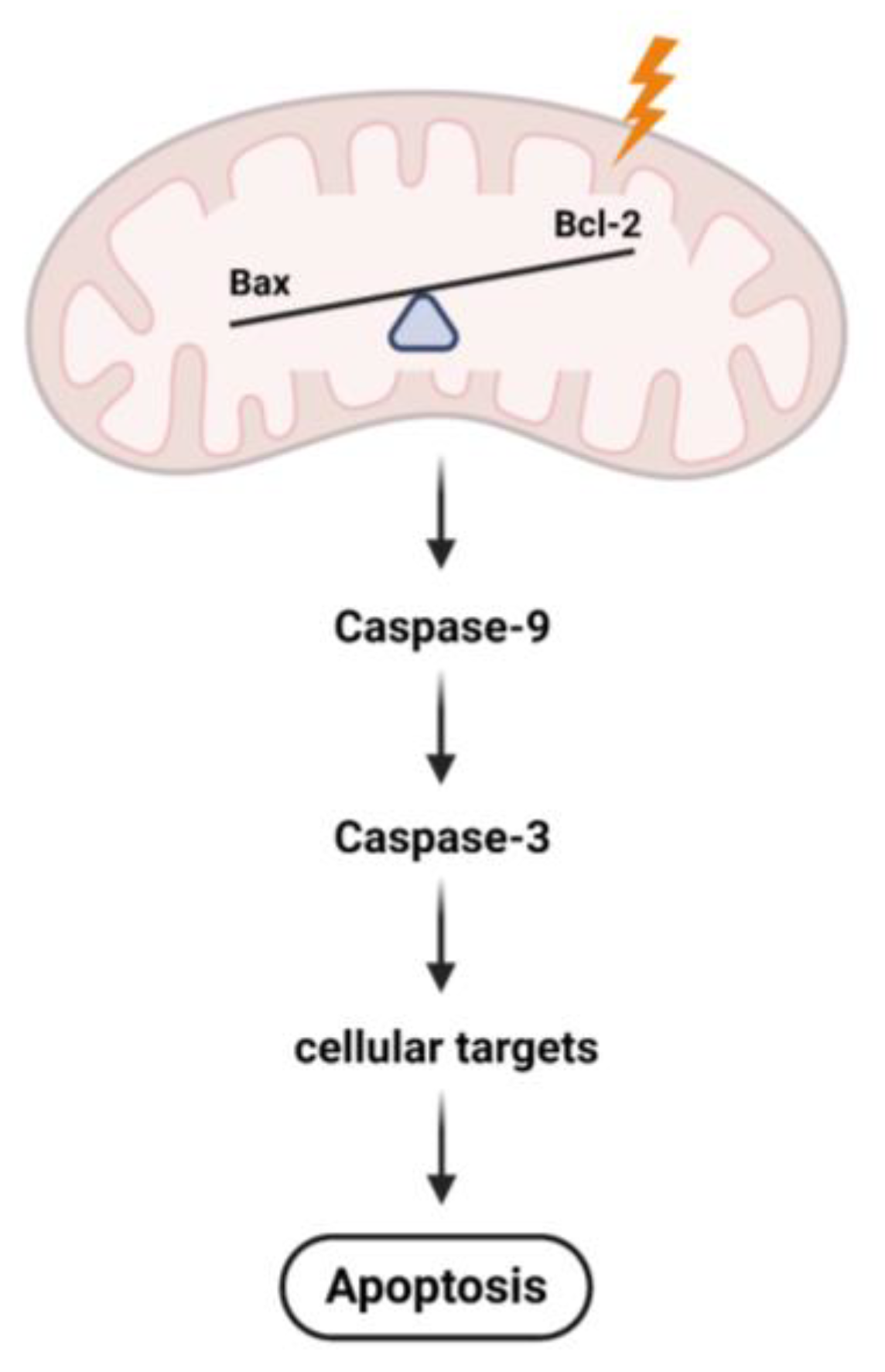
3. Skeletal Muscle Mitochondrial Disruption Leads to Apoptosis in CRC Cachexia
4. The Effect of Exercise on Skeletal Muscle Mitochondrial Function in CRC Cachexia
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mokdad, A.H.; Dwyer-Lindgren, L.; Fitzmaurice, C.; Stubbs, R.W.; Bertozzi-Villa, A.; Morozoff, C.; Charara, R.; Allen, C.; Naghavi, M.; Murray, C.J. Trends and Patterns of Disparities in Cancer Mortality Among US Counties, 1980-2014. JAMA 2017, 317, 388–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Xiong, Y.; Liu, Z.; Hou, S.; Zhou, T. Expression and prognostic significance of CBX2 in colorectal cancer: Database mining for CBX family members in malignancies and vitro analyses. Cancer Cell Int. 2021, 21, 402. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz. Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef]
- Fearon, K.C. Cancer cachexia and fat-muscle physiology. N. Engl. J. Med. 2011, 365, 565–567. [Google Scholar] [CrossRef]
- Vanhoutte, G.; van de Wiel, M.; Wouters, K.; Sels, M.; Bartolomeeussen, L.; De Keersmaecker, S.; Verschueren, C.; De Vroey, V.; De Wilde, A.; Smits, E.; et al. Cachexia in cancer: What is in the definition? BMJ Open Gastroenterol. 2016, 3, e000097. [Google Scholar] [CrossRef]
- Malavaki, C.J.; Sakkas, G.K.; Mitrou, G.I.; Kalyva, A.; Stefanidis, I.; Myburgh, K.H.; Karatzaferi, C. Skeletal muscle atrophy: Disease-induced mechanisms may mask disuse atrophy. J. Muscle Res. Cel.l Motil. 2015, 36, 405–421. [Google Scholar] [CrossRef]
- Donohoe, C.L.; Ryan, A.M.; Reynolds, J.V. Cancer cachexia: Mechanisms and clinical implications. Gastroenterol. Res. Pract. 2011, 2011, 601434. [Google Scholar] [CrossRef] [Green Version]
- Kurk, S.A.; Peeters, P.H.M.; Dorresteijn, B.; de Jong, P.A.; Jourdan, M.; Kuijf, H.J.; Punt, C.J.A.; Koopman, M.; May, A.M. Impact of different palliative systemic treatments on skeletal muscle mass in metastatic colorectal cancer patients. J. Cachexia Sarcopenia Muscle 2018, 9, 909–919. [Google Scholar] [CrossRef]
- Argiles, J.M.; Busquets, S.; Stemmler, B.; Lopez-Soriano, F.J. Cachexia and sarcopenia: Mechanisms and potential targets for intervention. Curr. Opin. Pharmacol. 2015, 22, 100–106. [Google Scholar] [CrossRef]
- Renfro, L.A.; Loupakis, F.; Adams, R.A.; Seymour, M.T.; Heinemann, V.; Schmoll, H.J.; Douillard, J.Y.; Hurwitz, H.; Fuchs, C.S.; Diaz-Rubio, E.; et al. Body Mass Index Is Prognostic in Metastatic Colorectal Cancer: Pooled Analysis of Patients From First-Line Clinical Trials in the ARCAD Database. J. Clin. Oncol. 2016, 34, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Pin, F.; Couch, M.E.; Bonetto, A. Preservation of muscle mass as a strategy to reduce the toxic effects of cancer chemotherapy on body composition. Curr. Opin. Support Palliat. Care 2018, 12, 420–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadeghi, M.; Keshavarz-Fathi, M.; Baracos, V.; Arends, J.; Mahmoudi, M.; Rezaei, N. Cancer cachexia: Diagnosis, assessment, and treatment. Crit. Rev. Oncol. Hematol. 2018, 127, 91–104. [Google Scholar] [CrossRef]
- Brown, J.L.; Lee, D.E.; Rosa-Caldwell, M.E.; Brown, L.A.; Perry, R.A.; Haynie, W.S.; Huseman, K.; Sataranatarajan, K.; Van Remmen, H.; Washington, T.A.; et al. Protein imbalance in the development of skeletal muscle wasting in tumour-bearing mice. J. Cachexia Sarcopenia Muscle 2018, 9, 987–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunz, H.E.; Dorschner, J.M.; Berent, T.E.; Meyer, T.; Wang, X.; Jatoi, A.; Kumar, R.; Lanza, I.R. Methylarginine metabolites are associated with attenuated muscle protein synthesis in cancer-associated muscle wasting. J. Biol. Chem. 2020, 295, 17441–17459. [Google Scholar] [CrossRef]
- Anderson, L.J.; Albrecht, E.D.; Garcia, J.M. Update on Management of Cancer-Related Cachexia. Curr. Oncol. Rep. 2017, 19, 3. [Google Scholar] [CrossRef]
- Solheim, T.S.; Laird, B.J.A.; Balstad, T.R.; Stene, G.B.; Bye, A.; Johns, N.; Pettersen, C.H.; Fallon, M.; Fayers, P.; Fearon, K.; et al. A randomized phase II feasibility trial of a multimodal intervention for the management of cachexia in lung and pancreatic cancer. J. Cachexia Sarcopenia Muscle 2017, 8, 778–788. [Google Scholar] [CrossRef]
- Hardee, J.P.; Counts, B.R.; Carson, J.A. Understanding the Role of Exercise in Cancer Cachexia Therapy. Am. J. Lifestyle Med. 2019, 13, 46–60. [Google Scholar] [CrossRef]
- White, J.P.; Baltgalvis, K.A.; Puppa, M.J.; Sato, S.; Baynes, J.W.; Carson, J.A. Muscle oxidative capacity during IL-6-dependent cancer cachexia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R201–R211. [Google Scholar] [CrossRef] [Green Version]
- Smuder, A.J.; Roberts, B.M.; Wiggs, M.P.; Kwon, O.S.; Yoo, J.K.; Christou, D.D.; Fuller, D.D.; Szeto, H.H.; Judge, A.R. Pharmacological targeting of mitochondrial function and reactive oxygen species production prevents colon 26 cancer-induced cardiorespiratory muscle weakness. Oncotarget 2020, 11, 3502–3514. [Google Scholar] [CrossRef]
- Neyroud, D.; Nosacka, R.L.; Judge, A.R.; Hepple, R.T. Colon 26 adenocarcinoma (C26)-induced cancer cachexia impairs skeletal muscle mitochondrial function and content. J. Muscle Res. Cell. Motil. 2019, 40, 59–65. [Google Scholar] [CrossRef]
- Penna, F.; Ballaro, R.; Martinez-Cristobal, P.; Sala, D.; Sebastian, D.; Busquets, S.; Muscaritoli, M.; Argiles, J.M.; Costelli, P.; Zorzano, A. Autophagy Exacerbates Muscle Wasting in Cancer Cachexia and Impairs Mitochondrial Function. J. Mol. Biol. 2019, 431, 2674–2686. [Google Scholar] [CrossRef]
- White, J.P.; Puppa, M.J.; Sato, S.; Gao, S.; Price, R.L.; Baynes, J.W.; Kostek, M.C.; Matesic, L.E.; Carson, J.A. IL-6 regulation on skeletal muscle mitochondrial remodeling during cancer cachexia in the ApcMin/+ mouse. Skelet. Muscle 2012, 2, 14. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.L.; Rosa-Caldwell, M.E.; Lee, D.E.; Blackwell, T.A.; Brown, L.A.; Perry, R.A.; Haynie, W.S.; Hardee, J.P.; Carson, J.A.; Wiggs, M.P.; et al. Mitochondrial degeneration precedes the development of muscle atrophy in progression of cancer cachexia in tumour-bearing mice. J. Cachexia Sarcopenia Muscle 2017, 8, 926–938. [Google Scholar] [CrossRef] [Green Version]
- Antunes, D.; Padrao, A.I.; Maciel, E.; Santinha, D.; Oliveira, P.; Vitorino, R.; Moreira-Goncalves, D.; Colaco, B.; Pires, M.J.; Nunes, C.; et al. Molecular insights into mitochondrial dysfunction in cancer-related muscle wasting. Biochim. Biophys. Acta 2014, 1841, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Shum, A.M.; Mahendradatta, T.; Taylor, R.J.; Painter, A.B.; Moore, M.M.; Tsoli, M.; Tan, T.C.; Clarke, S.J.; Robertson, G.R.; Polly, P. Disruption of MEF2C signaling and loss of sarcomeric and mitochondrial integrity in cancer-induced skeletal muscle wasting. Aging 2012, 4, 133–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballaro, R.; Beltra, M.; De Lucia, S.; Pin, F.; Ranjbar, K.; Hulmi, J.J.; Costelli, P.; Penna, F. Moderate exercise in mice improves cancer plus chemotherapy-induced muscle wasting and mitochondrial alterations. FASEB J. 2019, 33, 5482–5494. [Google Scholar] [CrossRef] [PubMed]
- Bonetto, A.; Rupert, J.E.; Barreto, R.; Zimmers, T.A. The Colon-26 Carcinoma Tumor-bearing Mouse as a Model for the Study of Cancer Cachexia. J. Vis. Exp. 2016. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.; Sui, H.; Fang, F.; Li, Q.; Li, B. The application of Apc(Min/+) mouse model in colorectal tumor researches. J. Cancer Res. Clin. Oncol. 2019, 145, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Puppa, M.J.; White, J.P.; Sato, S.; Cairns, M.; Baynes, J.W.; Carson, J.A. Gut barrier dysfunction in the Apc(Min/+) mouse model of colon cancer cachexia. Biochim. Biophys. Acta 2011, 1812, 1601–1606. [Google Scholar] [CrossRef]
- Kitaoka, Y.; Miyazaki, M.; Kikuchi, S. Voluntary exercise prevents abnormal muscle mitochondrial morphology in cancer cachexia mice. Physiol. Rep. 2021, 9, e15016. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.H.; Ross, J.A.; Kaasa, S.; Skorpen, F.; Fearon, K.C.; European Palliative Care Research, C. Identification of possible genetic polymorphisms involved in cancer cachexia: A systematic review. J. Genet 2011, 90, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Bentzinger, C.F.; Wang, Y.X.; Dumont, N.A.; Rudnicki, M.A. Cellular dynamics in the muscle satellite cell niche. EMBO Rep. 2013, 14, 1062–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perandini, L.A.; Chimin, P.; Lutkemeyer, D.D.S.; Camara, N.O.S. Chronic inflammation in skeletal muscle impairs satellite cells function during regeneration: Can physical exercise restore the satellite cell niche? FEBS J. 2018, 285, 1973–1984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonetto, A.; Penna, F.; Muscaritoli, M.; Minero, V.G.; Rossi Fanelli, F.; Baccino, F.M.; Costelli, P. Are antioxidants useful for treating skeletal muscle atrophy? Free Radic. Biol. Med. 2009, 47, 906–916. [Google Scholar] [CrossRef]
- Gomez-Cabrera, M.C.; Vina, J.; Ji, L.L. Interplay of oxidants and antioxidants during exercise: Implications for muscle health. Phys. Sportsmed. 2009, 37, 116–123. [Google Scholar] [CrossRef]
- Huot, J.R.; Novinger, L.J.; Pin, F.; Bonetto, A. HCT116 colorectal liver metastases exacerbate muscle wasting in a mouse model for the study of colorectal cancer cachexia. Dis. Model. Mech. 2020, 13. [Google Scholar] [CrossRef] [Green Version]
- Laine, A.; Iyengar, P.; Pandita, T.K. The role of inflammatory pathways in cancer-associated cachexia and radiation resistance. Mol. Cancer Res. 2013, 11, 967–972. [Google Scholar] [CrossRef] [Green Version]
- Bonetto, A.; Aydogdu, T.; Jin, X.; Zhang, Z.; Zhan, R.; Puzis, L.; Koniaris, L.G.; Zimmers, T.A. JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL-6 and in experimental cancer cachexia. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E410–E421. [Google Scholar] [CrossRef] [Green Version]
- Fischer, P.; Hilfiker-Kleiner, D. Survival pathways in hypertrophy and heart failure: The gp130-STAT axis. Basic Res. Cardiol. 2007, 102, 393–411. [Google Scholar] [CrossRef]
- Eskiler, G.G.; Bezdegumeli, E.; Ozman, Z.; Ozkan, A.D.; Bilir, C.; Kucukakca, B.N.; Ince, M.N.; Men, A.Y.; Aktas, O.; Horoz, Y.E.; et al. IL-6 mediated JAK/STAT3 signaling pathway in cancer patients with cachexia. Bratisl. Lek. Listy 2019, 66, 819–826. [Google Scholar] [CrossRef] [Green Version]
- Hulmi, J.J.; Penna, F.; Pollanen, N.; Nissinen, T.A.; Hentila, J.; Euro, L.; Lautaoja, J.H.; Ballaro, R.; Soliymani, R.; Baumann, M.; et al. Muscle NAD(+) depletion and Serpina3n as molecular determinants of murine cancer cachexia-the effects of blocking myostatin and activins. Mol. Metab. 2020, 41, 101046. [Google Scholar] [CrossRef] [PubMed]
- Asl, E.R.; Amini, M.; Najafi, S.; Mansoori, B.; Mokhtarzadeh, A.; Mohammadi, A.; Lotfinejad, P.; Bagheri, M.; Shirjang, S.; Lotfi, Z.; et al. Interplay between MAPK/ERK signaling pathway and MicroRNAs: A crucial mechanism regulating cancer cell metabolism and tumor progression. Life Sci. 2021, 278, 119499. [Google Scholar] [CrossRef] [PubMed]
- Au, E.D.; Desai, A.P.; Koniaris, L.G.; Zimmers, T.A. The MEK-Inhibitor Selumetinib Attenuates Tumor Growth and Reduces IL-6 Expression but Does Not Protect against Muscle Wasting in Lewis Lung Cancer Cachexia. Front. Physiol. 2016, 7, 682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glass, D.J. Signaling pathways perturbing muscle mass. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, T.L.; Martignoni, M.E.; Bachmann, J.; Fechtner, K.; Friess, H.; Kinscherf, R.; Hildebrandt, W. Activity of the Akt-dependent anabolic and catabolic pathways in muscle and liver samples in cancer-related cachexia. J. Mol. Med. 2007, 85, 647–654. [Google Scholar] [CrossRef]
- Rao, V.K.; Das, D.; Taneja, R. Cancer Cachexia: Signaling and Transcriptional Regulation of Muscle Catabolic Genes. Cancers 2022, 14, 4258. [Google Scholar] [CrossRef]
- Halle, J.L.; Pena, G.S.; Paez, H.G.; Castro, A.J.; Rossiter, H.B.; Visavadiya, N.P.; Whitehurst, M.A.; Khamoui, A.V. Tissue-specific dysregulation of mitochondrial respiratory capacity and coupling control in colon-26 tumor-induced cachexia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 317, R68–R82. [Google Scholar] [CrossRef]
- Pin, F.; Beltra, M.; Garcia-Castillo, L.; Pardini, B.; Birolo, G.; Matullo, G.; Penna, F.; Guttridge, D.; Costelli, P. Extracellular vesicles derived from tumour cells as a trigger of energy crisis in the skeletal muscle. J. Cachexia Sarcopenia Muscle 2022, 13, 481–494. [Google Scholar] [CrossRef]
- Hentila, J.; Nissinen, T.A.; Korkmaz, A.; Lensu, S.; Silvennoinen, M.; Pasternack, A.; Ritvos, O.; Atalay, M.; Hulmi, J.J. Activin Receptor Ligand Blocking and Cancer Have Distinct Effects on Protein and Redox Homeostasis in Skeletal Muscle and Liver. Front. Physiol. 2018, 9, 1917. [Google Scholar] [CrossRef]
- Boulton, D.P.; Caino, M.C. Mitochondrial Fission and Fusion in Tumor Progression to Metastasis. Front. Cell Dev. Biol. 2022, 10, 849962. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Gu, Y.; Sui, X.; Shen, L.; Han, J.; Wang, H.; Xi, Q.; Zhuang, Q.; Meng, Q.; Wu, G. Phosphorylation of Dynamin-Related Protein 1 (DRP1) Regulates Mitochondrial Dynamics and Skeletal Muscle Wasting in Cancer Cachexia. Front. Cell Dev. Biol. 2021, 9, 673618. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Cho, J.; Song, E.J. Ubiquitin-proteasome system (UPS) as a target for anticancer treatment. Arch. Pharm. Res. 2020, 43, 1144–1161. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jeon, J.H.; Lee, M.J. Docosahexaenoic Acid, a Potential Treatment for Sarcopenia, Modulates the Ubiquitin-Proteasome and the Autophagy-Lysosome Systems. Nutrients 2020, 12, 2597. [Google Scholar] [CrossRef] [PubMed]
- Tipton, K.D.; Hamilton, D.L.; Gallagher, I.J. Assessing the Role of Muscle Protein Breakdown in Response to Nutrition and Exercise in Humans. Sports Med. 2018, 48, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.; Zhao, L.; Chen, S.; Li, X. Inhibition of mitochondrial and cytosolic calpain attenuates atrophy in myotubes co-cultured with colon carcinoma cells. Oncol. Lett. 2021, 21, 124. [Google Scholar] [CrossRef]
- Zeng, X.; Chen, S.; Lin, Y.; Ke, Z. Acylated and unacylated ghrelin inhibit apoptosis in myoblasts cocultured with colon carcinoma cells. Oncol. Rep. 2018, 39, 1387–1395. [Google Scholar] [CrossRef] [Green Version]
- Miao, C.; Zhang, W.; Feng, L.; Gu, X.; Shen, Q.; Lu, S.; Fan, M.; Li, Y.; Guo, X.; Ma, Y.; et al. Cancer-derived exosome miRNAs induce skeletal muscle wasting by Bcl-2-mediated apoptosis in colon cancer cachexia. Mol. Ther. Nucleic Acids 2021, 24, 923–938. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, W.; Gu, X.; Miao, C.; Feng, L.; Shen, Q.; Liu, X.; Zhang, X. GDF-15 in tumor-derived exosomes promotes muscle atrophy via Bcl-2/caspase-3 pathway. Cell Death Discov. 2022, 8, 162. [Google Scholar] [CrossRef]
- Molinari, F.; Pin, F.; Gorini, S.; Chiandotto, S.; Pontecorvo, L.; Penna, F.; Rizzuto, E.; Pisu, S.; Musaro, A.; Costelli, P.; et al. The mitochondrial metabolic reprogramming agent trimetazidine as an ’exercise mimetic’ in cachectic C26-bearing mice. J. Cachexia Sarcopenia Muscle 2017, 8, 954–973. [Google Scholar] [CrossRef]
- Guillory, B.; Splenser, A.; Garcia, J. The role of ghrelin in anorexia-cachexia syndromes. Vitam. Horm. 2013, 92, 61–106. [Google Scholar] [CrossRef] [PubMed]
- Sorriento, D.; Di Vaia, E.; Iaccarino, G. Physical Exercise: A Novel Tool to Protect Mitochondrial Health. Front. Physiol. 2021, 12, 660068. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.W.; Hornsby, W.E.; Goetzinger, A.; Forbes, L.M.; Sherrard, E.L.; Quist, M.; Lane, A.T.; West, M.; Eves, N.D.; Gradison, M.; et al. Prognostic significance of functional capacity and exercise behavior in patients with metastatic non-small cell lung cancer. Lung Cancer 2012, 76, 248–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerritsen, J.K.; Vincent, A.J. Exercise improves quality of life in patients with cancer: A systematic review and meta-analysis of randomised controlled trials. Br. J. Sports Med. 2016, 50, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Lundby, C.; Jacobs, R.A. Adaptations of skeletal muscle mitochondria to exercise training. Exp. Physiol. 2016, 101, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Rockl, K.S.; Hirshman, M.F.; Brandauer, J.; Fujii, N.; Witters, L.A.; Goodyear, L.J. Skeletal muscle adaptation to exercise training: AMP-activated protein kinase mediates muscle fiber type shift. Diabetes 2007, 56, 2062–2069. [Google Scholar] [CrossRef] [Green Version]
- Ranjbar, K.; Ballaro, R.; Bover, Q.; Pin, F.; Beltra, M.; Penna, F.; Costelli, P. Combined Exercise Training Positively Affects Muscle Wasting in Tumor-Bearing Mice. Med. Sci. Sports Exerc. 2019, 51, 1387–1395. [Google Scholar] [CrossRef]
- Ballaro, R.; Penna, F.; Pin, F.; Gomez-Cabrera, M.C.; Vina, J.; Costelli, P. Moderate Exercise Improves Experimental Cancer Cachexia by Modulating the Redox Homeostasis. Cancers 2019, 11, 285. [Google Scholar] [CrossRef] [Green Version]
- Lamon, S.; Russell, A.P. The role and regulation of erythropoietin (EPO) and its receptor in skeletal muscle: How much do we really know? Front. Physiol. 2013, 4, 176. [Google Scholar] [CrossRef] [Green Version]
- Pin, F.; Busquets, S.; Toledo, M.; Camperi, A.; Lopez-Soriano, F.J.; Costelli, P.; Argiles, J.M.; Penna, F. Combination of exercise training and erythropoietin prevents cancer-induced muscle alterations. Oncotarget 2015, 6, 43202–43215. [Google Scholar] [CrossRef]
- McLean, J.B.; Moylan, J.S.; Andrade, F.H. Mitochondria dysfunction in lung cancer-induced muscle wasting in C2C12 myotubes. Front. Physiol. 2014, 5, 503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunz, H.E.; Port, J.D.; Kaufman, K.R.; Jatoi, A.; Hart, C.R.; Gries, K.J.; Lanza, I.R.; Kumar, R. Skeletal muscle mitochondrial dysfunction and muscle and whole body functional deficits in cancer patients with weight loss. J. Appl. Physiol. 2022, 132, 388–401. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van de Haterd, B.; Verboven, K.; Vandenabeele, F.; Agten, A. The Role of Skeletal Muscle Mitochondria in Colorectal Cancer Related Cachexia: Friends or Foes? Int. J. Mol. Sci. 2022, 23, 14833. https://doi.org/10.3390/ijms232314833
van de Haterd B, Verboven K, Vandenabeele F, Agten A. The Role of Skeletal Muscle Mitochondria in Colorectal Cancer Related Cachexia: Friends or Foes? International Journal of Molecular Sciences. 2022; 23(23):14833. https://doi.org/10.3390/ijms232314833
Chicago/Turabian Stylevan de Haterd, Britt, Kenneth Verboven, Frank Vandenabeele, and Anouk Agten. 2022. "The Role of Skeletal Muscle Mitochondria in Colorectal Cancer Related Cachexia: Friends or Foes?" International Journal of Molecular Sciences 23, no. 23: 14833. https://doi.org/10.3390/ijms232314833
APA Stylevan de Haterd, B., Verboven, K., Vandenabeele, F., & Agten, A. (2022). The Role of Skeletal Muscle Mitochondria in Colorectal Cancer Related Cachexia: Friends or Foes? International Journal of Molecular Sciences, 23(23), 14833. https://doi.org/10.3390/ijms232314833






