Spermidine and 1,3-Diaminopropane Have Opposite Effects on the Final Stage of Cephalosporin C Biosynthesis in High-Yielding Acremonium chrysogenum Strain
Abstract
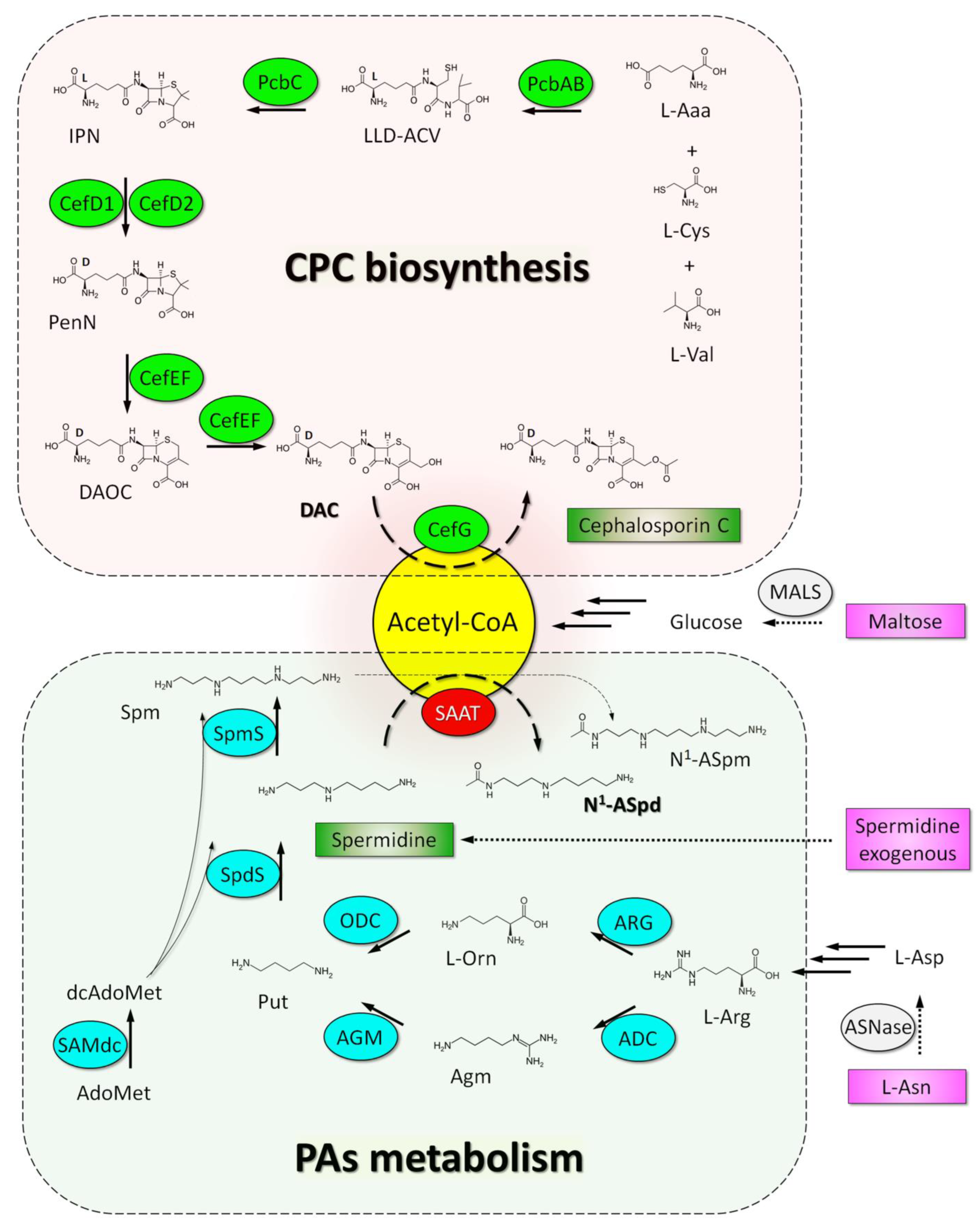
:1. Introduction
2. Results
2.1. Determining of Nitrogen (N) Source and Doses of PAs for Synthetic (SN) Medium
2.1.1. Determining N Source for Cultivation of A. chrysogenum HY
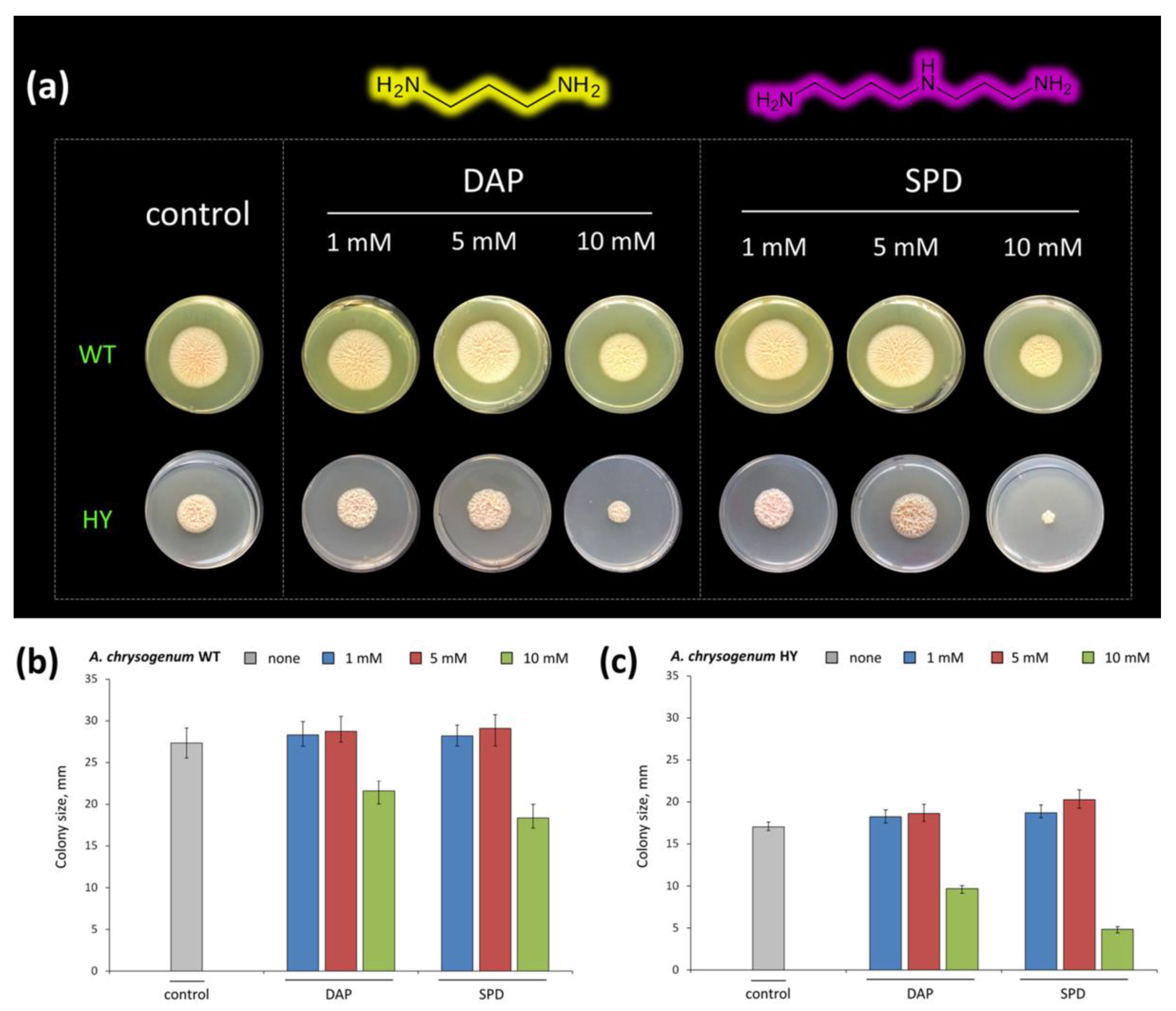
2.1.2. Determining Doses of PAs for Cultivation of A. chrysogenum HY on SN Medium with L-Asn
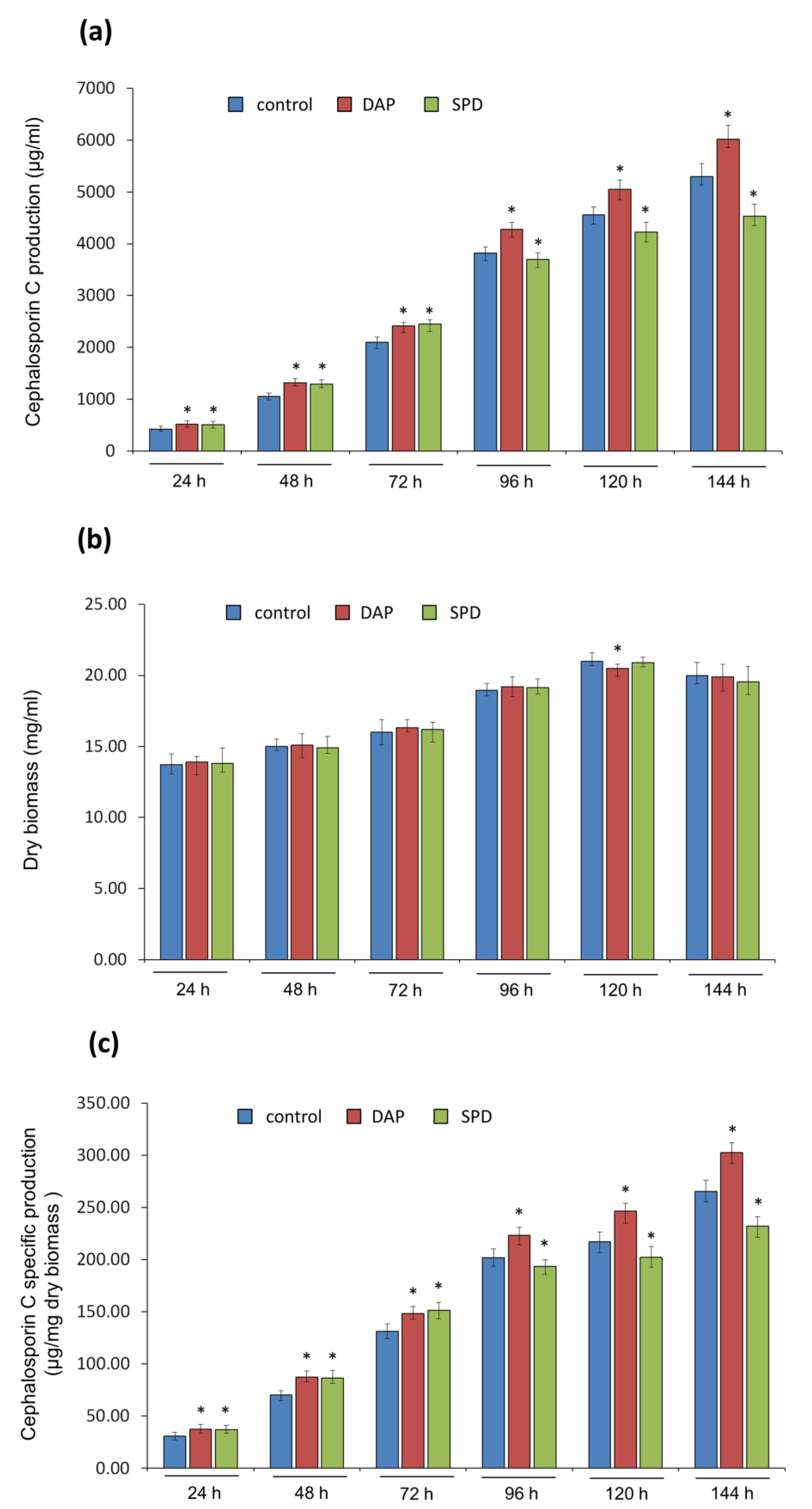
2.2. Submerged Fermentation of A. chrysogenum HY Strain with Exogenous PAs on SN and CP Media
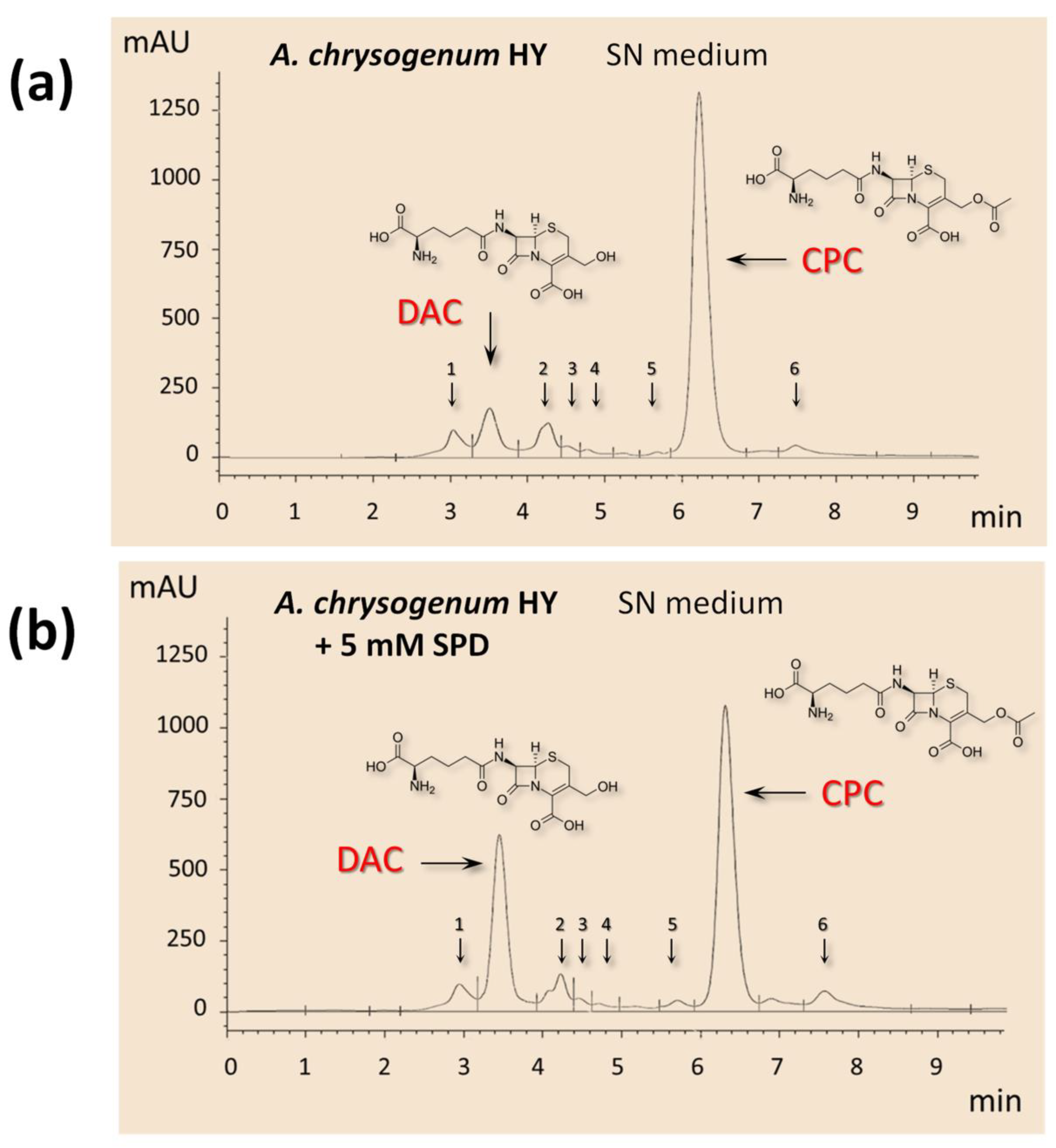
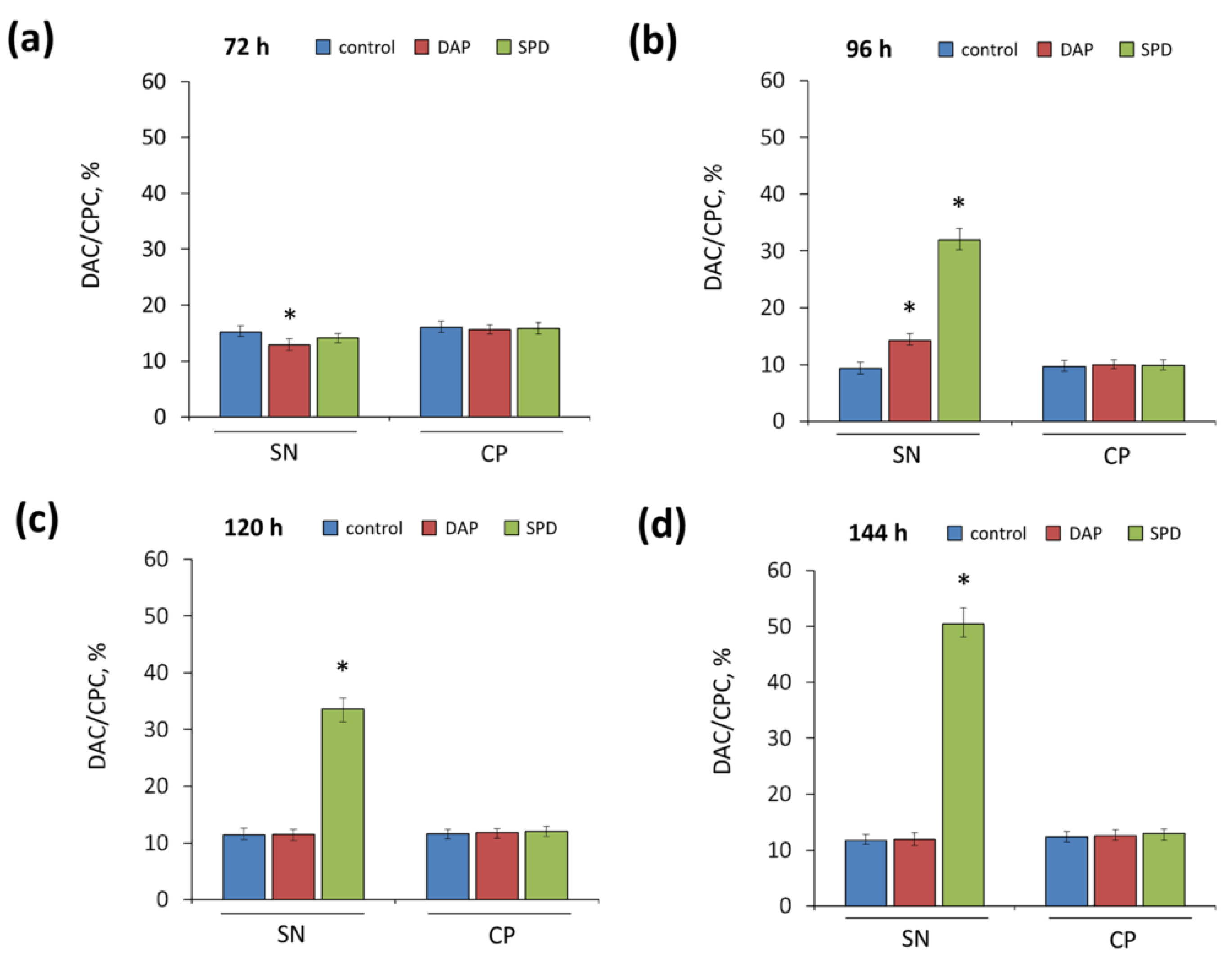
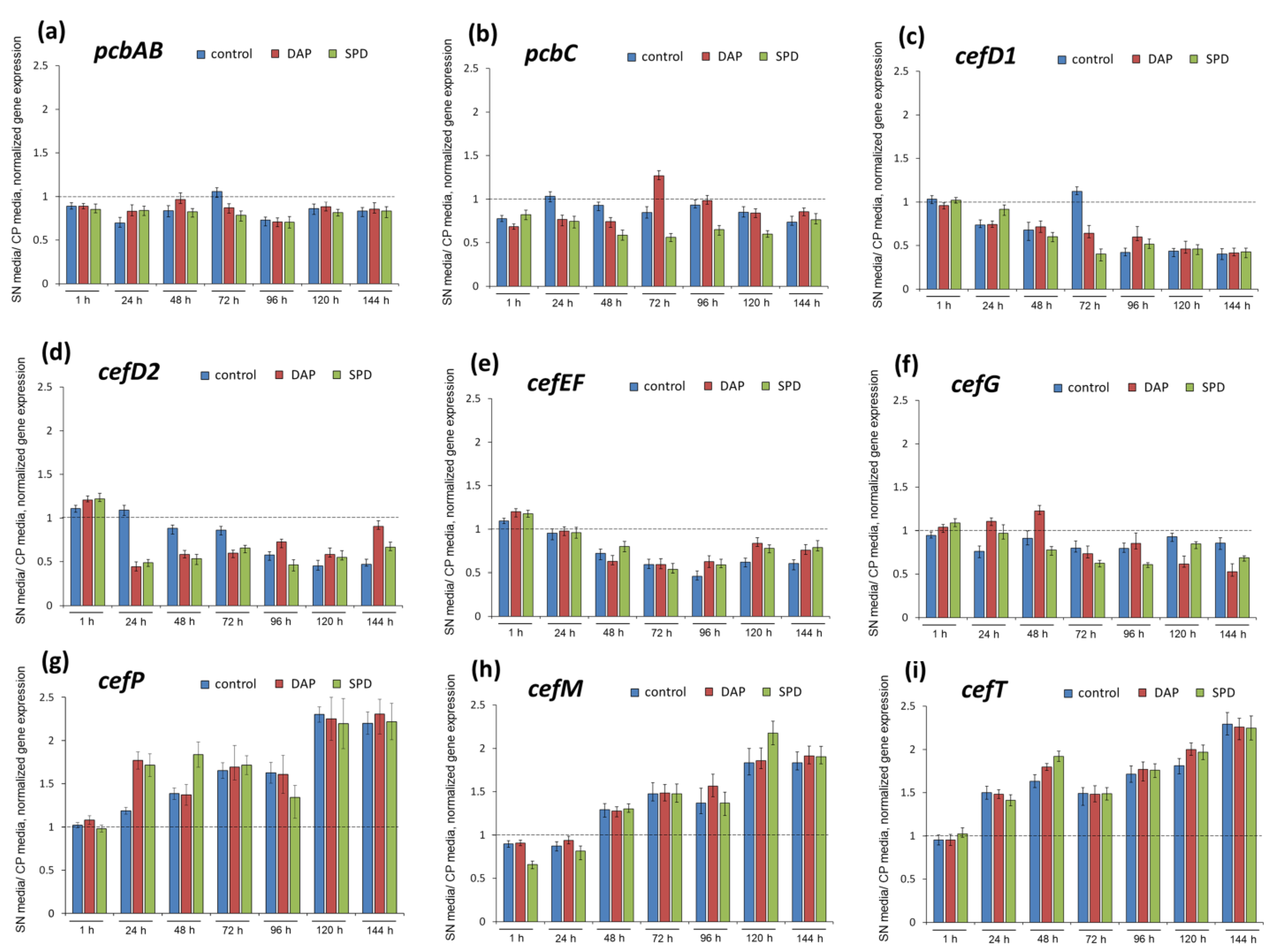
2.3. Effect of the Addition of Exogenous PAs to SN and CP Media on Cephems Ratio
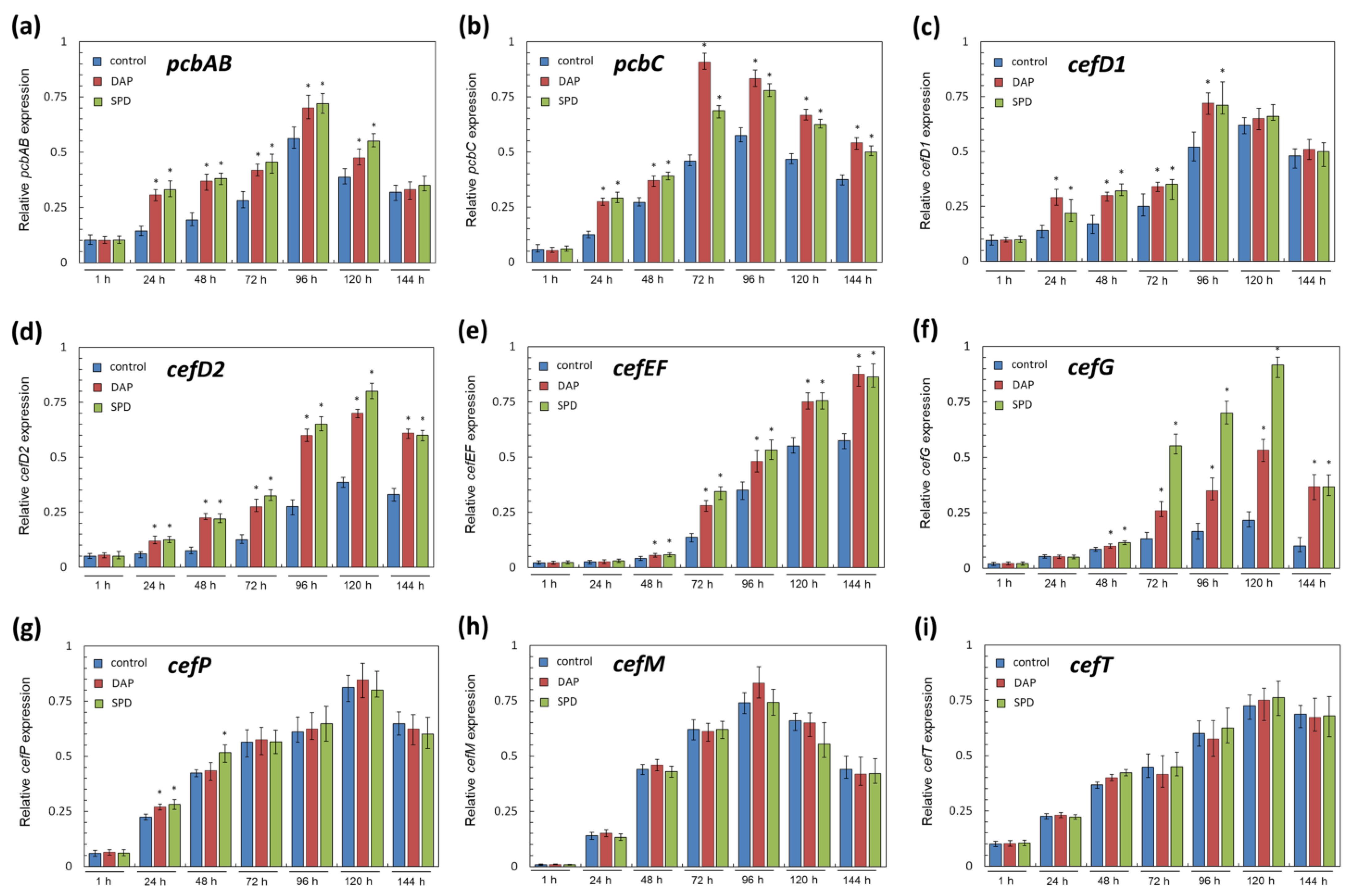
2.4. Analysis of the Expression Level of the Biosynthetic and Transport Genes from Beta-Lactam BGCs in A. chrysogenum HY after Fermentation on SN and CP Media with PAs
2.5. Effect of the Addition of Exogenous PAs to SN and CP Media on Acetyl-CoA Content
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Strains of Microorganisms
4.3. Cultivation of A. chrysogenum Strains on Agarized Media with PAs
4.4. Light Microscopy
4.5. Submerged Fermentation of A. chrysogenum HY Strain with Exogenous PAs
4.6. Determination of Dry Biomass
4.7. HPLC Analysis of Beta-Lactams
4.8. Determination of Cellular Acetyl-CoA
4.9. Preparation of Total RNA and cDNA Synthesis and qPCR Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Acetyl-CoA | Acetyl-coenzyme A |
| BGC | biosynthetic gene cluster |
| CAA | Agarized Czapek-L-Asn (medium) |
| CPC | Cephalosporin C |
| CDA | Agarized Czapek-Dox (medium) |
| CPA | Agarized complex (medium) |
| DAOC | Deacetoxycephalosporin C |
| 1,3-DAP | 1,3-diaminopropane |
| DAC | Deacetylcephalosporin C |
| LLD-ACV | δ-(L-α-aminoadipoyl)-L-cysteinyl-D-valine |
| HY | High-yielding |
| PAs | Polyamines |
| SN | Synthetic (medium) |
| CP | Complex (medium) |
| SPD | Spermidine |
| WT | Wild type |
References
- Bills, G.F.; Gloer, J.B. Biologically Active Secondary Metabolites from the Fungi. In The Fungal Kingdom; American Society of Microbiology: Washington, DC, USA, 2017; pp. 1087–1119. [Google Scholar]
- Bérdy, J. Thoughts and facts about antibiotics: Where we are now and where we are heading. J. Antibiot. 2012, 65, 385–395. [Google Scholar] [CrossRef] [Green Version]
- Backus, M.P.; Stauffer, J.F. The Production and Selection of a Family of Strains in Penicillium chrysogenum. Mycologia 1955, 47, 429–463. [Google Scholar] [CrossRef]
- Zhgun, A.A. Random Mutagenesis of Filamentous Fungi Stains for High-Yield Production of Secondary Metabolites: The Role of Polyamines. In Genotoxicity and Mutagenicity—Mechanisms and Test Methods, Chapter 2; Soloneski, S., Larramendy, M.L., Eds.; IntechOpen: London, UK, 2021; pp. 25–41. ISBN 978-1-83880-041-3. [Google Scholar]
- Bayer, E.A.; Fierro, F.; Vaca, I.; Castillo, N.I.; García-Rico, R.O.; Chávez, R. Penicillium chrysogenum, a Vintage Model with a Cutting-Edge Profile in Biotechnology. Microorg 2022, 10, 573. [Google Scholar] [CrossRef]
- Domratcheva, A.G.; Zhgun, A.A.; Novak, N.V.; Dzhavakhiya, V.V. The Influence of Chemical Mutagenesis on the Properties of the Cyclosporine a High-Producer Strain Tolypocladium inflatum VKM F-3630D. Appl. Biochem. Microbiol. 2018, 54, 53–57. [Google Scholar] [CrossRef]
- Bartoshevich, Y.; Novak, M.; Domratcheva, A.; Skrybin, K. Method of Cephalosporin C Biosynthesis by Using New Acremonium chrysogenum Strain RNCM NO F-4081D. Patent RU 2426793 C12P035/06, C07D501/02, C12R001/75, 20 August 2011. [Google Scholar]
- Zhgun, A.A.; Dumina, M.V.; Voinova, T.M.; Dzhavakhiya, V.V.; Eldarov, M.A. Role of acetyl-CoA Synthetase and LovE Regulator Protein of Polyketide Biosynthesis in Lovastatin Production by Wild-Type and Overproducing Aspergillus terreus Strains. Appl. Biochem. Microbiol. 2018, 54, 188–197. [Google Scholar] [CrossRef]
- Martín, J.; García-Estrada, C.; Kosalková, K.; Ullán, R.V.; Albillos, S.M.; Martín, J.-F. The inducers 1,3-diaminopropane and spermidine produce a drastic increase in the expression of the penicillin biosynthetic genes for prolonged time, mediated by the LaeA regulator. Fungal Genet. Biol. 2012, 49, 1004–1013. [Google Scholar] [CrossRef]
- Zhgun, A.A.; Nuraeva, G.K.; Dumina, M.V.; Voinova, T.M.; Dzhavakhiya, V.V.; Eldarov, M.A. 1,3-Diaminopropane and Spermidine Upregulate Lovastatin Production and Expression of Lovastatin Biosynthetic Genes in Aspergillus terreus via LaeA Regulation. Appl. Biochem. Microbiol. 2019, 55, 243–254. [Google Scholar] [CrossRef]
- Zhgun, A.A.; Eldarov, M.A. Polyamines Upregulate Cephalosporin C Production and Expression of β-Lactam Biosynthetic Genes in High-Yielding Acremonium chrysogenum Strain. Molecules 2021, 26, 6636. [Google Scholar] [CrossRef]
- Michael, A.J. Polyamines in Eukaryotes, Bacteria, and Archaea. J. Biol. Chem. 2016, 291, 14896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, S.S. A Guide to the Polyamines, 1st ed.; Oxford UP: New York, NY, USA, 1998; ISBN-10 0-19-511064-1/ISBN-13 978-0-19-511064-7. [Google Scholar]
- Liu, J.H.; Wang, W.; Wu, H.; Gong, X.; Moriguchi, T. Polyamines function in stress tolerance: From synthesis to regulation. Front. Plant Sci. 2015, 6, 827. [Google Scholar] [CrossRef] [PubMed]
- Dever, T.E.; Ivanov, I.P. Roles of polyamines in translation. J. Biol. Chem. 2018, 293, 18719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthews, H.R. Polyamines, chromatin structure and transcription. Bioessays 1993, 15, 561–566. [Google Scholar] [CrossRef]
- Minois, N.; Carmona-Gutierrez, D.; Madeo, F. Polyamines in aging and disease. Aging 2011, 3, 716–732. [Google Scholar] [CrossRef] [Green Version]
- Pottosin, I.; Shabala, S. Polyamines control of cation transport across plant membranes: Implications for ion homeostasis and abiotic stress signaling. Front. Plant Sci. 2014, 5, 154. [Google Scholar] [CrossRef] [Green Version]
- Dhara, M.; Matta, J.A.; Lei, M.; Knowland, D.; Yu, H.; Gu, S.; Bredt, D.S. Polyamine regulation of ion channel assembly and implications for nicotinic acetylcholine receptor pharmacology. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Miller-Fleming, L.; Olin-Sandoval, V.; Campbell, K.; Ralser, M. Remaining Mysteries of Molecular Biology: The Role of Polyamines in the Cell. J. Mol. Biol. 2015, 427, 3389–3406. [Google Scholar] [CrossRef]
- Nakanishi, S.; Cleveland, J.L. Polyamine Homeostasis in Development and Disease. Med. Sci. 2021, 9, 28. [Google Scholar] [CrossRef]
- Perez-Leal, O.; Merali, S. Regulation of polyamine metabolism by translational control. Amino Acids 2012, 42, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Alm, K.; Oredsson, S. Cells and polyamines do it cyclically. Essays Biochem. 2009, 46, 63–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willert, E.; Phillips, M.A. Regulation and function of polyamines in African trypanosomes. Trends Parasitol. 2012, 28, 66–72. [Google Scholar] [CrossRef]
- Pegg, A.E. Regulation of ornithine decarboxylase. J. Biol. Chem. 2006, 281, 14529–14532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shantz, L.M.; Holm, I.; Janne, O.A.; Pegg, A.E. Regulation of S-adenosylmethionine decarboxylase activity by alterations in the intracellular polyamine content. Biochem. J. 1992, 288, 511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heby, O.; Persson, L.; Smith, S.S. Polyamines, DNA methylation and cell differentiation. Adv. Exp. Med. Biol. 1988, 250, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Herrera, J. Polyamines, DNA methylation, and fungal differentiation. Crit. Rev. Microbiol. 1994, 20, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Tabor, H.; Rosenthal, S.M.; Tabor, C.W. The biosynthesis of spermidine and spermine from putrescine and methionine. J. Biol. Chem. 1958, 233, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E. Spermidine/spermine-N1-acetyltransferase: A key metabolic regulator. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E995–E1010. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Casero, R.A. Mammalian polyamine catabolism: A therapeutic target, a pathological problem, or both? J. Biochem. 2006, 139, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Ling, K.Q.; Sayre, L.M.; McIntire, W.S. Inhibition of murine N1-acetylated polyamine oxidase by an acetylenic amine and the allenic amine, MDL 72527. Biochem. Biophys. Res. Commun. 2005, 326, 483–490. [Google Scholar] [CrossRef]
- Pieper, M.; Schleich, H.; Gröger, H. General Synthesis of Industrial Cephalosporin-Based Antibiotics Through Amidation with Tosyl Chloride as a Coupling Reagent. Eur. J. Org. Chem. 2019, 2019, 3259–3263. [Google Scholar] [CrossRef]
- Martín, J.F. Transport systems, intracellular traffic of intermediates and secretion of β-lactam antibiotics in fungi. Fungal Biol. Biotechnol. 2020, 7, 6. [Google Scholar] [CrossRef]
- Dumina, M.V.; Zhgun, A.A.; Domracheva, A.G.; Novak, M.I.; El’darov, M.A. Chromosomal polymorphism of Acremonium chrysogenum strains producing cephalosporin C. Russ. J. Genet. 2012, 48, 778–784. [Google Scholar] [CrossRef]
- Dumina, M.V.; Zhgun, A.A.; Novak, M.I.; Domratcheva, A.G.; Petukhov, D.V.; Dzhavakhiya, V.V.; Eldarov, M.A.; Bartoshevitch, I.E. Comparative gene expression profiling reveals key changes in expression levels of cephalosporin C biosynthesis and transport genes between low and high-producing strains of Acremonium chrysogenum. World J. Microbiol. Biotechnol. 2014, 30, 2933–2941. [Google Scholar] [CrossRef]
- Zhgun, A.A.; Ivanova, M.A.; Domracheva, A.G.; Novak, M.I.; Elidarov, M.A.; Skryabin, K.G.; Bartoshevich, Y.E. Genetic transformation of the mycelium fungi Acremonium chrysogenum. Appl. Biochem. Microbiol. 2008, 44, 600–607. [Google Scholar] [CrossRef]
- Kalebina, T.S.; Selyakh, I.O.; Gorkovskii, A.A.; Bezsonov, E.E.; El’darov, M.A.; Novak, M.I.; Domracheva, A.G.; Bartoshevich, Y.E. Structure peculiarities of cell walls of Acremonium chrysogenum—An autotroph of cephalosporin C. Appl. Biochem. Microbiol. 2010, 46, 614–619. [Google Scholar] [CrossRef]
- Valiakhmetov, A.Y.; Trilisenko, L.V.; Vagabov, V.M.; Bartoshevich, Y.E.; Kulaev, I.S.; Novak, M.I.; Domracheva, A.G.; El’darov, M.A.; Skryabin, K.G. The concentration dynamics of inorganic polyphosphates during the cephalosporin C synthesis by Acremonium chrysogenum. Appl. Biochem. Microbiol. 2010, 46, 184–190. [Google Scholar] [CrossRef]
- Zhgun, A.; Dumina, M.; Valiakhmetov, A.; Eldarov, M. The critical role of plasma membrane H+-ATPase activity in cephalosporin C biosynthesis of Acremonium chrysogenum. PLoS ONE 2020, 15, e0238452. [Google Scholar] [CrossRef]
- Hyvönen, M.T.; Keinänen, T.A.; Nuraeva, G.K.; Yanvarev, D.V.; Khomutov, M.; Khurs, E.N.; Kochetkov, S.N.; Vepsäläinen, J.; Zhgun, A.A.; Khomutov, A.R. Hydroxylamine analogue of agmatine: Magic bullet for arginine decarboxylase. Biomolecules 2020, 10, 406. [Google Scholar] [CrossRef] [Green Version]
- Zhgun, A.A.; Nuraeva, G.K.; Eldarov, M.A. The Role of LaeA and LovE Regulators in Lovastatin Biosynthesis with Exogenous Polyamines in Aspergillus terreus. Appl. Biochem. Microbiol. 2019, 55, 639–648. [Google Scholar] [CrossRef]
- Gutiérrez, S.; Velasco, J.; Marcos, A.T.; Fernández, F.J.; Fierro, F.; Barredo, J.L.; Díez, B.; Martín, J.F. Expression of the the cefG gene is limiting for cephalosporin biosynthesis in Acremonium chrysogenum. Appl. Microbiol. Biotechnol. 1997, 48, 606–614. [Google Scholar] [CrossRef]
- Hacskaylo, J.; Lilly, V.G.; Barnett, H.L. Growth of Fungi on Three Sources of Nitrogen. Mycologia 2018, 46, 691–701. [Google Scholar] [CrossRef]
- Di Lonardo, D.P.; van der Wal, A.; Harkes, P.; de Boer, W. Effect of nitrogen on fungal growth efficiency. Plant Biosyst. 2020, 154, 433–437. [Google Scholar] [CrossRef]
- Paschalidis, K.; Tsaniklidis, G.; Wang, B.Q.; Delis, C.; Trantas, E.; Loulakakis, K.; Makky, M.; Sarris, P.F.; Ververidis, F.; Liu, J.H. The Interplay among Polyamines and Nitrogen in Plant Stress Responses. Plants 2019, 8, 315. [Google Scholar] [CrossRef] [Green Version]
- Tudzynski, B. Nitrogen regulation of fungal secondary metabolism in fungi. Front. Microbiol. 2014, 5, 656. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Pan, Y.; Liu, G. Disruption of the nitrogen regulatory gene AcareA in Acremonium chrysogenum leads to reduction of cephalosporin production and repression of nitrogen metabolism. Fungal Genet. Biol. 2013, 61, 69–79. [Google Scholar] [CrossRef]
- Guan, F.; Pan, Y.; Li, J.; Liu, G. A GATA-type transcription factor AcAREB for nitrogen metabolism is involved in regulation of cephalosporin biosynthesis in Acremonium chrysogenum. Sci. China Life Sci. 2017, 60, 958–967. [Google Scholar] [CrossRef]
- Dumina, M.V.; Zhgun, A.A.; Kerpichnikov, I.V.; Domracheva, A.G.; Novak, M.I.; Valiachmetov, A.Y.; Knorre, D.A.; Severin, F.F.; Eldarov, M.A.; Bartoshevich, Y.E. Functional analysis of MFS protein CefT involved in the transport of beta-lactam antibiotics in Acremonium chrysogenum and Saccharomyces cerevisiae. Appl. Biochem. Microbiol. 2013, 49, 368–377. [Google Scholar] [CrossRef]
- Hegde, S.S.; Chandler, J.; Vetting, M.W.; Yu, M.; Blanchard, J.S. Mechanistic and structural analysis of human spermidine/spermine N1-acetyltransferase. Biochemistry 2007, 46, 7187–7195. [Google Scholar] [CrossRef] [Green Version]
- Strijbis, K.; Distel, B. Intracellular Acetyl Unit Transport in Fungal Carbon Metabolism. Eukaryot. Cell 2010, 9, 1809. [Google Scholar] [CrossRef] [Green Version]
- Brock, M.; Buckel, W. On the mechanism of action of the antifungal agent propionate. Eur. J. Biochem. 2004, 271, 3227–3241. [Google Scholar] [CrossRef] [PubMed]
- Newton, G.G.; Abraham, E.P. Isolation of cephalosporin C, a penicillin-like antibiotic containing D-alpha-aminoadipic acid. Biochem. J. 1956, 62, 651–658. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, B.; Jiang, R. Improving acetyl-CoA biosynthesis in Saccharomyces cerevisiae via the overexpression of pantothenate kinase and PDH bypass. Biotechnol. Biofuels 2017, 10, 41. [Google Scholar] [CrossRef]



| Primer | Gene | Product, Function | Oligonucleotide (Sequence 5 → 3) | GenBank, Source |
|---|---|---|---|---|
| actq1 | act1 | γ-Actin, a major component of the cytoskeleton | CCGGTTTCGCCGGTGATGATGCT | JN836733.1, [50] |
| actq2 | TGCTCAATGGGGTAGCGCAG | |||
| pcbABq3 | pcbAB | δ-(L-α-Aminoadipyl)-L-Cysteinyl-D-Valine synthetase | AGGCATCGTCAGGTTGGCCG | E05192.1, [36] |
| pcbABq4 | CCGGAGGGGCCATACCACAT | |||
| pcbCq1 | pcbC | Isopenicillin N-synthase | CTAGGTCGCGACGAGGACTTCT | M33522.1, [36] |
| pcbCq2 | CACGTCGGACTGGTACAACACC | |||
| cefD1q1 | cefD1 | Isopenicillin N-CoA synthetase | CCCCGGTGAGGAAGATGCGT | AJ507632.2, [36] |
| cefD1q2 | TCGATCTCCGCCTTGGACGC | |||
| cefD2q1 | cefD2 | Isopenicillin N-CoA epimerase | ACAGGATGGAGAGGAGCACCTTG | |
| cefD2q2 | TCGTAGAGCTCGCGGGGCTA | |||
| cefEFq3 | cefEF | Deacetoxycephalosporin C synthetase/ hydroxylase | GTCGAGTGCGATCCCCTCCT | AJ404737.1, [40] |
| cefEFq4 | CGAATTCTCCGTCCACCTCG | |||
| cefGq3 | cefG | Deacetylcephalosporin-C acetyltransferase | CTCCTGGAGCCATATGGAAGCGC | M91649.1, [40] |
| cefGq4 | GGTGCGCAGCTTGGTTCGAGAC | |||
| cefPq1 | cefP | Multidrug efflux pump protein, putative role in isopenicillin N transport | AATGCGACCCCGAGGAGTACGT | AM231816.1, [36] |
| cefPq2 | CCATCCCAGGAATGTTGTCGGC | |||
| cefMq1 | cefM | Multidrug efflux pump protein, putative role in penicillin N transport | TTTATCCAGGAGGAGCGCGGTC | AM231815.1, [36] |
| cefMq2 | TGTCGTAGGCGGTTCACCTTGC | |||
| cefTq3 | cefT | Multidrug efflux pump protein | TGTTGTCGGATTCGGTGTCGG | AJ487683.1, [50] |
| cefTq4 | TTCCACATATCGGCAAGGGTGC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhgun, A.A.; Eldarov, M.A. Spermidine and 1,3-Diaminopropane Have Opposite Effects on the Final Stage of Cephalosporin C Biosynthesis in High-Yielding Acremonium chrysogenum Strain. Int. J. Mol. Sci. 2022, 23, 14625. https://doi.org/10.3390/ijms232314625
Zhgun AA, Eldarov MA. Spermidine and 1,3-Diaminopropane Have Opposite Effects on the Final Stage of Cephalosporin C Biosynthesis in High-Yielding Acremonium chrysogenum Strain. International Journal of Molecular Sciences. 2022; 23(23):14625. https://doi.org/10.3390/ijms232314625
Chicago/Turabian StyleZhgun, Alexander A., and Mikhail A. Eldarov. 2022. "Spermidine and 1,3-Diaminopropane Have Opposite Effects on the Final Stage of Cephalosporin C Biosynthesis in High-Yielding Acremonium chrysogenum Strain" International Journal of Molecular Sciences 23, no. 23: 14625. https://doi.org/10.3390/ijms232314625
APA StyleZhgun, A. A., & Eldarov, M. A. (2022). Spermidine and 1,3-Diaminopropane Have Opposite Effects on the Final Stage of Cephalosporin C Biosynthesis in High-Yielding Acremonium chrysogenum Strain. International Journal of Molecular Sciences, 23(23), 14625. https://doi.org/10.3390/ijms232314625







