Tissue Reactions and Mechanism in Cardiovascular Diseases Induced by Radiation
Abstract
:1. Introduction
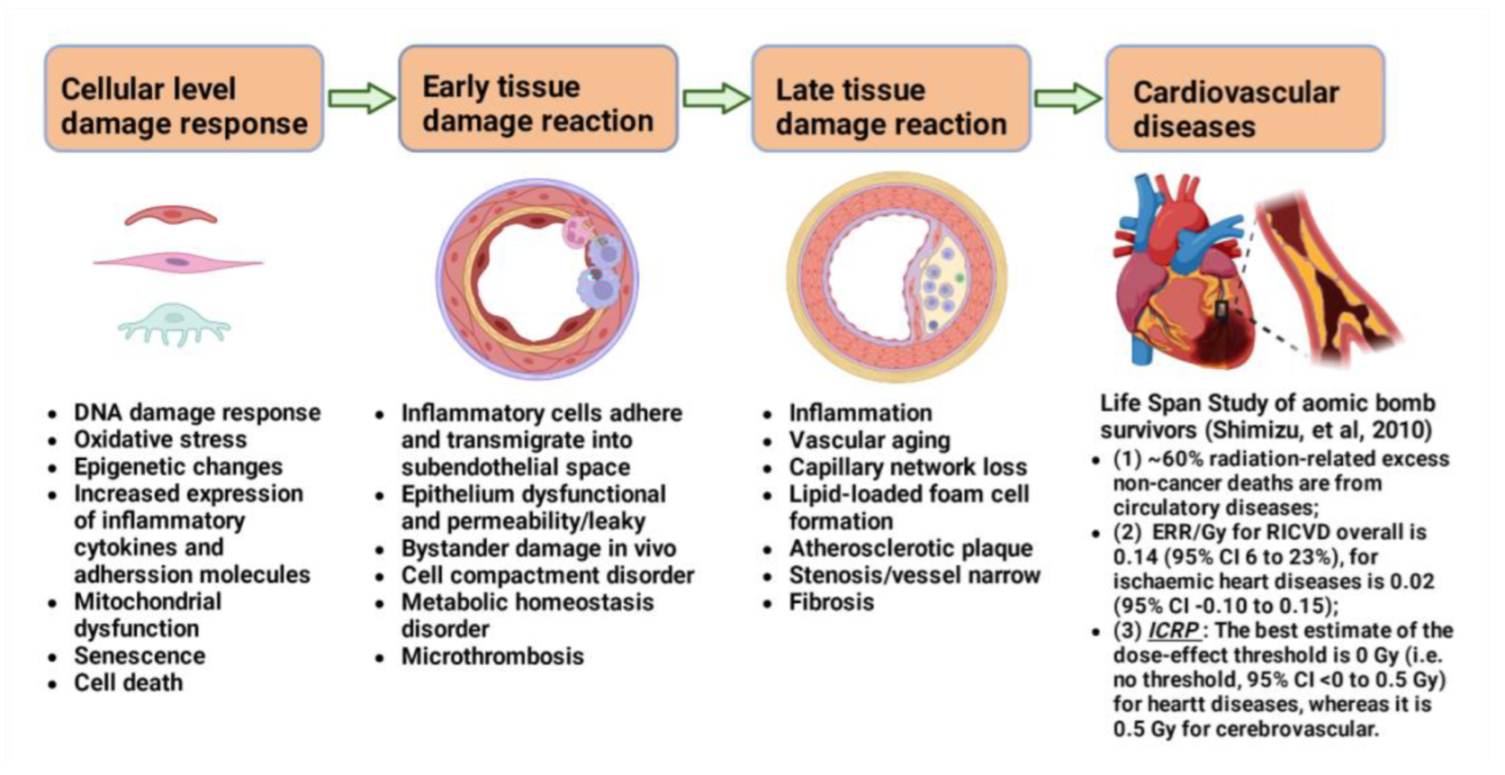
2. Radiation-Induced Injuries of Cardiovascular Tissues
2.1. Early Effects of Radiation on Vascular Cells
2.2. Late Effects of Radiation on Cardiovascular Tissues
3. Risks of Cardiovascular Diseases in Different Scenarios of Radiation Exposures
3.1. Risk of Cardiovascular Diseases Related to Radiotherapy
3.2. Risk of Cardiovascular Diseases by Low-Dose Radiation Exposure
4. Mechanisms of Radiation-Induced Cardiovascular Injuries
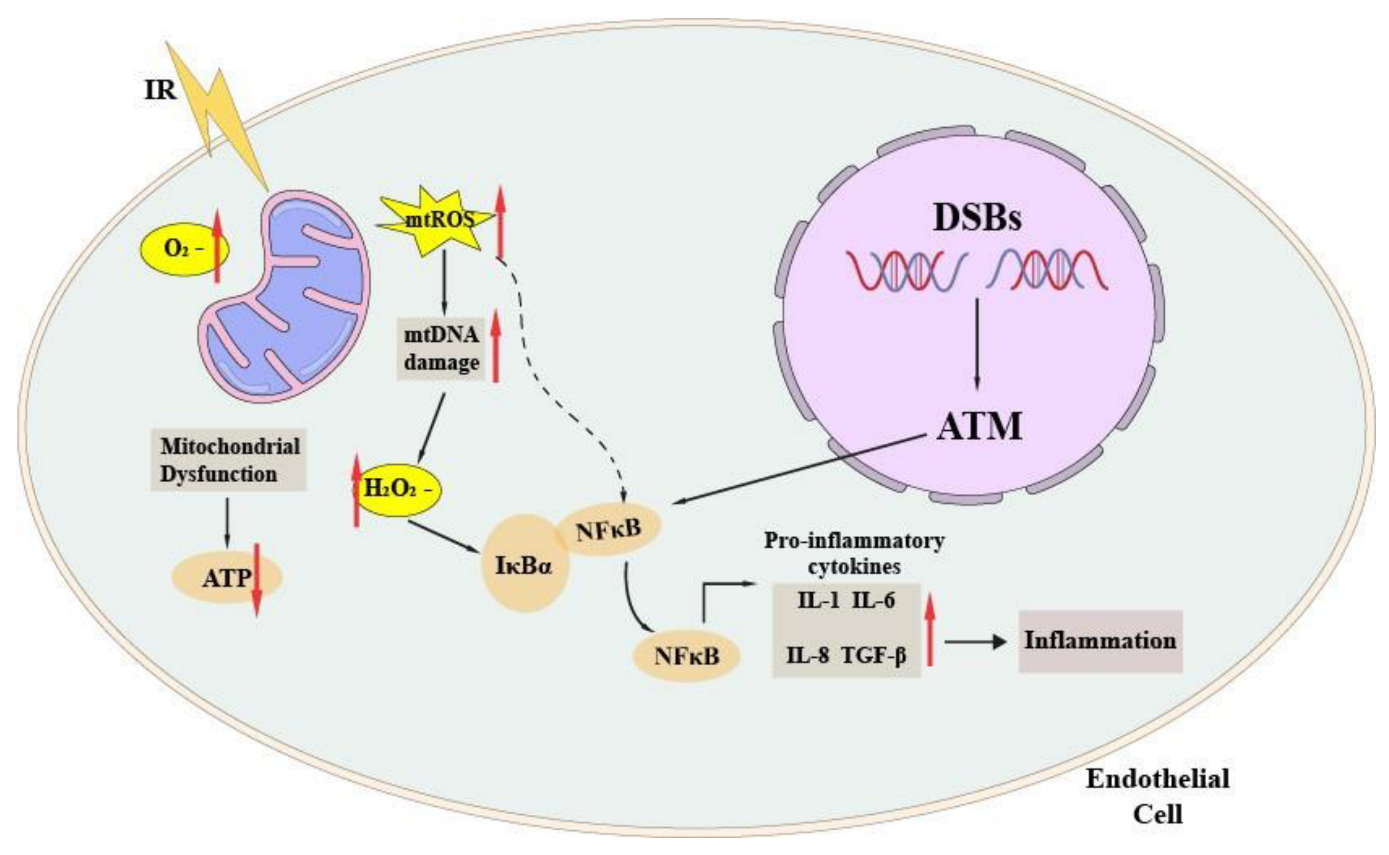
4.1. ROS and Endothelial Dysfunction
4.2. Mitochondrial Dysfunction
4.3. Endothelial Senescence and Inflammation in Atherosclerotic Plaque Formation
4.4. Late Tissue Reactions to Cardiovascular Diseases
4.4.1. Vascular Stenosis
4.4.2. Atherosclerosis
4.4.3. Fibrosis
4.4.4. Role of Tissue Level Bystander Effects in Cardiovascular Diseases
5. Prospective and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Delaney, G.; Jacob, S.; Featherstone, C.; Barton, M. The role of radiotherapy in cancer treatment: Estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer 2005, 104, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.-X.; Zhou, P.-K. DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer. Signal Transduct. Target. Ther. 2020, 5, 60. [Google Scholar] [CrossRef] [PubMed]
- Dauer, L.; Yorke, E.; Williamson, M.; Gao, Y.; Dauer, Z.; Miller, D.; Vañó, E. Radiotherapeutic implications of the updated ICRP thresholds for tissue reactions related to cataracts and circulatory diseases. Ann. ICRP 2018, 47, 196–213. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhou, Y.; Hu, S.; Ren, G.; Cui, F.; Zhou, P.-K. Radiotherapy Exposure in Cancer Patients and Subsequent Risk of Stroke: A Systematic Review and Meta-Analysis. Front. Neurol. 2019, 10, 233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef] [Green Version]
- Koutroumpakis, E.; Deswal, A.; Yusuf, S.W.; Abe, J.-I.; Nead, K.T.; Potter, A.S.; Liao, Z.; Lin, S.H.; Palaskas, N.L. Radiation-Induced Cardiovascular Disease: Mechanisms, Prevention, and Treatment. Curr. Oncol. Rep. 2022, 24, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.; Collins, R.; Darby, S.; Davies, C.; Elphinstone, P.; Evans, V.; Godwin, J.; Gray, R.; Hicks, C.; James, S.; et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005, 366, 2087–2106. [Google Scholar] [CrossRef] [PubMed]
- Paszat, L.F.; MacKillop, W.J.; A Groome, P.; Boyd, C.; Schulze, K.; Holowaty, E. Mortality from myocardial infarction after adjuvant radiotherapy for breast cancer in the surveillance, epidemiology, and end-results cancer registries. J. Clin. Oncol. 1998, 16, 2625–2631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinksman, C.A.; Haylock, R.G.E.; Gillies, M. Cerebrovascular Disease Mortality after occupational Radiation Exposure among the UK National Registry for Radiation Workers Cohort. Radiat. Res. 2022, 197, 459–470. [Google Scholar] [CrossRef]
- Cha, E.S.; Zablotska, L.B.; Bang, Y.J.; Lee, W.J. Occupational radiation exposure and morbidity of circulatory disease among diagnostic medical radiation workers in South Korea. Occup. Environ. Med. 2020, 77, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Hancock, S.L.; Tucker, M.A.; Hoppe, R.T. Factors affecting late mortality from heart disease after treatment of Hodgkin’s disease. JAMA 1993, 270, 1949–1955. [Google Scholar] [CrossRef]
- Hooning, M.J.; Botma, A.; Aleman, B.M.P.; Baaijens, M.H.A.; Bartelink, H.; Klijn, J.G.M.; Taylor, C.W.; Van Leeuwen, F.E. Long-Term Risk of Cardiovascular Disease in 10-Year Survivors of Breast Cancer. J. Natl. Cancer Inst. 2007, 99, 365–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Nimwegen, F.A.; Schaapveld, M.; Janus, C.P.M.; Krol, A.D.G.; Petersen, E.J.; Raemaekers, J.M.M.; Kok, W.E.M.; Aleman, B.M.P.; Van Leeuwen, F.E. Cardiovascular Disease After Hodgkin Lymphoma Treatment: 40-year disease risk. JAMA Intern. Med. 2015, 175, 1007–1017. [Google Scholar] [CrossRef]
- Veinot, J.P.; Edwards, W.D. Pathology of radiation-induced heart disease: A surgical and autopsy study of 27 cases. Hum. Pathol. 1996, 27, 766–773. [Google Scholar] [CrossRef]
- Al-Kindi, S.G.; Oliveira, G.H. Incidence and trends of cardiovascular mortality after common cancers in young adults: Analysis of surveillance, epidemiology and end-results program. World J. Cardiol. 2016, 8, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Rutqvist, L.E.; Rose, C.; Cavallin-Ståhl, E. A Systematic Overview of Radiation Therapy Effects in Breast Cancer. Acta Oncol. 2003, 42, 532–545. [Google Scholar] [CrossRef] [PubMed]
- Underberg, R.W.; Lagerwaard, F.J.; Slotman, B.J.; Cuijpers, J.P.; Senan, S. Benefit of respiration-gated stereotactic radiotherapy for stage I lung cancer: An analysis of 4DCT datasets. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Koutroumpakis, E.; Palaskas, N.L.; Lin, S.H.; Abe, J.-I.; Liao, Z.; Banchs, J.; Deswal, A.; Yusuf, S.W. Modern Radiotherapy and Risk of Cardiotoxicity. Chemotherapy 2020, 65, 65–76. [Google Scholar] [CrossRef]
- Yue, X.; Bai, C.; Xie, D.; Ma, T.; Zhou, P.-K. DNA-PKcs: A Multi-Faceted Player in DNA Damage Response. Front. Genet. 2020, 11, 607428. [Google Scholar] [CrossRef]
- Huang, R.; Zhou, P.-K. DNA damage repair: Historical perspectives, mechanistic pathways and clinical translation for targeted cancer therapy. Signal Transduct. Target. Ther. 2021, 6, 254. [Google Scholar] [CrossRef]
- Yusuf, S.W.; Sami, S.; Daher, I.N. Radiation-Induced Heart Disease: A Clinical Update. Cardiol. Res. Pract. 2011, 2011, 317659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laiakis, E.C.; Canadell, M.P.; Grilj, V.; Harken, A.D.; Garty, G.Y.; Astarita, G.; Brenner, D.J.; Smilenov, L.; Fornace, A.J., Jr. Serum lipidomic analysis from mixed neutron/X-ray radiation fields reveals a hyperlipidemic and pro-inflammatory phenotype. Sci. Rep. 2019, 9, 4539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paris, F.; Fuks, Z.; Kang, A.; Capodieci, P.; Juan, G.; Ehleiter, D.; Haimovitz-Friedman, A.; Cordon-Cardo, C.; Kolesnick, R. Endothelial Apoptosis as the Primary Lesion Initiating Intestinal Radiation Damage in Mice. Science 2001, 293, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Satyamitra, M.M.; DiCarlo, A.L.; Taliaferro, L. Understanding the Pathophysiology and Challenges of Development of Medical Countermeasures for Radiation-Induced Vascular/Endothelial Cell Injuries: Report of a NIAID Workshop, August 20, 2015. Radiat. Res. 2016, 186, 99–111. [Google Scholar] [CrossRef] [Green Version]
- Tang, N.; Bueno, M.; Meylan, S.; Perrot, Y.; Tran, H.N.; Freneau, A.; Dos Santos, M.; Vaurijoux, A.; Gruel, G.; Bernal, M.A.; et al. Assessment of Radio-Induced Damage in Endothelial Cells Irradiated with 40 kVp, 220 kVp, and 4 MV X-rays by Means of Micro and Nanodosimetric Calculations. Int. J. Mol. Sci. 2019, 20, 6204. [Google Scholar] [CrossRef] [Green Version]
- Cui, F.M.; Liu, L.; Zheng, L.L.; Bao, G.L.; Tu, Y.; Sun, L.; Zhu, W.; Cao, J.P.; Zhou, P.K.; Chen, Q.; et al. The Role of miR-34a in Tritiated Water Toxicity in Human Umbilical Vein Endothelial Cells. Dose-Response 2016, 14, 1559325816638585. [Google Scholar] [CrossRef] [Green Version]
- Hwang, H.J.; Lee, Y.-R.; Kang, D.; Lee, H.C.; Seo, H.R.; Ryu, J.-K.; Kim, Y.-N.; Ko, Y.-G.; Park, H.J.; Lee, J.-S. Endothelial cells under therapy-induced senescence secrete CXCL11, which increases aggressiveness of breast cancer cells. Cancer Lett. 2020, 490, 100–110. [Google Scholar] [CrossRef]
- Babini, G.; Baiocco, G.; Barbieri, S.; Morini, J.; Sangsuwan, T.; Haghdoost, S.; Yentrapalli, R.; Azimzadeh, O.; Rombouts, C.; Aerts, A.; et al. A systems radiation biology approach to unravel the role of chronic low-dose-rate gamma-irradiation in inducing premature senescence in endothelial cells. PLoS ONE 2022, 17, e0265281. [Google Scholar] [CrossRef]
- Ben Kacem, M.; Benadjaoud, M.A.; Dos Santos, M.; Buard, V.; Tarlet, G.; Le Guen, B.; François, A.; Guipaud, O.; Milliat, F.; Paget, V. Variation of 4 MV X-ray dose rate in fractionated irradiation strongly impacts biological endothelial cell response in vitro. Int. J. Radiat. Biol. 2022, 98, 50–59. [Google Scholar] [CrossRef]
- Soltani, B.; Bodaghabadi, N.; Mahpour, G.; Ghaemi, N.; Sadeghizadeh, M. Nanoformulation of curcumin protects HUVEC endothelial cells against ionizing radiation and suppresses their adhesion to monocytes: Potential in prevention of radiation-induced atherosclerosis. Biotechnol. Lett. 2016, 38, 2081–2088. [Google Scholar] [CrossRef]
- Hu, S.; Gao, Y.; Zhou, H.; Kong, F.; Xiao, F.; Zhou, P.; Chen, Y. New insight into mitochondrial changes in vascular endothelial cells irradiated by gamma ray. Int. J. Radiat. Biol. 2017, 93, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Sakata, K.; Kondo, T.; Mizuno, N.; Shoji, M.; Yasui, H.; Yamamori, T.; Inanami, O.; Yokoo, H.; Yoshimura, N.; Hattori, Y. Roles of ROS and PKC-βII in ionizing radiation-induced eNOS activation in human vascular endothelial cells. Vasc. Pharmacol. 2015, 70, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Segaran, R.C.; Chan, L.Y.; Aladresi, A.A.M.; Chinnathambi, A.; Alharbi, S.A.; Sethi, G.; Tang, F.R. Gamma Radiation-Induced Disruption of Cellular Junctions in HUVECs Is Mediated through Affecting MAPK/NF-κB Inflammatory Pathways. Oxidative Med. Cell. Longev. 2019, 2019, 1486232. [Google Scholar] [CrossRef] [Green Version]
- Kalamida, D.; Karagounis, I.V.; Giatromanolaki, A.; Koukourakis, M.I. Important Role of Autophagy in Endothelial Cell Response to Ionizing Radiation. PLoS ONE 2014, 9, e102408. [Google Scholar] [CrossRef]
- Shin, S.H.; Vincent, S.; Vallieres, B. Pulsatile Nature of Luteinizing Hormone Release Is Maintained during Luteinizing Hormone-Releasing Hormone Stimulation in Normal Male Rats. Horm. Res. 1988, 29, 223–228. [Google Scholar] [CrossRef]
- Ahmad, M.; Khurana, N.R.; Jaberi, J.E. Ionizing radiation decreases capillary-like structure formation by endothelial cells in vitro. Microvasc. Res. 2007, 73, 14–19. [Google Scholar] [CrossRef]
- González-González, A.; Rueda, N.; Alonso-González, C.; Menéndez-Menéndez, J.; Gómez-Arozamena, J.; Martínez-Campa, C.; Cos, S. Melatonin Enhances the Usefulness of Ionizing Radiation: Involving the Regulation of Different Steps of the Angiogenic Process. Front. Physiol. 2019, 10, 879. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.; Chen, Z.; Peng, Q.; Chen, G.; Yan, W.; Chen, X. Ku86 alleviates human umbilical vein endothelial cellular apoptosis and senescence induced by a low dose of ionizing radiation. J. Int. Med. Res. 2019, 47, 893–904. [Google Scholar] [CrossRef]
- Li, C.; Tian, M.; Gou, Q.; Jia, Y.R.; Su, X. Connexin43 Modulates X-Ray-Induced Pyroptosis in Human Umbilical Vein Endothelial Cells. Biomed. Environ. Sci. 2019, 32, 177–188. [Google Scholar]
- Xiao, F.-J.; Zhang, D.; Wu, Y.; Jia, Q.-H.; Zhang, L.; Li, Y.-X.; Yang, Y.-F.; Wang, H.; Wu, C.-T.; Wang, L.-S. miRNA-17-92 protects endothelial cells from erastin-induced ferroptosis through targeting the A20-ACSL4 axis. Biochem. Biophys. Res. Commun. 2019, 515, 448–454. [Google Scholar] [CrossRef]
- Luo, L.; Yan, C.; Fuchi, N.; Kodama, Y.; Zhang, X.; Shinji, G.; Miura, K.; Sasaki, H.; Li, T.-S. Mesenchymal stem cell-derived extracellular vesicles as probable triggers of radiation-induced heart disease. Stem Cell Res. Ther. 2021, 12, 422. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, L.; Chen, C.; Ming, P.; Huang, Q.; Li, C.; Cao, D.; Xu, X.; Ge, W. The extracellular vesicles secreted by lung cancer cells in radiation therapy promote endothelial cell angiogenesis by transferring miR-23a. PeerJ 2017, 5, e3627. [Google Scholar] [CrossRef] [Green Version]
- Feghhi, M.; Rezaie, J.; Mostafanezhad, K.; Jabbari, N. Bystander effects induced by electron beam-irradiated MCF-7 cells: A potential mechanism of therapy resistance. Breast Cancer Res. Treat. 2021, 187, 657–671. [Google Scholar] [CrossRef]
- Mo, F.; Xu, Y.; Zhang, J.; Zhu, L.; Wang, C.; Chu, X.; Pan, Y.; Bai, Y.; Shao, C.; Zhang, J. Effects of Hypoxia and Radiation-Induced Exosomes on Migration of Lung Cancer Cells and Angiogenesis of Umbilical Vein Endothelial Cells. Radiat. Res. 2020, 194, 71–80. [Google Scholar] [CrossRef]
- Mo, L.-J.; Song, M.; Huang, Q.-H.; Guan, H.; Liu, X.-D.; Xie, D.-F.; Huang, B.; Huang, R.-X.; Zhou, P.-K. Exosome-packaged miR-1246 contributes to bystander DNA damage by targeting LIG4. Br. J. Cancer 2018, 119, 492–502. [Google Scholar] [CrossRef] [Green Version]
- Lanza, V.; Fadda, P.; Iannone, C.; Negri, R. Low-Dose Ionizing Radiation Stimulates Transcription and Production of Endothelin by Human Vein Endothelial Cells. Radiat. Res. 2007, 168, 193–198. [Google Scholar] [CrossRef]
- Lanza, V.; Pretazzoli, V.; Olivieri, G.; Pascarella, G.; Panconesi, A.; Negri, R. Transcriptional Response of Human Umbilical Vein Endothelial Cells to Low Doses of Ionizing Radiation. J. Radiat. Res. 2005, 46, 265–276. [Google Scholar] [CrossRef] [Green Version]
- Najafi, M.; Fardid, R.; Najafi, M.; Salajegheh, A.; Kazemi, E.; Rezaeyan, A. Radiation-induced non-targeted effect in vivo: Evaluation of cyclooygenase-2 and endothelin-1 gene expression in rat heart tissues. J. Cancer Res. Ther. 2017, 13, 51–55. [Google Scholar] [CrossRef]
- Merlin, S.; Brock, G.; Begin, L.; Tim, F.H.; Macramalla, A.; Seyam, R.; Shenouda, G.; Dion, S. New insights into the role of endothelin-1 in radiation-associated impotence. Int. J. Impot. Res. 2001, 13, 104–109. [Google Scholar] [CrossRef] [Green Version]
- Papapetropoulos, A.; Burch, S.; Topouzis, S.; Catravas, J. Radiation-Induced Alterations in Angiotensin Converting Enzyme Activity in Cultured Bovine Pulmonary Arterial Endothelial Cell Monolayers. Toxicol. Appl. Pharmacol. 1993, 120, 96–105. [Google Scholar] [CrossRef]
- Wei, J.; Xu, H.; Liu, Y.; Li, B.; Zhou, F. Effect of captopril on radiation-induced TGF-β1 secretion in EA.Hy926 human umbilical vein endothelial cells. Oncotarget 2017, 8, 20842–20850. [Google Scholar] [CrossRef]
- Ward, W.F.; Kim, Y.T.; Molteni, A.; Solliday, N.H. Radiation-induced pulmonary endothelial dysfunction in rats: Modification by an inhibitor of angiotensin converting enzyme. Int. J. Radiat. Oncol. Biol. Phys. 1988, 15, 135–140. [Google Scholar] [CrossRef]
- Cao, S.; Wu, R. Expression of Angiotensin II and Aldosterone in Radiation-induced Lung Injury. Cancer Biol. Med. 2012, 9, 254–260. [Google Scholar] [CrossRef]
- Heckenkamp, J.; Leszczynski, D.; Schiereck, J.; Kung, J.; LaMuraglia, G.M. Different Effects of Photodynamic Therapy and γ-Irradiation on Vascular Smooth Muscle Cells and Matrix: Implications for inhibiting restenosis. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 2154–2161. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.-Y.; Park, K.-W.; Oh, S.; Cho, H.-J.; Cho, H.-J.; Park, J.-S.; Cho, Y.-S.; Koo, B.-K.; Chae, I.-H.; Choi, D.-J.; et al. NF-κB decoy potentiates the effects of radiation on vascular smooth muscle cells by enhancing apoptosis. Exp. Mol. Med. 2005, 37, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Keller, P.-F.; Verin, V.; Ziegler, T.; Mermillod, B.; Popowski, Y.; Delafontaine, P. Gamma-Irradiation Markedly Inhibits the Hydrated Collagen Gel Contraction by Arterial Smooth Muscle Cells. J. Investig. Med. 2001, 49, 258–264. [Google Scholar] [CrossRef]
- Ryu, J.; Jung, I.; Park, E.; Kim, K.; Kim, K.; Yeom, J.; Jung, J.; Lee, S. Radiation-induced C-reactive protein triggers apoptosis of vascular smooth muscle cells through ROS interfering with the STAT3/Ref-1 complex. J. Cell. Mol. Med. 2022, 26, 2104–2118. [Google Scholar] [CrossRef]
- Hallahan, D.; Kuchibhotla, J.; Wyble, C. Cell adhesion molecules mediate radiation-induced leukocyte adhesion to the vascular endothelium. Cancer Res. 1996, 56, 5150–5155. [Google Scholar]
- Quarmby, S.; Hunter, R.D.; Kumar, S. Irradiation induced expression of CD31, ICAM-1 and VCAM-1 in human microvascular endothelial cells. Anticancer Res. 2000, 20, 3375–3381. [Google Scholar]
- Shimizu, Y.; Kodama, K.; Nishi, N.; Kasagi, F.; Suyama, A.; Soda, M.; Grant, E.J.; Sugiyama, H.; Sakata, R.; Moriwaki, H.; et al. Radiation exposure and circulatory disease risk: Hiroshima and Nagasaki atomic bomb survivor data, 1950–2003. BMJ 2010, 340, b5349. [Google Scholar] [CrossRef] [Green Version]
- Marks, L.B.; Yu, X.; Prosnitz, R.G.; Zhou, S.-M.; Hardenbergh, P.H.; Blazing, M.; Hollis, D.; Lind, P.; Tisch, A.; Wong, T.Z.; et al. The incidence and functional consequences of RT-associated cardiac perfusion defects. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 214–223. [Google Scholar] [CrossRef]
- Darby, S.C.; Ewertz, M.; McGale, P.; Bennet, A.M.; Blom-Goldman, U.; Brønnum, D.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B.; et al. Risk of Ischemic Heart Disease in Women after Radiotherapy for Breast Cancer. N. Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef] [Green Version]
- Adams, M.; Hardenbergh, P.H.; Constine, L.S.; Lipshultz, S.E. Radiation-associated cardiovascular disease. Crit. Rev. Oncol. 2003, 45, 55–75. [Google Scholar] [CrossRef] [PubMed]
- Aleman, B.M.; van den Belt-Dusebout, A.W.; Klokman, W.J.; van’t Veer, M.B.; Bartelink, H.; van Leeuwen, F.E. Long-Term Cause-Specific Mortality of Patients Treated for Hodgkin’s Disease. J. Clin. Oncol. 2003, 21, 3431–3439. [Google Scholar] [CrossRef]
- Hu, J.-R.; Florido, R.; Lipson, E.J.; Naidoo, J.; Ardehali, R.; Tocchetti, C.G.; Lyon, A.R.; Padera, R.F.; Johnson, D.B.; Moslehi, J. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc. Res. 2019, 115, 854–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hennequin, C.; Barillot, I.; Azria, D.; Belkacémi, Y.; Bollet, M.; Chauvet, B.; Cowen, D.; Cutuli, B.; Fourquet, A.; Hannoun-Lévi, J.; et al. Radiothérapie du cancer du sein. Radiother. Breast Cancer 2016, 20, S139–S146. [Google Scholar] [CrossRef]
- Darby, S.C.; McGale, P.; Taylor, C.W.; Peto, R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: Prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol. 2005, 6, 557–565. [Google Scholar] [CrossRef]
- Dorresteijn, L.D.; Kappelle, A.C.; Boogerd, W.; Klokman, W.J.; Balm, A.J.; Keus, R.B.; Van Leeuwen, F.E.; Bartelink, H. Increased Risk of Ischemic Stroke After Radiotherapy on the Neck in Patients Younger Than 60 Years. J. Clin. Oncol. 2002, 20, 282–288. [Google Scholar] [CrossRef]
- Gustavsson, A.; Osterman, B.; Cavallin-Ståhl, E. A Systematic Overview of Radiation Therapy Effects in Non-Hodgkin’s Lymphoma. Acta Oncol. 2003, 42, 605–619. [Google Scholar] [CrossRef]
- Eriksson, F.; Gagliardi, G.; Liedberg, A.; Lax, I.; Lee, C.; Levitt, S.; Lind, B.; Rutqvist, L.-E. Long-term cardiac mortality following radiation therapy for Hodgkin’s disease: Analysis with the relative seriality model. Radiother. Oncol. 2000, 55, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Baselet, B.; Rombouts, C.; Benotmane, A.M.; Baatout, S.; Aerts, A. Cardiovascular diseases related to ionizing radiation: The risk of low-dose exposure (Review). Int. J. Mol. Med. 2016, 38, 1623–1641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Lee, D.N.; Jin, Y.W.; Cha, E.S.; Jang, W.-I.; Park, S.; Seo, S. Non-cancer disease prevalence and association with occupational radiation exposure among Korean radiation workers. Sci. Rep. 2021, 11, 22415. [Google Scholar] [CrossRef] [PubMed]
- Azizova, T.; Briks, K.; Bannikova, M.; Grigoryeva, E. Hypertension Incidence Risk in a Cohort of Russian Workers Exposed to Radiation at the Mayak Production Association Over Prolonged Periods. Hypertension 2019, 73, 1174–1184. [Google Scholar] [CrossRef]
- Zamorano, J.L.; Lancellotti, P.; Muñoz, D.R.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. J. Heart Fail. 2016, 19, 9–42. [Google Scholar] [CrossRef] [PubMed]
- Yahalom, J.; Portlock, C.S. Long-Term Cardiac and Pulmonary Complications of Cancer Therapy. Hematol. Clin. N. Am. 2008, 22, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Wennstig, A.-K.; Wadsten, C.; Garmo, H.; Fredriksson, I.; Blomqvist, C.; Holmberg, L.; Nilsson, G.; Sund, M. Long-term risk of ischemic heart disease after adjuvant radiotherapy in breast cancer: Results from a large population-based cohort. Breast Cancer Res. 2020, 22, 10. [Google Scholar] [CrossRef] [PubMed]
- Di Mattéo, J.; Vacheron, A.; Heulin, A.; Meeus, L.; Di Mattéo, G.; Gilles, R.; Delage, F.; de Ratuld, A. Cardiac complications of thoracic radiotherapy. Arch. Mal. Coeur. Vaiss. 1978, 71, 447–452. [Google Scholar] [PubMed]
- Zheng, Z.; Zhao, Q.; Wei, J.; Wang, B.; Wang, H.; Meng, L.; Xin, Y.; Jiang, X. Medical prevention and treatment of radiation-induced carotid injury. Biomed. Pharmacother. 2020, 131, 110664. [Google Scholar] [CrossRef]
- Sutton, E.J.; Tong, R.T.; Gillis, A.M.; Henning, T.D.; Weinberg, V.A.; Boddington, S.; Haas-Kogan, D.A.; Matthay, K.; Sha, V.; Gooding, C.; et al. Decreased aortic growth and middle aortic syndrome in patients with neuroblastoma after radiation therapy. Pediatr. Radiol. 2009, 39, 1194–1202. [Google Scholar] [CrossRef] [Green Version]
- Kralik, S.F.; Watson, G.A.; Shih, C.-S.; Ho, C.Y.; Finke, W.; Buchsbaum, J. Radiation-Induced Large Vessel Cerebral Vasculopathy in Pediatric Patients With Brain Tumors Treated With Proton Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 817–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Benhawy, S.A.; Sadek, N.A.; Kamel, M.M.; Sharaf, A.M.; Abderhman, I.G.; Morsi, M.I.; Abobakr, A. Study the relationship of endothelial damage / dysfunction due to occupational exposure to low dose ionizing radiation versus high dose exposure during radiotherapy. Cancer Treat. Res. Commun. 2020, 25, 100215. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H. The nuclear factor-kappa B pathway and response to treatment in breast cancer. Pharmacogenomics 2017, 18, 1697–1709. [Google Scholar] [CrossRef] [PubMed]
- Dorresteijn, L.D.; Kappelle, A.C.; Scholz, N.M.; Munneke, M.; Scholma, J.T.; Balm, A.J.; Bartelink, H.; Boogerd, W. Increased carotid wall thickening after radiotherapy on the neck. Eur. J. Cancer 2005, 41, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Alvarez, V.; López, F.; Suárez, C.; Strojan, P.; Eisbruch, A.; Silver, C.E.; Mendenhall, W.M.; Langendijk, J.A.; Rinaldo, A.; Lee, A.W.M.; et al. Radiation-induced carotid artery lesions. Strahlenther. Onkol. 2018, 194, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Boivin, J.-F.; Hutchison, G.B.; Lubin, J.H.; Mauch, P. Coronary artery disease mortality in patients treated for hodgkin’s disease. Cancer 1992, 69, 1241–1247. [Google Scholar] [CrossRef]
- Fidler, M.M.; Reulen, R.C.; Henson, K.; Kelly, J.; Cutter, D.; Levitt, G.A.; Frobisher, C.; Winter, D.L.; Hawkins, M.M. Population-Based Long-Term Cardiac-Specific Mortality Among 34 489 Five-Year Survivors of Childhood Cancer in Great Britain. Circulation 2017, 135, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Guldner, L.; Haddy, N.; Pein, F.; Diallo, I.; Shamsaldin, A.; Dahan, M.; Lebidois, J.; Merlet, P.; Villain, E.; Sidi, D.; et al. Radiation dose and long term risk of cardiac pathology following radiotherapy and anthracyclin for a childhood cancer. Radiother. Oncol. 2006, 81, 47–56. [Google Scholar] [CrossRef] [PubMed]
- McGale, P.; Darby, S.C.; Hall, P.; Adolfsson, J.; Bengtsson, N.-O.; Bennet, A.M.; Fornander, T.; Gigante, B.; Jensen, M.-B.; Peto, R.; et al. Incidence of heart disease in 35,000 women treated with radiotherapy for breast cancer in Denmark and Sweden. Radiother. Oncol. 2011, 100, 167–175. [Google Scholar] [CrossRef] [PubMed]
- A Heidenreich, P.; Hancock, S.L.; Lee, B.K.; Mariscal, C.S.; Schnittger, I. Asymptomatic cardiac disease following mediastinal irradiation. J. Am. Coll. Cardiol. 2003, 42, 743–749. [Google Scholar] [CrossRef] [Green Version]
- Loap, P.; De Marzi, L.; Mirandola, A.; Dendale, R.; Iannalfi, A.; Vitolo, V.; Barcellini, A.; Filippi, A.; Jereczek-Fossa, B.; Kirova, Y.; et al. Development and Implementation of Proton Therapy for Hodgkin Lymphoma: Challenges and Perspectives. Cancers 2021, 13, 3744. [Google Scholar] [CrossRef]
- Toltz, A.; Shin, N.; Mitrou, E.; Laude, C.; Freeman, C.R.; Seuntjens, J.; Parker, W.; Roberge, D. Late radiation toxicity in Hodgkin lymphoma patients: Proton therapy’s potential. J. Appl. Clin. Med. Phys. 2015, 16, 167–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Velzen, S.G.; Gal, R.; Teske, A.J.; van der Leij, F.; Bongard, D.H.V.D.; Viergever, M.A.; Verkooijen, H.M.; Išgum, I. AI-Based Radiation Dose Quantification for Estimation of Heart Disease Risk in Breast Cancer Survivors After Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2021, 112, 621–632. [Google Scholar] [CrossRef]
- Cervelli, T.; Panetta, D.; Navarra, T.; Andreassi, M.G.; Basta, G.; Galli, A.; Salvadori, P.A.; Picano, E.; Del Turco, S. Effects of single and fractionated low-dose irradiation on vascular endothelial cells. Atherosclerosis 2014, 235, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Baselet, B.; Belmans, N.; Coninx, E.; Lowe, D.; Janssen, A.; Michaux, A.; Tabury, K.; Raj, K.; Quintens, R.; Benotmane, M.A.; et al. Functional Gene Analysis Reveals Cell Cycle Changes and Inflammation in Endothelial Cells Irradiated with a Single X-ray Dose. Front. Pharmacol. 2017, 8, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, Y.; Pierce, D.A.; Preston, D.L.; Mabuchi, K. Studies of the mortality of atomic bomb survivors. Report 12, part II. Noncancer mortality: 1950–1990. Radiat. Res. 1999, 152, 374. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, V.K.; Maksioutov, M.A.; Chekin, S.Y.; Kruglova, Z.G.; Petrov, A.V.; Tsyb, A.F. Radiation-Epidemiological Analysis of Incidence of Non-Cancer Diseases among the Chernobyl Liquidators. Health Phys. 2000, 78, 495–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trivedi, A.; Hannan, M.A. Radiation and Cardiovascular Diseases. J. Environ. Pathol. Toxicol. Oncol. 2004, 23, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Sakata, R.; Grant, E.J.; Ozasa, K. Long-term follow-up of atomic bomb survivors. Maturitas 2012, 72, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Ozasa, K.; Shimizu, Y.; Suyama, A.; Kasagi, F.; Soda, M.; Grant, E.J.; Sakata, R.; Sugiyama, H.; Kodama, K. Studies of the Mortality of Atomic Bomb Survivors, Report 14, 1950–2003: An Overview of Cancer and Noncancer Diseases. Radiat. Res. 2012, 177, 229–243. [Google Scholar] [CrossRef] [Green Version]
- Little, M.P.; Azizova, T.V.; Bazyka, D.; Bouffler, S.D.; Cardis, E.; Chekin, S.; Chumak, V.V.; Cucinotta, F.A.; De Vathaire, F.; Hall, P.; et al. Systematic Review and Meta-analysis of Circulatory Disease from Exposure to Low-Level Ionizing Radiation and Estimates of Potential Population Mortality Risks. Environ. Health Perspect. 2012, 120, 1503–1511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Little, M.P.; Azizova, T.V.; Hamada, N. Low- and moderate-dose non-cancer effects of ionizing radiation in directly exposed individuals, especially circulatory and ocular diseases: A review of the epidemiology. Int. J. Radiat. Biol. 2021, 97, 782–803. [Google Scholar] [CrossRef]
- Sadetzki, S.; Chetrit, A.; Boursi, B.; Luxenburg, O.; Novikov, I.; Cohen, A. Childhood Exposure to Low to Moderate Doses of Ionizing Radiation and the Risk of Vascular Diseases. Am. J. Epidemiol. 2020, 190, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Zuchi, C.; Tritto, I.; Carluccio, E.; Mattei, C.; Cattadori, G.; Ambrosio, G. Role of endothelial dysfunction in heart failure. Heart Fail. Rev. 2020, 25, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Alexander, Y.; Osto, E.; Schmidt-Trucksäss, A.; Shechter, M.; Trifunovic, D.; Duncker, D.J.; Aboyans, V.; Bäck, M.; Badimon, L.; Cosentino, F.; et al. Endothelial function in cardiovascular medicine: A consensus paper of the European Society of Cardiology Working Groups on Atherosclerosis and Vascular Biology, Aorta and Peripheral Vascular Diseases, Coronary Pathophysiology and Microcirculation, and Thrombosis. Cardiovasc. Res. 2021, 117, 29–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Triggle, C.R.; Samuel, S.M.; Ravishankar, S.; Marei, I.; Arunachalam, G.; Ding, H. The endothelium: Influencing vascular smooth muscle in many ways. Can. J. Physiol. Pharmacol. 2012, 90, 713–738. [Google Scholar] [CrossRef] [PubMed]
- Van Hinsbergh, V.W.M. Endothelium—Role in regulation of coagulation and inflammation. Semin. Immunopathol. 2012, 34, 93–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalal, P.J.; Muller, W.A.; Sullivan, D.P. Endothelial Cell Calcium Signaling during Barrier Function and Inflammation. Am. J. Pathol. 2020, 190, 535–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filippini, A.; D’Amore, A.; D’Alessio, A. Calcium Mobilization in Endothelial Cell Functions. Int. J. Mol. Sci. 2019, 20, 4525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, S.; Zhang, J.; Ning, R.; Du, Z.; Liu, J.; Batibawa, J.W.; Duan, J.; Sun, Z. The critical role of endothelial function in fine particulate matter-induced atherosclerosis. Part. Fibre Toxicol. 2020, 17, 61. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.M.; Channon, K.M. Free radicals and redox signalling in cardiovascular disease. Heart 2004, 90, 486–487. [Google Scholar] [CrossRef] [Green Version]
- Joffre, J.; Hellman, J. Oxidative Stress and Endothelial Dysfunction in Sepsis and Acute Inflammation. Antioxid. Redox Signal. 2021, 35, 1291–1307. [Google Scholar] [CrossRef] [PubMed]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ping, Z.; Peng, Y.; Lang, H.; Xinyong, C.; Zhiyi, Z.; Xiaocheng, W.; Hong, Z.; Liang, S. Oxidative Stress in Radiation-Induced Cardiotoxicity. Oxidative Med. Cell. Longev. 2020, 2020, 3579143. [Google Scholar] [CrossRef] [PubMed]
- Pavlakou, P.; Dounousi, E.; Roumeliotis, S.; Eleftheriadis, T.; Liakopoulos, V. Oxidative Stress and the Kidney in the Space Environment. Int. J. Mol. Sci. 2018, 19, 3176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leach, J.K.; Van Tuyle, G.; Lin, P.S.; Schmidt-Ullrich, R.; Mikkelsen, R.B. Ionizing radiation-induced, mitochondria-dependent generation of reactive oxygen/nitrogen. Cancer Res. 2001, 61, 3894–3901. [Google Scholar]
- Prithivirajsingh, S.; Story, M.D.; A Bergh, S.; Geara, F.B.; Ang, K.K.; Ismail, S.M.; Stevens, C.W.; A Buchholz, T.; A Brock, W. Accumulation of the common mitochondrial DNA deletion induced by ionizing radiation. FEBS Lett. 2004, 571, 227–232. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Kuwahara, Y.; Li, L.; Baba, T.; Shin, R.-W.; Ohkubo, Y.; Ono, K.; Fukumoto, M. Analysis of Common Deletion (CD) and a novel deletion of mitochondrial DNA induced by ionizing radiation. Int. J. Radiat. Biol. 2007, 83, 433–442. [Google Scholar] [CrossRef]
- Schilling-Tóth, B.; Sándor, N.; Kis, E.; Kadhim, M.; Sáfrány, G.; Hegyesi, H. Analysis of the common deletions in the mitochondrial DNA is a sensitive biomarker detecting direct and non-targeted cellular effects of low dose ionizing radiation. Mutat. Res. Mol. Mech. Mutagen. 2011, 716, 33–39. [Google Scholar] [CrossRef]
- Azzam, E.I.; Jay-Gerin, J.-P.; Pain, D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012, 327, 48–60. [Google Scholar] [CrossRef] [Green Version]
- Yahyapour, R.; Motevaseli, E.; Rezaeyan, A.; Abdollahi, H.; Farhood, B.; Cheki, M.; Rezapoor, S.; Shabeeb, D.; Musa, A.E.; Najafi, M.; et al. Reduction–oxidation (redox) system in radiation-induced normal tissue injury: Molecular mechanisms and implications in radiation therapeutics. Clin. Transl. Oncol. 2018, 20, 975–988. [Google Scholar] [CrossRef]
- Ghosh-Choudhary, S.K.; Liu, J.; Finkel, T. The role of mitochondria in cellular senescence. FASEB J. 2021, 35, e21991. [Google Scholar] [CrossRef]
- Andrieux, P.; Chevillard, C.; Cunha-Neto, E.; Nunes, J.P.S. Mitochondria as a Cellular Hub in Infection and Inflammation. Int. J. Mol. Sci. 2021, 22, 11338. [Google Scholar] [CrossRef]
- Fan, P.-C.; Zhang, Y.; Wang, Y.; Wei, W.; Zhou, Y.-X.; Xie, Y.; Wang, X.; Qi, Y.-Z.; Chang, L.; Jia, Z.-P.; et al. Quantitative proteomics reveals mitochondrial respiratory chain as a dominant target for carbon ion radiation: Delayed reactive oxygen species generation caused DNA damage. Free Radic. Biol. Med. 2019, 130, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Lafargue, A.; Degorre, C.; Corre, I.; Alves-Guerra, M.-C.; Gaugler, M.-H.; Vallette, F.; Pecqueur, C.; Paris, F. Ionizing radiation induces long-term senescence in endothelial cells through mitochondrial respiratory complex II dysfunction and superoxide generation. Free. Radic. Biol. Med. 2017, 108, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra, C.J.; Hernandez-Resendiz, S.; Crespo-Avilan, G.E.; Lin, Y.-H.; Hausenloy, D.J. Mitochondria in acute myocardial infarction and cardioprotection. eBioMedicine 2020, 57, 102884. [Google Scholar] [CrossRef] [PubMed]
- Kirkman, D.L.; Robinson, A.T.; Rossman, M.J.; Seals, D.R.; Edwards, D.G. Mitochondrial contributions to vascular endothelial dysfunction, arterial stiffness, and cardiovascular diseases. Am. J. Physiol. Circ. Physiol. 2021, 320, H2080–H2100. [Google Scholar] [CrossRef]
- Shao, Y.; Li, X.; Wood, J.W.; Ma, J.-X. Mitochondrial dysfunctions, endothelial progenitor cells and diabetic retinopathy. J. Diabetes Its Complicat. 2018, 32, 966–973. [Google Scholar] [CrossRef]
- Peng, W.; Cai, G.; Xia, Y.; Chen, J.; Wu, P.; Wang, Z.; Li, G.; Wei, D. Mitochondrial Dysfunction in Atherosclerosis. DNA Cell Biol. 2019, 38, 597–606. [Google Scholar] [CrossRef]
- Nagane, M.; Yasui, H.; Kuppusamy, P.; Yamashita, T.; Inanami, O. DNA damage response in vascular endothelial senescence: Implication for radiation-induced cardiovascular diseases. J. Radiat. Res. 2021, 62, 564–573. [Google Scholar] [CrossRef]
- Casella, G.; Munk, R.; Kim, K.M.; Piao, Y.; De, S.; Abdelmohsen, K.; Gorospe, M. Transcriptome signature of cellular senescence. Nucleic Acids Res. 2019, 47, 7294–7305. [Google Scholar] [CrossRef] [Green Version]
- Vávrová, J.; Rezacova, M. The importance of senescence in ionizing radiation-induced tumour suppression. Folia Biol. 2011, 57, 41–46. [Google Scholar]
- Lundsgaard, N.U.; Cramp, R.L.; Franklin, C.E. Early exposure to UV radiation causes telomere shortening and poorer condition later in life. J. Exp. Biol. 2022, 225, jeb243924. [Google Scholar] [CrossRef] [PubMed]
- Arsenis, N.C.; You, T.; Ogawa, E.F.; Tinsley, G.M.; Zuo, L. Physical activity and telomere length: Impact of aging and potential mechanisms of action. Oncotarget 2017, 8, 45008–45019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.-W.; Park, J.-E.; Kim, S.-R.; Sim, M.-K.; Kang, C.-M.; Kim, K.S. Metformin alleviates ionizing radiation-induced senescence by restoring BARD1-mediated DNA repair in human aortic endothelial cells. Exp. Gerontol. 2022, 160, 111706. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-B.; Heo, J.-I.; Kim, H.; Kim, K.S. Acetylation of PGC1α by Histone Deacetylase 1 Downregulation Is Implicated in Radiation-Induced Senescence of Brain Endothelial Cells. J. Gerontol. Ser. A 2018, 74, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Sermsathanasawadi, N.; Ishii, H.; Igarashi, K.; Miura, M.; Yoshida, M.; Inoue, Y.; Iwai, T. Enhanced adhesion of early endothelial progenitor cells to radiation-induced senescence-like vascular endothelial cells in vitro. J. Radiat. Res. 2009, 50, 469–475. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.S.; Kim, J.E.; Choi, K.J.; Bae, S. Characterization of DNA damage-induced cellular senescence by ionizing radiation in endothelial cells. Int. J. Radiat. Biol. 2014, 90, 71–80. [Google Scholar] [CrossRef]
- Baselet, B.; Sonveaux, P.; Baatout, S.; Aerts, A. Pathological effects of ionizing radiation: Endothelial activation and dysfunction. Cell. Mol. Life Sci. 2019, 76, 699–728. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, J.; Scipione, C.A.; Hyduk, S.J.; Althagafi, M.G.; Atif, J.; Dick, S.A.; Rajora, M.; Jang, E.; Emoto, T.; Murakami, J.; et al. Radiation Impacts Early Atherosclerosis by Suppressing Intimal LDL Accumulation. Circ. Res. 2021, 128, 530–543. [Google Scholar] [CrossRef]
- Glasow, A.; Patties, I.; Priest, N.D.; Mitchel, R.E.J.; Hildebrandt, G.; Manda, K. Dose and Dose Rate-Dependent Effects of Low-Dose Irradiation on Inflammatory Parameters in ApoE-Deficient and Wild Type Mice. Cells 2021, 10, 3251. [Google Scholar] [CrossRef]
- Linton, M.F.; Babaev, V.R.; Huang, J.; Linton, E.F.; Tao, H.; Yancey, P.G. Macrophage Apoptosis and Efferocytosis in the Pathogenesis of Atherosclerosis. Circ. J. 2016, 80, 2259–2268. [Google Scholar] [CrossRef] [Green Version]
- Kreuzer, M.; Auvinen, A.; Cardis, E.; Hall, J.; Jourdain, J.-R.; Laurier, D.; Little, M.P.; Peters, A.; Raj, K.; Russell, N.S.; et al. Low-dose ionising radiation and cardiovascular diseases—Strategies for molecular epidemiological studies in Europe. Mutat. Res. Mutat. Res. 2015, 764, 90–100. [Google Scholar] [CrossRef]
- Yentrapalli, R.; Azimzadeh, O.; Barjaktarovic, Z.; Sarioglu, H.; Wojcik, A.; Harms-Ringdahl, M.; Atkinson, M.J.; Haghdoost, S.; Tapio, S. Quantitative proteomic analysis reveals induction of premature senescence in human umbilical vein endothelial cells exposed to chronic low-dose rate gamma radiation. Proteomics 2013, 13, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Yentrapalli, R.; Azimzadeh, O.; Sriharshan, A.; Malinowsky, K.; Merl, J.; Wojcik, A.; Harms-Ringdahl, M.; Atkinson, M.; Becker, K.-F.; Haghdoost, S.; et al. The PI3K/Akt/mTOR Pathway Is Implicated in the Premature Senescence of Primary Human Endothelial Cells Exposed to Chronic Radiation. PLoS ONE 2013, 8, e70024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Boerma, M.; Zhou, D. Ionizing Radiation-Induced Endothelial Cell Senescence and Cardiovascular Diseases. Radiat. Res. 2016, 186, 153–161. [Google Scholar] [CrossRef]
- Subramanian, V.; Seemann, I.; Merl-Pham, J.; Hauck, S.M.; Stewart, F.A.; Atkinson, M.J.; Tapio, S.; Azimzadeh, O. Role of TGF Beta and PPAR Alpha Signaling Pathways in Radiation Response of Locally Exposed Heart: Integrated Global Transcriptomics and Proteomics Analysis. J. Proteome Res. 2017, 16, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Sniegon, I.; Prieß, M.; Heger, J.; Schulz, R.; Euler, G. Endothelial Mesenchymal Transition in Hypoxic Microvascular Endothelial Cells and Paracrine Induction of Cardiomyocyte Apoptosis Are Mediated via TGFβ1/SMAD Signaling. Int. J. Mol. Sci. 2017, 18, 2290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Low, E.L.; Baker, A.H.; Bradshaw, A.C. TGFβ, smooth muscle cells and coronary artery disease: A review. Cell Signal. 2019, 53, 90–101. [Google Scholar] [CrossRef]
- Tonomura, S.; Shimada, K.; Funatsu, N.; Kakehi, Y.; Shimizu, H.; Takahashi, N. Pathologic Findings of Symptomatic Carotid Artery Stenosis Several Decades after Radiation Therapy: A Case Report. J. Stroke Cerebrovasc. Dis. 2018, 27, e39–e41. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; von Birgelen, C.; Zhang, S.; Ding, D.; Huang, J.; Tu, S. Simultaneous evaluation of plaque stability and ischemic potential of coronary lesions in a fluid–structure interaction analysis. Int. J. Cardiovasc. Imaging 2019, 35, 1563–1572. [Google Scholar] [CrossRef] [PubMed]
- de Groot, C.; Beukema, J.C.; Langendijk, J.A.; van der Laan, H.P.; van Luijk, P.; van Melle, J.P.; Muijs, C.T.; Prakken, N.H. Radiation-Induced Myocardial Fibrosis in Long-Term Esophageal Cancer Survivors. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Asaithamby, A. Ionizing radiation and heart risks. Semin. Cell Dev. Biol. 2016, 58, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Tapio, S.; Little, M.P.; Kaiser, J.C.; Impens, N.; Hamada, N.; Georgakilas, A.G.; Simar, D.; Salomaa, S. Ionizing radiation-induced circulatory and metabolic diseases. Environ. Int. 2021, 146, 106235. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.; Kanisicak, O.; Prasad, V.; Correll, R.N.; Fu, X.; Schips, T.; Vagnozzi, R.J.; Liu, R.; Huynh, T.; Lee, S.-J.; et al. Fibroblast-specific TGF-β–Smad2/3 signaling underlies cardiac fibrosis. J. Clin. Investig. 2017, 127, 3770–3783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bickelhaupt, S.; Erbel, C.; Timke, C.; Wirkner, U.; Dadrich, M.; Flechsig, P.; Tietz, A.; Pföhler, J.; Gross, W.; Peschke, P.; et al. Effects of CTGF Blockade on Attenuation and Reversal of Radiation-Induced Pulmonary Fibrosis. JNCI J. Natl. Cancer Inst. 2017, 109, djw339. [Google Scholar] [CrossRef]
- Taunk, N.K.; Haffty, B.G.; Kostis, J.B.; Egoyal, S. Radiation-Induced Heart Disease: Pathologic Abnormalities and Putative Mechanisms. Front. Oncol. 2015, 5, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ejaz, A.; Greenberger, J.S.; Rubin, P.J. Understanding the mechanism of radiation induced fibrosis and therapy options. Pharmacol. Ther. 2019, 204, 107399. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.-X.; Zhao, X.-K.; Li, Y.-D. New therapeutic insights into radiation-induced myocardial fibrosis. Ther. Adv. Chronic Dis. 2019, 10, 2040622319868383. [Google Scholar] [CrossRef]
- Czubryt, M.P. Cardiac Fibroblast to Myofibroblast Phenotype Conversion—An Unexploited Therapeutic Target. J. Cardiovasc. Dev. Dis. 2019, 6, 28. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Yu, K.; Hou, J.; Liu, Q.; Han, W. Radiation-induced bystander effect: Early process and rapid assessment. Cancer Lett. 2015, 356, 137–144. [Google Scholar] [CrossRef] [PubMed]
- A Lorimore, S.; Coates, P.J.; E Scobie, G.; Milne, G.; Wright, E.G. Inflammatory-type responses after exposure to ionizing radiation in vivo: A mechanism for radiation-induced bystander effects? Oncogene 2001, 20, 7085–7095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulsen, K.L.; Olivero-Verbel, J.; Beggs, K.M.; Ganey, P.E.; Roth, R.A. Trovafloxacin Enhances Lipopolysaccharide-Stimulated Production of Tumor Necrosis Factor-α by Macrophages: Role of the DNA Damage Response. J. Pharmacol. Exp. Ther. 2014, 350, 164–170. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Du, S.; Liu, L.; Gan, F.; Jiang, X.; Wangrao, K.; Lyu, P.; Gong, P.; Yao, Y. Radiation-Induced Bystander Effect can be Transmitted Through Exosomes Using miRNAs as Effector Molecules. Radiat. Res. 2020, 194, 89–100. [Google Scholar] [CrossRef]
- Cai, S.; Shi, G.-S.; Cheng, H.-Y.; Zeng, Y.-N.; Li, G.; Zhang, M.; Song, M.; Zhou, P.-K.; Tian, Y.; Cui, F.-M.; et al. Exosomal miR-7 Mediates Bystander Autophagy in Lung after Focal Brain Irradiation in Mice. Int. J. Biol. Sci. 2017, 13, 1287–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Ma, L.; Ye, Z.; Shi, W.; Zhang, L.; Wang, J.; Yang, H. Radiation-induced bystander effects may contribute to radiation-induced cognitive impairment. Int. J. Radiat. Biol. 2021, 97, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Ma, H.; Lv, C.; Lan, F.; Wang, Y.; Deng, Y. Exosomes and exosomal microRNA in non-targeted radiation bystander and abscopal effects in the central nervous system. Cancer Lett. 2021, 499, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Bellin, G.; Gardin, C.; Ferroni, L.; Chachques, J.C.; Rogante, M.; Mitrečić, D.; Ferrari, R.; Zavan, B. Exosome in Cardiovascular Diseases: A Complex World Full of Hope. Cells 2019, 8, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henning, R.J. Cardiovascular Exosomes and MicroRNAs in Cardiovascular Physiology and Pathophysiology. J. Cardiovasc. Transl. Res. 2021, 14, 195–212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.-R.; Huang, W.-Q. Angiogenic Exosome-Derived microRNAs: Emerging Roles in Cardiovascular Disease. J. Cardiovasc. Transl. Res. 2021, 14, 824–840. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.-C.; Zhou, P.-K. Tissue Reactions and Mechanism in Cardiovascular Diseases Induced by Radiation. Int. J. Mol. Sci. 2022, 23, 14786. https://doi.org/10.3390/ijms232314786
Liu X-C, Zhou P-K. Tissue Reactions and Mechanism in Cardiovascular Diseases Induced by Radiation. International Journal of Molecular Sciences. 2022; 23(23):14786. https://doi.org/10.3390/ijms232314786
Chicago/Turabian StyleLiu, Xiao-Chang, and Ping-Kun Zhou. 2022. "Tissue Reactions and Mechanism in Cardiovascular Diseases Induced by Radiation" International Journal of Molecular Sciences 23, no. 23: 14786. https://doi.org/10.3390/ijms232314786
APA StyleLiu, X.-C., & Zhou, P.-K. (2022). Tissue Reactions and Mechanism in Cardiovascular Diseases Induced by Radiation. International Journal of Molecular Sciences, 23(23), 14786. https://doi.org/10.3390/ijms232314786







