Enzymatically Modified Isoquercitrin: Production, Metabolism, Bioavailability, Toxicity, Pharmacology, and Related Molecular Mechanisms
Abstract
:1. Introduction
2. Structure, Production, and Properties
2.1. Structure
2.2. Production
2.3. Physicochemical Properties
3. Application in the Food Industry
4. Metabolism, Absorption, and Bioavailability
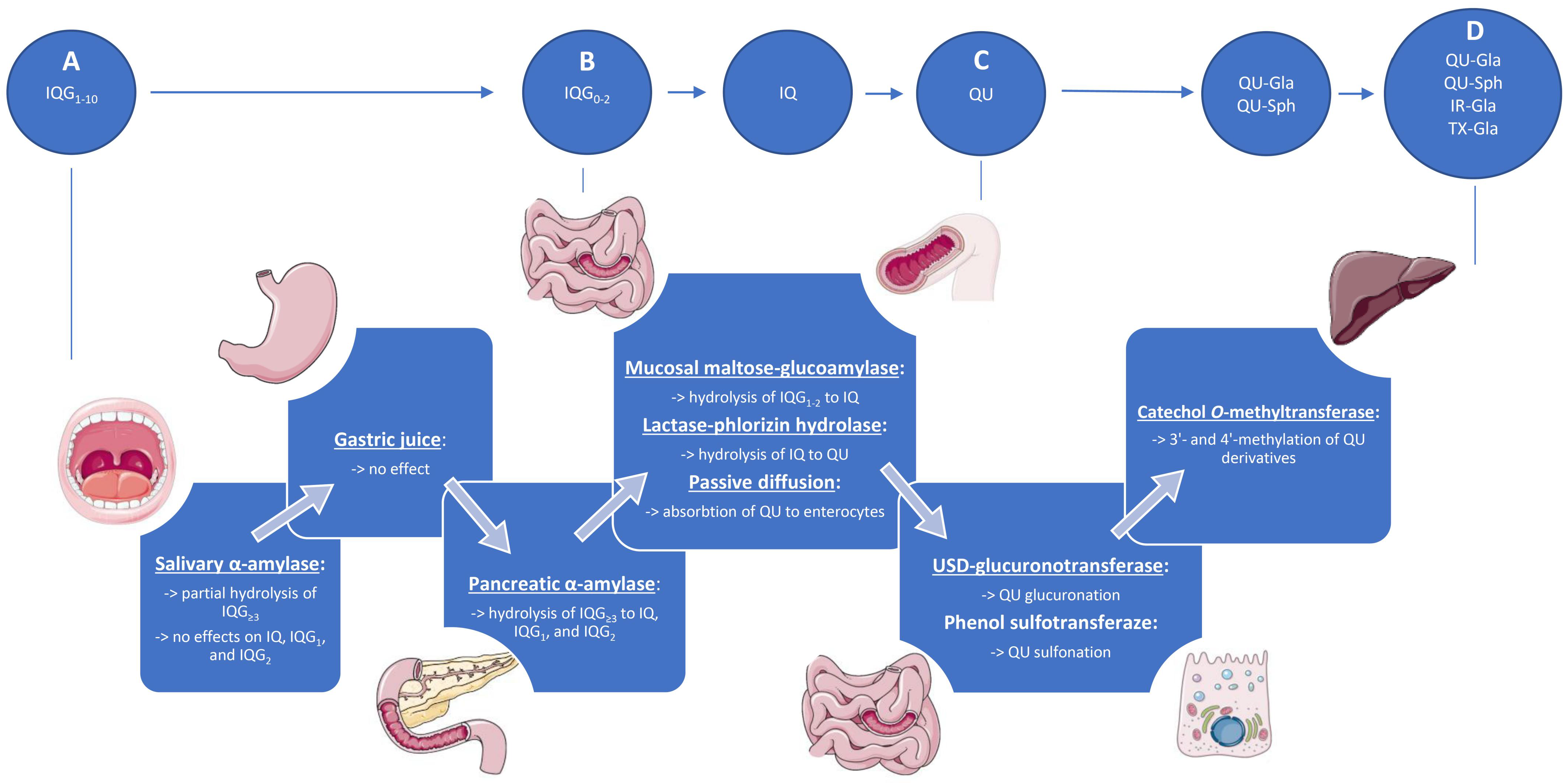
4.1. Upper Gastrointestinal Tract
4.2. Lower Gastrointestinal Tract
4.3. Plasma Metabolites and Bioavailability
4.4. Elimination and Accumulation
5. Safety
5.1. General Toxicity
5.2. Genotoxicity
| Model | Sample Size | Dosage | Treatment Time | Effects | Reference |
|---|---|---|---|---|---|
| Acute toxicity | |||||
| Four-week-old male and female Sprague-Dawley rats | 10/group/sex | 16, 20, 25 mg/kg p.o. | Single dose + 14-day follow up | No mortality related to the treatment No toxic effects related to the treatment | [15] |
| Subacute toxicity | |||||
| Juvenile Göttingen minipigs | 5/group | 100, 300, 1000 mg/kg/day p.o. | 10 days | No toxic effects related to the treatment | [46] |
| 7/group | 100, 300, 1000 mg/kg/day p.o. | 4 weeks | No clinical signs nor mortality related to the treatment Tendency for lower body weight gain Darker urine Yellow coloration of bones (femur and calvarium) | ||
| 4-week-old male and female F344/DuCrj rats | 5/group/sex | 0.625, 1.25, 2.5, 5% in the diet | 4 weeks | No clinical signs nor mortality related to the treatment Yellow coloration of bones (cranium, forelimb, and hindlimb bone) Tendency for lower body weight (>2.5%) Tendency for lower food and water consumption (>2.5%, males) No impact on organ weights | [15] |
| Subchronic toxicity | |||||
| 4-week-old male and female F344 rats | 10/group/sex | 0.3, 0.625, 1.25, 2.5% in the diet | 13 weeks | No clinical signs nor mortality related to the treatment Tendency for lower body weight (2.5%, females) No impact on food and water consumption Yellow coloration of the urine Higher ketones level in the urine (2.5%, males) Yellow coloration of bones (femur and cranium) Higher reticulocytes count Increased serum levels of γ-GTP and BUN Mild changes in various organ weights without morphological alterations | [47] |
| 5-week-old male and female Sprague-Dawley rats | 10/group/sex | 0.5, 1.5, 3, 5% in the diet | 90 days | No clinical signs nor mortality related to the treatment No impact on body weight No impact on food consumption Yellow coloration of bones (femur, calvarium, maxilla) Mild changes in various organ weights without microscopic abnormalities No changes in urinalysis No changes in automated motor activity | [45] |
| Chronic toxicity/Carcinogenicity | |||||
| 5-week-old male and female F344/DuCrj rats | 50/group | 0.5, 1.5% in the diet | 104 weeks | No clinical signs nor mortality related to treatment No impact on body weight No impact on food and water consumption Decreased serum levels of γ-GTP Changes in various organ weight unrelated to neoplasia No evidence of carcinogenicity | [48] |
| 5-week-old male and female rasH2 mice | 25/group/sex | 1.5, 3, 5% in the diet | 6 months | No clinical signs nor mortality related to the treatment Lower body weight (females, 3%) Higher food consumption (males, 5%) Mild changes in various organ weights without pathologic findings Sporadic non-dose dependent hematological changes Yellow coloration of bones (femur, calvarium) No changes in urinalysis related to the treatment No evidence of carcinogenicity | [49] |
| Maternal toxicity and embryotoxicity | |||||
| New Zealand white female rabbits | 22/group | 250, 500, 1000 mg/kg/day p.o. | 6–28 gestation day | No maternal toxicity No embryotoxic effects No teratogenic effects No effect on reproductive parameters | [52] |
| 48/group | 500, 1000 mg/kg/day p.o. | ||||
| Genotoxicity | |||||
| Male and female B6C3F1 mice Male and female Sprague Dawley rats | 5/group | 1000, 1500, 2000 mg/kg/day p.o. | 3 days | No clinical signs related to the treatment Slight mass loss in male animals (−3.3%) No biologically relevant increase in the frequency of MN-RET No biologically relevant increase in DNA damage in liver, duodenum, and stomach tissue | [51] |
| Male transgenic mice (MutaTM Mouse) | 5/group | 5%, 1.5%, 0.5% in diet | 28 days | Unnaturally yellow urine No other changes in clinical signs No impact on body weight, food consumption No differences in the organ weights and relative organ weights | [51] |
| Allergenic effects | |||||
| 7-week-old female CBA/J mice | 5/group | 10%, 25%, 50% in DMF topically | 3 days | No abnormal clinical observations, no mortality No significant dermal irritation symptoms No impact on the size of lymph nodes No sensitizing effects found by Local Lymph Node assay | [53] |
5.3. Embryotoxicity
5.4. Allergenic Effects
6. Bioactivity
6.1. Cardioprotective and Metabolic Effects
6.2. Anti-Allergic and Anti-Inflammatory Effects
6.3. Chemopreventive Effects
| Animal | Model | Treatments | Sample Size | Effects of EMIQ Treatment | Reference |
|---|---|---|---|---|---|
| Cardioprotective and metabolic effects | |||||
| 4-week-old male SHR/Izm rats | Spontaneous hypertension | EMIQ (3, 26 mg/kg p.o.), QU (1.2, 10.4 mg/kg p.o.), diltiazem (120 mg/kg p.o.) | 10/group | ↓ systolic blood pressure No effect on diastolic blood pressure No effect on heart rate | [20] |
| 6-week-old male apoE-deficient mice | Diet-induced atherosclerosis | EMIQ (0.026% in the diet) | 7–10/group | No effect of food intake, body weight, and lipid profile ↓ (~50%) area of aortic atherosclerotic lesions ↓ (~24%) plaque area in the aortic sinus ↓ lipid peroxidation product (4-HNE) in the plaque area ↓ macrophage accumulation in the plaque ↑ plaque stabilization (↑ collagen levels and smooth muscle cell accumulation) | [21] |
| 4-week-old male ICR mice | Diet-induced obesity and hyperglycemia | EMIQ, QU, IQ, RT (0.1% in the diet) | 5/group | ↓ weight gain ↓ glucose, insulin, ↓ HOMA-IR ↓ total cholesterol, triglycerides, NEFA ↑ AMPK phosphorylation, no effect on AMPK expression ↑ GLUT4 translocation to the plasma membrane of skeletal muscle No effect on JAK/STAT-pathways ↑ ACC phosphorylation in white adipose tissue and the liver ↓ adipocyte differentiation ↓ UCP1, PGC-1α, PRDM 16 expression in white adipose tissue ↓ FAS, SREBP1, ↑ CPT1, PPARα expression in the liver | [54] |
| 5-week-old male Wistar-ST rats | Diet-induced obesity and hyperglycemia | EMIQ (0.7% in the diet) Soybean fiber (5% in the diet) EMIQ + soybean fiber (0.7% + 5% in the diet) | 7/group | ↓ visceral fat (soybean fiber) ↓ glucose, insulin, HOMA-IR (EMIQ + soybean fiber) | [56] |
| 6-week-old male C57BL/6 mice | Diet-induced obesity | EMIQ + heat transformed green tea (50 + 50 mg/kg, 100 + 100 mg/kg p.o.) Mirabegron (10 mg/kg p.o.) | 6/group | ↓ body weight and fat mass ↓ adipocyte size No effect on fecal fat content ↑ VO2, VCO2, and energy expenditure ↑ brown adipocyte markers and mitochondrial proteins (UCP1, COXIV) ↑ MCAD in brown and white adipose tissue ↑ glucose tolerance | [55] |
| Anti-inflammatory and anti-allergic effects | |||||
| 6-week-old male Balb/c mice | Sensitization with ovalbumin | EMIQ | 7–8/group | ↓ passive cutaneous anaphylaxis (4 mmol/kg) | [18] |
| 8-week-old male Swiss albino mice | Histamine-induced paw edema | EMIQ (50, 100, 200 mg/kg p.o.); sulfasalazine (100 mg/kg p.o.) | 5/group | ↓ paw edema (at 50, 100 mg/kg) comparable to SSZ ↓ paw edema (at 200 mg/kg) better than SSZ | |
| Cpd 48/80-induced local paw edema | EMIQ (50, 100 mg/kg p.o.); sulfasalazine (100 mg/kg p.o.) | 5/group | ↓ paw edema (at 50, 100 mg/kg) comparable to SSZ | [19] | |
| Cpd 48/80-induced systemic anaphylaxis | EMIQ (50, 100 mg/kg p.o.); sulfasalazine (100 mg/kg p.o.) | 10/group | ↑ survival to 100% (at 100 mg/kg) ↓ histamine release (at 50, 100 mg/kg) ↓ mast cells’ degranulation (at 50, 100 mg/kg) | ||
| WRS-induced acute gastric ulcer | EMIQ (50, 100 mg/kg p.o.); sulfasalazine (100 mg/kg p.o.) | 5/group | Protection of gastric mucosa, ↓ hemorrhage, ↓ pathological changes, ↓ loss of superficial mucous cells ↑ reduced glutathione ↓ malondialdehyde, nitric oxide | ||
| 4-week-old female BALB/ cAnNCrlCrlj mice | Acute colitis induced by dextran sodium sulphate | EMIQ (1.5% in the diet) | 4/group | ↓ DSS-mediated decrease in BrdU-positive cell number ↓ colitis severity | [58] |
| 4-week-old female BALB/ cAnNCrlCrlj mice | Acute colitis induced by dextran sodium sulphate | EMIQ (1.5% in the diet) | 12/group | ↓ diarrhea ↓ IL-6, TNF-α, keratinocyte-derived cytokine ↓ mucosal injury, ↑ mucinous production | [31] |
| Chemopreventive effects | |||||
| 5-week-old male F344/NSlc rats | Liver cancer induced by N-diethylnitrosamine and oxfendazole | EMIQ (0.2% in drinking water) Melatonin (0.01% in drinking water) | 5–12/group | ↓ number of GST-P-positive foci ↓ transcription of Cyp2b2 and Me1 | [23] |
| 6-week-old male F344/N rats | Liver cancer induced by N-diethylnitrosamine and β-naphtoflavone | EMIQ (0.2% in drinking water) | 11–12/group | ↓ area and number of GST-P-positive foci ↓ COX-2-positive cells ↓ mRNA expression of Gstm1, Serpine1, Cox2 and Nfkbia ↑ mRNA expression of Yc2 | [24] |
| 4-week-old male F344/DuCrlCrlj rats | Liver cancer induced by N-diethylnitrosamine | EMIQ (1, 0.1, 0.01% in the diet) IQ (1, 0.1, 0.01% in diet) Purple corn color (1, 0.1, 0.01% in diet) | 16–22/group | No effect on body weight and liver weight Negative dose–effect correlation for number of GST-P-positive foci ↑ antioxidant capacity of serum (at 1% EMIQ in the diet) | [32] |
| 5-week-old male F344/N rats | Liver cancer induced by N-diethylnitrosamine and phenobarbital | EMIQ (0.2% in drinking water) | 11–15/group | ↓ area and number of GST-P-positive foci ↓ PCNA-positive (proliferating) liver cells No changes in ROS, TBARS, and 8-OHdG ↓ transcription of Mapkapk3 and Mrp2 ↓ nuclear translocation of CAR | [26] |
| 5-week-old male F344/NSlc rats | Liver cancer induced by N-diethylnitrosamine and thioacetamide | EMIQ (0.5% in the diet) | 11–12/group | ↓ area and number of GST-P-positive foci ↓ PCNA-positive (proliferating) liver cells ↓ ED2-, COX-2-, and HO-1-positive hepatic macrophages ↓ CD3-positive lymphocytes ↑ TUNEL-, DR5-, and 4-HNE-positive liver cells inside GST-P-positive foci ↓ TUNEL- and DR5-positive liver cells outside GST-P-positive foci ↓ transcription for Tnfrsf10b No changes in the transcription level of antioxidant enzymes (Aldh1a1, Gstm1) | [27] |
| 5-week-old male F344/NSlc rats | Liver cancer induced by N-diethylnitrosamine and piperonyl butoxide | EMIQ (0.2% in drinking water) | 11–12/group | ↓ area and number of GST-P-positive foci ↓ Ki-67-positive (proliferating) liver cells ↓ transcription for Cyp1a1 (no statistical significance) ↑ transcription for Mapk8, Mapk14, and Tp53 No impact on microsomal ROS production ↓ TBARS in the liver | [29] |
| 5-week-old male F344/N rats | Liver cancer induced by N-diethylnitrosamine and malachite green + high-fat diet | EMIQ (0.5% in drinking water) | 9–13/group | ↑ liver weight ↓ total cholesterol and ALP No impact on aera and number of GST-P-positive foci No impact on proliferating liver cells (Ki-67-positive) ↓ p22phox-positive cells in GST-P-positive foci | [30] |
| 4-week-old male F344/NSlc rats | Ochratoxin A-induced renal carcinogenesis | EMIQ (0.2% in drinking water) | 15–16/group | No effect on the number of karyomegalic cells No effect on the number of immunoreactive cells for any marker ↓ transcription of Mapk8 and Txn1 | [61] |
| 4-week-old female BALB/cAnNCrlCrlj mice | Inflammation associated colon carcinogenesis induced by azoxymethane and dextran sodium sulphate | EMIQ (1.5% in the diet) | 8/group | ↓ colon weight ↓ lesions with mucin-depleted foci and aberrant crypt foci ↓ multiplicity of the lesions ↑ β-catenin expression in the proliferating cell ↓ Iba1 and cyclin D1 scores | [58] |
| Neurological effects | |||||
| Mated female Slc:SD rats and their offspring | Developmental stage of rats | EMIQ (0.5% in the diet) | 19/group | No impact on short-term spatial memory (Y-maze test) ↑ fear extinction learning (↓ freezing time in the 3rd trial) ↑ FOS-positive cells in the granule cell layer (hippocampus) ↑ transcription of Chrm2, Slc17a6, Fos, Ntrk2, and Kif21b in the hippocampal dentate gyrus ↑ transcription of Grin2d in amygdala ↑ transcription of Chrna7, Kif21b in infralimbic cortex | [62] |
| Mated female Slc:SD rats and their offspring | Developmental stage of rats | EMIQ (0.5% in the diet) | 10/group | No differences in open-field test and object recognition test ↑ fear extinction learning (↓ freezing time) ↑ FOS- and p-ERK1/2-positive cells in the infralimbic cortex ↑ p-ERK1/2-positive cells in the prelimbic cortex Significant changes in genes transcription in hippocampal dentate gyrus, prelimbic and infralimbic cortex, and amygdala (Ephs/Ephrins, glutamate receptors, glutamate transporters, nitric oxide synthases, angiogenesis-related proteins, and others) | [63] |
| Mated female Slc:SD rats and their offspring | LPS-induced autism-like behaviors and disruptive hippocampal neurogenesis | EMIQ (0.25, 0.5% in the diet) | 10/group | ↑ moving distance in social interaction test during adolescent stage ↑ freezing time in contextual fear conditioning test ↓ number of GFAP-positive astrocytes ↑ recovery of mature granule cells Amelioration of fear memory acquisition ↓ neuroinflammation | [64] |
| Musculotropic effects | |||||
| 6-week-old male ICR mice | Overload-induced hypertrophy initiated by ablation of the synergistic gastrocnemius and soleus muscles | EMIQ (4 mg/kg/day p.o.) | 9/group | ↑ cross-sectional area of the plantaris muscle ↑ minimal fiber diameter of the plantaris muscle | [65] |
| 19-month old female C57BL/6J mice | Old age | EMIQ (0.3% in the diet) | 7–9/group | No effect on muscles weight ↑ fat oxidation and energy consumption during exercise No effect on the glycolytic metabolites in the muscles ↑ antioxidant capacity of plasma ↑ transcription of Gpx ↓ carbonylated protein content in the muscles | [66] |
6.4. Neurological Effects
6.5. Musculotropic Effects
7. Methods
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 4-HNE | 4-hydroxy-2-nonenal |
| β3-AR | beta-3 adrenergic receptor |
| 8-OHdG | 8-hydroxydeoxyguanosine |
| ACC | acetyl-CoA carboxylase |
| Aldh1a1 | aldehyde dehydrogenase family 1 member A1 |
| AGIQ | alpha-glycosyl isoquercitrin |
| ALP | alkaline phosphatase |
| AMP | adenosine monophosphate |
| AMPK | 5′AMP-activated protein kinase |
| apoE | apolipoprotein E |
| AUC | area under the plasma concentration–time curve |
| BHT | butylated hydroxytoluene |
| BrdU | 5-bromo-20-deoxyuridine |
| BUN | blood urea nitrogen |
| CAR | constitutive androstane receptor |
| CD3 | cluster of differentiation 3 |
| Chrm2 | cholinergic receptor, muscarinic 2 |
| Chrna7 | cholinergic receptor nicotinic alpha 7 subunit |
| Cmax | maximum plasma concentration |
| Cox2 | cyclooxygenase-2; prostaglandin-endoperoxide synthase 2 |
| COXIV | cytochrome c oxidase subunit 4 |
| Cpd | compound |
| CPT1 | carnitine palmitoyltransferase 1 |
| Cyp1a1 | cytochrome P450, family 1, subfamily a, polypeptide 1 |
| Cyp2b2 | cytochrome P450, family 2, subfamily b, polypeptide 2 |
| DEN | N-diethylnitrosamine |
| DMF | N,N-dimethyl formamide |
| DR5 | death receptor 5 |
| DSS | dextran sulphate sodium |
| ED2 | liver macrophages of the ED2 phenotype |
| EFSA | European Food Safety Authority |
| ERK | extracellular signal-regulated protein kinase |
| EMIQ | enzymatically modified isoquercitrin |
| FAS | fatty acid synthase |
| FOS | Fos proto-oncogene |
| GFAP | glial fibrillary acidic protein |
| GLP-1 | glucagon-like peptide-1 |
| GLUT4 | glucose transporter type 4 |
| GMP | good manufacturing practices |
| Gpx | glutathione peroxidase |
| GRAS | generally recognized as safe |
| Grin2d | glutamate ionotropic receptor NMDA type subunit 2D |
| Gstm1 | glutathione S-transferase, mu 1 |
| GST-P | glutathione S-tranferase placental form |
| γ-GTP | gamma-glutamyl transpeptidase |
| HO-1 | heme oxygenase-1 |
| HOMA-IR | Homeostatic Model Assessment-Insulin Resistance; homeostatic model assessment for insulin resistance |
| Iba1 | ionized calcium-binding adapter molecule 1 |
| IFN-γ | type II interferon |
| IgE | immunoglobulin E |
| IL | interleukin |
| IQ | isoquercitrin |
| IQG | isoquercitrin with α-glucose moiety |
| IQGn | isoquercitrin win n α-glucose moieties |
| JAK | Janus kinase |
| LD50 | median lethal dose |
| LPH | lactase-phlorizin hydrolase |
| LPS | lipopolysaccharide |
| Kif21b | kinesin family member 21B |
| MAPK | mitogen-activated protein kinase |
| Mapk8 | mitogen-activated protein kinase 8 |
| Mapk14 | mitogen-activated protein kinase 14 |
| Mapkapk3 | mitogen-activated protein kinase-activated proteinkinase 3 |
| MCAD | medium-chain acyl-CoA dehydrogenase |
| MGAM | mucosal maltose-glucoamylase |
| Me1 | malic enzyme 1 |
| MN-RET | micronucleated reticulocyte(s) |
| Mrp2 | multidrug resistance-associated protein 2; ATP-binding cassette, subfamily C, member 2 |
| NADPH | reduced form of nicotinamide adenine dinucleotide phosphate |
| NEFA | non-esterified fatty acid |
| Nfkbia | nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha |
| NOAEL | no-observed-adverse-effect level |
| NOX | NADPH oxidase |
| Ntrk2 | neurotrophic receptor tyrosine kinase 2 |
| OECD | Organisation for Economic Cooperation and Development |
| p.o. | per os; oral administration |
| PCNA | proliferating cell nuclear antigen |
| p-ERK1/2 | phospho-ERK1/2 |
| PGC-1α | PPAR-γ coactivator 1α |
| PPARα | peroxisome proliferator-activated receptor α |
| PRDM 16 | PR-domain containing protein 16 |
| QU | quercetin |
| ROS | reactive oxygen species |
| RT | rutin |
| Serpine1 | serine (or cysteine) peptidase inhibitor, clade E, member 1 |
| SGLT 1 | sodium-dependent glucose transporter 1 |
| SHR | spontaneously hypertensive rat |
| Slc17a6 | solute carrier family 17 member 6 |
| SREBP1 | sterol regulatory element-binding protein 1 |
| SSZ | sulfasalazine |
| STAT | signal transducer and activator of transcription proteins |
| TBARS | thiobarbituric acid reactive substances |
| TGF-β | transforming growth factor β |
| Tmax | time to reach Cmax |
| TNF-α | tumor necrosis factor α |
| Tnfrsf10b | tumor necrosis factor receptor superfamily, member 10b |
| Tp53 | tumor protein p53 |
| TUNEL | terminal deoxynucleotidyl transferase-mediated nick end labelining |
| Txn1 | thioredoxin 1 |
| UCP 1 | uncoupling protein 1 |
| UDP | uridine 5′-diphosphate |
| U.S. FDA | The United States Food and Drug Administration |
| UV-C | ultraviolet C light |
| VCO2 | carbon dioxide production |
| VO2 | oxygen consumption |
| WRS | water-restraint stress |
| Yc2 | glutathione S-transferase Yc2 subunit |
References
- Brodowska, K.M. Natural Flavonoids: Classification, Potential Role, and Application of Flavonoid Analogues. Eur. J. Biol. Res. 2017, 7, 108–123. [Google Scholar] [CrossRef]
- Santos, E.L.; Maia, B.H.L.N.S.; Ferriani, A.P.; Teixeira, S.D. Flavonoids: Classification, Biosynthesis and Chemical Ecology. In Flavonoids—From Biosynthesis to Human Health; Justino, G.C., Ed.; InTechOpen: London, UK, 2017; ISBN 978-953-51-3423-7. [Google Scholar]
- Frutos, M.J.; Rincón-Frutos, L.; Valero-Cases, E. Rutin. In Nonvitamin and Nonmineral Nutritional Supplements; Elsevier: Amsterdam, The Netherlands, 2019; pp. 111–117. [Google Scholar]
- Phenol-Explorer 3.6. Available online: http://phenol-explorer.eu (accessed on 12 August 2022).
- Perez-Vizcaino, F.; Duarte, J. Flavonols and cardiovascular disease. Mol. Asp. Med. 2010, 31, 478–494. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [Green Version]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosseini, A.; Razavi, B.M.; Banach, M.; Hosseinzadeh, H. Quercetin and metabolic syndrome: A review. Phytother. Res. 2021, 35, 5352–5364. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Woo, J.-B.; Ryu, S.-I.; Moon, S.-K.; Han, N.S.; Lee, S.-B. Glucosylation of flavonol and flavanones by Bacillus cyclodextrin glucosyltransferase to enhance their solubility and stability. Food Chem. 2017, 229, 75–83. [Google Scholar] [CrossRef]
- Makino, T.; Shimizu, R.; Kanemaru, M.; Suzuki, Y.; Moriwaki, M.; Mizukami, H. Enzymatically Modified Isoquercitrin, α-Oligoglucosyl Quercetin 3-O-Glucoside, Is Absorbed More Easily than Other Quercetin Glycosides or Aglycone after Oral Administration in Rats. Biol. Pharm. Bull. 2009, 32, 2034–2040. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Meng, X.; de Leeuw, T.C.; Poele, E.M.T.; Pijning, T.; Dijkhuizen, L.; Liu, W. Enzymatic glucosylation of polyphenols using glucansucrases and branching sucrases of glycoside hydrolase family 70. Crit. Rev. Food Sci. Nutr. 2021, 1–21. [Google Scholar] [CrossRef]
- Gupta, A.; Kagliwal, L.D.; Singhal, R.S. Biotransformation of Polyphenols for Improved Bioavailability and Processing Stability. Adv. Food Nutr. Res. 2013, 69, 183–217. [Google Scholar] [CrossRef]
- Murota, K.; Matsuda, N.; Kashino, Y.; Fujikura, Y.; Nakamura, T.; Kato, Y.; Shimizu, R.; Okuyama, S.; Tanaka, H.; Koda, T.; et al. α-Oligoglucosylation of a sugar moiety enhances the bioavailability of quercetin glucosides in humans. Arch. Biochem. Biophys. 2010, 501, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health Labour and Welfare Japan’s Specifications and Standards for Food Additives 2018. Available online: https://www.ffcr.or.jp/en/upload/Japan%27s%20Specifications%20and%20Standards%20for%20Food%20Additives%208th%20Edition.pdf (accessed on 22 October 2022).
- US FDA Agency Response Letter GRAS Notice No. GRN0-220 [Alpha-Glucosyl Isoquercitrin]. Available online: https://www.accessdata.fda.gov/scripts/fdcc/index.cfm?set=GrASNotices&id=2 (accessed on 22 October 2022).
- Kawai, M.; Hirano, T.; Arimitsu, J.; Higa, S.; Kuwahara, Y.; Hagihara, K.; Shima, Y.; Narazaki, M.; Ogata, A.; Koyanagi, M.; et al. Effect of Enzymatically Modified Isoquercitrin, a Flavonoid, on Symptoms of Japanese Cedar Pollinosis: A Randomized Double-Blind Placebo-Controlled Trial. Int. Arch. Allergy Immunol. 2009, 149, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Kawai, M.; Arimitsu, J.; Ogawa, M.; Kuwahara, Y.; Hagihara, K.; Shima, Y.; Narazaki, M.; Ogata, A.; Koyanagi, M.; et al. Preventative Effect of a Flavonoid, Enzymatically Modified Isoquercitrin on Ocular Symptoms of Japanese Cedar Pollinosis. Allergol. Int. 2009, 58, 373–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makino, T.; Kanemaru, M.; Okuyama, S.; Shimizu, R.; Tanaka, H.; Mizukami, H. Anti-allergic effects of enzymatically modified isoquercitrin (α-oligoglucosyl quercetin 3-O-glucoside), quercetin 3-O-glucoside, α-oligoglucosyl rutin, and quercetin, when administered orally to mice. J. Nat. Med. 2013, 67, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Waheed, G.; Ramadan, G.; Mohammed, H.A. Sodium R-lipoate and enzymatically-modified isoquercitrin suppressed IgE-independent anaphylactic reactions and stress-induced gastric ulceration in mice. Int. Immunopharmacol. 2021, 97, 107735. [Google Scholar] [CrossRef]
- Emura, K.; Yokomizo, A.; Toyoshi, T.; Moriwaki, M. Effect of Enzymatically Modified Isoquercitrin in Spontaneously Hypertensive Rats. J. Nutr. Sci. Vitaminol. 2007, 53, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Motoyama, K.; Koyama, H.; Moriwaki, M.; Emura, K.; Okuyama, S.; Sato, E.; Inoue, M.; Shioi, A.; Nishizawa, Y. Atheroprotective and plaque-stabilizing effects of enzymatically modified isoquercitrin in atherogenic apoE-deficient mice. Nutrition 2009, 25, 421–427. [Google Scholar] [CrossRef]
- Bondonno, N.P.; Bondonno, C.P.; Ward, N.C.; Woodman, R.J.; Hodgson, J.M.; Croft, K.D. Enzymatically modified isoquercitrin improves endothelial function in volunteers at risk of cardiovascular disease. Br. J. Nutr. 2020, 123, 182–189. [Google Scholar] [CrossRef]
- Nishimura, J.; Saegusa, Y.; Dewa, Y.; Jin, M.; Kawai, M.; Kemmochi, S.; Harada, T.; Hayashi, S.-M.; Shibutani, M.; Mitsumori, K. Antioxidant enzymatically modified isoquercitrin or melatonin supplementation reduces oxidative stress-mediated hepatocellular tumor promotion of oxfendazole in rats. Arch. Toxicol. 2010, 84, 143–153. [Google Scholar] [CrossRef]
- Shimada, Y.; Dewa, Y.; Ichimura, R.; Suzuki, T.; Mizukami, S.; Hayashi, S.-M.; Shibutani, M.; Mitsumori, K. Antioxidant enzymatically modified isoquercitrin suppresses the development of liver preneoplastic lesions in rats induced by β-naphthoflavone. Toxicology 2010, 268, 213–218. [Google Scholar] [CrossRef]
- Kuwata, K.; Shibutani, M.; Hayashi, H.; Shimamoto, K.; Hayashi, S.-M.; Suzuki, K.; Mitsumori, K. Concomitant apoptosis and regeneration of liver cells as a mechanism of liver-tumor promotion by β-naphthoflavone involving TNFα-signaling due to oxidative cellular stress in rats. Toxicology 2011, 283, 8–17. [Google Scholar] [CrossRef]
- Morita, R.; Shimamoto, K.; Ishii, Y.; Kuwata, K.; Ogawa, B.-I.; Imaoka, M.; Hayashi, S.-M.; Suzuki, K.; Shibutani, M.; Mitsumori, K. Suppressive effect of enzymatically modified isoquercitrin on phenobarbital-induced liver tumor promotion in rats. Arch. Toxicol. 2011, 85, 1475–1484. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Kimura, M.; Ishii, Y.; Yamamoto, R.; Morita, R.; Hayashi, S.-M.; Suzuki, K.; Shibutani, M. Effect of enzymatically modified isoquercitrin on preneoplastic liver cell lesions induced by thioacetamide promotion in a two-stage hepatocarcinogenesis model using rats. Toxicology 2013, 305, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Fujii, Y.; Yamamoto, R.; Yafune, A.; Hayashi, S.-M.; Suzuki, K.; Shibutani, M. Involvement of multiple cell cycle aberrations in early preneoplastic liver cell lesions by tumor promotion with thioacetamide in a two-stage rat hepatocarcinogenesis model. Exp. Toxicol. Pathol. 2013, 65, 979–988. [Google Scholar] [CrossRef]
- Hara, S.; Morita, R.; Ogawa, T.; Segawa, R.; Takimoto, N.; Suzuki, K.; Hamadate, N.; Hayashi, S.-M.; Odachi, A.; Ogiwara, I.; et al. Tumor suppression effects of bilberry extracts and enzymatically modified isoquercitrin in early preneoplastic liver cell lesions induced by piperonyl butoxide promotion in a two-stage rat hepatocarcinogenesis model. Exp. Toxicol. Pathol. 2014, 66, 225–234. [Google Scholar] [CrossRef]
- Yoshida, T.; Murayama, H.; Kawashima, M.; Nagahara, R.; Kangawa, Y.; Mizukami, S.; Kimura, M.; Abe, H.; Hayashi, S.-M.; Shibutani, M. Apocynin and enzymatically modified isoquercitrin suppress the expression of a NADPH oxidase subunit p22phox in steatosis-related preneoplastic liver foci of rats. Exp. Toxicol. Pathol. 2017, 69, 9–16. [Google Scholar] [CrossRef]
- Kangawa, Y.; Yoshida, T.; Abe, H.; Seto, Y.; Miyashita, T.; Nakamura, M.; Kihara, T.; Hayashi, S.-M.; Shibutani, M. Anti-inflammatory effects of the selective phosphodiesterase 3 inhibitor, cilostazol, and antioxidants, enzymatically-modified isoquercitrin and α-lipoic acid, reduce dextran sulphate sodium-induced colorectal mucosal injury in mice. Exp. Toxicol. Pathol. 2017, 69, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Yokohira, M.; Yamakawa, K.; Saoo, K.; Matsuda, Y.; Hosokawa, K.; Hashimoto, N.; Kuno, T.; Imaida, K. Antioxidant Effects of Flavonoids Used as Food Additives (Purple Corn Color, Enzymatically Modified Isoquercitrin, and Isoquercitrin) on Liver Carcinogenesis in a Rat Medium-Term Bioassay. J. Food Sci. 2008, 73, C561–C568. [Google Scholar] [CrossRef]
- Akiyama, T.; Washino, T.; Yamada, T.; Koda, T.; Maitani, T. Constituents of Enzymatically Modified Isoquercitrin and Enzymatically Modified Rutin (Extract). Food Hyg. Saf. Sci. (Shokuhin Eiseigaku Zasshi) 2000, 41, 54–60_1. [Google Scholar] [CrossRef] [Green Version]
- San-Ei Gen, F.F.I. EMIQ (Antioxidant of Natural Origin). Available online: http://www.saneigen.com/emiq.html (accessed on 22 October 2022).
- Maronpot, R.R.; Hobbs, C.A.; Davis, J.; Swartz, C.; Boyle, M.; Koyanagi, M.; Hayashi, S.-M. Genetic and rat toxicity studies of cyclodextrin glucanotransferase. Toxicol. Rep. 2016, 3, 381–392. [Google Scholar] [CrossRef] [Green Version]
- Davis, J.P.; Koyanagi, M.; Maronpot, R.R.; Recio, L.; Hayashi, S.-M. Identification of compound causing yellow bone discoloration following alpha-glycosyl isoquercitrin exposure in Sprague–Dawley rats. Arch. Toxicol. 2020, 94, 2413–2421. [Google Scholar] [CrossRef]
- Yan, Q.; Zhang, L.; Zhang, X.; Liu, X.; Yuan, F.; Hou, Z.; Gao, Y. Stabilization of grape skin anthocyanins by copigmentation with enzymatically modified isoquercitrin (EMIQ) as a copigment. Food Res. Int. 2013, 50, 603–609. [Google Scholar] [CrossRef]
- Liu, S.; Liu, F.; Xue, Y.; Gao, Y. Evaluation on oxidative stability of walnut beverage emulsions. Food Chem. 2016, 203, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Tamura, S.; Tsuji, K.; Moriwaki, M.; Murakami, N. Metabolic Bahavior of Enzymatially Modified Isoquercitrin by α-Amylase and Gastric Juices. Jpn. J. Food Chem. Saf. 2005, 12, 152–156. [Google Scholar] [CrossRef]
- Dhital, S.; Lin, A.H.-M.; Hamaker, B.R.; Gidley, M.J.; Muniandy, A. Mammalian Mucosal α-Glucosidases Coordinate with α-Amylase in the Initial Starch Hydrolysis Stage to Have a Role in Starch Digestion beyond Glucogenesis. PLoS ONE 2013, 8, e62546. [Google Scholar] [CrossRef] [Green Version]
- Day, A.J.; Cañada, F.J.; Díaz, J.C.; Kroon, P.; Mclauchlan, R.; Faulds, C.B.; Plumb, G.W.; Morgan, M.R.A.; Williamson, G. Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett. 2000, 468, 166–170. [Google Scholar] [CrossRef] [Green Version]
- Crespy, V.; Morand, C.; Besson, C.; Manach, C.; Démigné, C.; Rémésy, C. Comparison of the Intestinal Absorption of Quercetin, Phloretin and Their Glucosides in Rats. J. Nutr. 2001, 131, 2109–2114. [Google Scholar] [CrossRef] [Green Version]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef] [Green Version]
- Murota, K.; Terao, J. Antioxidative flavonoid quercetin: Implication of its intestinal absorption and metabolism. Arch. Biochem. Biophys. 2003, 417, 12–17. [Google Scholar] [CrossRef]
- Nyska, A.; Hayashi, S.-M.; Koyanagi, M.; Davis, J.P.; Jokinen, M.P.; Ramot, Y.; Maronpot, R.R. Ninety-day toxicity and single-dose toxicokinetics study of alpha-glycosyl isoquercitrin in Sprague-Dawley rats. Food Chem. Toxicol. 2016, 97, 354–366. [Google Scholar] [CrossRef]
- Maronpot, R.R.; Ramot, Y.; Koyanagi, M.; Dias, N.; Cameron, D.; Eniola, S.; Nyska, A.; Hayashi, S.-M. Ten-day and four-week toxicity and toxicokinetics studies of alpha-glycosyl isoquercitrin in juvenile Göttingen minipigs. Toxicol. Res. Appl. 2019, 3, 239784731985508. [Google Scholar] [CrossRef]
- Tamano, S.; Hatahara, Y.; Sano, M.; Hagiwara, A.; Nakamura, M.; Washino, T.; Imaida, K. 13-Week Oral Toxicity and 4-Week Recovery Study of Enzymatically Modified Lsoquercitrin in F344/DuCrj Rats. Jpn. J. Food Chem. Saf. 2002, 8, 161–167. [Google Scholar]
- Salim, E.I.; Kaneko, M.; Wanibuchi, H.; Morimura, K.; Fukushima, S. Lack of carcinogenicity of enzymatically modified isoquercitrin in F344/DuCrj rats. Food Chem. Toxicol. 2004, 42, 1949–1969. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, D.; Donahue, D.A.; Nyska, A.; Hayashi, S.-M.; Koyanagi, M.; Maronpot, R.R. alpha-Glycosyl Isoquercitrin (AGIQ) and its lack of carcinogenicity in rasH2 mice. Food Chem. Toxicol. 2021, 151, 112103. [Google Scholar] [CrossRef] [PubMed]
- Omi, N.; Shiba, H.; Nishimura, E.; Tsukamoto, S.; Maruki-Uchida, H.; Oda, M.; Morita, M. Effects of enzymatically modified isoquercitrin in supplementary protein powder on athlete body composition: A randomized, placebo-controlled, double-blind trial. J. Int. Soc. Sports Nutr. 2019, 16, 39. [Google Scholar] [CrossRef] [Green Version]
- Hobbs, C.A.; Koyanagi, M.; Swartz, C.; Davis, J.; Kasamoto, S.; Maronpot, R.; Recio, L.; Hayashi, S.-M. Comprehensive evaluation of the flavonol anti-oxidants, alpha-glycosyl isoquercitrin and isoquercitrin, for genotoxic potential. Food Chem. Toxicol. 2018, 113, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Maronpot, R.R.; Leggett, A.M.; Donahue, D.A.; Hayashi, S.M.; Breslin, W. Embryo-fetal developmental toxicity study of alpha-glycosyl isoquercitrin administered orally to New Zealand White rabbits. Toxicol. Res. Appl. 2020, 4, 239784732096490. [Google Scholar] [CrossRef]
- Vij, P.; Donahue, D.A.; Burke, K.P.; Hayashi, S.-M.; Maronpot, R.R. Lack of skin sensitization hazard potential for alpha-glycosyl isoquercitrin (AGIQ) utilizing the Local Lymph Node Assay. Toxicol. Rep. 2022, 9, 1291–1296. [Google Scholar] [CrossRef]
- Jiang, H.; Horiuchi, Y.; Hironao, K.-Y.; Kitakaze, T.; Yamashita, Y.; Ashida, H. Prevention effect of quercetin and its glycosides on obesity and hyperglycemia through activating AMPKα in high-fat diet-fed ICR mice. J. Clin. Biochem. Nutr. 2020, 67, 75–83. [Google Scholar] [CrossRef]
- Im, H.; Lee, J.; Kim, K.; Son, Y.; Lee, Y.-H. Anti-obesity effects of heat-transformed green tea extract through the activation of adipose tissue thermogenesis. Nutr. Metab. 2022, 19, 14. [Google Scholar] [CrossRef]
- Trakooncharoenvit, A.; Hara, H.; Hira, T. Combination of α-Glycosyl-Isoquercitrin and Soybean Fiber Promotes Quercetin Bioavailability and Glucagon-like Peptide-1 Secretion and Improves Glucose Homeostasis in Rats Fed a High-Fat High-Sucrose Diet. J. Agric. Food Chem. 2021, 69, 5907–5916. [Google Scholar] [CrossRef]
- Manandhar, B.; Ahn, J.-M. Glucagon-like Peptide-1 (GLP-1) Analogs: Recent Advances, New Possibilities, and Therapeutic Implications. J. Med. Chem. 2015, 58, 1020–1037. [Google Scholar] [CrossRef] [PubMed]
- Kangawa, Y.; Yoshida, T.; Maruyama, K.; Okamoto, M.; Kihara, T.; Nakamura, M.; Ochiai, M.; Hippo, Y.; Hayashi, S.-M.; Shibutani, M. Cilostazol and enzymatically modified isoquercitrin attenuate experimental colitis and colon cancer in mice by inhibiting cell proliferation and inflammation. Food Chem. Toxicol. 2017, 100, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Elcombe, C.R.; Peffer, R.C.; Wolf, D.C.; Bailey, J.; Bars, R.; Bell, D.; Cattley, R.C.; Ferguson, S.S.; Geter, D.; Goetz, A.; et al. Mode of action and human relevance analysis for nuclear receptor-mediated liver toxicity: A case study with phenobarbital as a model constitutive androstane receptor (CAR) activator. Crit. Rev. Toxicol. 2014, 44, 64–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wada, T.; Gao, J.; Xie, W. PXR and CAR in energy metabolism. Trends Endocrinol. Metab. 2009, 20, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Taniai, E.; Yafune, A.; Nakajima, M.; Hayashi, S.-M.; Nakane, F.; Itahashi, M.; Shibutani, M. Ochratoxin A induces karyomegaly and cell cycle aberrations in renal tubular cells without relation to induction of oxidative stress responses in rats. Toxicol. Lett. 2014, 224, 64–72. [Google Scholar] [CrossRef]
- Okada, R.; Masubuchi, Y.; Tanaka, T.; Nakajima, K.; Masuda, S.; Nakamura, K.; Maronpot, R.R.; Yoshida, T.; Koyanagi, M.; Hayashi, S.-M.; et al. Continuous exposure to α-glycosyl isoquercitrin from developmental stage facilitates fear extinction learning in rats. J. Funct. Foods 2019, 55, 312–324. [Google Scholar] [CrossRef]
- Masubuchi, Y.; Nakahara, J.; Kikuchi, S.; Okano, H.; Takahashi, Y.; Takashima, K.; Koyanagi, M.; Maronpot, R.R.; Yoshida, T.; Hayashi, S.-M.; et al. Continuous exposure to α-glycosyl isoquercitrin from developmental stages to adulthood is necessary for facilitating fear extinction learning in rats. J. Toxicol. Pathol. 2020, 33, 247–263. [Google Scholar] [CrossRef]
- Okano, H.; Takashima, K.; Takahashi, Y.; Ojiro, R.; Tang, Q.; Ozawa, S.; Ogawa, B.; Koyanagi, M.; Maronpot, R.R.; Yoshida, T.; et al. Ameliorating effect of continuous alpha-glycosyl isoquercitrin treatment starting from late gestation in a rat autism model induced by postnatal injection of lipopolysaccharides. Chem. Biol. Interact. 2022, 351, 109767. [Google Scholar] [CrossRef]
- Kohara, A.; Machida, M.; Setoguchi, Y.; Ito, R.; Sugitani, M.; Maruki-Uchida, H.; Inagaki, H.; Ito, T.; Omi, N.; Takemasa, T. Enzymatically modified isoquercitrin supplementation intensifies plantaris muscle fiber hypertrophy in functionally overloaded mice. J. Int. Soc. Sports Nutr. 2017, 14, 32. [Google Scholar] [CrossRef] [Green Version]
- Tsukamoto-Sen, S.; Kawakami, S.; Maruki-Uchida, H.; Ito, R.; Matsui, N.; Komiya, Y.; Mita, Y.; Morisasa, M.; Goto-Inoue, N.; Furuichi, Y.; et al. Effect of antioxidant supplementation on skeletal muscle and metabolic profile in aging mice. Food Funct. 2021, 12, 825–833. [Google Scholar] [CrossRef]


| Model | Treatment | Detected Metabolites | Tmax | Cmax | AUC | Reference |
|---|---|---|---|---|---|---|
| Male Wistar ST rats | 50 µmol/kg p.o. | quercetin conjugates tamarixetin conjugates isorhamnetin conjugates | 15 min 15–30 min 6 h | 10.7 µM 1 µM 4.8 µM | 46.0 µMh 11.2 µMh 55.1 µMh | [10] |
| ddY mice | 4 mmol/kg, p.o. | isoquercitrin quercetin glucuronide quercetin | 30 min a | 600 µM 95 µM n.d. | - - - | [18] |
| Harlan Sprague-Dawley rats | 1000 mg/kg p.o. | quercetin quercetin glucuronide isoquercitrin | 1 h 1 h - | 1.0 µg/mL 9.7 µg/mL <LOQ | 3.3 h·µg/mL 3.3 h·µg/mL - | [45] |
| Göttingen minipigs | 1000 mg/kg p.o. | quercetin isoquercitrin quercetin glucuronide | 30 min–2 h 30 min–2 h 30 min–2 h | 0.24 µg/mL 0.13 µg/mL 0.59 µg/mL | 0.60 h·µg/mL 0.42 h·µ/mL 2.95 h·µg/mL | [46] |
| Human volunteers | 2 mg aglycone equivalent/kg p.o. | quercetin 3-glucuronide | 1.8 h b | 1.84 µM b | 5.99 µM b | [13] |
| quercetin 3′-sulphate | ||||||
| isorhamnetin 3-glucuronide | ~1.5 h | ~0.75 µM | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owczarek-Januszkiewicz, A.; Magiera, A.; Olszewska, M.A. Enzymatically Modified Isoquercitrin: Production, Metabolism, Bioavailability, Toxicity, Pharmacology, and Related Molecular Mechanisms. Int. J. Mol. Sci. 2022, 23, 14784. https://doi.org/10.3390/ijms232314784
Owczarek-Januszkiewicz A, Magiera A, Olszewska MA. Enzymatically Modified Isoquercitrin: Production, Metabolism, Bioavailability, Toxicity, Pharmacology, and Related Molecular Mechanisms. International Journal of Molecular Sciences. 2022; 23(23):14784. https://doi.org/10.3390/ijms232314784
Chicago/Turabian StyleOwczarek-Januszkiewicz, Aleksandra, Anna Magiera, and Monika Anna Olszewska. 2022. "Enzymatically Modified Isoquercitrin: Production, Metabolism, Bioavailability, Toxicity, Pharmacology, and Related Molecular Mechanisms" International Journal of Molecular Sciences 23, no. 23: 14784. https://doi.org/10.3390/ijms232314784
APA StyleOwczarek-Januszkiewicz, A., Magiera, A., & Olszewska, M. A. (2022). Enzymatically Modified Isoquercitrin: Production, Metabolism, Bioavailability, Toxicity, Pharmacology, and Related Molecular Mechanisms. International Journal of Molecular Sciences, 23(23), 14784. https://doi.org/10.3390/ijms232314784






