Dynamics of SARS-CoV-2 Major Genetic Lineages in Moscow in the Context of Vaccine Prophylaxis
Abstract
1. Introduction
2. Results
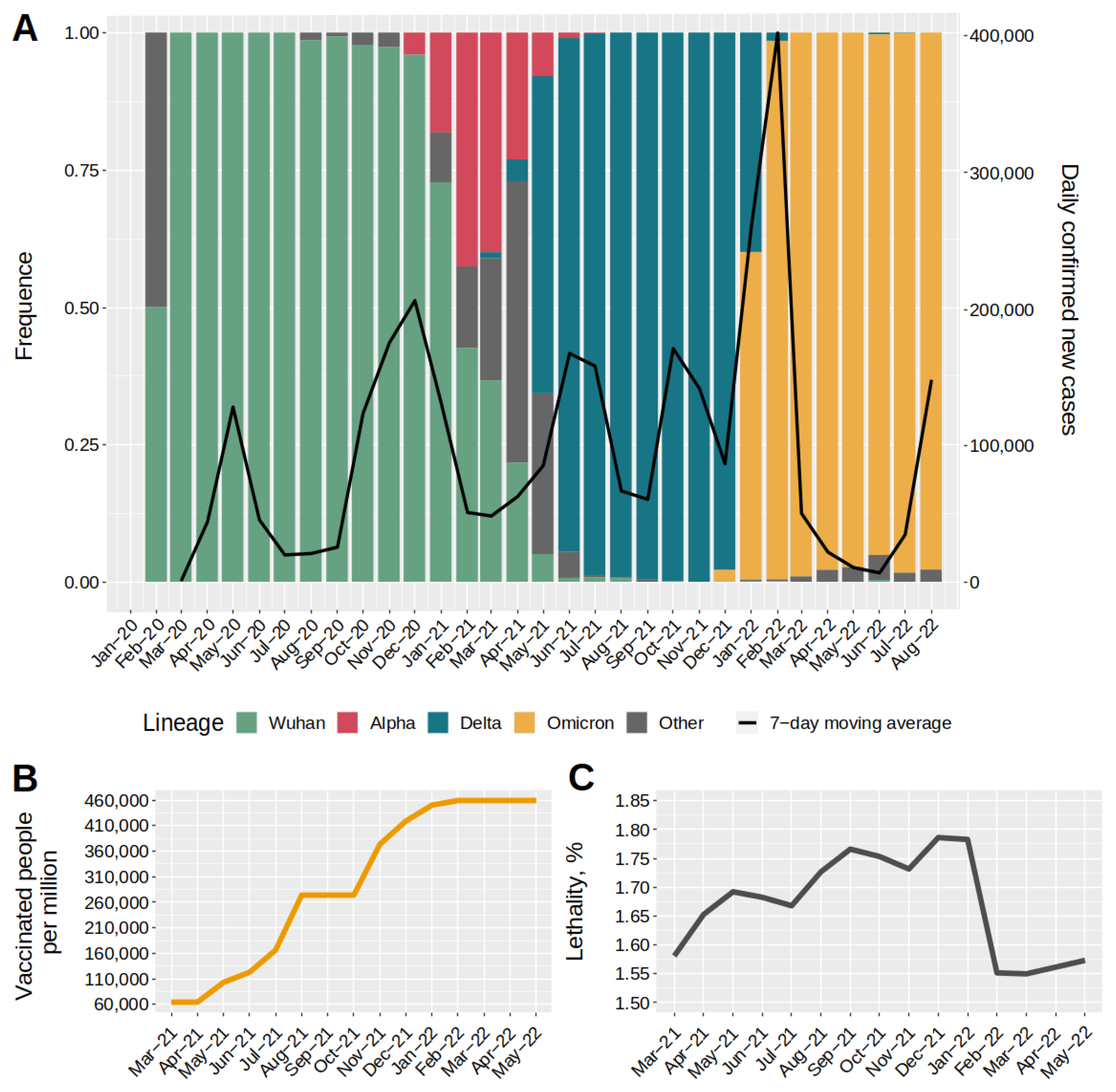
2.1. Dynamics of SARS-CoV-2 Major Genetic Lineages in Moscow, COVID-19 Incidence, and Level Immunity
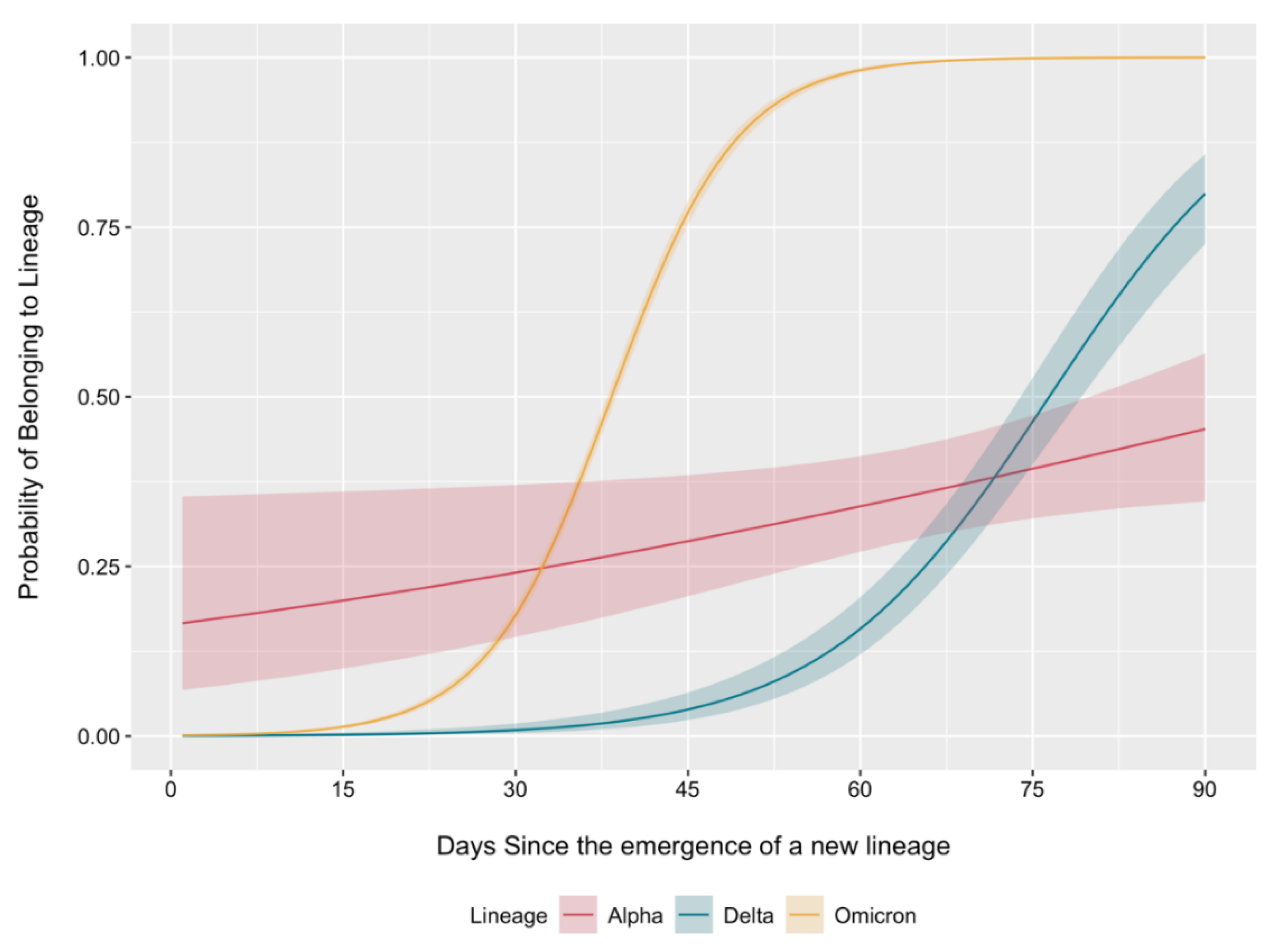
2.2. Dynamics of Circulating Variant Displacement by Primary SARS-CoV-2 Genetic Lineages
2.3. Effective Reproduction Numbers of Major SARS-CoV-2 Genetic Lineages
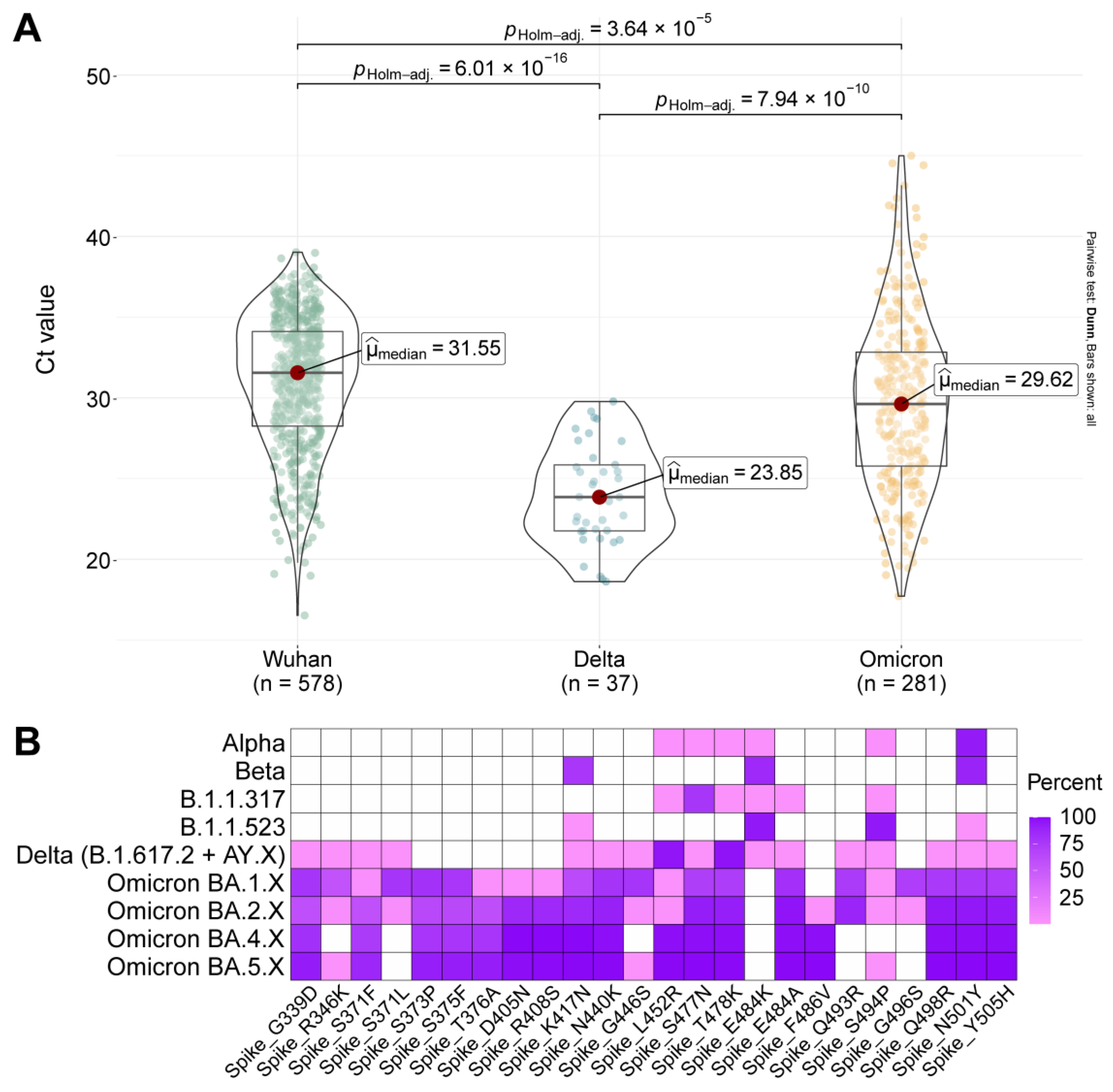
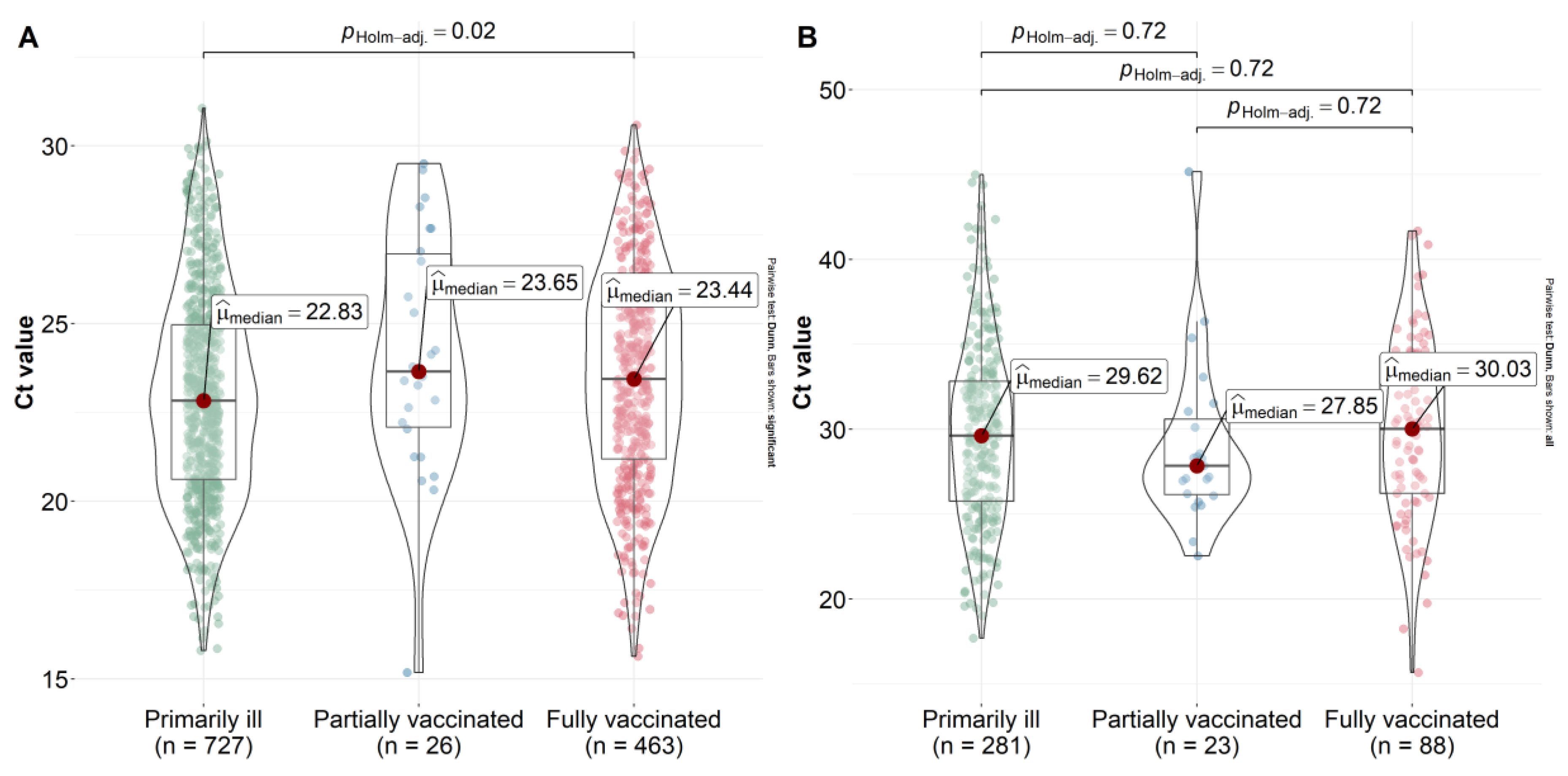
2.4. Viral Load and Mutation Composition of the S Protein in the Main Genetic Lineages of SARS-CoV-2
2.5. Effect of Vaccination on Viral Load Reduction
3. Discussion
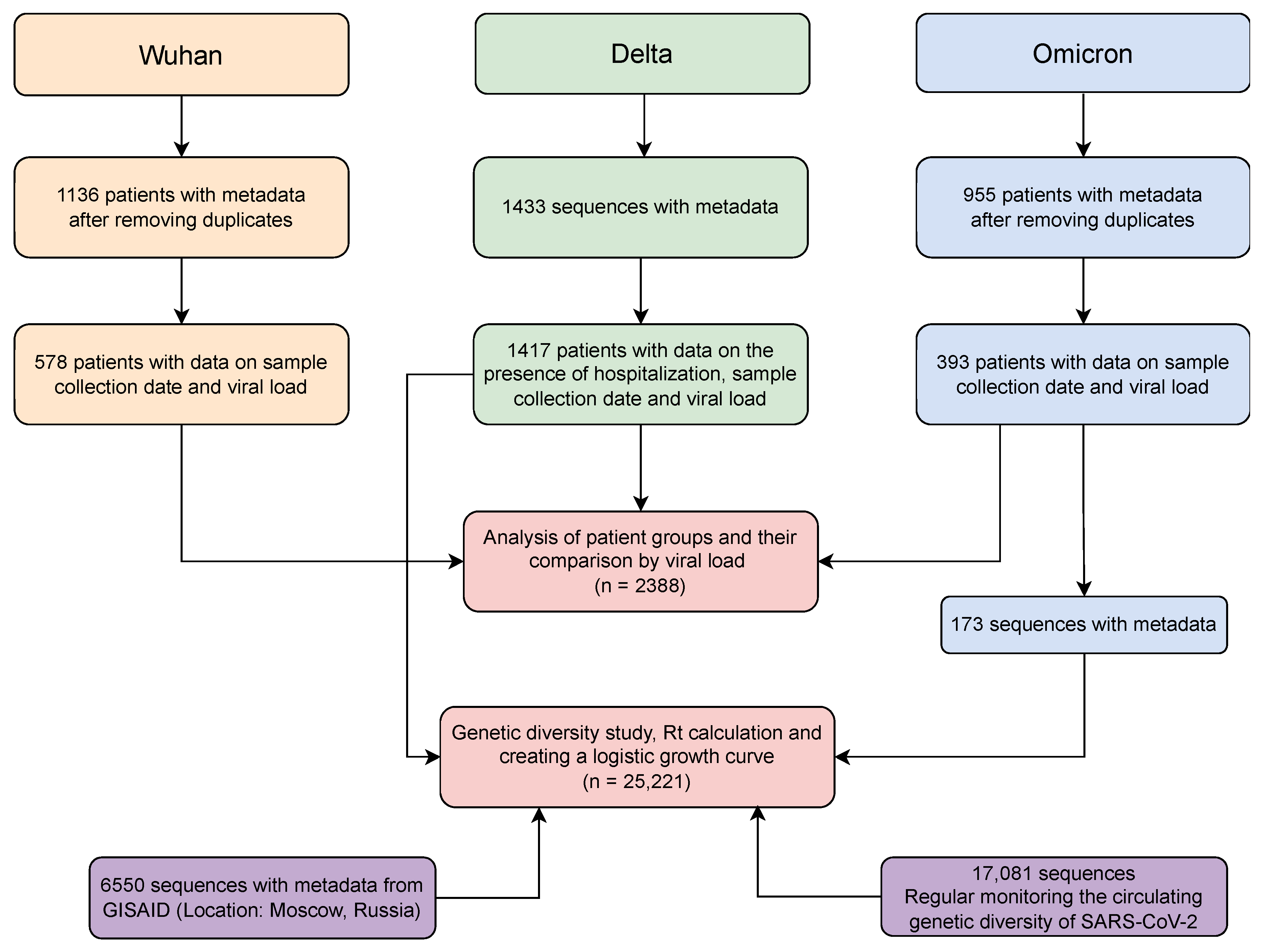
4. Materials and Methods
4.1. Ethics
4.2. Sample Collection and RT-PCR Testing (Laboratory 1)
4.3. Sample Collection and RT-PCR Testing (Laboratory 2)
4.4. Library Preparation and Sequencing (Ion Torrent)
4.5. Library Preparation and Sequencing (Illumina)
4.6. NGS Data Analysis (Ion Torrent)
4.7. NGS Data Analysis (Illumina)
4.8. Characterisation of the Genetic Landscape of SARS-CoV-2 Lines
4.9. Logistic Growth Rates
4.10. Estimation of Reproductive Number for Different Lines SARS-CoV-2
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Worobey, M. Dissecting the early COVID-19 cases in Wuhan. Science 2021, 374, 1202–1204. [Google Scholar] [CrossRef] [PubMed]
- Pekar, J.E.; Magee, A.; Parker, E.; Moshiri, N.; Izhikevich, K.; Havens, J.L.; Gangavarapu, K.; Mariana, L.; Serrano, M.; Crits-Christoph, A.; et al. The molecular epidemiology of multiple zoonotic origins of SARS-CoV-2. Science 2022, 377, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Kadkhoda, K. Herd Immunity to COVID-19. Am. J. Clin. Pathol. 2021, 155, 471–472. [Google Scholar] [CrossRef]
- Mohapatra, R.K.; Kuppili, S.; Kumar Suvvari, T.; Kandi, V.; Behera, A.; Verma, S.; Biswal, S.K.; Al-Noor, T.H.; El-ajaily, M.M.; Sarangi, A.K.; et al. SARS-CoV-2 and its variants of concern including Omicron: A never ending pandemic. Chem. Biol. Drug Des. 2022, 99, 769–788. [Google Scholar] [CrossRef] [PubMed]
- Hart, W.S.; Miller, E.; Andrews, N.J.; Waight, P.; Maini, P.K.; Funk, S.; Thompson, R.N. Generation time of the alpha and delta SARS-CoV-2 variants: An epidemiological analysis. Lancet Infect. Dis. 2022, 22, 603–610. [Google Scholar] [CrossRef]
- Earnest, R.; Uddin, R.; Matluk, N.; Renzette, N.; Turbett, S.E.; Siddle, K.J.; Loreth, C.; Adams, G.; Tomkins-Tinch, C.H.; Petrone, M.E.; et al. Comparative transmissibility of SARS-CoV-2 variants Delta and Alpha in New England, USA. Cell Rep. Med. 2022, 3, 100583. [Google Scholar] [CrossRef]
- Bian, L.; Gao, Q.; Gao, F.; Wang, Q.; He, Q.; Wu, X.; Mao, Q.; Xu, M.; Liang, Z. Impact of the Delta variant on vaccine efficacy and response strategies. Expert Rev. Vaccines 2021, 20, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Thambiraja, T.S.; Karuppanan, K.; Subramaniam, G. Omicron and Delta variant of SARS-CoV-2: A comparative computational study of spike protein. J. Med. Virol. 2022, 94, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Ye, Z.W.; Liang, R.; Tang, K.; Zhang, A.J.; Lu, G.; Ong, C.P.; Poon, V.K.M.; Chan, C.C.S.; Mok, B.W.Y.; et al. Pathogenicity, transmissibility, and fitness of SARS-CoV-2 Omicron in Syrian hamsters. Science 2022, 377, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, M.; Mahmud, S. Omicron SARS-CoV-2 variant of concern. Medicine (Baltimore) 2022, 101, e29165. [Google Scholar] [CrossRef] [PubMed]
- Plesner Lyngse, F.; Hvas Mortensen, L.; Denwood, M.J.; Engbo Christiansen, L.; Holten Møller, C.; Leo Skov, R.; Spiess, K.; Fomsgaard, A.; Magdalena Lassaunière, M.; Rasmussen, M.; et al. SARS-CoV-2 Omicron VOC Transmission in Danish Households. medRxiv 2021. [Google Scholar] [CrossRef]
- Tukhvatulin, A.I.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; Botikov, A.G.; et al. An open, non-randomised, phase 1/2 trial on the safety, tolerability, and immunogenicity of single-dose vaccine “Sputnik Light” for prevention of coronavirus infection in healthy adults. Lancet Reg. Health-Eur. 2021, 11, 100241. [Google Scholar] [CrossRef]
- Official Website Vaccine against Coronavirus Sputnik V. 2022. Available online: https://sputnikvaccine.com/about-vaccine (accessed on 16 November 2022).
- Yandex DataLens. 2022. Available online: https://datalens.yandex/7o7is1q6ikh23?tab=X1 (accessed on 16 August 2022).
- GOGOV. 2022. Available online: https://gogov.ru/covid-v-stats/msk (accessed on 16 August 2022).
- Dong, M.; He, F.; Deng, Y. How to Understand Herd Immunity in the Context of COVID-19. Viral Immunol. 2021, 34, 174–181. [Google Scholar] [CrossRef]
- Gong, W.; Parkkila, S.; Wu, X.; Aspatwar, A. SARS-CoV-2 variants and COVID-19 vaccines: Current challenges and future strategies. Int. Rev. Immunol. 2022, 1–22. [Google Scholar] [CrossRef]
- Yogesh, R.; Srivastava, N.; Abbas Bukhari, S.N. Abbas Bukhari Syed Nasir COVID-19 Challenge: A Quest for Effective Vaccine Strategies Against Circulating and Emerging SARS-CoV-2 Variants. Curr. Pharm. Des. 2022, 28, 2901–2913. [Google Scholar] [CrossRef]
- World Health Organization. 2022. Available online: https://www.who.int/ru/news/item/17-06-2022-interim-statement-on--the-composition-of-current-COVID-19-vaccines (accessed on 17 June 2022).
- Medical Xpress. 2022. Available online: https://medicalxpress.com/news/2022-08-eu-eyes-autumn-pfizer-jab.html (accessed on 10 August 2022).
- Pfizer. 2022. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-announce-omicron-adapted-covid-19 (accessed on 25 June 2022).
- Moderna. 2022. Available online: https://investors.modernatx.com/news/news-details/2022/Moderna-And-the-European-Commission-EC-Amend-Covid-19-Vaccine-Agreement-to-Supply-Omicron-Containing-Bivalent-Candidates-EC-Purchases-Additional-15-million-Doses/default.aspx (accessed on 9 August 2022).
- Campbell, F.; Archer, B.; Laurenson-Schafer, H.; Jinnai, Y.; Konings, F.; Batra, N.; Pavlin, B.; Vandemaele, K.; Van Kerkhove, M.D.; Jombart, T.; et al. Increased transmissibility and global spread of SARSCoV-2 variants of concern as at June 2021. Eurosurveillance 2021, 26, 1–6. [Google Scholar] [CrossRef]
- Cao, Y.; Yisimayi, A.; Jian, F.; Song, W.; Xiao, T.; Wang, L.; Du, S.; Wang, J.; Li, Q.; Chen, X.; et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature 2022, 608, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wei, P.; Zhang, Q.; Aviszus, K.; Linderberger, J.; Yang, J.; Liu, J.; Chen, Z.; Waheed, H.; Reynoso, L.; et al. The Lambda variant of SARS-CoV-2 has a better chance than the Delta variant to escape vaccines. bioRxiv 2021. [Google Scholar] [CrossRef]
- Motozono, C.; Toyoda, M.; Zahradnik, J.; Saito, A.; Nasser, H.; Tan, T.S.; Ngare, I.; Kimura, I.; Uriu, K.; Kosugi, Y.; et al. SARS-CoV-2 spike L452R variant evades cellular immunity and increases infectivity. Cell Host Microbe 2021, 29, 1124–1136.e11. [Google Scholar] [CrossRef] [PubMed]
- Tegally, H.; Moir, M.; Everatt, J.; Giovanetti, M.; Scheepers, C.; Wilkinson, E.; Subramoney, K.; Makatini, Z.; Moyo, S.; Amoako, D.G.; et al. Emergence of SARS-CoV-2 Omicron lineages BA.4 and BA.5 in South Africa. Nat. Med. 2022, 28, 1785–1790. [Google Scholar] [CrossRef]
- Li, B.; Deng, A.; Li, K.; Hu, Y.; Li, Z.; Shi, Y.; Xiong, Q.; Liu, Z.; Guo, Q.; Zou, L.; et al. Viral infection and transmission in a large, well-traced outbreak caused by the SARS-CoV-2 Delta variant. Nat. Commun. 2022, 13, 460. [Google Scholar] [CrossRef]
- Planas, D.; Veyer, D.; Baidaliuk, A.; Staropoli, I.; Guivel-Benhassine, F.; Rajah, M.M.; Planchais, C.; Porrot, F.; Robillard, N.; Puech, J.; et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 2021, 596, 276–280. [Google Scholar] [CrossRef]
- Kislaya, I.; Rodrigues, E.F.; Borges, V.; Gomes, J.P.; Sousa, C.; Almeida, J.P.; Peralta-Santos, A.; Nunes, B. Comparative Effectiveness of Coronavirus Vaccine in Preventing Breakthrough Infections among Vaccinated Persons Infected with Delta and Alpha Variants. Emerg. Infect. Dis. 2022, 28, 331–337. [Google Scholar] [CrossRef]
- Monticelli, M.; Mele, B.H.; Andreotti, G.; Cubellis, M.V.; Riccio, G. Why does SARS-CoV-2 hit in different ways? Host genetic factors can influence the acquisition or the course of COVID-19. Eur. J. Med. Genet. 2021, 64, 104227. [Google Scholar] [CrossRef]
- Serpeloni, J.M.; Lima Neto, Q.A.; Lucio, L.C.; Ramão, A.; Carvalho de Oliveira, J.; Gradia, D.F.; Malheiros, D.; Ferrasa, A.; Marchi, R.; Figueiredo, D.L.A.; et al. Genome interaction of the virus and the host genes and non-coding RNAs in SARS-CoV-2 infection. Immunobiology 2021, 226, 152130. [Google Scholar] [CrossRef]
- Feng, S.; Song, F.; Guo, W.; Tan, J.; Zhang, X.; Qiao, F.; Guo, J.; Zhang, L.; Jia, X. Potential Genes Associated with COVID-19 and Comorbidity. Int. J. Med. Sci. 2022, 19, 402–415. [Google Scholar] [CrossRef]
- Kleymenov, D.A.; Bykonia, E.N.; Popova, L.I.; Mazunina, E.P.; Gushchin, V.A.; Kolobukhina, L.V.; Burgasova, O.A.; Kruzhkova, I.S.; Kuznetsova, N.A.; Shidlovskaya, E.V.; et al. A Deep Look Into COVID-19 Severity Through Dynamic Changes in Blood Cytokine Levels. Front. Immunol. 2021, 12, 4508. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.P.; Flaxman, S.; Gallinat, A.S.; Kinosian, S.P.; Stemkovski, M.; Juliette, H.; Watson, O.J.; Whittaker, C.; Cattarino, L.; Dorigatti, I.; et al. Temperature and population density influence SARS-CoV-2 transmission in the absence of nonpharmaceutical interventions. Proc. Natl. Acad. Sci. USA 2021, 118, e2019284118. [Google Scholar] [CrossRef] [PubMed]
- Gushchin, V.A.; Dolzhikova, I.V.; Shchetinin, A.M.; Odintsova, A.S.; Siniavin, A.E.; Nikiforova, M.A.; Pochtovyi, A.A.; Shidlovskaya, E.V.; Kuznetsova, N.A.; Burgasova, O.A.; et al. Neutralizing Activity of Sera from Sputnik V-Vaccinated People against Variants of Concern (VOC: B.1.1.7, B.1.351, P.1, B.1.617.2, B.1.617.3) and Moscow Endemic SARS-CoV-2 Variants. Vaccines 2021, 9, 779. [Google Scholar] [CrossRef] [PubMed]
- Barchuk, A.; Skougarevskiy, D.; Kouprianov, A.; Shirokov, D.; Dudkina, O.; Tursun-zade, R.; Sergeeva, M.; Tychkova, V.; Komissarov, A.; Zheltukhina, A.; et al. COVID-19 pandemic in Saint Petersburg, Russia: Combining population-based serological study and surveillance data. PLoS ONE 2022, 17, e0266945. [Google Scholar] [CrossRef]
- Barchuk, A.; Cherkashin, M.; Bulina, A.; Berezina, N.; Rakova, T.; Kuplevatskaya, D.; Stanevich, O.; Skougarevskiy, D.; Okhotin, A. Vaccine Effectiveness against Referral to Hospital and Severe Lung Injury Associated with COVID-19: A Population-Based Case-Control Study in St. Petersburg, Russia. medRxiv 2021. [Google Scholar] [CrossRef]
- Simon-Loriere, E.; Schwartz, O. Towards SARS-CoV-2 serotypes? Nat. Rev. Microbiol. 2022, 20, 187–188. [Google Scholar] [CrossRef]
- Lapa, D.; Grousova, D.M.; Matusali, G.; Meschi, S.; Colavita, F.; Bettini, A.; Gramigna, G.; Francalancia, M.; Garbuglia, A.R.; Girardi, E.; et al. Retention of Neutralizing Response against SARS-CoV-2 Omicron Variant in Sputnik V-Vaccinated Individuals. Vaccines 2022, 10, 817. [Google Scholar] [CrossRef]
- Shkoda, A.S.; Gushchin, V.A.; Ogarkova, D.A.; Stavitskaya, S.V.; Orlova, O.E.; Kuznetsova, N.A.; Keruntu, E.N.; Pochtovyi, A.A.; Pukhov, A.V.; Kleymenov, D.A.; et al. Sputnik V Effectiveness against Hospitalization with COVID-19 during Omicron Dominance. Vaccines 2022, 10, 938. [Google Scholar] [CrossRef]
- Klink, G.V.; Safina, K.; Nabieva, E.; Shvyrev, N.; Garushyants, S.; Alekseeva, E.; Komissarov, A.B.; Danilenko, D.M.; Pochtovyi, A.A.; Divisenko, E.V.; et al. The rise and spread of the SARS-CoV-2 AY.122 lineage in Russia. Virus Evol. 2022, 8, veac017. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt Removes Adapter Sequences from High- throughput Sequencing Reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 2016, e2584. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Garrison, E.; Marth, G. Haplotype-based variant detection from short-read sequencing. arXiv 2012, arXiv:1207.3907. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Quinlan, A.R.; Hall, I.M. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Li, H. Minimap2: Pairwise alignment for nucleotide sequences. Bioinformatics 2018, 34, 3094–3100. [Google Scholar] [CrossRef]
- Depristo, M.A.; Banks, E.; Poplin, R.; Garimella, K.V.; Maguire, J.R.; Hartl, C.; Philippakis, A.A.; Del Angel, G.; Rivas, M.A.; Hanna, M.; et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 2011, 43, 491–501. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer: New York, NY, USA, 2009. [Google Scholar] [CrossRef]
- Cori, A.; Ferguson, N.M.; Fraser, C.; Cauchemez, S. A new framework and software to estimate time-varying reproduction numbers during epidemics. Am. J. Epidemiol. 2013, 178, 1505–1512. [Google Scholar] [CrossRef]
- Patil, I. Visualizations with statistical details: The “ggstatsplot” approach. J. Open Source Softw. 2021, 6, 3167. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gushchin, V.A.; Pochtovyi, A.A.; Kustova, D.D.; Ogarkova, D.A.; Tarnovetskii, I.Y.; Belyaeva, E.D.; Divisenko, E.V.; Vasilchenko, L.A.; Shidlovskaya, E.V.; Kuznetsova, N.A.; et al. Dynamics of SARS-CoV-2 Major Genetic Lineages in Moscow in the Context of Vaccine Prophylaxis. Int. J. Mol. Sci. 2022, 23, 14670. https://doi.org/10.3390/ijms232314670
Gushchin VA, Pochtovyi AA, Kustova DD, Ogarkova DA, Tarnovetskii IY, Belyaeva ED, Divisenko EV, Vasilchenko LA, Shidlovskaya EV, Kuznetsova NA, et al. Dynamics of SARS-CoV-2 Major Genetic Lineages in Moscow in the Context of Vaccine Prophylaxis. International Journal of Molecular Sciences. 2022; 23(23):14670. https://doi.org/10.3390/ijms232314670
Chicago/Turabian StyleGushchin, Vladimir A., Andrei A. Pochtovyi, Daria D. Kustova, Darya A. Ogarkova, Ivan Y. Tarnovetskii, Elizaveta D. Belyaeva, Elizaveta V. Divisenko, Lyudmila A. Vasilchenko, Elena V. Shidlovskaya, Nadezhda A. Kuznetsova, and et al. 2022. "Dynamics of SARS-CoV-2 Major Genetic Lineages in Moscow in the Context of Vaccine Prophylaxis" International Journal of Molecular Sciences 23, no. 23: 14670. https://doi.org/10.3390/ijms232314670
APA StyleGushchin, V. A., Pochtovyi, A. A., Kustova, D. D., Ogarkova, D. A., Tarnovetskii, I. Y., Belyaeva, E. D., Divisenko, E. V., Vasilchenko, L. A., Shidlovskaya, E. V., Kuznetsova, N. A., Tkachuk, A. P., Slutskiy, E. A., Speshilov, G. I., Komarov, A. G., Tsibin, A. N., Zlobin, V. I., Logunov, D. Y., & Gintsburg, A. L. (2022). Dynamics of SARS-CoV-2 Major Genetic Lineages in Moscow in the Context of Vaccine Prophylaxis. International Journal of Molecular Sciences, 23(23), 14670. https://doi.org/10.3390/ijms232314670








