Uptake and Metabolization of Serotonin by Granulosa Cells Form a Functional Barrier in the Mouse Ovary
Abstract
1. Introduction
2. Results
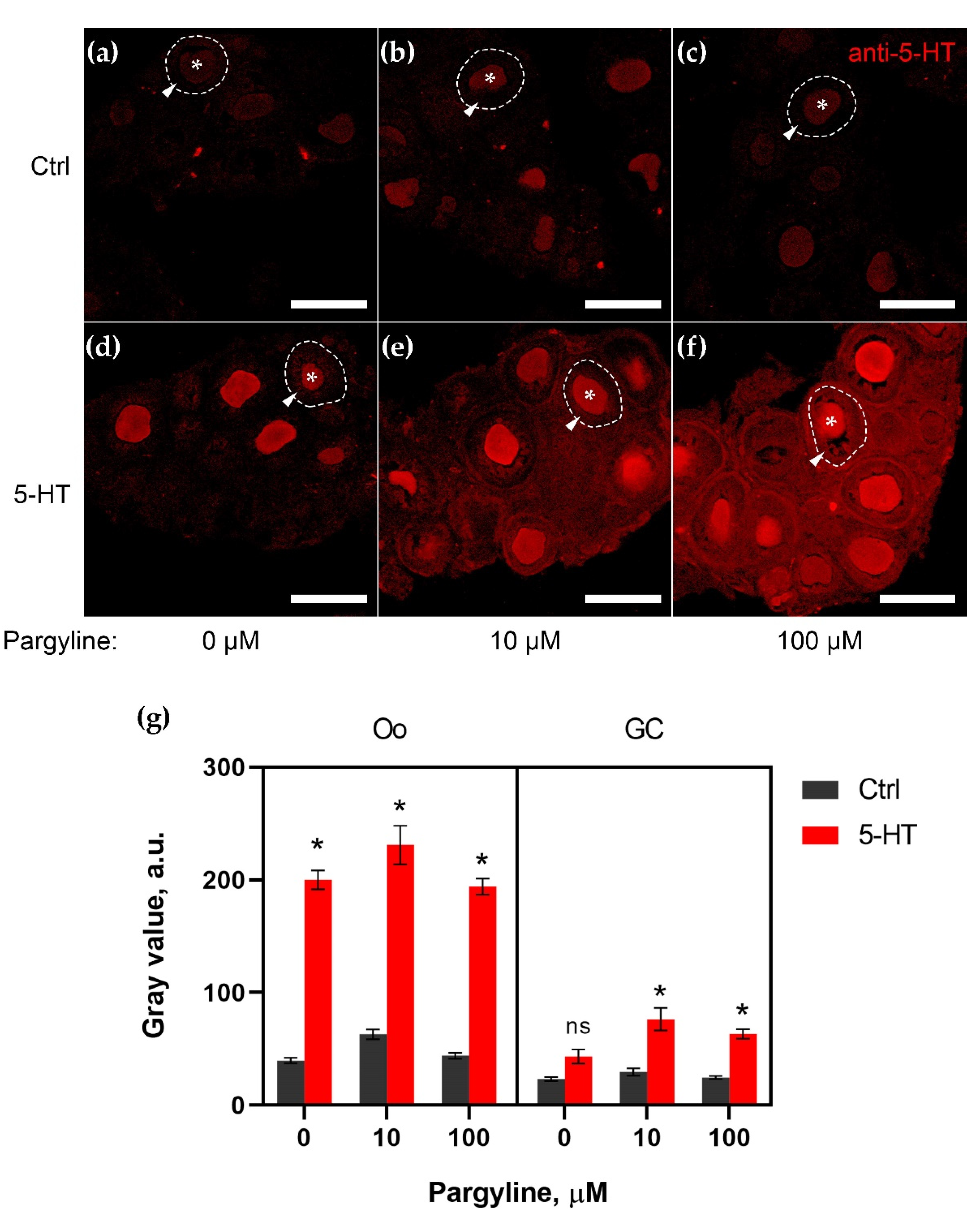
2.1. MAO Shows Its Activity in Granulosa Cells of Growing Follicles
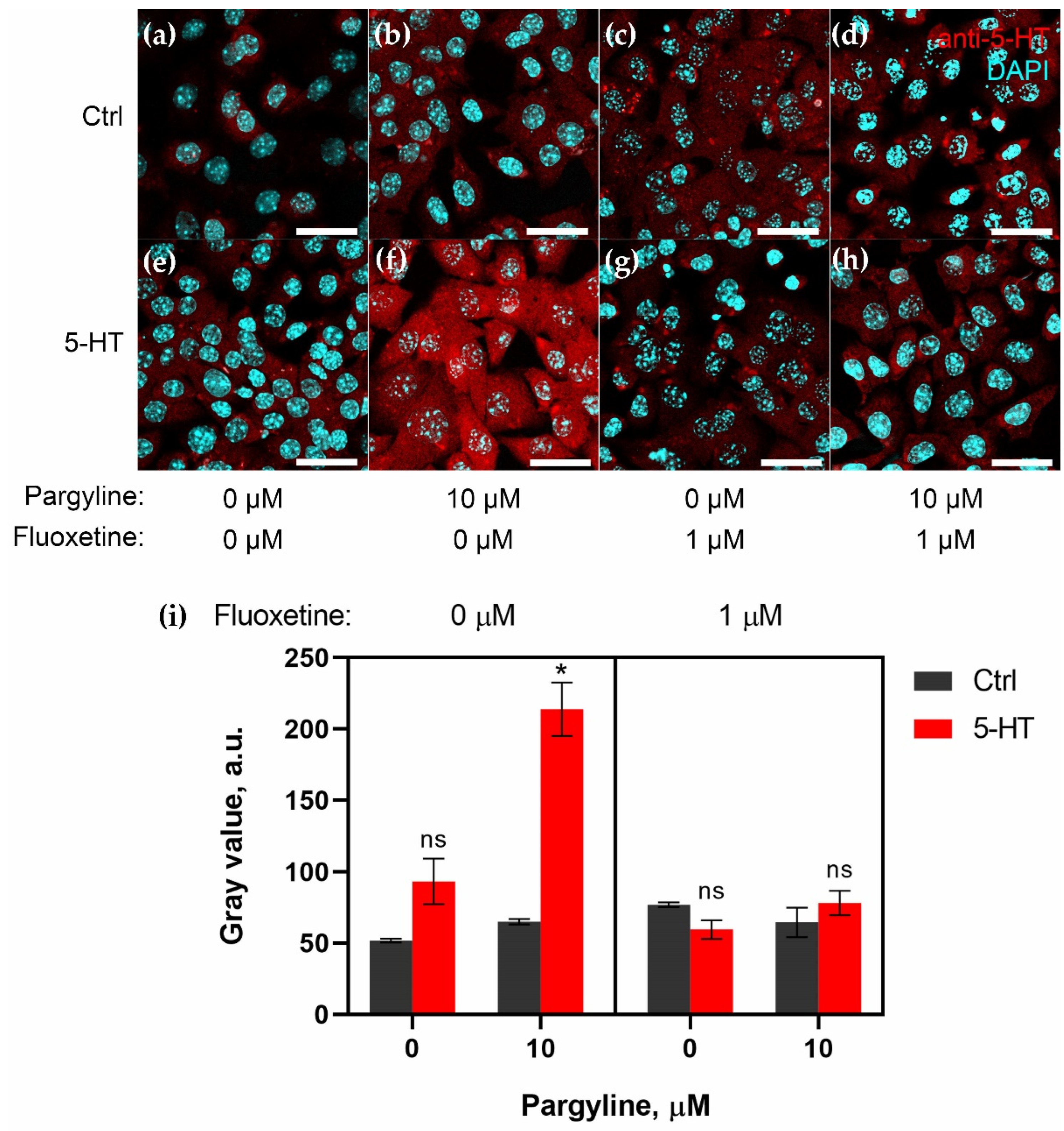
2.2. Granulosa Cells Uptake 5-HT via SERT Activity
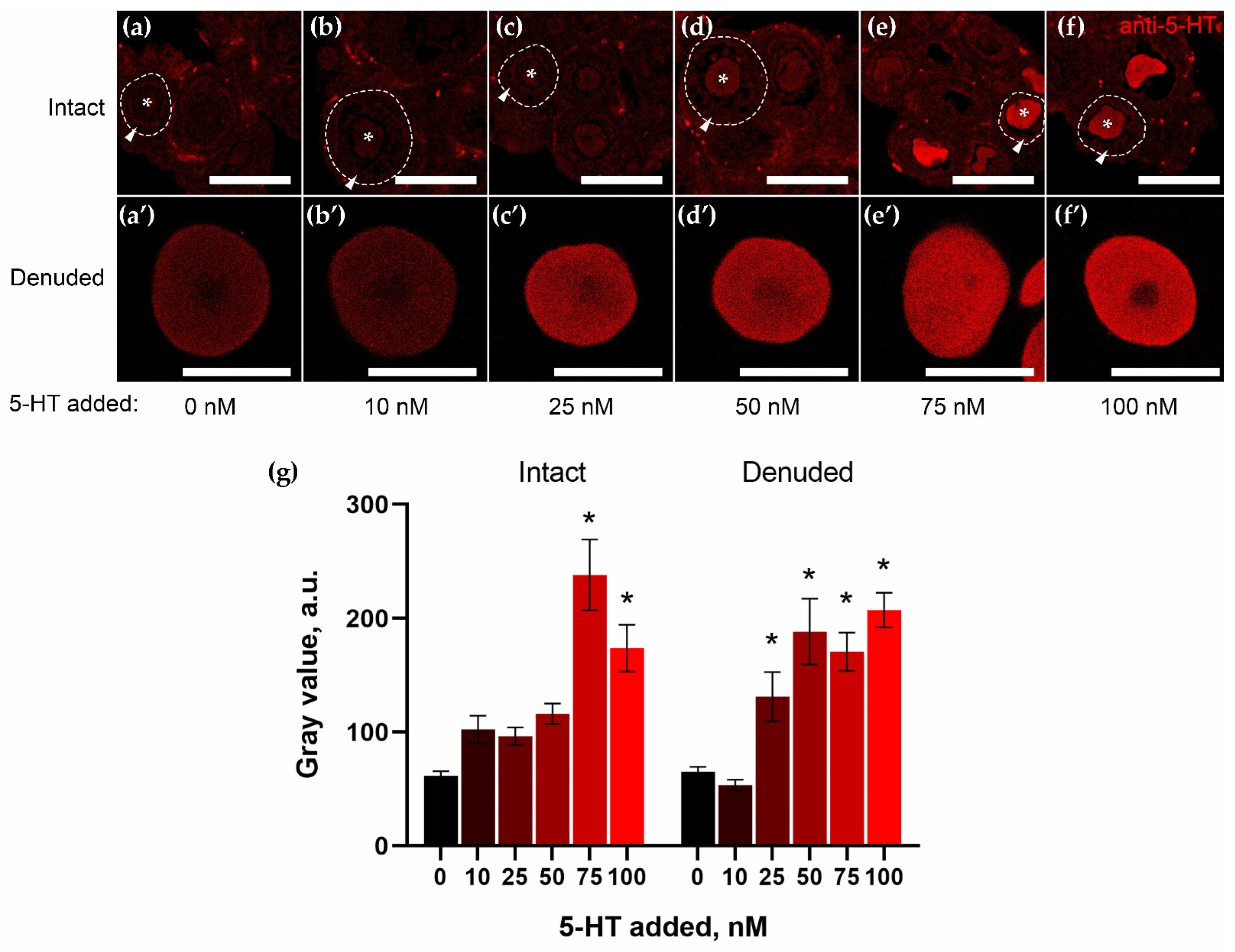
2.3. Granulosa Cells of Growing Follicles Act as a Functional Barrier for 5-HT
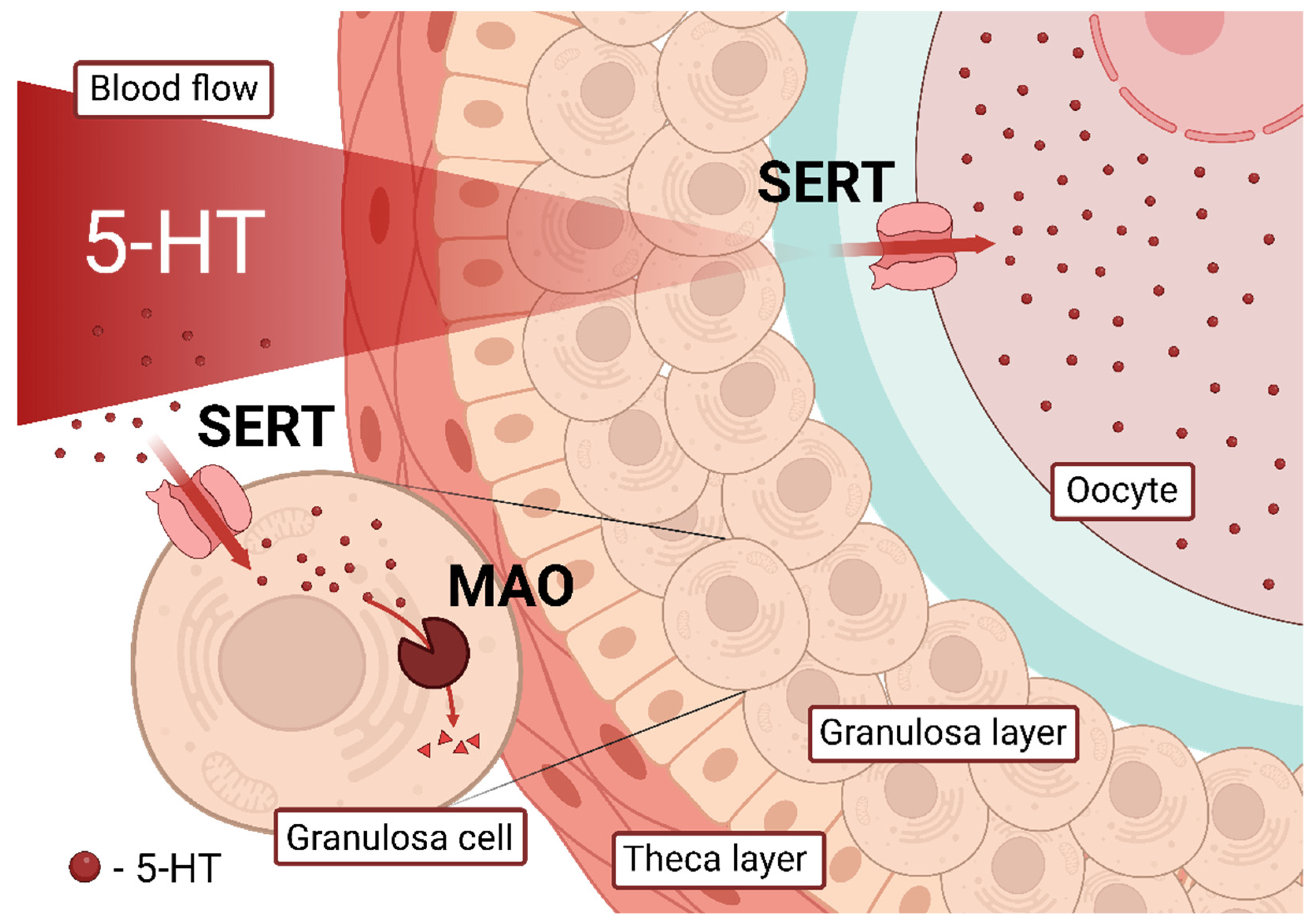
3. Discussion
4. Materials and Methods
4.1. Experimental Animals and Chemicals
4.2. Ovary Fragments and Denuded Oocytes Incubation Experiments
4.3. Granulosa Cells Incubation Experiments
4.4. Immunohistochemistry
4.5. Image Analysis and Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Merahbi, R.; Löffler, M.; Mayer, A.; Sumara, G. The roles of peripheral serotonin in metabolic homeostasis. FEBS Lett. 2015, 589, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Spohn, S.N.; Mawe, G.M. Non-conventional features of peripheral serotonin signalling—The gut and beyond. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Wang, L.; Liu, X.S.J.S.; Montplaisir, V.; Tiberi, M.; Baltz, J.M.; Liu, X.S.J.S. A serotonin receptor antagonist induces oocyte maturation in both frogs and mice: Evidence that the same G protein ptor is responsible for maintaining meiosis arrest in both species. J. Cell. Physiol. 2005, 202, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Dubé, F.; Amireault, P. Local serotonergic signaling in mammalian follicles, oocytes and early embryos. Life Sci. 2007, 81, 1627–1637. [Google Scholar] [CrossRef]
- Shmukler, Y.B.; Nikishin, D.A. Non-Neuronal Transmitter Systems in Bacteria, Non-Nervous Eukaryotes, and Invertebrate Embryos. Biomolecules 2022, 12, 271. [Google Scholar] [CrossRef]
- Perez, V.; Bel, N.; Celada, P.; Ortiz, J.; Alvarez, E.; Artigas, F. Relationship Between Blood Serotonergic Variables, Melancholic Traits, and Response to Antidepressant Treatments. J. Clin. Psychopharmacol. 1998, 18, 222–230. [Google Scholar] [CrossRef]
- Nikishin, D.A.; Khramova, Y.V.; Bagayeva, T.S.; Semenova, M.L.; Shmukler, Y.B. Expression of Components of the Serotonergic System in Folliculogenesis and Preimplantation Development in Mice. Russ. J. Dev. Biol. 2018, 49, 184–192. [Google Scholar] [CrossRef]
- Nikishin, D.A.; Alyoshina, N.M.; Semenova, M.L.; Shmukler, Y.B. Analysis of Expression and Functional Activity of Aromatic L-Amino Acid Decarboxylase (DDC) and Serotonin Transporter (SERT) as Potential Sources of Serotonin in Mouse Ovary. Int. J. Mol. Sci. 2019, 20, 3070. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Georgieva, M.G.; Atanasov, A.G.; Tzvetkov, N.T. Monoamine Oxidases (MAOs) as Privileged Molecular Targets in Neuroscience: Research Literature Analysis. Front. Mol. Neurosci. 2019, 12, 143. [Google Scholar] [CrossRef]
- Chen, K.; Shih, J.C. Monoamine oxidase A and B: Structure, function, and behavior. Adv. Pharmacol. 1998, 42, 292–296. [Google Scholar] [CrossRef]
- Pickar, D.; Murphy, D.L.; Cohen, R.M.; Campbell, I.C.; Lipper, S. Selective and nonselective monoamine oxidase inhibitors: Behavioral disturbances during their administration to depressed patients. Arch. Gen. Psychiatry 1982, 39, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.L.; Renner, K.J.; Luine, V.N. Pargyline-induced increase in serotonin levels: Correlation with inhibition of lordosis in rats. Pharmacol. Biochem. Behav. 1993, 45, 837–841. [Google Scholar] [CrossRef] [PubMed]
- Cecconi, S.; Ciccarelli, C.; Barberi, M.; Macchiarelli, G.; Canipari, R. Granulosa cell-oocyte interactions. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 115, S19–S22. [Google Scholar] [CrossRef] [PubMed]
- Čikoš, Š.; Fabian, D.D.; Makarevich, A.V.; Chrenek, P.; Koppel, J.; Cikos, S.; Fabian, D.D.; Makarevich, A.V.; Chrenek, P.; Koppel, J. Biogenic monoamines in preimplantation development. Hum. Reprod. 2011, 26, 2296–2305. [Google Scholar] [CrossRef]
- Terranova, P.F.; Uilenbroek, J.T.; Saville, L.; Horst, D.; Nakamura, Y. Serotonin enhances oestradiol production by hamster preovulatory follicles in vitro: Effects of experimentally induced atresia. J. Endocrinol. 1990, 125, 433–438. [Google Scholar] [CrossRef]
- Buznikov, G.A.; Nikitina, L.A.; Galanov AYu; Malchenko, L.A.; Trubnikova, O.B. The control of oocyte maturation in the starfish and amphibians by serotonin and its antagonists. Int. J. Dev. Biol. 1993, 37, 363–364. [Google Scholar]
- Tanaka, E.; Baba, N.; Toshida, K.; Suzuki, K. Serotonin stimulates steroidogenesis in rat preovulatory follicles: Involvement of 5-HT2 receptor. Life Sci. 1993, 53, 563–570. [Google Scholar] [CrossRef]
- Cerdà, J.; Subhedar, N.; Reich, G.; Wallace, R.A.; Selman, K. Oocyte sensitivity to serotonergic regulation during the follicular cycle of the teleost Fundulus heteroclitus. Biol. Reprod. 1998, 59, 53–61. [Google Scholar] [CrossRef]
- Zatylny, C.; Durantou, F.; Boucaud-Camou, E.; Henry, J. Evidence of 5-hydroxytryptamine synthesis in the follicles of Sepia officinalis and direct involvement in the control of egg-laying. Mol. Reprod. Dev. 2000, 55, 182–188. [Google Scholar] [CrossRef]
- Tinikul, Y.; Joffre Mercier, A.; Soonklang, N.; Sobhon, P. Changes in the levels of serotonin and dopamine in the central nervous system and ovary, and their possible roles in the ovarian development in the giant freshwater prawn, Macrobrachium rosenbergii. Gen. Comp. Endocrinol. 2008, 158, 250–258. [Google Scholar] [CrossRef]
- Stricker, S.A.; Smythe, T.L. 5-HT causes an increase in cAMP that stimulates, rather than inhibits, oocyte maturation in marine nemertean worms. Development 2001, 128, 1415–1427. [Google Scholar] [CrossRef]
- Lister, A.; Regan, C.; Van Zwol, J.; Van Der Kraak, G. Inhibition of egg production in zebrafish by fluoxetine and municipal effluents: A mechanistic evaluation. Aquat. Toxicol. 2009, 95, 320–329. [Google Scholar] [CrossRef]
- Wang, Q.; He, M. Molecular characterization and analysis of a putative 5-HT receptor involved in reproduction process of the pearl oyster Pinctada fucata. Gen. Comp. Endocrinol. 2014, 204, 71–79. [Google Scholar] [CrossRef]
- Amireault, P.; Dubé, F. Serotonin and its antidepressant-sensitive transport in mouse cumulus-oocyte complexes and early embryos. Biol. Reprod. 2005, 73, 358–365. [Google Scholar] [CrossRef]
- Bòdis, J.; Bognàr, Z.; Hartmann, G.; Török, A.; Csaba, I.F. Measurement of noradrenaline, dopamine and serotonin contents in follicular fluid of human graafian follicles after superovulation treatment. Gynecol. Obstet. Investig. 1992, 33, 165–167. [Google Scholar] [CrossRef]
- Graveleau, C.; Paust, H.J.; Schmidt-Grimminger, D.; Mukhopadhyay, A.K. Presence of a 5-HT7 receptor positively coupled to adenylate cyclase activation in human granulosa-lutein cells. J. Clin. Endocrinol. Metab. 2000, 85, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Koppan, M.; Bodis, J.; Verzar, Z.; Tinneberg, H.-R.; Torok, A. Serotonin may alter the pattern of gonadotropin-induced progesterone release of human granulosa cells in superfusion system. Endocrine 2004, 24, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.D.; Klett, D.; Combarnous, Y. Fluoxetine affects cytosolic cAMP, ATP, Ca 2+ responses to forskolin, and survival of human ovarian granulosa tumor COV434 cells. Korean J. Physiol. Pharmacol. 2021, 25, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Romero-Reyes, J.; Cárdenas, M.; Damián-Matsumura, P.; Domínguez, R.; Ayala, M.E. Inhibition of serotonin reuptake in the prepubertal rat ovary by fluoxetine and effects on ovarian functions. Reprod. Toxicol. 2016, 59, 80–88. [Google Scholar] [CrossRef]
- Ulker, N.; Yardimci, A.; Kaya Tektemur, N.; Colakoglu, N.; Ozcan, M.; Canpolat, S.; Kelestimur, H. Chronic exposure to paroxetine or bupropion modulates the pubertal maturation and the reproductive system in female rats. Reprod. Biol. 2020, 20, 154–163. [Google Scholar] [CrossRef]
- Moore, C.J.; DeLong, N.E.; Chan, K.A.; Holloway, A.C.; Petrik, J.J.; Sloboda, D.M. Perinatal Administration of a Selective Serotonin Reuptake Inhibitor Induces Impairments in Reproductive Function and Follicular Dynamics in Female Rat Offspring. Reprod. Sci. 2015, 22, 1297–1311. [Google Scholar] [CrossRef]
- Zha, W.; Ho, H.T.B.; Hu, T.; Hebert, M.F.; Wang, J. Serotonin transporter deficiency drives estrogen-dependent obesity and glucose intolerance. Sci. Rep. 2017, 7, 1137. [Google Scholar] [CrossRef]
- Zha, W.; Hu, T.; Hebert, M.F.; Wang, J. Effect of Pregnancy on Paroxetine-Induced Adiposity and Glucose Intolerance in Mice. J. Pharmacol. Exp. Ther. 2019, 371, 113–120. [Google Scholar] [CrossRef]
- Rojas, J.; Chávez, M.; Olivar, L.; Rojas, M.; Morillo, J.; Mejías, J.; Calvo, M.; Bermúdez, V. Polycystic ovary syndrome, insulin resistance, and obesity: Navigating the pathophysiologic labyrinth. Int. J. Reprod. Med. 2014, 2014, 719050. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, Z.; Qin, Q.; Nisenblat, V.; Chang, H.M.; Yu, Y.; Wang, T.; Lu, C.; Yang, M.; Yang, S.; et al. Transcriptome Landscape of Human Folliculogenesis Reveals Oocyte and Granulosa Cell Interactions. Mol. Cell 2018, 72, 1021–1034. [Google Scholar] [CrossRef]
- Holzbauer, M.; Youdim, M.B. The oestrous cycle and monoamine oxidase activity. Br. J. Pharmacol. 1973, 48, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Mihalik, J.; Maslankova, J.; Hodorova, I.; Ferenc, P.; Rybarova, S.; Marekova, M. Relationship between serotonin and norepinephrine levels and the preimplantation embryo development in rat. Bratisl. Lek. Listy 2011, 112, 52–57. [Google Scholar] [PubMed]
- Cawthon, R.M.; Breakefield, X.O. Differences in A and B forms of monoamine oxidase revealed by limited proteolysis and peptide mapping. Nature 1979, 281, 692–694. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.L.; Karoum, F.; Pickar, D.; Cohen, R.M.; Lipper, S.; Mellow, A.M.; Tariot, P.N.; Sunderland, T. Differential trace amine alterations in individuals receiving acetylenic inhibitors of MAO-A (clorgyline) or MAO-B (selegiline and pargyline). J. Neural Transm. Suppl. 1998, 52, 39–48. [Google Scholar] [CrossRef]
- Siu, M.K.Y.; Cheng, C.Y. The blood-follicle barrier (BFB) in disease and in ovarian function. Adv. Exp. Med. Biol. 2012, 763, 186–192. [Google Scholar] [CrossRef]
- Cran, D.G.; Moor, R.M.; Hay, M.F. Permeability of ovarian follicles to electron-dense macromolecules. Acta Endocrinol. (Copenh) 1976, 82, 631–636. [Google Scholar] [CrossRef]
- Hess, K.A.; Chen, L.; Larsen, W.J. The ovarian blood follicle barrier is both charge- and size-selective in mice. Biol. Reprod. 1998, 58, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Kéreveur, A.; Callebert, J.; Humbert, M.; Hervé, P.; Simonneau, G.; Launay, J.M.; Drouet, L. High plasma serotonin levels in primary pulmonary hypertension. Effect of long-term epoprostenol (prostacyclin) therapy. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2233–2239. [Google Scholar] [CrossRef] [PubMed]
- Duffy, D.M.; Ko, C.; Jo, M.; Brannstrom, M.; Curry, T.E. Ovulation: Parallels With Inflammatory Processes. Endocr. Rev. 2019, 40, 369–416. [Google Scholar] [CrossRef]
- Amenta, F.; Vega, J.A.; Ricci, A.; Collier, W.L. Localization of 5-hydroxytryptamine-like immunoreactive cells and nerve fibers in the rat female reproductive system. Anat. Rec. 1992, 233, 478–484. [Google Scholar] [CrossRef]
- Sèdes, L.; Leclerc, A.; Moindjie, H.; Cate, R.L.; Picard, J.Y.; Clemente, N.D.; Jamin, S.P. Anti-Müllerian Hormone Recruits BMPR-IA in Immature Granulosa Cells. PLoS ONE 2013, 8, 81551. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alyoshina, N.M.; Tkachenko, M.D.; Malchenko, L.A.; Shmukler, Y.B.; Nikishin, D.A. Uptake and Metabolization of Serotonin by Granulosa Cells Form a Functional Barrier in the Mouse Ovary. Int. J. Mol. Sci. 2022, 23, 14828. https://doi.org/10.3390/ijms232314828
Alyoshina NM, Tkachenko MD, Malchenko LA, Shmukler YB, Nikishin DA. Uptake and Metabolization of Serotonin by Granulosa Cells Form a Functional Barrier in the Mouse Ovary. International Journal of Molecular Sciences. 2022; 23(23):14828. https://doi.org/10.3390/ijms232314828
Chicago/Turabian StyleAlyoshina, Nina M., Maria D. Tkachenko, Lyudmila A. Malchenko, Yuri B. Shmukler, and Denis A. Nikishin. 2022. "Uptake and Metabolization of Serotonin by Granulosa Cells Form a Functional Barrier in the Mouse Ovary" International Journal of Molecular Sciences 23, no. 23: 14828. https://doi.org/10.3390/ijms232314828
APA StyleAlyoshina, N. M., Tkachenko, M. D., Malchenko, L. A., Shmukler, Y. B., & Nikishin, D. A. (2022). Uptake and Metabolization of Serotonin by Granulosa Cells Form a Functional Barrier in the Mouse Ovary. International Journal of Molecular Sciences, 23(23), 14828. https://doi.org/10.3390/ijms232314828




