Inhibition of MZF1/c-MYC Axis by Cantharidin Impairs Cell Proliferation in Glioblastoma
Abstract
:1. Introduction
2. Results
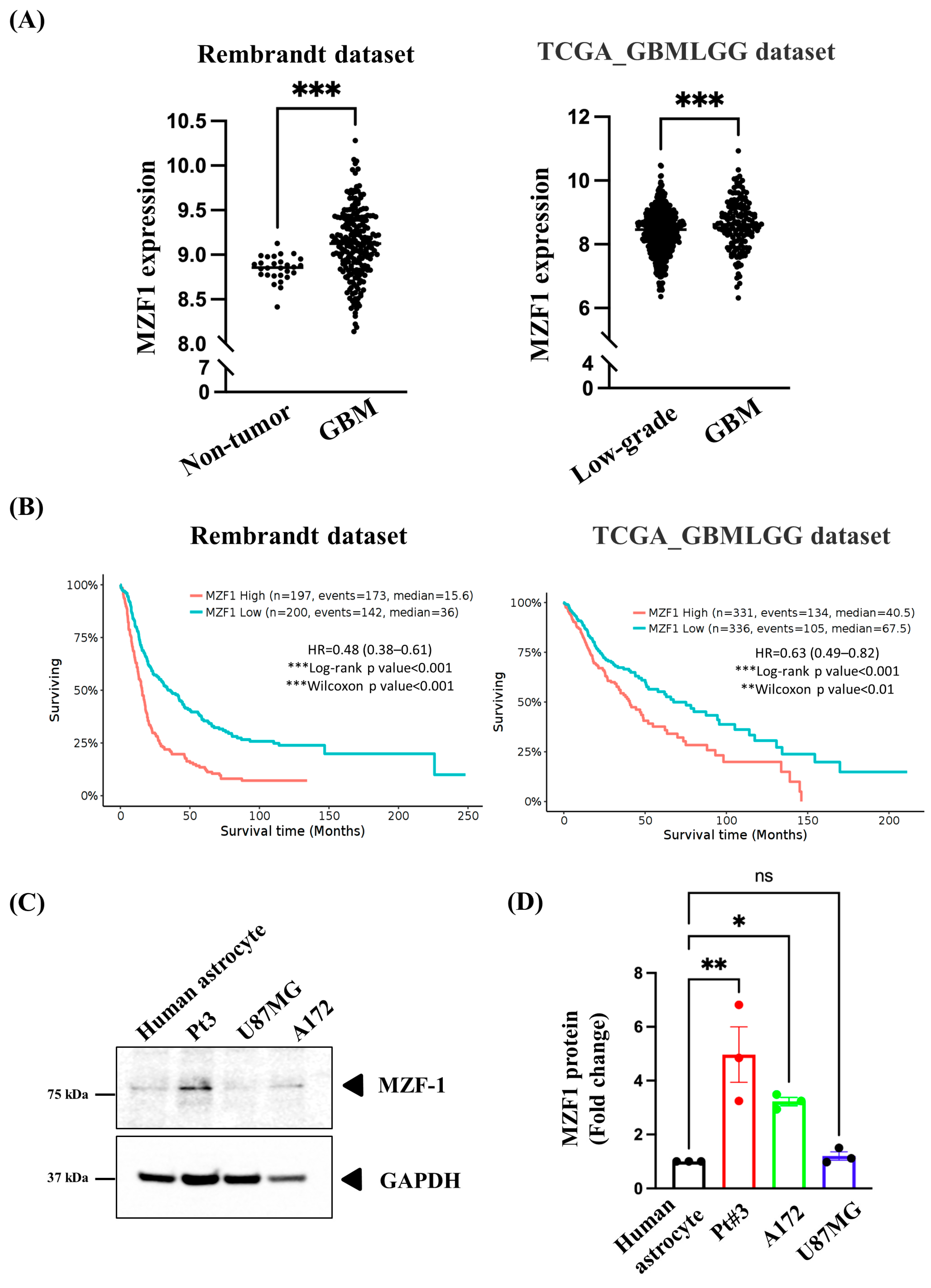
2.1. Overexpression of MZF1 Is Correlated with Poor Prognosis among Patients with GBM
2.2. MZF1 Overexpression Promotes Cell Proliferation and Regulates c-MYC Expression in PT#3 and A172 Glioma Cells
2.3. Antiproliferative Effect of Cantharidin on Gliomas
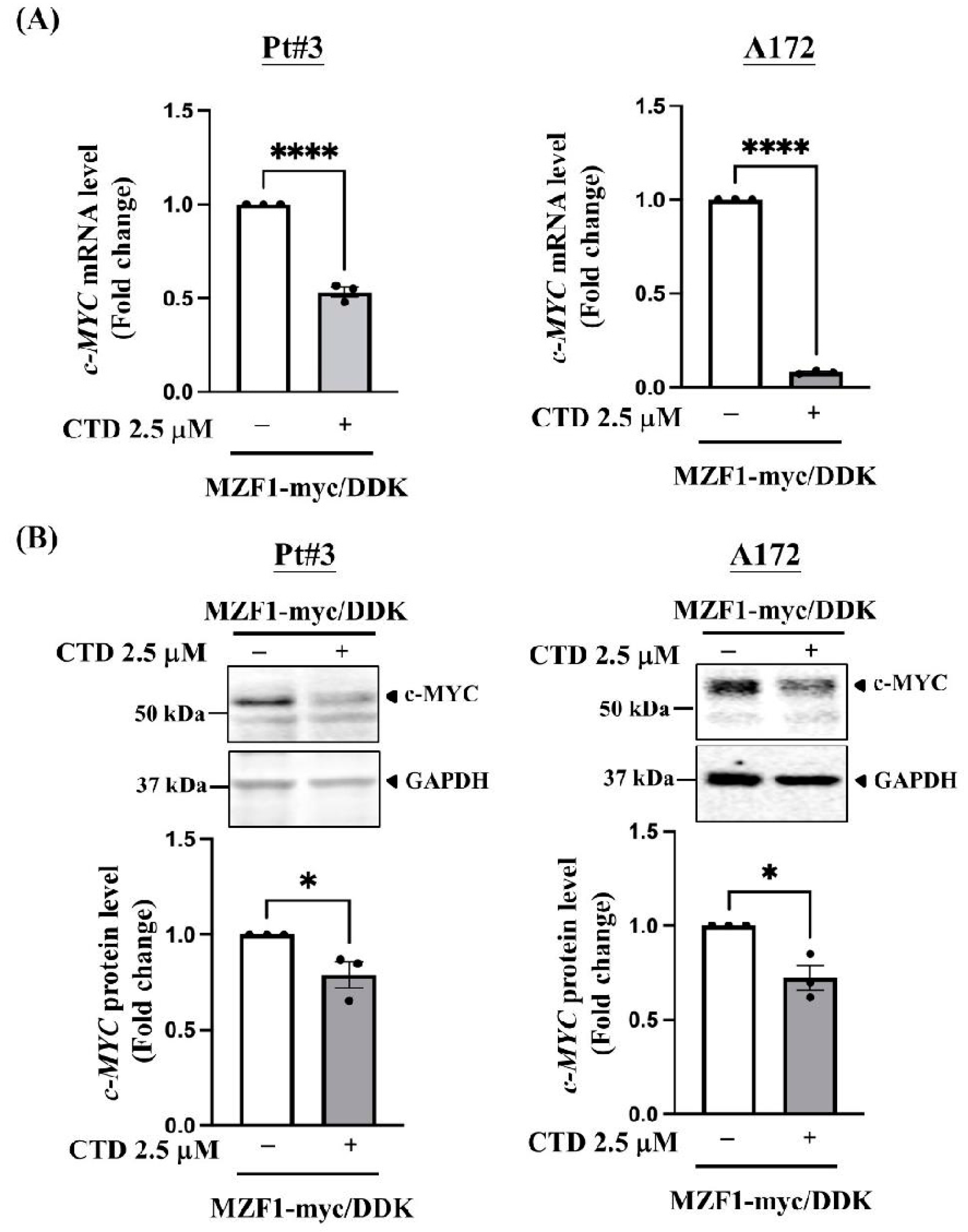
2.4. Cantharidin Disrupts MZF1-Induced c-MYC Expression in PT#3 and A172 Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Transfection
4.2. Bioinformatic Analysis
4.3. CCK-8 Assay
4.4. Foci Assay
4.5. Western Blot Analysis
4.6. RT-qPCR for Quantifying Gene Expression
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, A.C.; Ashley, D.M.; Lopez, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of glioblastoma: State of the art and future directions. CA Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Gersey, Z.C.; Rodriguez, G.A.; Barbarite, E.; Sanchez, A.; Walters, W.M.; Ohaeto, K.C.; Komotar, R.J.; Graham, R.M. Curcumin decreases malignant characteristics of glioblastoma stem cells via induction of reactive oxygen species. BMC Cancer 2017, 17, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bausart, M.; Preat, V.; Malfanti, A. Immunotherapy for glioblastoma: The promise of combination strategies. J. Exp. Clin. Cancer Res. 2022, 41, 35. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liao, T.; Liu, H.; Yuan, H.; Ouyang, T.; Wang, J.; Chai, S.; Li, J.; Chen, J.; Li, X.; et al. Hypoxic Glioma Stem Cell-Derived Exosomes Containing Linc01060 Promote Progression of Glioma by Regulating the MZF1/c-Myc/HIF1alpha Axis. Cancer Res. 2021, 81, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.M.; Lin, W.C.; Lin, H.Y.; Chen, Y.L.; Ko, C.Y.; Wang, J.M. CCAAT/Enhancer-binding protein delta mediates glioma stem-like cell enrichment and ATP-binding cassette transporter ABCA1 activation for temozolomide resistance in glioblastoma. Cell Death Discov. 2021, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.M.; Lin, H.Y.; Chen, Y.L.; Hsu, T.I.; Chuang, J.Y.; Kao, T.J.; Ko, C.Y. CCAAT/enhancer-binding protein delta regulates the stemness of glioma stem-like cells through activating PDGFA expression upon inflammatory stimulation. J. Neuroinflamm. 2019, 16, 146. [Google Scholar] [CrossRef]
- Brix, D.M.; Bundgaard Clemmensen, K.K.; Kallunki, T. Zinc Finger Transcription Factor MZF1-A Specific Regulator of Cancer Invasion. Cells 2020, 9, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Gao, Q.; Wang, S. Kinase GSK3beta functions as a suppressor in colorectal carcinoma through the FTO-mediated MZF1/c-Myc axis. J. Cell. Mol. Med. 2021, 25, 2655–2665. [Google Scholar] [CrossRef]
- Tsai, L.H.; Wu, J.Y.; Cheng, Y.W.; Chen, C.Y.; Sheu, G.T.; Wu, T.C.; Lee, H. The MZF1/c-MYC axis mediates lung adenocarcinoma progression caused by wild-type lkb1 loss. Oncogene 2015, 34, 1641–1649. [Google Scholar] [CrossRef]
- Cimmino, F.; Scoppettuolo, M.N.; Carotenuto, M.; De Antonellis, P.; Dato, V.D.; De Vita, G.; Zollo, M. Norcantharidin impairs medulloblastoma growth by inhibition of Wnt/beta-catenin signaling. J. Neurooncol. 2012, 106, 59–70. [Google Scholar] [CrossRef]
- Hu, S.; Chang, J.; Ruan, H.; Zhi, W.; Wang, X.; Zhao, F.; Ma, X.; Sun, X.; Liang, Q.; Xu, H.; et al. Cantharidin inhibits osteosarcoma proliferation and metastasis by directly targeting miR-214-3p/DKK3 axis to inactivate beta-catenin nuclear translocation and LEF1 translation. Int. J. Biol. Sci. 2021, 17, 2504–2522. [Google Scholar] [CrossRef]
- He, T.; Wang, Q.; Ao, J.; Chen, K.; Li, X.; Zhang, J.; Duan, C. Endoplasmic reticulum stress contributes to autophagy and apoptosis in cantharidin-induced nephrotoxicity. Food Chem. Toxicol. 2022, 163, 112986. [Google Scholar] [CrossRef] [PubMed]
- Nazim, U.M.; Yin, H.; Park, S.Y. Downregulation of cFLIP and upregulation of DR5 by cantharidin sensitizes TRAILmediated apoptosis in prostate cancer cells via autophagy flux. Int. J. Mol. Med. 2020, 46, 280–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.M.; Mackintosh, C.; Casida, J.E. Protein phosphatase 2A and its [3H]cantharidin/[3H]endothall thioanhydride binding site. Inhibitor specificity of cantharidin and ATP analogues. Biochem. Pharmacol. 1993, 46, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, L.; Wang, C.; He, H.; Liu, G.; Ma, H.; Pang, L.; Jiang, M.; Lu, Q.; Li, P.; et al. Cantharidin Induces Apoptosis and Promotes Differentiation of AML Cells Through Nuclear Receptor Nur77-Mediated Signaling Pathway. Front. Pharmacol. 2020, 11, 1321. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Shi, X.; Gong, Z.; Su, Q.; Yu, R.; Wang, B.; Yang, T.; Dai, B.; Zhan, Y.; Zhang, D.; et al. Cantharidin treatment inhibits hepatocellular carcinoma development by regulating the JAK2/STAT3 and PI3K/Akt pathways in an EphB4-dependent manner. Pharmacol. Res. 2020, 158, 104868. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Yang, K.; Tang, X.; Bi, Y.; Ding, Y.; Deng, M.; Xia, D.; Zhao, Y.; Chen, T. Norcantharidin Nanostructured Lipid Carrier (NCTD-NLC) Suppresses the Viability of Human Hepatocellular Carcinoma HepG2 Cells and Accelerates the Apoptosis. J. Immunol. Res. 2022, 2022, 3851604. [Google Scholar] [CrossRef]
- Kim, Y. Regulation of cell proliferation and migration in glioblastoma: New therapeutic approach. Front. Oncol. 2013, 3, 53. [Google Scholar] [CrossRef] [Green Version]
- Eguchi, T.; Prince, T.L.; Tran, M.T.; Sogawa, C.; Lang, B.J.; Calderwood, S.K. MZF1 and SCAND1 Reciprocally Regulate CDC37 Gene Expression in Prostate Cancer. Cancers 2019, 11, 792. [Google Scholar] [CrossRef] [Green Version]
- Yue, C.H.; Huang, C.Y.; Tsai, J.H.; Hsu, C.W.; Hsieh, Y.H.; Lin, H.; Liu, J.Y. MZF-1/Elk-1 Complex Binds to Protein Kinase Calpha Promoter and Is Involved in Hepatocellular Carcinoma. PLoS ONE 2015, 10, e0127420. [Google Scholar] [CrossRef]
- Qi, Z.; Wang, J.; Li, Y.; Xu, Y. MZF1 Transcriptionally Activated MicroRNA-328-3p Suppresses the Malignancy of Stomach Adenocarcinoma via Inhibiting CD44. J. Immunol. Res. 2022, 2022, 5819295. [Google Scholar] [CrossRef]
- Zhou, J.; Ren, Y.; Tan, L.; Song, X.; Wang, M.; Li, Y.; Cao, Z.; Guo, C. Norcantharidin: Research advances in pharmaceutical activities and derivatives in recent years. Biomed. Pharmacother. 2020, 131, 110755. [Google Scholar] [CrossRef]
- Wang, S.M.; Lee, Y.C.; Ko, C.Y.; Lai, M.D.; Lin, D.Y.; Pao, P.C.; Chi, J.Y.; Hsiao, Y.W.; Liu, T.L.; Wang, J.M. Increase of zinc finger protein 179 in response to CCAAT/enhancer binding protein delta conferring an antiapoptotic effect in astrocytes of Alzheimer’s disease. Mol. Neurobiol. 2015, 51, 370–382. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.M.; Wu, H.E.; Yasui, Y.; Geva, M.; Hayden, M.; Maurice, T.; Cozzolino, M.; Su, T.P. Nucleoporin POM121 signals TFEB-mediated autophagy via activation of SIGMAR1/sigma-1 receptor chaperone by pridopidine. Autophagy 2022, 1–26. [Google Scholar] [CrossRef]
- Li, W.; Xie, L.; Chen, Z.; Zhu, Y.; Sun, Y.; Miao, Y.; Xu, Z.; Han, X. Cantharidin, a potent and selective PP2A inhibitor, induces an oxidative stress-independent growth inhibition of pancreatic cancer cells through G2/M cell-cycle arrest and apoptosis. Cancer Sci. 2010, 101, 1226–1233. [Google Scholar] [CrossRef]
- Bonness, K.; Aragon, I.V.; Rutland, B.; Ofori-Acquah, S.; Dean, N.M.; Honkanen, R.E. Cantharidin-induced mitotic arrest is associated with the formation of aberrant mitotic spindles and lagging chromosomes resulting, in part, from the suppression of PP2Aalpha. Mol. Cancer Ther. 2006, 5, 2727–2736. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.A.; Kim, Y.; Kwon, B.M.; Han, D.C. The natural compound cantharidin induces cancer cell death through inhibition of heat shock protein 70 (HSP70) and Bcl-2-associated athanogene domain 3 (BAG3) expression by blocking heat shock factor 1 (HSF1) binding to promoters. J. Biol. Chem. 2013, 288, 28713–28726. [Google Scholar] [CrossRef] [Green Version]
- Morfini, G.; Szebenyi, G.; Brown, H.; Pant, H.C.; Pigino, G.; DeBoer, S.; Beffert, U.; Brady, S.T. A novel CDK5-dependent pathway for regulating GSK3 activity and kinesin-driven motility in neurons. EMBO J. 2004, 23, 2235–2245. [Google Scholar] [CrossRef] [Green Version]
- Hong, C.Y.; Huang, S.C.; Lin, S.K.; Lee, J.J.; Chueh, L.L.; Lee, C.H.; Lin, J.H.; Hsiao, M. Norcantharidin-induced post-G(2)/M apoptosis is dependent on wild-type p53 gene. Biochem. Biophys. Res. Commun. 2000, 276, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Niu, W.; Mu, M.; Hu, S.; Niu, C. Long non-coding RNA LPP-AS2 promotes glioma tumorigenesis via miR-7-5p/EGFR/PI3K/AKT/c-MYC feedback loop. J. Exp. Clin. Cancer Res. 2020, 39, 196. [Google Scholar] [CrossRef] [PubMed]
- Fukasawa, K.; Kadota, T.; Horie, T.; Tokumura, K.; Terada, R.; Kitaguchi, Y.; Park, G.; Ochiai, S.; Iwahashi, S.; Okayama, Y.; et al. CDK8 maintains stemness and tumorigenicity of glioma stem cells by regulating the c-MYC pathway. Oncogene 2021, 40, 2803–2815. [Google Scholar] [CrossRef]
- Chen, X.; Yang, F.; Zhang, T.; Wang, W.; Xi, W.; Li, Y.; Zhang, D.; Huo, Y.; Zhang, J.; Yang, A.; et al. MiR-9 promotes tumorigenesis and angiogenesis and is activated by MYC and OCT4 in human glioma. J. Exp. Clin. Cancer Res. 2019, 38, 99. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Wang, L.; Zhu, L.; Xu, Z.; Liu, Y.; Li, Z.; Zhou, J.; Luo, F. Exosomes as Drug Carriers in Anti-Cancer Therapy. Front. Cell Dev. Biol. 2022, 10, 728616. [Google Scholar] [CrossRef]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine 2016, 12, 655–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katakowski, M.; Buller, B.; Zheng, X.; Lu, Y.; Rogers, T.; Osobamiro, O.; Shu, W.; Jiang, F.; Chopp, M. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013, 335, 201–204. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-H.; Wu, H.-C.; Hsu, C.-W.; Chang, Y.-W.; Ko, C.-Y.; Hsu, T.-I.; Chuang, J.-Y.; Tseng, T.-H.; Wang, S.-M. Inhibition of MZF1/c-MYC Axis by Cantharidin Impairs Cell Proliferation in Glioblastoma. Int. J. Mol. Sci. 2022, 23, 14727. https://doi.org/10.3390/ijms232314727
Wang C-H, Wu H-C, Hsu C-W, Chang Y-W, Ko C-Y, Hsu T-I, Chuang J-Y, Tseng T-H, Wang S-M. Inhibition of MZF1/c-MYC Axis by Cantharidin Impairs Cell Proliferation in Glioblastoma. International Journal of Molecular Sciences. 2022; 23(23):14727. https://doi.org/10.3390/ijms232314727
Chicago/Turabian StyleWang, Chie-Hong, Hsuan-Cheng Wu, Chen-Wei Hsu, Yun-Wei Chang, Chiung-Yuan Ko, Tsung-I Hsu, Jian-Ying Chuang, Tsui-Hwa Tseng, and Shao-Ming Wang. 2022. "Inhibition of MZF1/c-MYC Axis by Cantharidin Impairs Cell Proliferation in Glioblastoma" International Journal of Molecular Sciences 23, no. 23: 14727. https://doi.org/10.3390/ijms232314727
APA StyleWang, C.-H., Wu, H.-C., Hsu, C.-W., Chang, Y.-W., Ko, C.-Y., Hsu, T.-I., Chuang, J.-Y., Tseng, T.-H., & Wang, S.-M. (2022). Inhibition of MZF1/c-MYC Axis by Cantharidin Impairs Cell Proliferation in Glioblastoma. International Journal of Molecular Sciences, 23(23), 14727. https://doi.org/10.3390/ijms232314727





