Mitochondrial Dysfunction and Induction of Apoptosis in Hepatocellular Carcinoma and Cholangiocarcinoma Cell Lines by Thymoquinone
Abstract
1. Introduction
2. Results
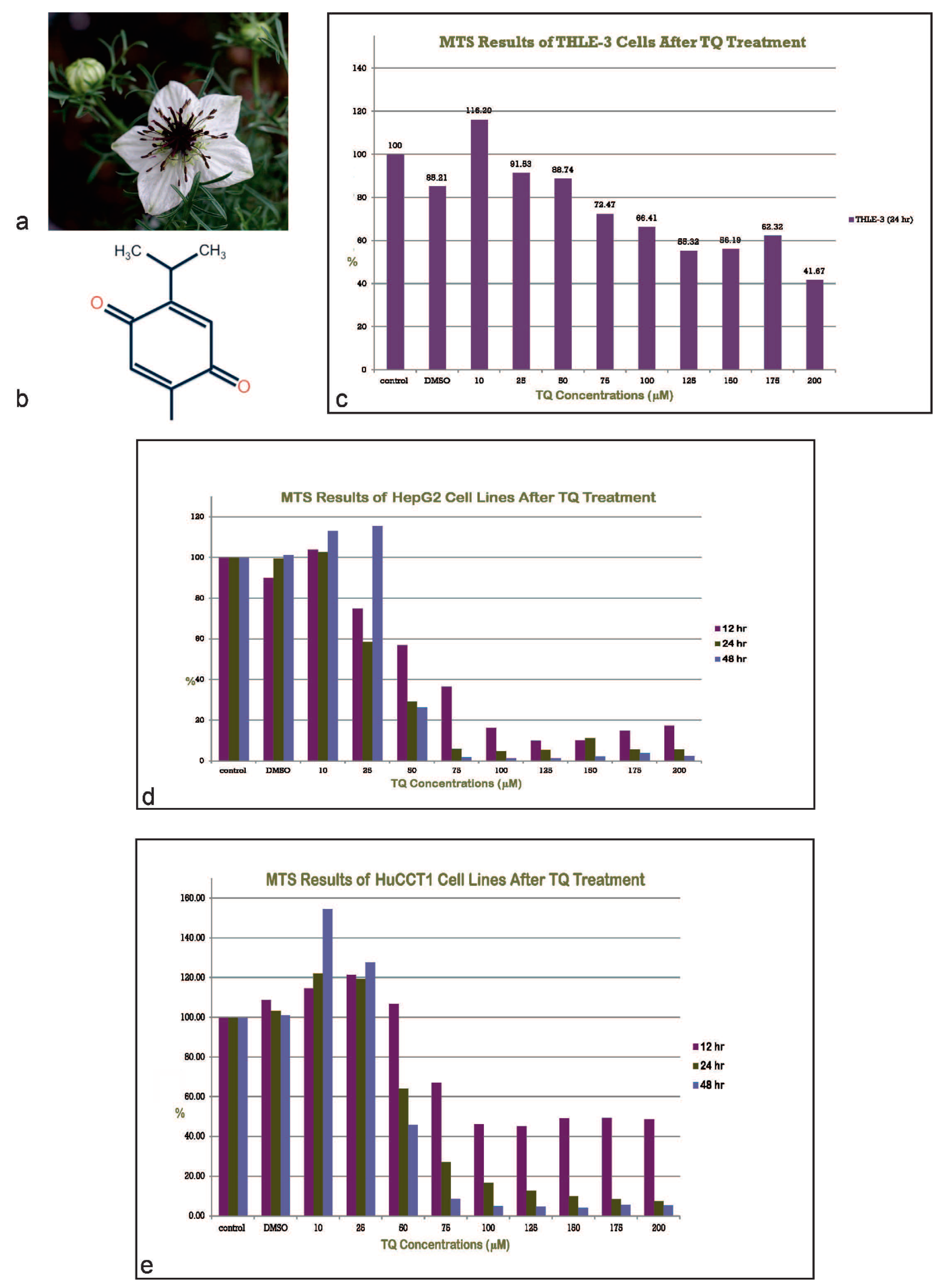
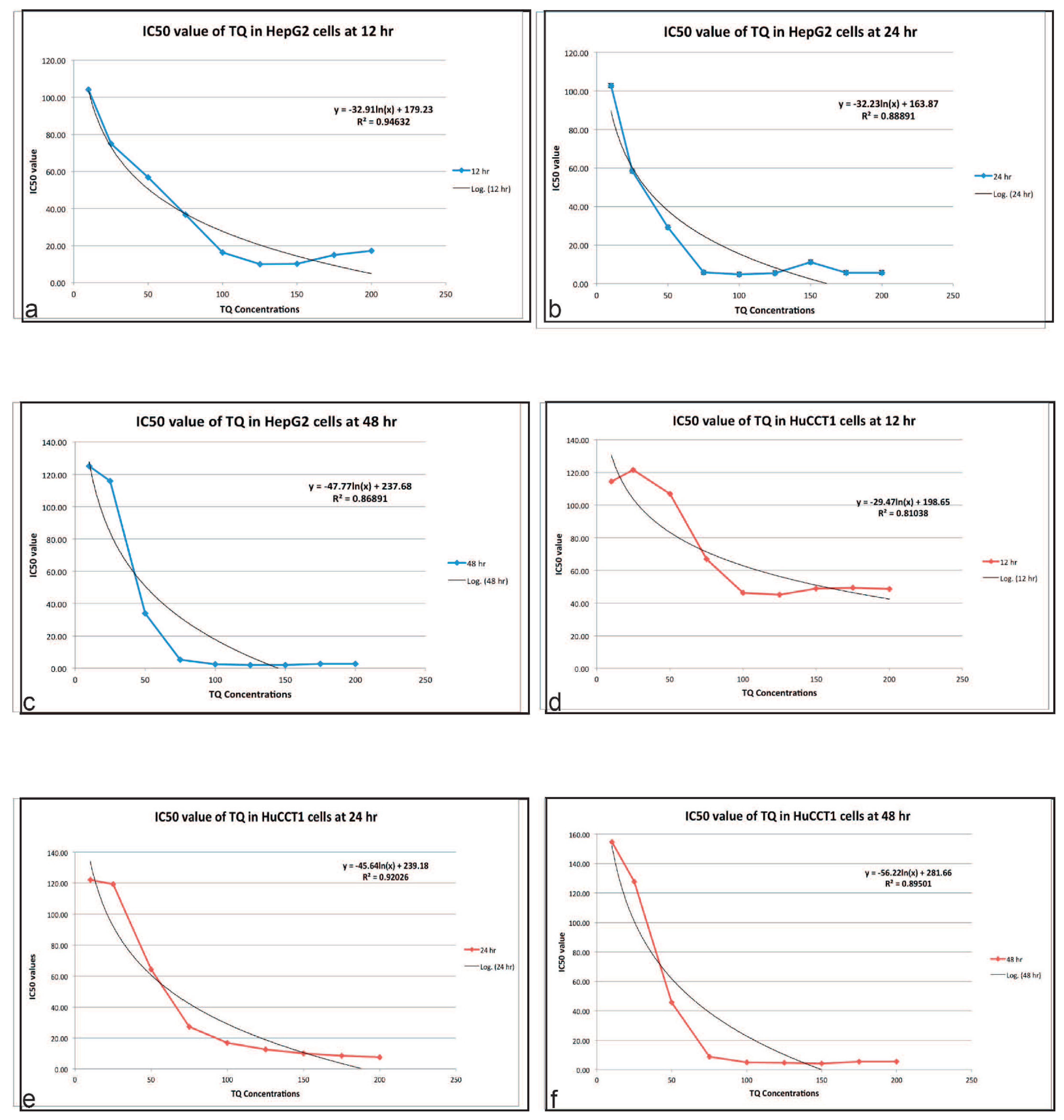
2.1. TQ Inhibits the Proliferation of Tumor Cell Lines
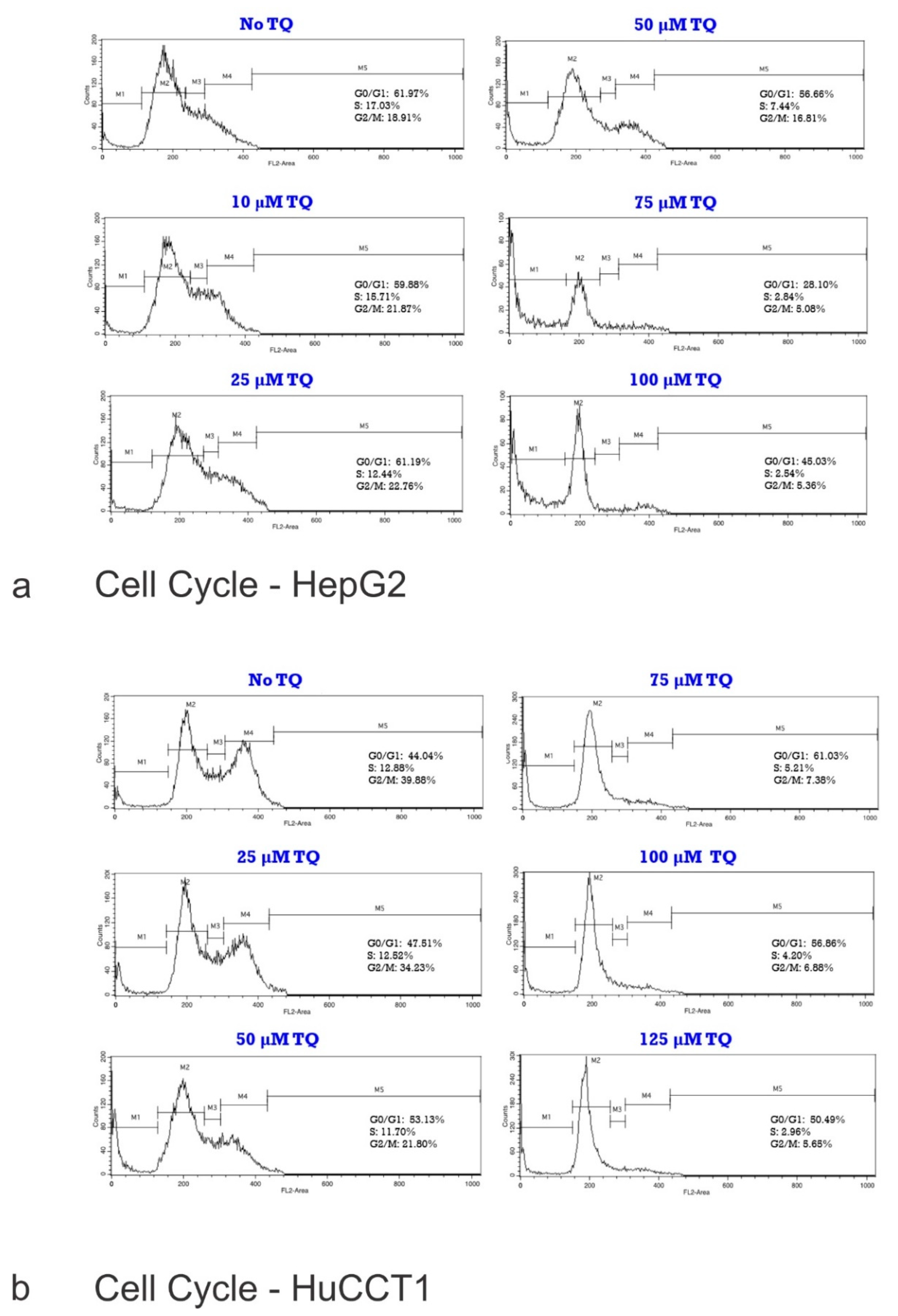
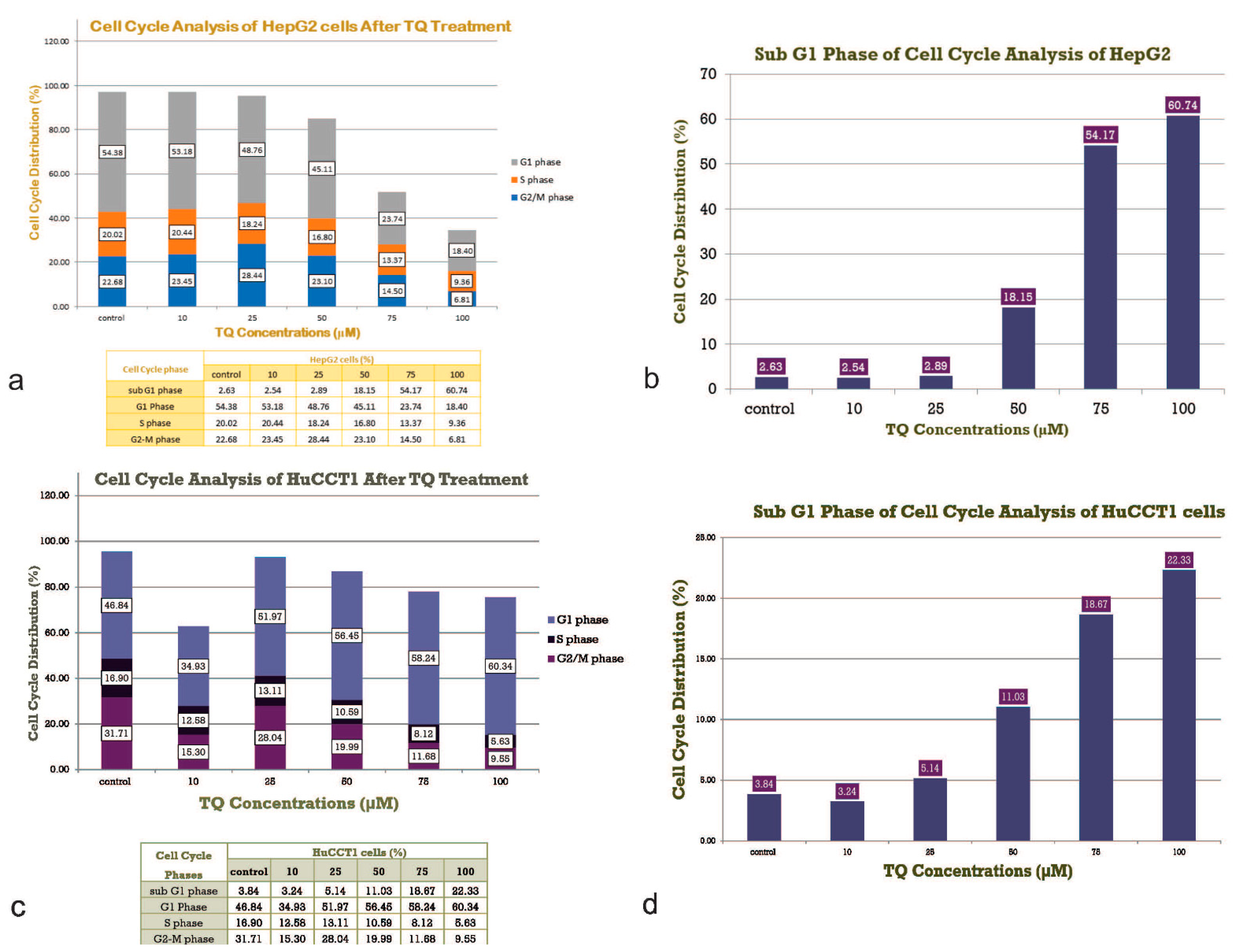
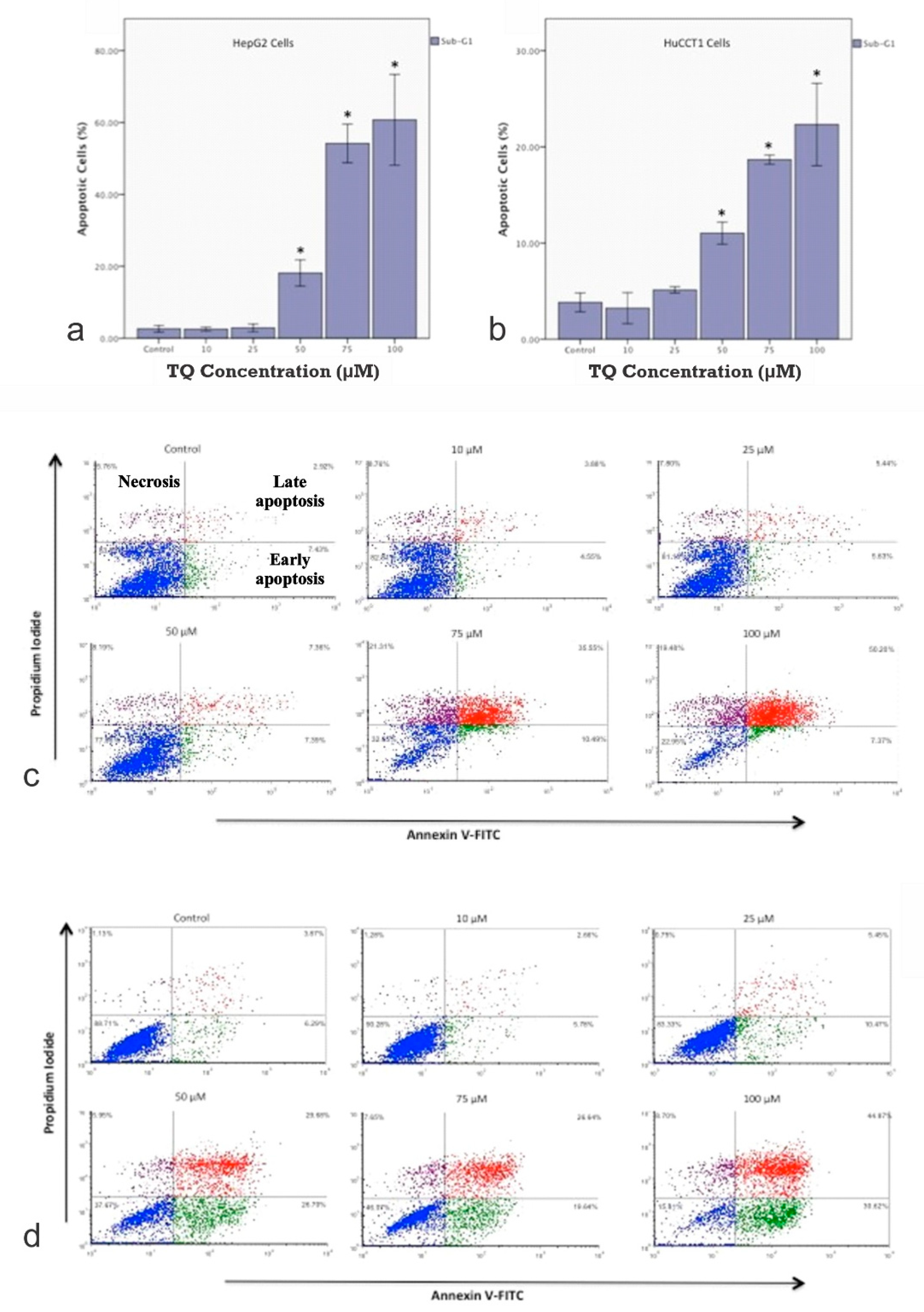
2.2. Effect of TQ on Cell Cycle Distribution
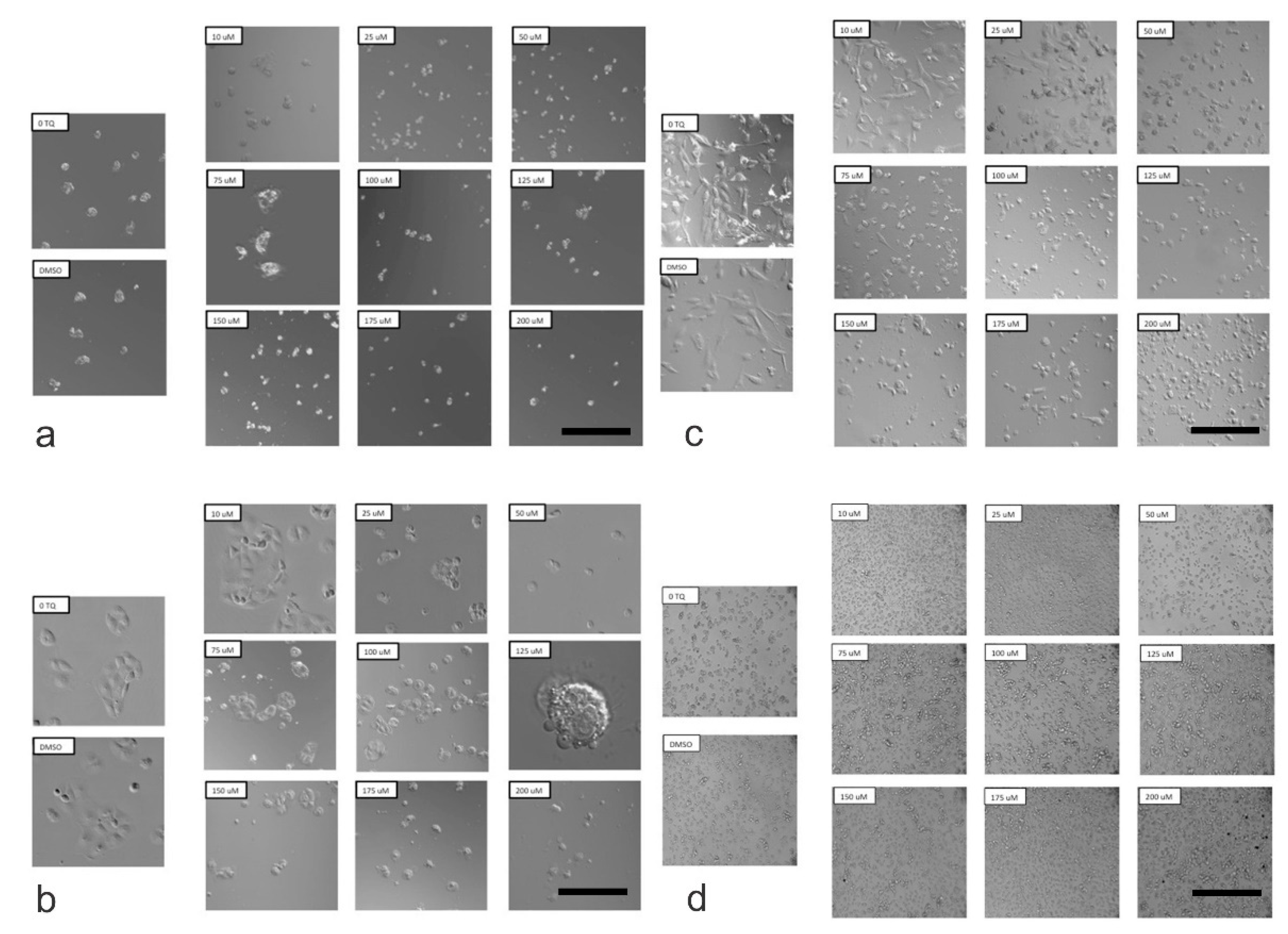
2.3. Determination of Morphological Changes in Cells Treated with TQ
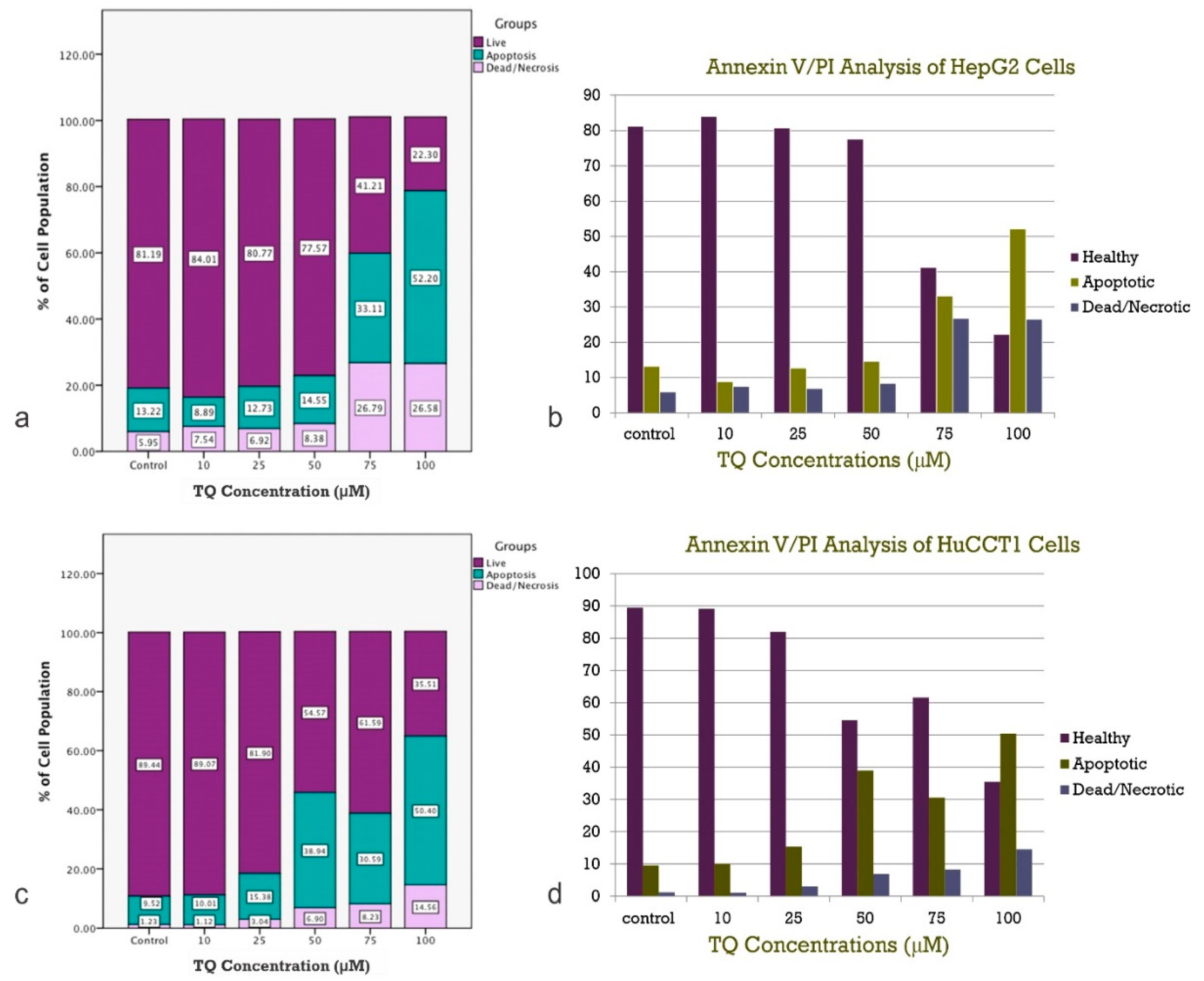
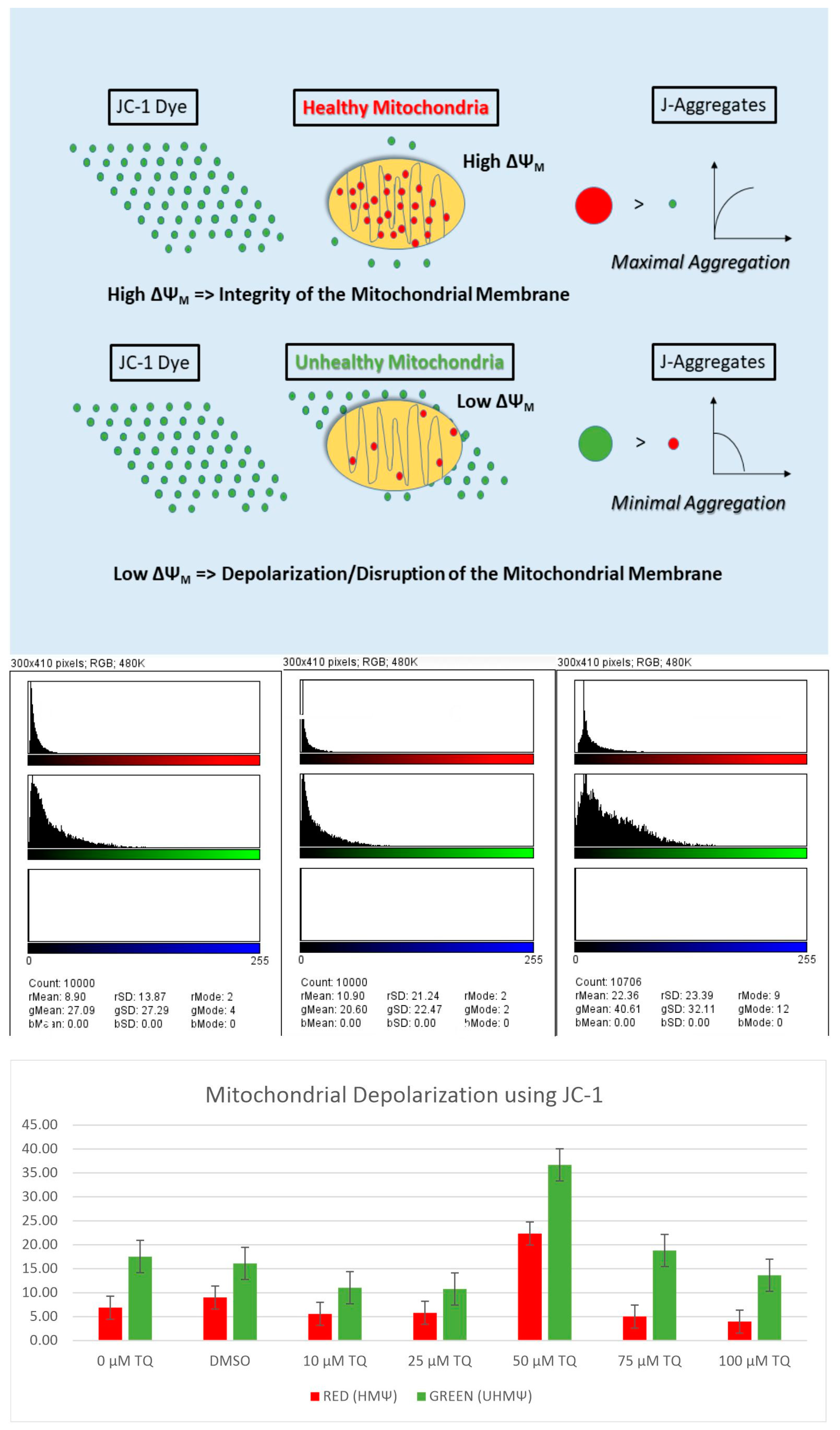
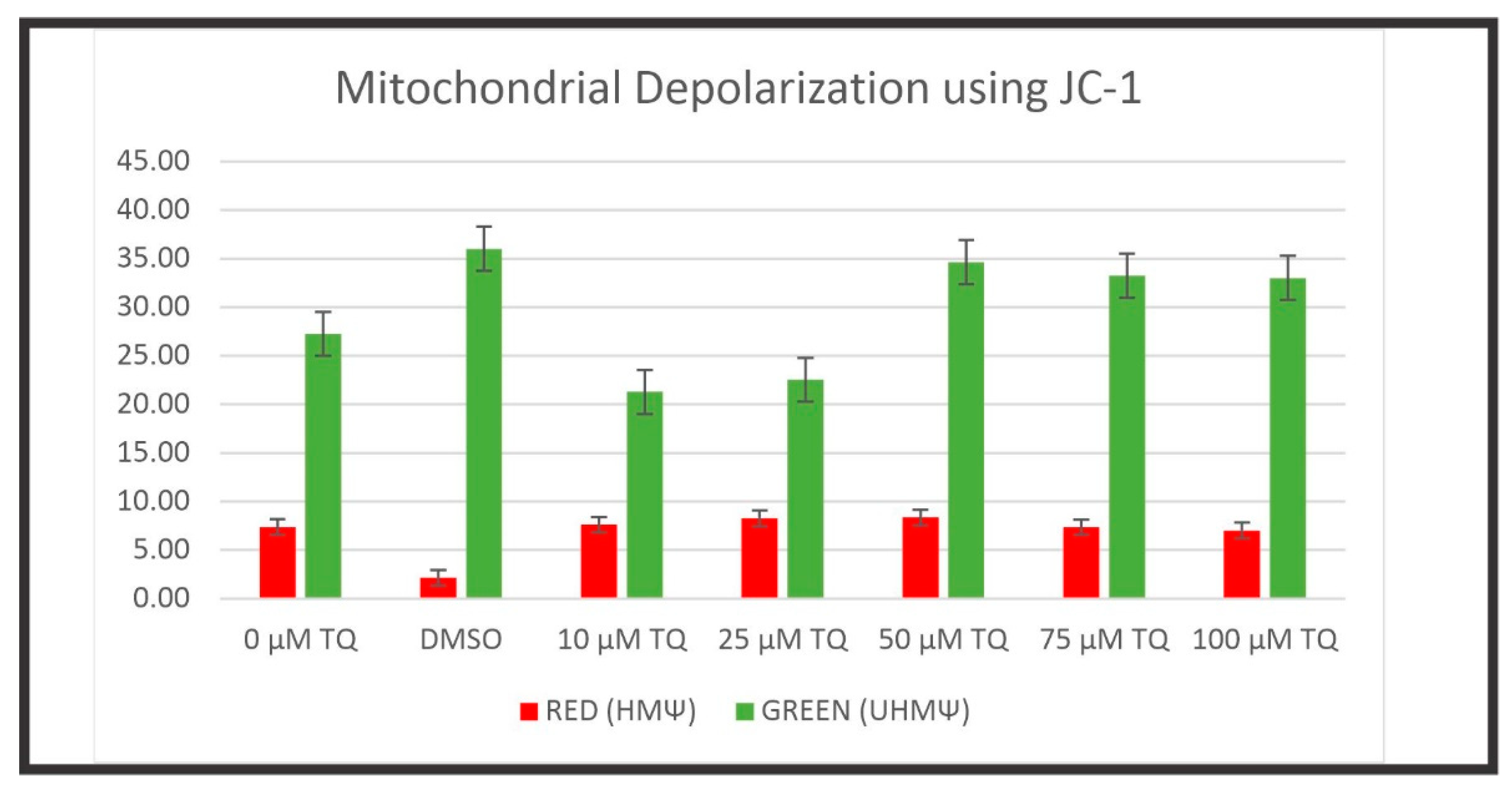
2.4. TQ Protective Effect Is Trustworthy
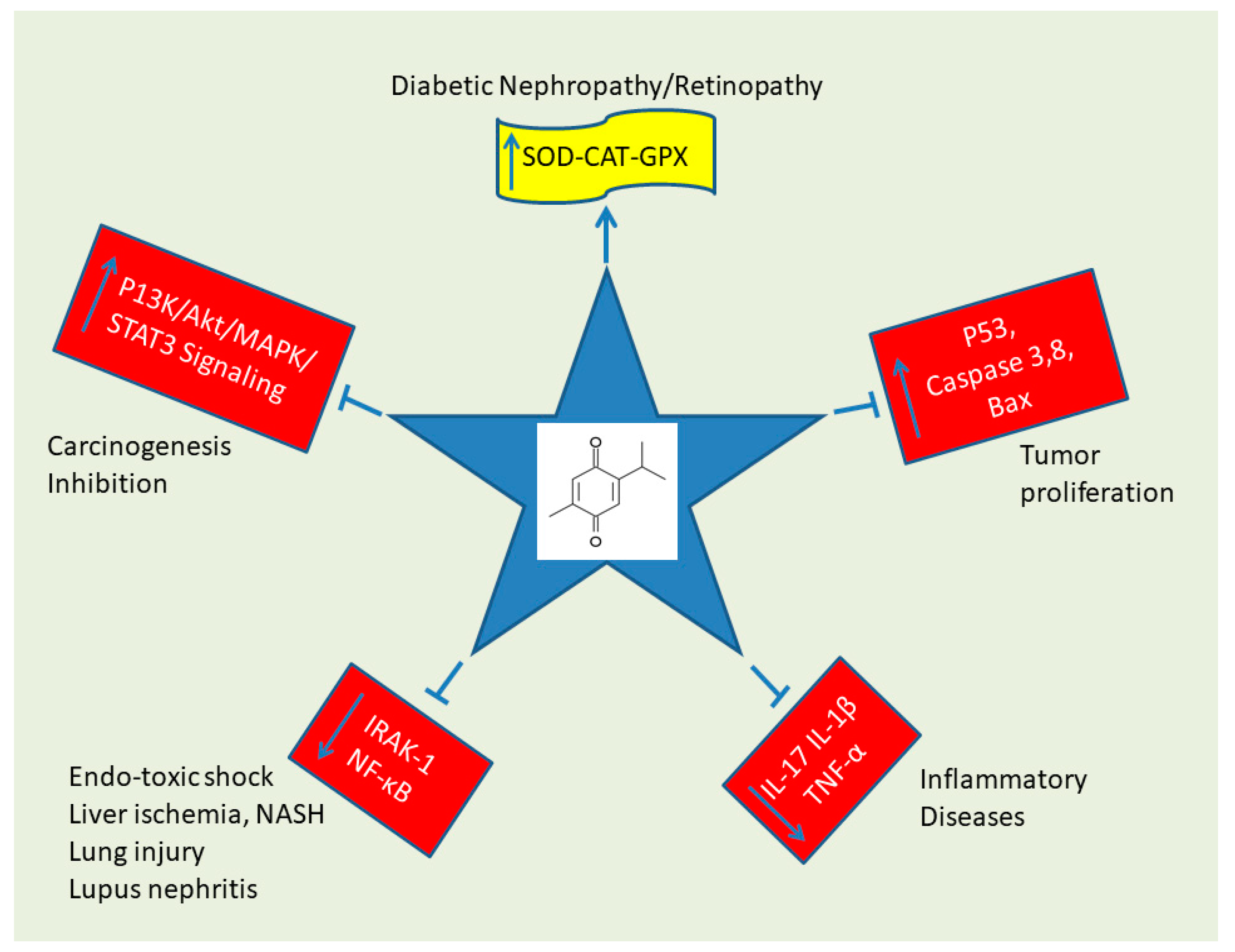
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Culture Conditions
4.2. TQ Preparation
4.3. Cell Proliferation Assay
4.4. Cell Cycle Analysis
4.5. Apoptosis and Cell Necrosis Assay
4.6. Morphological Assessment
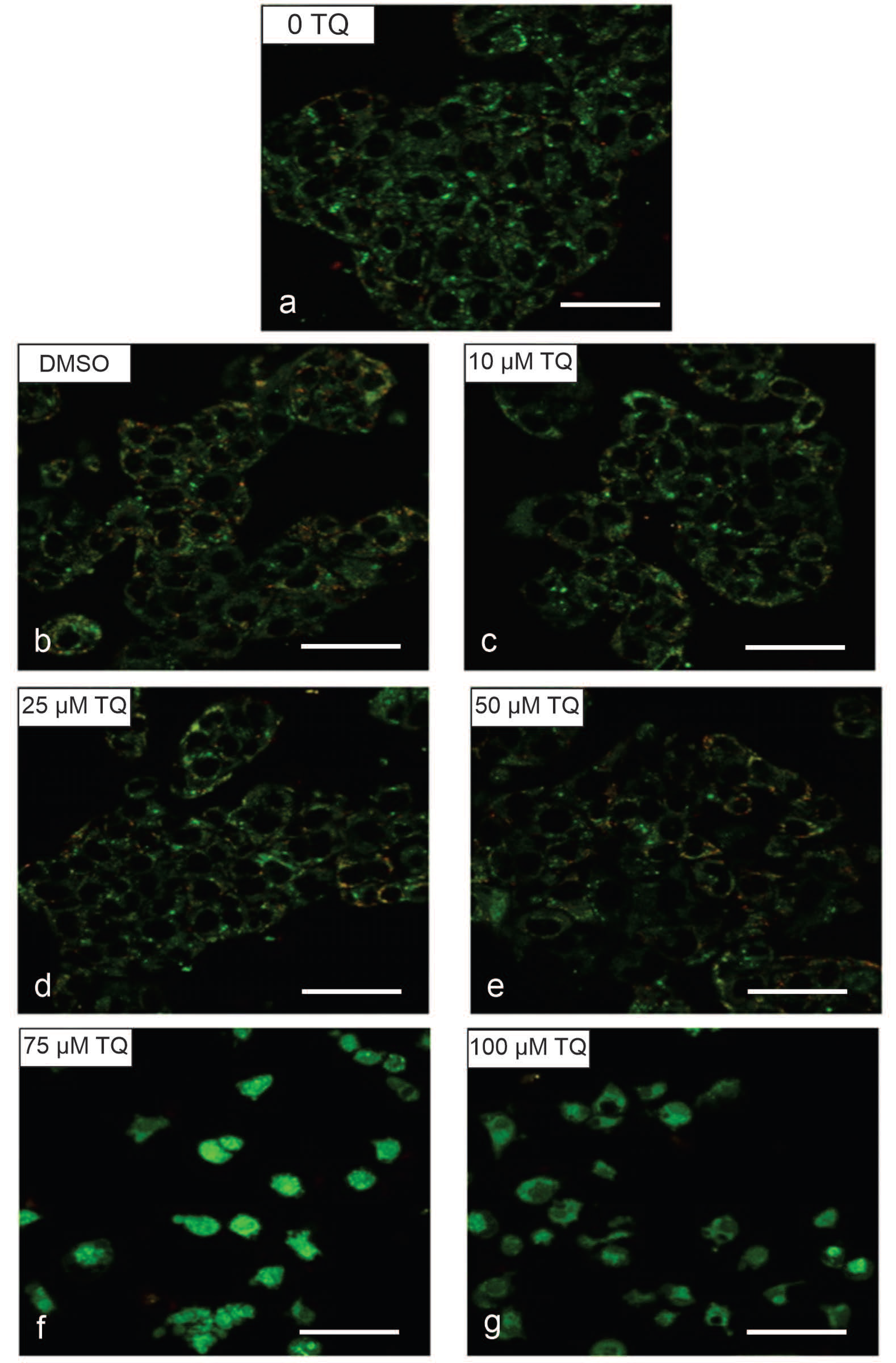
4.7. Mitochondrial Membrane Potential
4.8. Statistical Analysis
4.9. Systematic Review
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boyle, D.A. Hepatocellular carcinoma: Implications for Asia-Pacific oncology nurses. Asia Pac. J. Oncol. Nurs. 2017, 4, 98–103. [Google Scholar] [CrossRef]
- Sergi, C.M. Hepatocellular carcinoma, fibrolamellar variant: Diagnostic pathologic criteria and molecular pathology update. A primer. Diagnostics 2015, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Al-Bahrani, R.; Nagamori, S.; Leng, R.; Petryk, A.; Sergi, C. Differential expression of sonic hedgehog protein in human hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Pathol. Oncol. Res. 2015, 21, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Waziry, R.; Hajarizadeh, B.; Grebely, J.; Amin, J.; Law, M.; Danta, M.; George, J.; Dore, G.J. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: A systematic review, meta-analyses, and meta-regression. J. Hepatol. 2017, 67, P1204–P1212. [Google Scholar] [CrossRef]
- Le, A.K.; Zhao, C.; Hoang, J.K.; Tran, S.A.; Chang, C.Y.; Jin, M.; Nguyen, N.H.; Yasukawa, L.A.; Zhang, J.Q.; Weber, S.C.; et al. Ethnic disparities in progression to advanced liver disease and overall survival in patients with chronic hepatitis C: Impact of a sustained virological response. Aliment. Pharmacol. Ther. 2017, 46, 605–616. [Google Scholar] [CrossRef]
- Ghouri, Y.A.; Mian, I.; Rowe, J.H. Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis. J. Carcinog. 2017, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Hallouch, O.; Chernyak, V.; Kamaya, A.; Sirlin, C.B. Epidemiology of hepatocellular carcinoma: Target population for surveillance and diagnosis. Abdom. Radiol. 2017, 43, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Akiel, M.; Guo, C.; Li, X.; Rajasekaran, D.; Mendoza, R.G.; Robertson, C.L.; Jariwala, N.; Yuan, F.; Subler, M.A.; Windle, J.; et al. IGFBP7 deletion promotes hepatocellular carcinoma. Cancer Res. 2017, 77, 4014–4025. [Google Scholar] [CrossRef]
- Jin, L.; Shen, F.; Weinfeld, M.; Sergi, C. Insulin growth factor binding protein 7 (IGFBP7)-related cancer and IGFBP3 and IGFBP7 crosstalk. Front. Oncol. 2020, 10, 727. [Google Scholar] [CrossRef] [PubMed]
- Sergi, C.; Shen, F.; Liu, S.M. Insulin/IGF-1R, SIRT1, and FOXOs pathways-an intriguing interaction platform for bone and osteosarcoma. Front. Endocrinol. 2019, 10, 93. [Google Scholar] [CrossRef] [PubMed]
- Ressler, S.; Radhi, J.; Aigner, T.; Loo, C.; Zwerschke, W.; Sergi, C. Insulin-like growth factor-binding protein-3 in osteosarcomas and normal bone tissues. Anticancer Res. 2009, 29, 2579–2587. [Google Scholar] [PubMed]
- Santer, F.R.; Bacher, N.; Moser, B.; Morandell, D.; Ressler, S.; Firth, S.M.; Spoden, G.A.; Sergi, C.; Baxter, R.C.; Jansen-Durr, P.; et al. Nuclear insulin-like growth factor binding protein-3 induces apoptosis and is targeted to ubiquitin/proteasome-dependent proteolysis. Cancer Res. 2006, 66, 3024–3033. [Google Scholar] [CrossRef] [PubMed][Green Version]
- He, B.; Yin, J.; Gong, S.; Gu, J.; Xiao, J.; Shi, W.; Ding, W.; He, Y. Bioinformatics analysis of key genes and pathways for hepatocellular carcinoma transformed from cirrhosis. Medicine 2017, 96, e6938. [Google Scholar] [CrossRef]
- Perez, P.; Rodriguez-Peralvarez, M.; Guerrero, L.; Gonzalez, V.; Sanchez, R.; Centeno, M.; Poyato, A.; Briceno, J.; Sanchez-Frias, M.; Montero, J.L.; et al. Incidental hepatocellular carcinoma after liver transplantation: Prevalence, histopathological features and prognostic impact. PLoS ONE 2017, 12, e0175010. [Google Scholar] [CrossRef] [PubMed]
- West, J.; Card, T.R.; Aithal, G.P.; Fleming, K.M. Risk of hepatocellular carcinoma among individuals with different aetiologies of cirrhosis: A population-based cohort study. Aliment. Pharmacol. Ther. 2017, 45, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Al-Bahrani, R.; Tuertcher, D.; Zailaie, S.; Abuetabh, Y.; Nagamori, S.; Zetouni, N.; Bahitham, W.; Sergi, C. Differential SIRT1 expression in hepatocellular carcinomas and cholangiocarcinoma of the liver. Ann. Clin. Lab. Sci. 2015, 45, 3–9. [Google Scholar] [PubMed]
- Al-Bahrani, R.; Abuetabh, Y.; Zeitouni, N.; Sergi, C. Cholangiocarcinoma: Risk factors, environmental influences and oncogenesis. Ann. Clin. Lab. Sci. 2013, 43, 195–210. [Google Scholar]
- Sahu, S.; Sun, W. Targeted therapy in biliary tract cancers-current limitations and potentials in the future. J. Gastrointest. Oncol. 2017, 8, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Blechacz, B. Cholangiocarcinoma: Current knowledge and new developments. Gut Liver 2017, 11, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Bahitham, W.; Liao, X.; Peng, F.; Bamforth, F.; Chan, A.; Mason, A.; Stone, B.; Stothard, P.; Sergi, C. Mitochondriome and cholangiocellular carcinoma. PLoS ONE 2014, 9, e104694. [Google Scholar] [CrossRef] [PubMed]
- Yeesoonsang, S.; Bilheem, S.; McNeil, E.; Iamsirithaworn, S.; Jiraphongsa, C.; Sriplung, H. Estimation of the incidence of hepatocellular carcinoma and cholangiocarcinoma in Songkhla, Thailand, 1989–2013, using multiple imputation method. Cancer Res. Treat. 2017, 49, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Sripa, B.; Echaubard, P. Prospects and challenges towards sustainable liver fluke control. Trends Parasitol. 2017, 33, P799–P812. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, B.A.; Echaubard, P. Balancing biomedical and ecological perspectives in research framing of liver fluke and cholangiocarcinoma in NE Thailand. Parasitol. Int. 2017, 66, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, O.S.; Kovshirina, Y.V.; Kovshirina, A.E.; Fedotova, M.M.; Deev, I.A.; Petrovskiy, F.I.; Filimonov, A.V.; Dmitrieva, A.I.; Kudyakov, L.A.; Saltykova, I.V.; et al. Opisthorchis felineus infection and cholangiocarcinoma in the Russian Federation: A review of medical statistics. Parasitol. Int. 2017, 66, 365–371. [Google Scholar] [CrossRef]
- Callea, F.; Sergi, C.; Fabbretti, G.; Brisigotti, M.; Cozzutto, C.; Medicina, D. Precancerous lesions of the biliary tree. J. Surg. Oncol. Suppl. 1993, 3, 131–133. [Google Scholar] [CrossRef]
- Benbrahim-Tallaa, L.; Lauby-Secretan, B.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; El Ghissassi, F.; Bouvard, V.; Guha, N.; Mattock, H.; Straif, K.; et al. Carcinogenicity of perfluorooctanoic acid, tetrafluoroethylene, dichloromethane, 1,2-dichloropropane, and 1,3-propane sultone. Lancet Oncol. 2014, 15, 924–925. [Google Scholar] [CrossRef]
- Dorn, L.; Menezes, L.F.; Mikuz, G.; Otto, H.F.; Onuchic, L.F.; Sergi, C. Immunohistochemical detection of polyductin and co-localization with liver progenitor cell markers during normal and abnormal development of the intrahepatic biliary system and in adult hepatobiliary carcinomas. J. Cell Mol. Med. 2009, 13, 1279–1290. [Google Scholar] [CrossRef][Green Version]
- Sergi, C.; Kahl, P.; Otto, H.F. Contribution of apoptosis and apoptosis-related proteins to the malformation of the primitive intrahepatic biliary system in Meckel syndrome. Am. J. Pathol. 2000, 156, 1589–1598. [Google Scholar] [CrossRef]
- Sergi, C.; Adam, S.; Kahl, P.; Otto, H.F. Study of the malformation of ductal plate of the liver in Meckel syndrome and review of other syndromes presenting with this anomaly. Pediatr. Dev. Pathol. 2000, 3, 568–583. [Google Scholar] [CrossRef]
- Chan, B.Y.H.; Roczkowsky, A.; Cho, W.J.; Poirier, M.; Sergi, C.; Keschrumrus, V.; Churko, J.M.; Granzier, H.; Schulz, R. MMP inhibitors attenuate doxorubicin cardiotoxicity by preventing intracellular and extracellular matrix remodelling. Cardiovasc. Res. 2021, 117, 188–200. [Google Scholar] [CrossRef]
- Abdualmjid, R.J.; Sergi, C. Hepatotoxic botanicals—An evidence-based systematic review. J. Pharm. Pharm. Sci. 2013, 16, 376–404. [Google Scholar] [CrossRef] [PubMed]
- Sultan, M.T.; Butt, M.S.; Qayyum, M.M.; Suleria, H.A. Immunity: Plants as effective mediators. Crit. Rev. Food Sci. Nutr. 2014, 54, 1298–1308. [Google Scholar] [CrossRef]
- Khan, M.A. Chemical composition and medicinal properties of Nigella sativa Linn. Inflammopharmacology 1999, 7, 15–35. [Google Scholar] [CrossRef] [PubMed]
- Sayed, M.D. Traditional medicine in health care. J. Ethnopharmacol. 1980, 2, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Tariq, M. Nigella sativa seeds: Folklore treatment in modern day medicine. Saudi J. Gastroenterol. 2008, 14, 105–106. [Google Scholar] [CrossRef]
- Staub, P.O.; Casu, L.; Leonti, M. Back to the roots: A quantitative survey of herbal drugs in Dioscorides’ De Materia Medica (ex Matthioli, 1568). Phytomedicine 2016, 23, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, W.M. Upregulation of chemoprotective enzymes and glutathione by Nigella sativa (black seed) and thymoquinone in CCl4-intoxicated rats. Int. J. Toxicol. 2011, 30, 707–714. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Kroh, L.W.; Morsel, J.T. Radical scavenging activity of black cumin (Nigella sativa L.), coriander (Coriandrum sativum L.), and niger (Guizotia abyssinica Cass.) crude seed oils and oil fractions. J. Agric. Food Chem. 2003, 51, 6961–6969. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Morsel, J.T. Characterization of phospholipid composition of black cumin (Nigella sativa L.) seed oil. Nahrung 2002, 46, 240–244. [Google Scholar] [CrossRef]
- Ali, B.H.; Blunden, G. Pharmacological and toxicological properties of Nigella sativa. Phytother. Res. 2003, 17, 299–305. [Google Scholar] [CrossRef]
- Salem, M.L. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int. Immunopharmacol. 2005, 5, 1749–1770. [Google Scholar] [CrossRef] [PubMed]
- Dajani, E.Z.; Shahwan, T.G.; Dajani, N.E. Overview of the preclinical pharmacological properties of Nigella sativa (black seeds): A complementary drug with historical and clinical significance. J. Physiol. Pharmacol. 2016, 67, 801–817. [Google Scholar] [PubMed]
- El-Dakhakhny, M. Studies on the Egyptian Nigella sativa L. IV. Some pharmacological properties of the seeds’ active principle in comparison to its dihydro compound and its polymer. Arzneimittelforschung 1965, 15, 1227–1229. [Google Scholar]
- Mostofa, A.G.M.; Hossain, M.K.; Basak, D.; Bin Sayeed, M.S. Thymoquinone as a potential adjuvant therapy for cancer treatment: Evidence from preclinical studies. Front. Pharmacol. 2017, 8, 295. [Google Scholar] [CrossRef]
- Cascella, M.; Palma, G.; Barbieri, A.; Bimonte, S.; Amruthraj, N.J.; Muzio, M.R.; Del Vecchio, V.; Rea, D.; Falco, M.; Luciano, A.; et al. Role of Nigella sativa and its constituent thymoquinone on chemotherapy-induced nephrotoxicity: Evidences from experimental animal studies. Nutrients 2017, 9, 625. [Google Scholar] [CrossRef]
- Ijaz, H.; Tulain, U.R.; Qureshi, J.; Danish, Z.; Musayab, S.; Akhtar, M.F.; Saleem, A.; Khan, K.K.; Zaman, M.; Waheed, I.; et al. Review: Nigella sativa (prophetic medicine): A review. Pak. J. Pharm. Sci. 2017, 30, 229–234. [Google Scholar]
- Majdalawieh, A.F.; Fayyad, M.W.; Nasrallah, G.K. Anti-cancer properties and mechanisms of action of thymoquinone, the major active ingredient of Nigella sativa. Crit. Rev. Food Sci. Nutr. 2017, 57, 3911–3928. [Google Scholar] [CrossRef]
- Arshad Malik, M.S.; Al Jaouni, S.K.; Harakeh, S.M.; Arshad Malik, M.F. Review—Therapeutic implications of Nigella sativa against cancer metastasis. Pak. J. Pharm. Sci. 2016, 29, 1881–1884. [Google Scholar] [PubMed]
- Elmaci, I.; Altinoz, M.A. Thymoquinone: An edible redox-active quinone for the pharmacotherapy of neurodegenerative conditions and glial brain tumors. A short review. Biomed. Pharmacother. 2016, 83, 635–640. [Google Scholar] [CrossRef]
- Gali-Muhtasib, H.; Kuester, D.; Mawrin, C.; Bajbouj, K.; Diestel, A.; Ocker, M.; Habold, C.; Foltzer-Jourdainne, C.; Schoenfeld, P.; Peters, B.; et al. Thymoquinone triggers inactivation of the stress response pathway sensor CHEK1 and contributes to apoptosis in colorectal cancer cells. Cancer Res. 2008, 68, 5609–5618. [Google Scholar] [CrossRef]
- Gali-Muhtasib, H.; Roessner, A.; Schneider-Stock, R. Thymoquinone: A promising anti-cancer drug from natural sources. Int. J. Biochem. Cell Biol. 2006, 38, 1249–1253. [Google Scholar] [CrossRef] [PubMed]
- Woo, C.C.; Kumar, A.P.; Sethi, G.; Tan, K.H. Thymoquinone: Potential cure for inflammatory disorders and cancer. Biochem. Pharmacol. 2012, 83, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Roepke, M.; Diestel, A.; Bajbouj, K.; Walluscheck, D.; Schonfeld, P.; Roessner, A.; Schneider-Stock, R.; Gali-Muhtasib, H. Lack of p53 augments thymoquinone-induced apoptosis and caspase activation in human osteosarcoma cells. Cancer Biol. Ther. 2007, 6, 160–169. [Google Scholar] [CrossRef]
- Shoieb, A.M.; Elgayyar, M.; Dudrick, P.S.; Bell, J.L.; Tithof, P.K. In vitro inhibition of growth and induction of apoptosis in cancer cell lines by thymoquinone. Int. J. Oncol. 2003, 22, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Gali-Muhtasib, H.; Diab-Assaf, M.; Boltze, C.; Al-Hmaira, J.; Hartig, R.; Roessner, A.; Schneider-Stock, R. Thymoquinone extracted from black seed triggers apoptotic cell death in human colorectal cancer cells via a p53-dependent mechanism. Int. J. Oncol. 2004, 25, 857–866. [Google Scholar]
- Sayed-Ahmed, M.M.; Aleisa, A.M.; Al-Rejaie, S.S.; Al-Yahya, A.A.; Al-Shabanah, O.A.; Hafez, M.M.; Nagi, M.N. Thymoquinone attenuates diethylnitrosamine induction of hepatic carcinogenesis through antioxidant signaling. Oxid. Med. Cell Longev. 2010, 3, 254–261. [Google Scholar] [CrossRef]
- Worthen, D.R.; Ghosheh, O.A.; Crooks, P.A. The in vitro anti-tumor activity of some crude and purified components of blackseed, Nigella sativa L. Anticancer Res. 1998, 18, 1527–1532. [Google Scholar]
- Gali-Muhtasib, H.U.; Abou Kheir, W.G.; Kheir, L.A.; Darwiche, N.; Crooks, P.A. Molecular pathway for thymoquinone-induced cell-cycle arrest and apoptosis in neoplastic keratinocytes. Anticancer Drugs 2004, 15, 389–399. [Google Scholar] [CrossRef]
- Kaseb, A.O.; Chinnakannu, K.; Chen, D.; Sivanandam, A.; Tejwani, S.; Menon, M.; Dou, Q.P.; Reddy, G.P. Androgen receptor and E2F-1 targeted thymoquinone therapy for hormone-refractory prostate cancer. Cancer Res. 2007, 67, 7782–7788. [Google Scholar] [CrossRef]
- Gurung, R.L.; Lim, S.N.; Khaw, A.K.; Soon, J.F.; Shenoy, K.; Mohamed Ali, S.; Jayapal, M.; Sethu, S.; Baskar, R.; Hande, M.P. Thymoquinone induces telomere shortening, DNA damage and apoptosis in human glioblastoma cells. PLoS ONE 2010, 5, e12124. [Google Scholar] [CrossRef]
- Richards, L.R.; Jones, P.; Hughes, J.; Benghuzzi, H.; Tucci, M. The physiological effect of conventional treatment with epigallocatechin-3-gallate, thymoquinone, and tannic acid on the LNCaP cell line. Biomed. Sci. Instrum. 2006, 42, 357–362. [Google Scholar] [PubMed]
- El-Mahdy, M.A.; Zhu, Q.; Wang, Q.E.; Wani, G.; Wani, A.A. Thymoquinone induces apoptosis through activation of caspase-8 and mitochondrial events in p53-null myeloblastic leukemia HL-60 cells. Int. J. Cancer 2005, 117, 409–417. [Google Scholar] [CrossRef]
- Bimonte, S.; Albino, V.; Barbieri, A.; Tamma, M.L.; Nasto, A.; Palaia, R.; Molino, C.; Bianco, P.; Vitale, A.; Schiano, R.; et al. Dissecting the roles of thymoquinone on the prevention and the treatment of hepatocellular carcinoma: An overview on the current state of knowledge. Infect. Agent Cancer 2019, 14, 10. [Google Scholar] [CrossRef]
- Tekbas, A.; Huebner, J.; Settmacher, U.; Dahmen, U. Plants and surgery: The protective effects of thymoquinone on hepatic injury—A systematic review of in vivo studies. Int. J. Mol. Sci. 2018, 19, 1085. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Sriramulu, S.; Catanzaro, R.; He, F.; Chabria, Y.; Balakrishnan, B.; Hari, S.; Ayala, A.; Munoz, M.; Pathak, S.; et al. Natural compounds as integrative therapy for liver protection against inflammatory and carcinogenic mechanisms: From induction to molecular biology advancement. Curr. Mol. Med. 2022, 23, 216–231. [Google Scholar] [CrossRef]
- Abdel-Wahab, W.M. Protective effect of thymoquinone on sodium fluoride-induced hepatotoxicity and oxidative stress in rats. J. Basic Appl. Zool. 2013, 66, 263–270. [Google Scholar] [CrossRef]
- Nagi, M.N.; Almakki, H.A.; Sayed-Ahmed, M.M.; Al-Bekairi, A.M. Thymoquinone supplementation reverses acetaminophen-induced oxidative stress, nitric oxide production and energy decline in mice liver. Food Chem. Toxicol. 2010, 48, 2361–2365. [Google Scholar] [CrossRef]
- Ojha, S.; Azimullah, S.; Mohanraj, R.; Sharma, C.; Yasin, J.; Arya, D.S.; Adem, A. Thymoquinone protects against myocardial ischemic injury by mitigating oxidative stress and inflammation. Evid. Based Complement. Alternat. Med. 2015, 2015, 143629. [Google Scholar] [CrossRef]
- Alam, M.F.; Khan, G.; Safhi, M.M.; Alshahrani, S.; Siddiqui, R.; Sivagurunathan Moni, S.; Anwer, T. Thymoquinone ameliorates doxorubicin-induced cardiotoxicity in Swiss albino mice by modulating oxidative damage and cellular inflammation. Cardiol. Res. Pract. 2018, 2018, 1483041. [Google Scholar] [CrossRef] [PubMed]
- Karabulut, D.; Ozturk, E.; Kaymak, E.; Akin, A.T.; Yakan, B. Thymoquinone attenuates doxorubicin-cardiotoxicity in rats. J. Biochem. Mol. Toxicol. 2021, 35, e22618. [Google Scholar] [CrossRef] [PubMed]
- Mehri, S.; Shahi, M.; Razavi, B.M.; Hassani, F.V.; Hosseinzadeh, H. Neuroprotective effect of thymoquinone in acrylamide-induced neurotoxicity in Wistar rats. Iran. J. Basic Med. Sci. 2014, 17, 1007–1011. [Google Scholar] [PubMed]
- Kundu, J.K.; Liu, L.; Shin, J.W.; Surh, Y.J. Thymoquinone inhibits phorbol ester-induced activation of NF-kappaB and expression of COX-2, and induces expression of cytoprotective enzymes in mouse skin in vivo. Biochem. Biophys. Res. Commun. 2013, 438, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Umar, S.; Zargan, J.; Umar, K.; Ahmad, S.; Katiyar, C.K.; Khan, H.A. Modulation of the oxidative stress and inflammatory cytokine response by thymoquinone in the collagen induced arthritis in Wistar rats. Chem. Biol. Interact. 2012, 197, 40–46. [Google Scholar] [CrossRef] [PubMed]
- El-Aarag, B.; Hussein, W.; Ibrahim, W.; Zahran, M. Thymoquinone improves anti-diabetic activity of metformin in streptozotocininduced diabetic male rats. J. Diabetes Metab. 2017, 8, 780. [Google Scholar]
- Rani, R.; Dahiya, S.; Dhingra, D.; Dilbaghi, N.; Kaushik, A.; Kim, K.H.; Kumar, S. Antidiabetic activity enhancement in streptozotocin + nicotinamide-induced diabetic rats through combinational polymeric nanoformulation. Int. J. Nanomed. 2019, 14, 4383–4395. [Google Scholar] [CrossRef]
- Sankaranarayanan, C.; Pari, L. Thymoquinone ameliorates chemical induced oxidative stress and beta-cell damage in experimental hyperglycemic rats. Chem. Biol. Interact. 2011, 190, 148–154. [Google Scholar] [CrossRef]
- Vaillancourt, F.; Silva, P.; Shi, Q.; Fahmi, H.; Fernandes, J.C.; Benderdour, M. Elucidation of molecular mechanisms underlying the protective effects of thymoquinone against rheumatoid arthritis. J. Cell Biochem. 2011, 112, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.; Prasad, S.; Sung, B.; Krishnan, S.; Guha, S. Prevention and treatment of colorectal cancer by natural agents from mother nature. Curr. Colorectal. Cancer Rep. 2013, 9, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, P.; Govindarajan, R. Cancer—An ayurvedic perspective. Pharmacol. Res. 2005, 51, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Ivankovic, S.; Stojkovic, R.; Jukic, M.; Milos, M.; Milos, M.; Jurin, M. The antitumor activity of thymoquinone and thymohydroquinone in vitro and in vivo. Exp. Oncol. 2006, 28, 220–224. [Google Scholar]
- Banerjee, S.; Padhye, S.; Azmi, A.; Wang, Z.; Philip, P.A.; Kucuk, O.; Sarkar, F.H.; Mohammad, R.M. Review on molecular and therapeutic potential of thymoquinone in cancer. Nutr. Cancer 2010, 62, 938–946. [Google Scholar] [CrossRef] [PubMed]
- El Arafa, S.A.; Zhu, Q.; Shah, Z.I.; Wani, G.; Barakat, B.M.; Racoma, I.; El-Mahdy, M.A.; Wani, A.A. Thymoquinone up-regulates PTEN expression and induces apoptosis in doxorubicin-resistant human breast cancer cells. Mutat. Res. 2011, 706, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Alhosin, M.; Abusnina, A.; Achour, M.; Sharif, T.; Muller, C.; Peluso, J.; Chataigneau, T.; Lugnier, C.; Schini-Kerth, V.B.; Bronner, C.; et al. Induction of apoptosis by thymoquinone in lymphoblastic leukemia Jurkat cells is mediated by a p73-dependent pathway which targets the epigenetic integrator UHRF1. Biochem. Pharmacol. 2010, 79, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Rajendran, P.; Sethi, G. Thymoquinone inhibits proliferation, induces apoptosis and chemosensitizes human multiple myeloma cells through suppression of signal transducer and activator of transcription 3 activation pathway. Br. J. Pharmacol. 2010, 161, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Sethi, G.; Ahn, K.S.; Aggarwal, B.B. Targeting nuclear factor-kappa B activation pathway by thymoquinone: Role in suppression of antiapoptotic gene products and enhancement of apoptosis. Mol. Cancer Res. 2008, 6, 1059–1070. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.P.; Ponnusamy, M.P.; Chakraborty, S.; Smith, L.M.; Das, S.; Arafat, H.A.; Batra, S.K. Effects of thymoquinone in the expression of mucin 4 in pancreatic cancer cells: Implications for the development of novel cancer therapies. Mol. Cancer Ther. 2010, 9, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Gali-Muhtasib, H.; Ocker, M.; Kuester, D.; Krueger, S.; El-Hajj, Z.; Diestel, A.; Evert, M.; El-Najjar, N.; Peters, B.; Jurjus, A.; et al. Thymoquinone reduces mouse colon tumor cell invasion and inhibits tumor growth in murine colon cancer models. J. Cell Mol. Med. 2008, 12, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Badary, O.A.; Al-Shabanah, O.A.; Nagi, M.N.; Al-Rikabi, A.C.; Elmazar, M.M. Inhibition of benzo(a)pyrene-induced forestomach carcinogenesis in mice by thymoquinone. Eur. J. Cancer Prev. 1999, 8, 435–440. [Google Scholar] [CrossRef]
- Raghunandhakumar, S.; Paramasivam, A.; Senthilraja, S.; Naveenkumar, C.; Asokkumar, S.; Binuclara, J.; Jagan, S.; Anandakumar, P.; Devaki, T. Thymoquinone inhibits cell proliferation through regulation of G1/S phase cell cycle transition in N-nitrosodiethylamine-induced experimental rat hepatocellular carcinoma. Toxicol. Lett. 2013, 223, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Badary, O.A. Thymoquinone attenuates ifosfamide-induced Fanconi syndrome in rats and enhances its antitumor activity in mice. J. Ethnopharmacol. 1999, 67, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Badary, O.A.; Gamal El-Din, A.M. Inhibitory effects of thymoquinone against 20-methylcholanthrene-induced fibrosarcoma tumorigenesis. Cancer Detect. Prev. 2001, 25, 362–368. [Google Scholar] [PubMed]
- Badary, O.A.; Nagi, M.N.; al-Shabanah, O.A.; al-Sawaf, H.A.; al-Sohaibani, M.O.; al-Bekairi, A.M. Thymoquinone ameliorates the nephrotoxicity induced by cisplatin in rodents and potentiates its antitumor activity. Can. J. Physiol. Pharmacol. 1997, 75, 1356–1361. [Google Scholar] [CrossRef]
- Al-Shabanah, O.A.; Badary, O.A.; Nagi, M.N.; al-Gharably, N.M.; al-Rikabi, A.C.; al-Bekairi, A.M. Thymoquinone protects against doxorubicin-induced cardiotoxicity without compromising its antitumor activity. J. Exp. Clin. Cancer Res. 1998, 17, 193–198. [Google Scholar]
- Nagi, M.N.; Mansour, M.A. Protective effect of thymoquinone against doxorubicin-induced cardiotoxicity in rats: A possible mechanism of protection. Pharmacol. Res. 2000, 41, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.A. Protective effects of thymoquinone and desferrioxamine against hepatotoxicity of carbon tetrachloride in mice. Life Sci. 2000, 66, 2583–2591. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Kaseb, A.O.; Wang, Z.; Kong, D.; Mohammad, M.; Padhye, S.; Sarkar, F.H.; Mohammad, R.M. Antitumor activity of gemcitabine and oxaliplatin is augmented by thymoquinone in pancreatic cancer. Cancer Res. 2009, 69, 5575–5583. [Google Scholar] [CrossRef] [PubMed]
- Jafri, S.H.; Glass, J.; Shi, R.; Zhang, S.; Prince, M.; Kleiner-Hancock, H. Thymoquinone and cisplatin as a therapeutic combination in lung cancer: In vitro and in vivo. J. Exp. Clin. Cancer Res. 2010, 29, 87. [Google Scholar] [CrossRef]
- ElKhoely, A.; Hafez, H.F.; Ashmawy, A.M.; Badary, O.; Abdelaziz, A.; Mostafa, A.; Shouman, S.A. Chemopreventive and therapeutic potentials of thymoquinone in HepG2 cells: Mechanistic perspectives. J. Nat. Med. 2015, 69, 313–323. [Google Scholar] [CrossRef]
- Hassan, M.I.; Mabrouk, G.M.; Shehata, H.H.; Aboelhussein, M.M. Antineoplastic effects of bee honey and Nigella sativa on hepatocellular carcinoma cells. Integr. Cancer Ther. 2012, 11, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Attoub, S.; Sperandio, O.; Raza, H.; Arafat, K.; Al-Salam, S.; Al Sultan, M.A.; Al Safi, M.; Takahashi, T.; Adem, A. Thymoquinone as an anticancer agent: Evidence from inhibition of cancer cells viability and invasion in vitro and tumor growth in vivo. Fundam. Clin. Pharmacol. 2013, 27, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Ashour, A.E.; Abd-Allah, A.R.; Korashy, H.M.; Attia, S.M.; Alzahrani, A.Z.; Saquib, Q.; Bakheet, S.A.; Abdel-Hamied, H.E.; Jamal, S.; Rishi, A.K. Thymoquinone suppression of the human hepatocellular carcinoma cell growth involves inhibition of IL-8 expression, elevated levels of TRAIL receptors, oxidative stress and apoptosis. Mol. Cell Biochem. 2014, 389, 85–98. [Google Scholar] [CrossRef]
- Xu, D.; Ma, Y.; Zhao, B.; Li, S.; Zhang, Y.; Pan, S.; Wu, Y.; Wang, J.; Wang, D.; Pan, H.; et al. Thymoquinone induces G2/M arrest, inactivates PI3K/Akt and nuclear factor-kappaB pathways in human cholangiocarcinomas both in vitro and in vivo. Oncol. Rep. 2014, 31, 2063–2070. [Google Scholar] [CrossRef] [PubMed]
- El-Najjar, N.; Chatila, M.; Moukadem, H.; Vuorela, H.; Ocker, M.; Gandesiri, M.; Schneider-Stock, R.; Gali-Muhtasib, H. Reactive oxygen species mediate thymoquinone-induced apoptosis and activate ERK and JNK signaling. Apoptosis 2010, 15, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Humeau, J.; Bravo-San Pedro, J.M.; Vitale, I.; Nunez, L.; Villalobos, C.; Kroemer, G.; Senovilla, L. Calcium signaling and cell cycle: Progression or death. Cell Calcium 2017, 70, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Miles, S.; Breeden, L. A common strategy for initiating the transition from proliferation to quiescence. Curr. Genet. 2017, 63, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.B.; McNeely, S.C.; Beckmann, R.P. Achieving precision death with cell-cycle inhibitors that target DNA replication and repair. Clin. Cancer Res. 2017, 23, 3232–3240. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.A.; Ahmed, W.A.; Galeb, F.M.; El-Taweel, M.A.; Abu-Bedair, F.A. In vitro challenge using thymoquinone on hepatocellular carcinoma (HepG2) cell line. Iran. J. Pharm. Res 2008, 7, 283–290. [Google Scholar]
- Arafa, N.M.; Abdel-Rahman, M.; El-khadragy, M.F.; Kassab, R.B. Evaluation of the possible epileptogenic activity of ciprofloxacin: The role of Nigella sativa on amino acids neurotransmitters. Neurochem. Res. 2013, 38, 174–185. [Google Scholar] [CrossRef]
- Sutton, K.M.; Greenshields, A.L.; Hoskin, D.W. Thymoquinone, a bioactive component of black caraway seeds, causes G1 phase cell cycle arrest and apoptosis in triple-negative breast cancer cells with mutant p53. Nutr. Cancer 2014, 66, 408–418. [Google Scholar] [CrossRef]
- Hussain, A.R.; Ahmed, M.; Ahmed, S.; Manogaran, P.; Platanias, L.C.; Alvi, S.N.; Al-Kuraya, K.S.; Uddin, S. Thymoquinone suppresses growth and induces apoptosis via generation of reactive oxygen species in primary effusion lymphoma. Free Radic. Biol. Med. 2011, 50, 978–987. [Google Scholar] [CrossRef]
- Yi, T.; Cho, S.G.; Yi, Z.; Pang, X.; Rodriguez, M.; Wang, Y.; Sethi, G.; Aggarwal, B.B.; Liu, M. Thymoquinone inhibits tumor angiogenesis and tumor growth through suppressing AKT and extracellular signal-regulated kinase signaling pathways. Mol. Cancer Ther. 2008, 7, 1789–1796. [Google Scholar] [CrossRef] [PubMed]
- Tabasi, N.; Mahmoudi, M.; Rastin, M.; Sadeghnia, H.R.; Hossein Pour Mashhadi, M.; Zamani Taghizade Rabe, S.; Khajavi Rad, A. Cytotoxic and apoptogenic properties of Nigella sativa and thymoquinone, its constituent, in human renal cell carcinoma are comparable with cisplatin. Food Agric. Immunol. 2015, 26, 138–156. [Google Scholar] [CrossRef][Green Version]
- Rooney, S.; Ryan, M.F. Modes of action of alpha-hederin and thymoquinone, active constituents of Nigella sativa, against HEp-2 cancer cells. Anticancer Res. 2005, 25, 4255–4259. [Google Scholar]
- Rooney, S.; Ryan, M.F. Effects of alpha-hederin and thymoquinone, constituents of Nigella sativa, on human cancer cell lines. Anticancer Res. 2005, 25, 2199–2204. [Google Scholar] [PubMed]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J. Hormesis mediates acquired resilience: Using plant-derived chemicals to enhance health. Annu. Rev. Food Sci. Technol. 2021, 12, 355–381. [Google Scholar] [CrossRef]
- Murakami, A. Dose-dependent functionality and toxicity of green tea polyphenols in experimental rodents. Arch. Biochem. Biophys. 2014, 557, 3–10. [Google Scholar] [CrossRef]
- Sutton, K.M.; Hoskin, D.W. Thymoquinone causes mitochondrial membrane disruption and potentiates chemotherapeutic drug- and radiation induced cytotoxicity in breast cancer cells. Mol. Cancer Ther. 2009, 8, B154. [Google Scholar] [CrossRef]
- Jehan, S.; Zhong, C.; Li, G.; Zulqarnain Bakhtiar, S.; Li, D.; Sui, G. Thymoquinone selectively induces hepatocellular carcinoma cell apoptosis in synergism with clinical therapeutics and dependence of p53 status. Front. Pharmacol. 2020, 11, 555283. [Google Scholar] [CrossRef]
- Auld, F.M.; Sergi, C.M.; Leng, R.; Shen, F. The Role of N(6)-Methyladenosine in the Promotion of Hepatoblastoma: A Critical Review. Cells 2022, 11, 1516. [Google Scholar] [CrossRef]

| Component | Chemistry (IUPAC) | % of Volatile Oil |
|---|---|---|
| Thymoquinone (TQ) | 2-Isopropyl-5-methylbenzo-1,4-quinone | 28–57 |
| ρ-cymene | 1-Methyl-4-(propan-2-yl)benzene | 7.1–15.5 |
| Carvacrol (cymophenol) | 2-Methyl-5-(propan-2-yl)phenol | 5.8–11.6 |
| 4-Terpineol | 2-(4-Methyl-1-cyclohex-3-enyl)propan-2-ol | 2.0–6.6 |
| Longifolene | 4,8,8-trimethyl-9-methylidenedecahydro-1,4-methanoazulene | 1.0–8.0 |
| T-Anethole | 1-Methoxy-4-[(1E)-prop-1-en-1-yl]benzene | 0.25–2.3 |
| Cell Lines | Cell Cycle Phases | Control Group (%) | TQ-Treated Group (%) | ||||
|---|---|---|---|---|---|---|---|
| 0 TQ | 10 μM | 25 μM | 50 μM | 75 μM | 100 μM | ||
| HepG2 | G1 | 54.3 | 53.1 | 48.7 | 45.1 | 23.7 | 18.4 |
| S | 20.0 | 20.4 | 18.2 | 16.8 | 13.3 | 9.3 | |
| G2/M | 22.6 | 23.4 | 28.4 | 23.1 | 14.5 | 6.8 | |
| SubG1 | 2.6 | 2.4 | 2.8 | 18.1 | 54.1 | 60.7 | |
| HuCCT1 | G1 | 46.8 | 34.9 | 51.9 | 56.4 | 58.2 | 60.3 |
| S | 16.9 | 12.5 | 13.1 | 10.5 | 8.1 | 5.6 | |
| G2/M | 31.7 | 15.3 | 28.0 | 19.9 | 11.8 | 9.5 | |
| SubG1 | 3.8 | 3.2 | 5.1 | 11.0 | 18.7 | 22.3 | |
| Cell Lines | 12 h | 24 h | 48 h |
|---|---|---|---|
| HepG2 | 50.74 | 34.23 | 50.84 |
| HuCCT1 | 155.1 | 63.12 | 61.59 |
| Model | Ref# | Carrier | TQ Dosage | Parameters | Mechanism | Potential |
|---|---|---|---|---|---|---|
| Mouse | [63] | APAP | 0.5–2 mg/kg | ALT, NO2−/NO3− LPX, GSH, ATP | ↑AOE=>↓OXS, NO2−/NO3− stress | Hep-Prot. |
| Mouse | [65] | DXR | 10–20 mg/kg | AOE, MNM, LPO | ↑AOE=>↓OXS, ↓ Inflammation | Cardio-Prot. |
| Mouse | [68] | TPA | 1–5 μM | ↑NFκB and COX-2 | ↓NFκB=>↓COX2 | Anti-Infl. |
| Rats | [69] | Collagen | 5 mg/kg | AOE and IMs | ↑AOE=>↓OXS | Anti-Infl. |
| Rat | [62] | NaF | 10 mg/kg | LFT, AOE | ↑AOE=>↓OXS | Hep-Prot. |
| Rat | [92] | CCl4 | 5 mg/kg | ALT, GSSG, AO-mRNAs | ↑AOE=>↓OXS | Hep-Prot. |
| Rats | [64] | IPN | 20 mg/kg | OSM, MNM | Mitigation OXS, ↑AOE, ↑Tissue integrity | Cardio-Prot. |
| Rats | [66] | DXR | 10 mg/kg | AOE, HSP70, HSP90, GRP78, caspase-3, ANP, NT-proBNP | ↑AOE=>↓OXS, ↑ tissue integrity | Cardio-Prot. |
| Rats | [67] | AM | 2.5–10 mg/kg | Gait score, MDA, GSH | ↑AOE | Neuro-Prot. |
| Rats | [70] | STZ | 80 mg/kg | Glu, TC, TG, HBA1c, MDA, TAC, Glut2 mRNA | ↑ Glut-3 and IR | Anti-DM |
| Rats | [71] | STZ+NA | 10 mg/kg | Glu, Lipids, HBA1c | Unclear | Anti-DM |
| Rats | [72] | STZ+NA | 80 mg/kg | Glu, insulin, GSH, CAT, SOD, GPX, GST, LPO | ↑AOE=>↓OXS | Anti-DM |
| Rats | [73] | FLS | 0–10 μM | IMs and MP13 | ↓ NFκB and MAPK signaling pathway | AI-Prot. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdualmjid, R.J.; Sergi, C.M. Mitochondrial Dysfunction and Induction of Apoptosis in Hepatocellular Carcinoma and Cholangiocarcinoma Cell Lines by Thymoquinone. Int. J. Mol. Sci. 2022, 23, 14669. https://doi.org/10.3390/ijms232314669
Abdualmjid RJ, Sergi CM. Mitochondrial Dysfunction and Induction of Apoptosis in Hepatocellular Carcinoma and Cholangiocarcinoma Cell Lines by Thymoquinone. International Journal of Molecular Sciences. 2022; 23(23):14669. https://doi.org/10.3390/ijms232314669
Chicago/Turabian StyleAbdualmjid, Reem J., and Consolato M. Sergi. 2022. "Mitochondrial Dysfunction and Induction of Apoptosis in Hepatocellular Carcinoma and Cholangiocarcinoma Cell Lines by Thymoquinone" International Journal of Molecular Sciences 23, no. 23: 14669. https://doi.org/10.3390/ijms232314669
APA StyleAbdualmjid, R. J., & Sergi, C. M. (2022). Mitochondrial Dysfunction and Induction of Apoptosis in Hepatocellular Carcinoma and Cholangiocarcinoma Cell Lines by Thymoquinone. International Journal of Molecular Sciences, 23(23), 14669. https://doi.org/10.3390/ijms232314669







