BocODD1 and BocODD2 Regulate the Biosynthesis of Progoitrin Glucosinolate in Chinese Kale
Abstract
:1. Introduction
2. Results
2.1. Determination of Glucosinolate Content in Chinese Kale
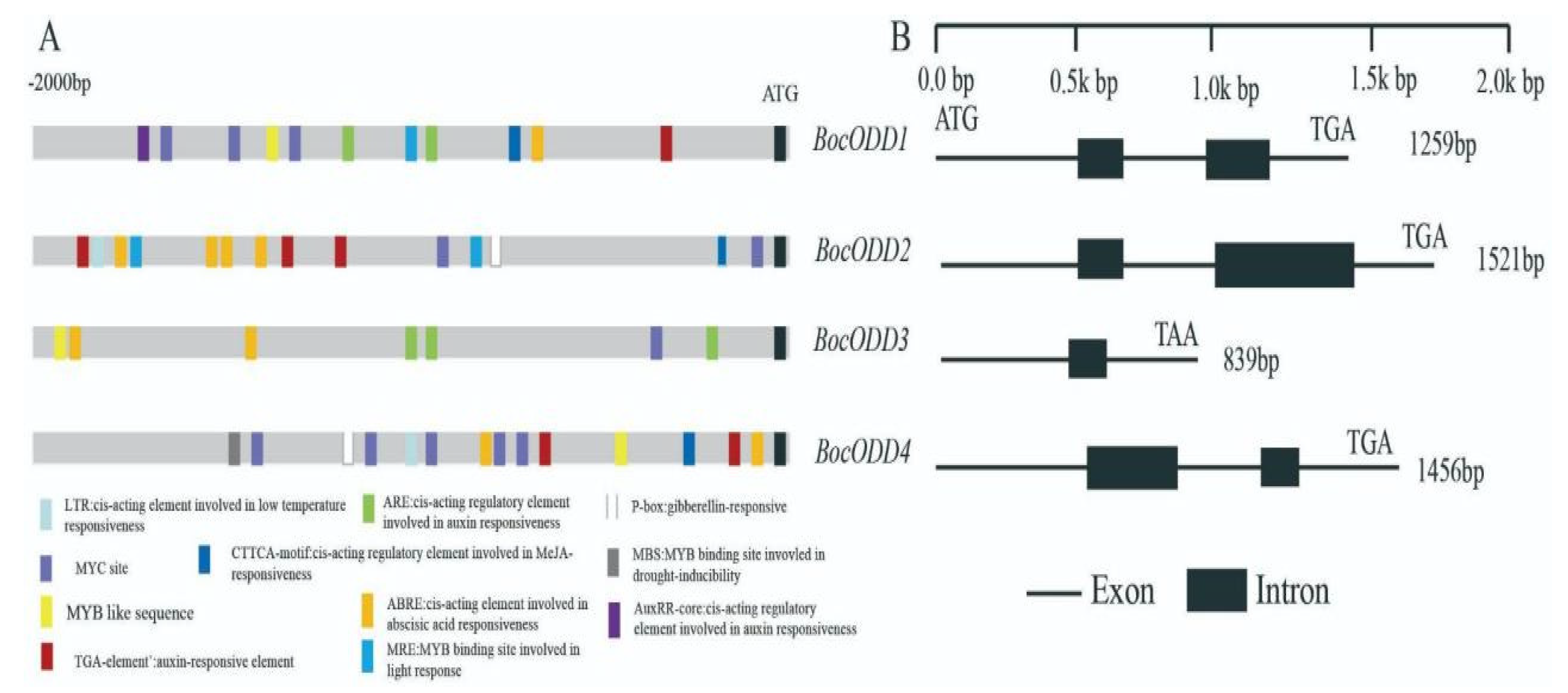
2.2. Isolation of ODD Homologues and the Promoters from Chinese Kale
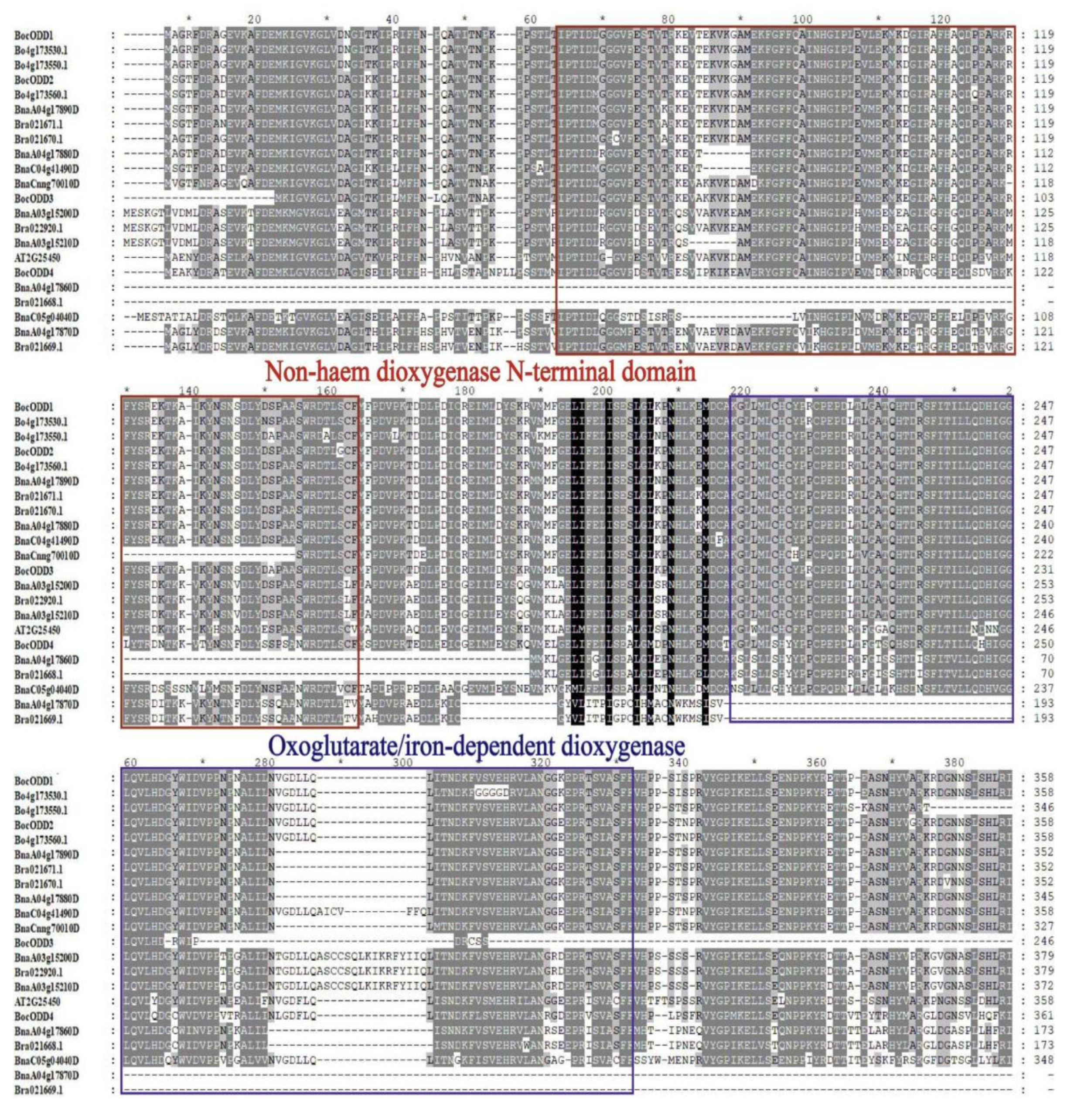
2.3. Amino Acid Sequence Analysis of BocODD Genes
2.4. Expression Analysis of BocODD1/2 in Chinese Kale
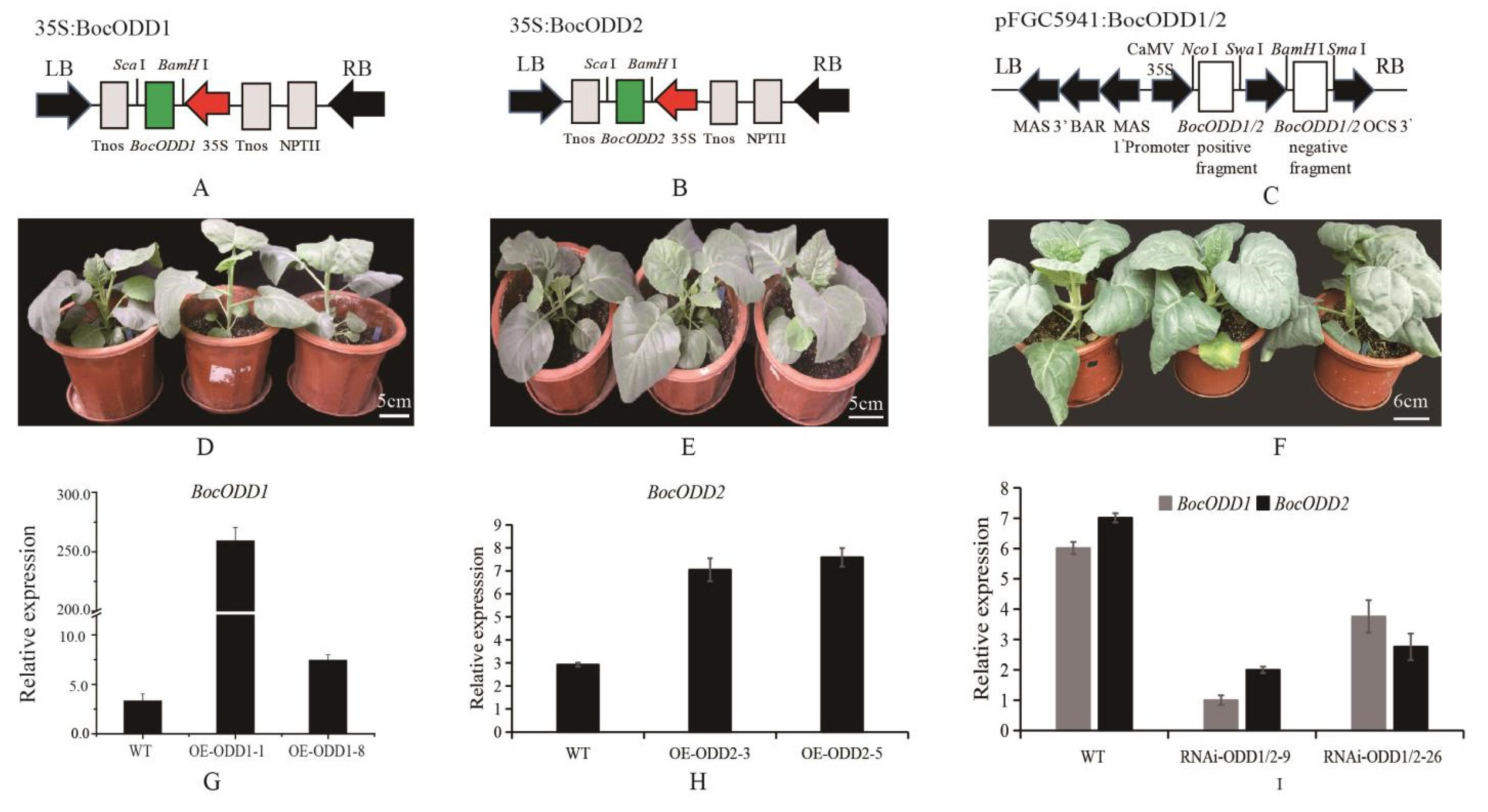
2.5. Function Analysis of BocODD1/2 in the Conversion of 3-Butenyl Glucosinolate to 2-Hydroxy-3-Butenyl Glucosinolate
3. Discussion
4. Materials and Methods
4.1. Gene Cloning and Sequence Analysis
4.2. Expression Analysis
4.3. Glucosinolate Extraction and Analysis
4.4. Transformation of BocODD1 and BocODD2 in Chinese Kale
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, Y.; Li, X.; Kim, S.-J.; Kim, H.; Lee, J.; Kim, H.; Park, S. MYB Transcription Factors Regulate Glucosinolate Biosynthesis in Different Organs of Chinese Cabbage (Brassica Rapa Ssp. Pekinensis). Molecules 2013, 18, 8682–8695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vela-Corcía, D.; Aditya Srivastava, D.; Dafa-Berger, A.; Rotem, N.; Barda, O.; Levy, M. MFS Transporter from botrytis Cinerea Provides Tolerance to blucosinolate-breakdown products and is required for pathogenicity. Nat. Commun. 2019, 10, 2886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heidel-Fischer, H.M.; Kirsch, R.; Reichelt, M.; Ahn, S.-J.; Wielsch, N.; Baxter, S.W.; Heckel, D.G.; Vogel, H.; Kroymann, J. An Insect Counteradaptation against Host Plant Defenses Evolved through Concerted Neofunctionalization. Mol. Biol. Evol. 2019, 36, 930–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abuyusuf, M.; Robin, A.H.K.; Lee, J.-H.; Jung, H.-J.; Kim, H.-T.; Park, J.-I.; Nou, I.-S. Glucosinolate Profiling and Expression Analysis of Glucosinolate Biosynthesis Genes Differentiate White Mold Resistant and Susceptible Cabbage Lines. Int. J. Mol. Sci. 2018, 19, 4037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.-Y.; Wang, Y.; Shen, W.-T.; Zhou, P. Content Determination of Benzyl Glucosinolate and Anti-Cancer Activity of Its Hydrolysis Product in Carica Papaya L. Asian Pac. J. Trop. Med. 2012, 5, 231–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.-Q.; Ho, S.C.; Mo, X.-F.; Lin, F.-Y.; Huang, W.-Q.; Luo, H.; Huang, J.; Zhang, C.-X. Glucosinolate and Isothiocyanate Intakes Are Inversely Associated with Breast Cancer Risk: A Case-Control Study in China. Br. J. Nutr. 2018, 119, 957–964. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Gu, H.; Yu, H.; Zhao, Z.; Sheng, X.; Zhang, X. Genotypic Variation of Glucosinolates in Broccoli (Brassica Oleracea Var. Italica) Florets from China. Food Chem. 2012, 133, 735–741. [Google Scholar]
- Fenwick, G.R.; Roger Fenwick, G.; Heaney, R.K.; John Mullin, W.; VanEtten, C.H. Glucosinolates and Their Breakdown Products in Food and Food Plants. CRC Crit. Rev. Food Sci. Nutr. 1983, 18, 123–201. [Google Scholar] [CrossRef]
- Agerbirk, N.; Olsen, C.E. Glucosinolate Structures in Evolution. Phytochemistry 2012, 77, 16–45. [Google Scholar] [CrossRef]
- Grubb, C.D.; Douglas Grubb, C.; Abel, S. Glucosinolate Metabolism and Its Control. Trends Plant Sci. 2006, 11, 89–100. [Google Scholar] [CrossRef]
- Urbancsok, J.; Bones, A.M.; Kissen, R. Glucosinolate-Derived Isothiocyanates Inhibit Arabidopsis Growth and the Potency Depends on Their Side Chain Structure. Int. J. Mol. Sci. 2017, 18, 2372. [Google Scholar] [CrossRef] [PubMed]
- Kliebenstein, D.J. A Quantitative Genetics and Ecological Model System: Understanding the Aliphatic Glucosinolate Biosynthetic Network via QTLs. Phytochem. Rev. 2009, 8, 243–254. [Google Scholar] [CrossRef]
- Augustine, R.; Bisht, N.C. Biofortification of Oilseed Brassica Juncea with the Anti-Cancer Compound Glucoraphanin by Suppressing GSL-ALK Gene Family. Sci. Rep. 2015, 5, 18005. [Google Scholar] [CrossRef] [Green Version]
- Sønderby, I.E.; Geu-Flores, F.; Halkier, B.A. Biosynthesis of Glucosinolates—Gene Discovery and beyond. Trends Plant Sci. 2010, 15, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Parkin, I.; Magrath, R.; Keith, D.; Sharpe, A.; Mithen, R.; Lydiate, D. Genetics of Aliphatic Glucosinolates. II. Hydroxylation of Alkenyl Glucosinolates in Brassica Napus. Heredity 1994, 72, 594–598. [Google Scholar] [CrossRef] [Green Version]
- Hansen, B.G.; Kerwin, R.E.; Ober, J.A.; Lambrix, V.M.; Mitchell-Olds, T.; Gershenzon, J.; Halkier, B.A.; Kliebenstein, D.J. A Novel 2-Oxoacid-Dependent Dioxygenase Involved in the Formation of the Goiterogenic 2-Hydroxybut-3-Enyl Glucosinolate and Generalist Insect Resistance in Arabidopsis. Plant Physiol. 2008, 148, 2096–2108. [Google Scholar] [CrossRef] [Green Version]
- Lei, J.; Jianjun, L.E.I.; Chen, G.; Chen, C.; Bihao, C.A.O. Germplasm Diversity of Chinese Kale in China. Hortic. Plant J. 2017, 3, 101–104. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Hu, L.-P.; Liu, G.-M.; Zhang, D.-S.; He, H.-J. Evaluation of the Nutritional Quality of Chinese Kale (Brassica Alboglabra Bailey) Using UHPLC-Quadrupole-Orbitrap MS/MS-Based Metabolomics. Molecules 2017, 22, 1262. [Google Scholar] [CrossRef] [Green Version]
- Zou, L.; Yu, B.; Ma, X.-L.; Cao, B.; Chen, G.; Chen, C.; Lei, J. Cloning and Expression Analysis of the BocMBF1c Gene Involved in Heat Tolerance in Chinese Kale. Int. J. Mol. Sci. 2019, 20, 5637. [Google Scholar] [CrossRef] [Green Version]
- Qian, H.; Sun, B.; Miao, H.; Cai, C.; Xu, C.; Wang, Q. Variation of Glucosinolates and Quinone Reductase Activity among Different Varieties of Chinese Kale and Improvement of Glucoraphanin by Metabolic Engineering. Food Chem. 2015, 168, 321–326. [Google Scholar] [CrossRef]
- Si, Y.; Chen, G.; Cao, B.; Feng, E.; Lei, J. Analysis of Components and Contents of Chinese Kale Glucosinolates in Different Genotypes. China Veg. 2009, 6, 7–13. (In Chinese) [Google Scholar]
- Chen, X. Investigation of Glucosinolates and Their Effect Factors in Chinese Brassica Vegetables. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2006. [Google Scholar]
- Blažević, I.; Montaut, S.; Burčul, F.; Olsen, C.E.; Burow, M.; Rollin, P.; Agerbirk, N. Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry 2020, 169, 112100. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J.; Nikmaram, N.; Roohinejad, S.; Khelfa, A.; Zhu, Z.; Koubaa, M. Bioavailability of Glucosinolates and Their Breakdown Products: Impact of Processing. Front. Nutr. 2016, 3, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collett, M.G.; Matthews, Z.M.; Parton, K.H. Hepatotoxicity of Two Progoitrin-Derived Nitriles in New Zealand White Rabbits. Toxins 2020, 12, 695. [Google Scholar] [CrossRef] [PubMed]
- Araújo, W.L.; Martins, A.O.; Fernie, A.R.; Tohge, T. 2-Oxoglutarate: Linking TCA Cycle Function with Amino Acid, Glucosinolate, Flavonoid, Alkaloid, and Gibberellin Biosynthesis. Front. Plant Sci. 2014, 5, 552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prescott, A.G.; John, P. Dioxygenases: Molecular Structure and Role in Plant Metabolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 245–271. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Li, G.; Chen, G.; Lei, J.; Cao, B.; Chen, C. Genome-Wide Identification and Expression Profiling of 2OGD Superfamily Genes from Three Plants. Genes 2021, 12, 1399. [Google Scholar] [CrossRef]
- Li, Y.; Sawada, Y.; Hirai, A.; Sato, M.; Kuwahara, A.; Yan, X.; Hirai, M.Y. Novel Insights into the Function of Arabidopsis R2R3-MYB Transcription Factors Regulating Aliphatic Glucosinolate Biosynthesis. Plant Cell Physiol. 2013, 54, 1335–1344. [Google Scholar] [CrossRef] [Green Version]
- Klopsch, R.; Witzel, K.; Börner, A.; Schreiner, M.; Hanschen, F.S. Metabolic Profiling of Glucosinolates and Their Hydrolysis Products in a Germplasm Collection of Brassica Rapa Turnips. Food Res. Int. 2017, 100, 392–403. [Google Scholar] [CrossRef]
- Tsunoda, T.; Grosser, K.; Dam, N.M. Locally and Systemically Induced Glucosinolates Follow Optimal Defence Allocation Theory upon Root Herbivory. Funct. Ecol. 2018, 32, 2127–2137. [Google Scholar] [CrossRef]
- Wu, S.; Lei, J.; Chen, G.; Chen, H.; Cao, B.; Chen, C. De Novo Transcriptome Assembly of Chinese Kale and Global Expression Analysis of Genes Involved in Glucosinolate Metabolism in Multiple Tissues. Front. Plant Sci. 2017, 8, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, E.D.; Gallagher, T.F. Characterization of Auxin-Induced ARRO-1 Expression in the Primary Root of Malus Domestica. J. Exp. Bot. 2000, 51, 1765–1766. [Google Scholar] [CrossRef] [PubMed]
- Butler, E.D.; Gallagher, T.F. Isolation and Characterization of a cDNA Encoding a Novel 2-Oxoacid-Dependent Dioxygenase Which Is up-Regulated during Adventitious Root Formation in Apple (Malus Domestica “Jork 9”) Stem Discs. J. Exp. Bot. 1999, 50, 551–552. [Google Scholar]
- Guo, L.; Yang, R.; Zhou, Y.; Gu, Z. Heat and Hypoxia Stresses Enhance the Accumulation of Aliphatic Glucosinolates and Sulforaphane in Broccoli Sprouts. Eur. Food Res. Technol. 2016, 242, 107–116. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [Green Version]
- Yin, L.; Chen, H.; Cao, B.; Lei, J.; Chen, G. Molecular Characterization of MYB28 Involved in Aliphatic Glucosinolate Biosynthesis in Chinese Kale (Var. Bailey). Front. Plant Sci. 2017, 8, 1083. [Google Scholar] [CrossRef] [Green Version]
- Doheny-Adams, T.; Redeker, K.; Kittipol, V.; Bancroft, I.; Hartley, S.E. Development of an efficient glucosinolate extraction method. Plant Methods 2017, 13, 17. [Google Scholar] [CrossRef]
- ISO 9167-1-1992 NA 057-05-05 AA—Joint committee of DIN and DGF for the Analysis of Fats, Oils and Products Thereof, Related and Primary Products. (2012): Rapeseed—Determination of Glucosinolate Content—Part 1: Method Using High-Performance Liquid Chromatography (ISO 9167–1:1992/DAM 1:2012), German Version EN ISO 9167-1:1995/prA1: 2012.). Available online: https://standards.iteh.ai/catalog/standards/cen/f3b8075e-e195-4ec2-a750-6245faa27809/en-iso-9167-2019 (accessed on 23 October 2022).
- Lin, L.; MingMing, K.; FaYin, Y.; GuoHua, Z. Effects of blanching on the content of glucosinolates and their indole degradation products in four selected Brassica vegetables. Food Ferment. Ind. 2018, 44, 160–165. (In Chinese) [Google Scholar]




| Strain | Generation | PRO (mg/g DW) | NAP (mg/g DW) | The Ratio of PRO/NAP |
|---|---|---|---|---|
| WT1 | 0.20 | 7.44 | 0.03 | |
| OE-ODD1-1-2 | T1 | 0.62 ** | 8.73 | 0.07 ** |
| OE-ODD1-8-11 | T1 | 1.17 ** | 8.77 | 0.13 ** |
| OE-ODD2-3-11 | T1 | 1.40 ** | 6.47 | 0.19 ** |
| OE-ODD2-5-8 | T1 | 1.18 ** | 6.34 | 0.22 ** |
| WT2 | 0.08 | 0.99 | 0.08 | |
| RNAi-9 | T0 | 0.04 ** | 2.47 ** | 0.02 ** |
| RNAi-26 | T0 | 0.04 ** | 3.36 ** | 0.01 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Zhang, T.; Wang, Y.; Chen, M.; Yang, J.; Li, F.; Deng, Y.; Zhu, Z.; Lei, J.; Chen, G.; et al. BocODD1 and BocODD2 Regulate the Biosynthesis of Progoitrin Glucosinolate in Chinese Kale. Int. J. Mol. Sci. 2022, 23, 14781. https://doi.org/10.3390/ijms232314781
Wu S, Zhang T, Wang Y, Chen M, Yang J, Li F, Deng Y, Zhu Z, Lei J, Chen G, et al. BocODD1 and BocODD2 Regulate the Biosynthesis of Progoitrin Glucosinolate in Chinese Kale. International Journal of Molecular Sciences. 2022; 23(23):14781. https://doi.org/10.3390/ijms232314781
Chicago/Turabian StyleWu, Shuanghua, Ting Zhang, Yudan Wang, Muxi Chen, Jianguo Yang, Fei Li, Ying Deng, Zhangsheng Zhu, Jianjun Lei, Guoju Chen, and et al. 2022. "BocODD1 and BocODD2 Regulate the Biosynthesis of Progoitrin Glucosinolate in Chinese Kale" International Journal of Molecular Sciences 23, no. 23: 14781. https://doi.org/10.3390/ijms232314781
APA StyleWu, S., Zhang, T., Wang, Y., Chen, M., Yang, J., Li, F., Deng, Y., Zhu, Z., Lei, J., Chen, G., Cao, B., & Chen, C. (2022). BocODD1 and BocODD2 Regulate the Biosynthesis of Progoitrin Glucosinolate in Chinese Kale. International Journal of Molecular Sciences, 23(23), 14781. https://doi.org/10.3390/ijms232314781






