Human Orphan Cytochrome P450 2U1 Catalyzes the ω-Hydroxylation of Leukotriene B4
Abstract
1. Introduction
2. Results
2.1. Screening of Databases
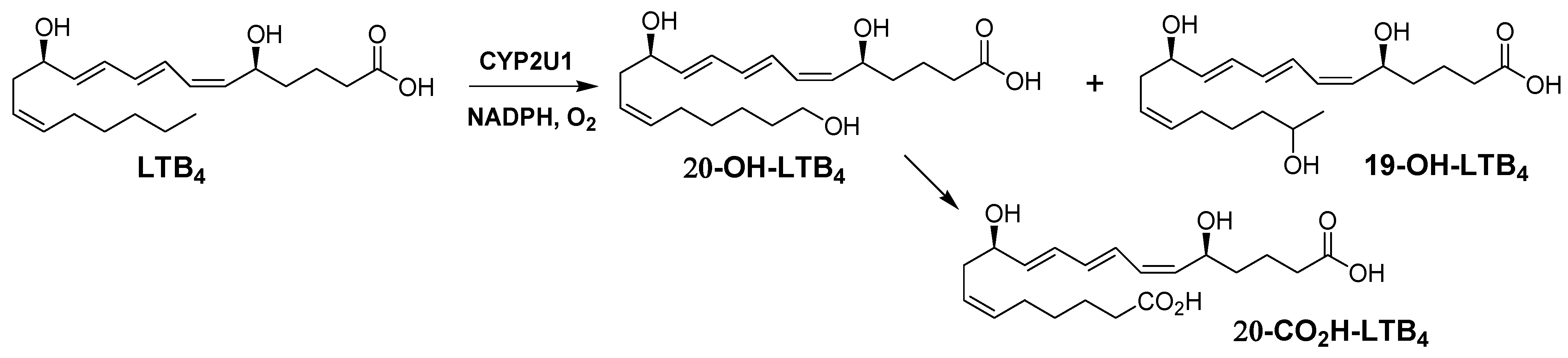
2.2. LTB4 Metabolism by Yeast Microsomes Expressing CYP2U1
2.3. Kinetic Analysis of LTB4 Metabolism by CYP2U1
2.4. Competition with Fatty Acid Derivatives
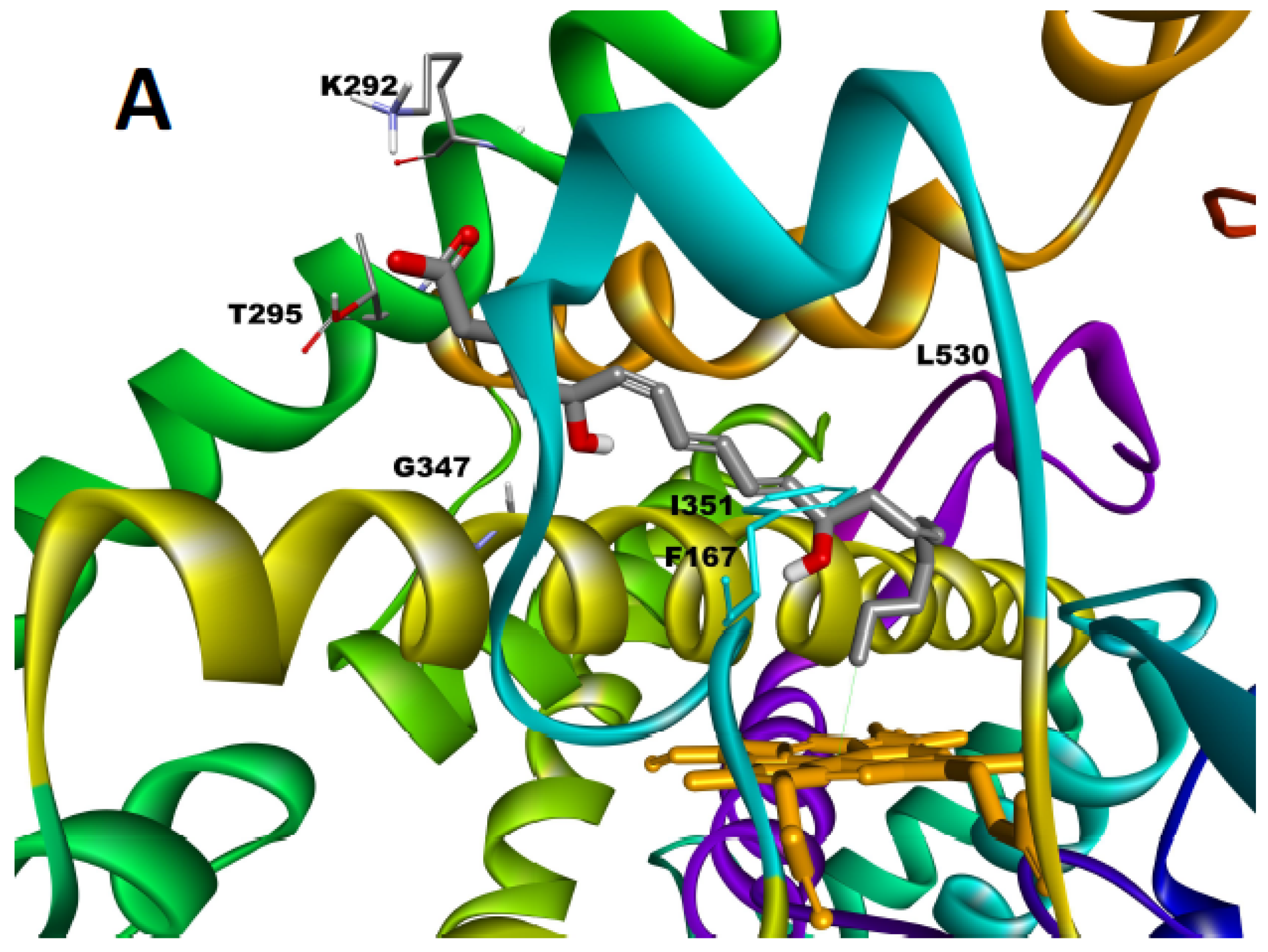
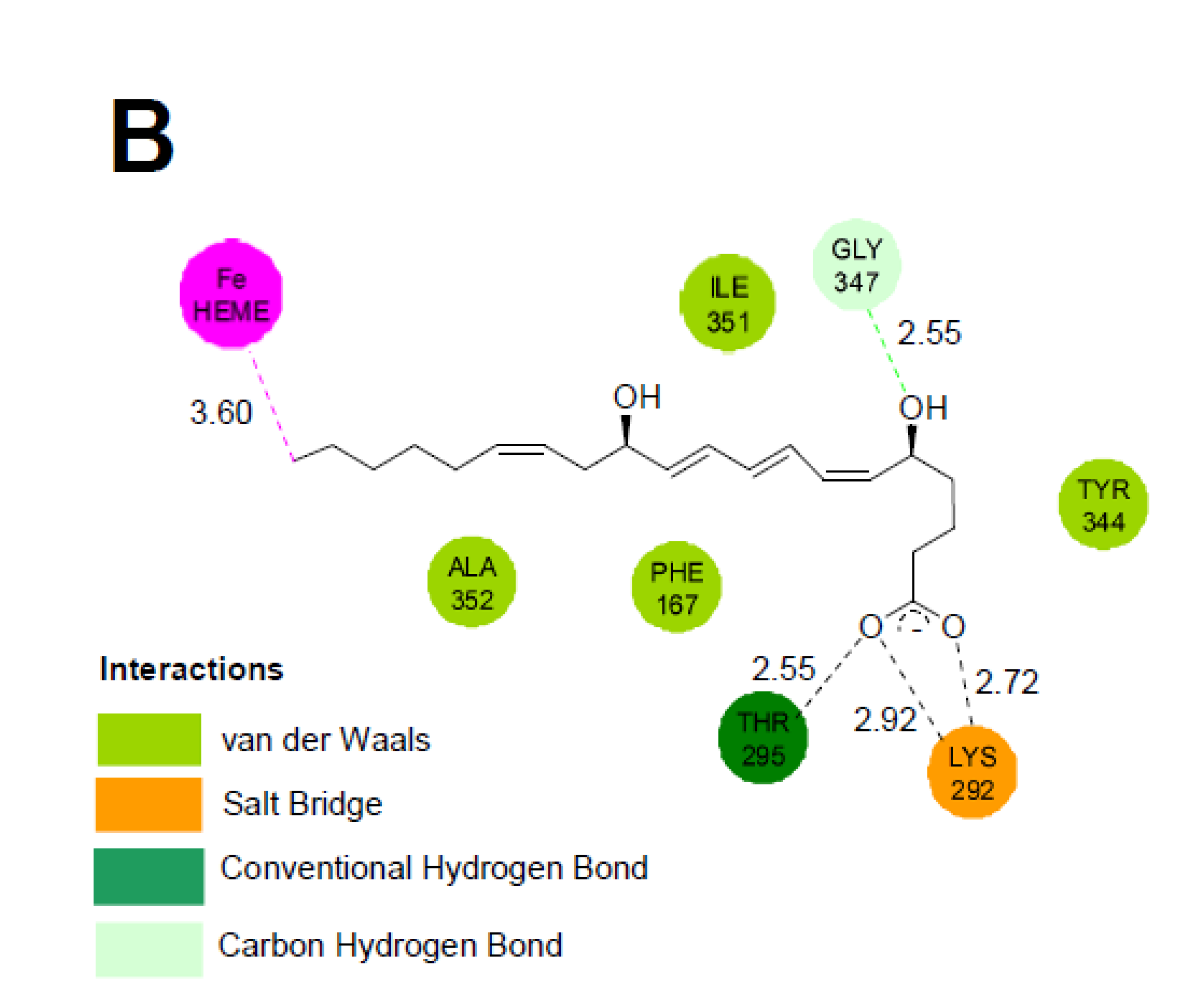
2.5. Molecular Docking of LTB4 at the Active Site
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Expression of CYP2U1 in Yeast
4.3. LTB4 Metabolism
4.4. Computational
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | arachidonic acid |
| Bz-ImH | N-benzyl-imidazole |
| CYP | cytochrome P450 |
| CYP2U1 | cytochrome P450 2U1 |
| CPR | cytochrome P450 reductase |
| HMDB | Human Metabolome Database |
| LTB4 | leukotriene B4 (5S,12R-dihydroxy-6Z,8E,10E,14Z-eicosatetraenoic acid) |
| LTD4 | leukotriene D4; LTE4, leukotriene E4 |
| 5-HETE | 5(S)-hydroxy-6E,8Z,11Z,14Z-eicosatetraenoic acid |
| 12-HETE | 12(S)-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid |
| PGB2 | prostaglandin B2 |
| Palm-Ser | palmitoylserotonin |
| 1-oleoyl-LPA | 1-O-9Z-octadecenoyl-sn-glyceryl-3-phosphoric acid |
References
- Guengerich, F.P. Human Cytochrome P450 Enzymes. In Cytochrome P450: Structure, Mechanism and Biochemistry, 3rd ed.; Ortiz de Montellano, P.R., Ed.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2005; pp. 377–530. [Google Scholar]
- Karlgren, M.; Backlund, M.; Johansson, L.; Oscarson, M.; Ingelman-Sundberg, M. Characterization and Tissue Distribution of a Novel Human Cytochrome P450-CYP2U1. Biochem. Biophys. Res. Commun. 2004, 315, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Karlgren, M.; Miura, M.; Ingelman-Sundberg, M. Novel Extra Hepatic Cytochrome P450s. Toxicol. Appl. Pharmacol. 2005, 207, 57–61. [Google Scholar] [CrossRef]
- Dhers, L.; Ducassou, L.; Boucher, J.L.; Mansuy, D. Cytochrome P450 2U1, a Very Peculiar Member of the Human P450s Family. Cell. Mol. Life Sci. 2017, 74, 1859–1869. [Google Scholar] [CrossRef] [PubMed]
- Devos, A.; Lino-Cardenas, C.L.; Glowacki, F.; Engels, A.; Lo-Guidice, J.M.; Chevalier, D.; Allorge, D.; Broly, F.; Cauffiez, C. Genetic Polymorphism of CYP2U1, a Cytochrome P450 Involved in Fatty Acids Hydroxylation. Prostaglandins Leukot. Essent. Fatty Acids 2010, 83, 105–110. [Google Scholar] [CrossRef]
- Toselli, F.; Booth Depaz, I.M.; Worrall, S.; Etheridge, N.; Dodd, P.R.; Wilce, P.A.; Gillam, E.M. Expression of CYP2E1 and CYP2U1 Proteins in Amygdala and Prefrontal Cortex: Influence of Alcoholism and Smoking. Alcohol Clin. Exp. Res. 2015, 39, 790–797. [Google Scholar] [CrossRef]
- Nelson, D.R. Comparison of P450s from Human and Fugu: 420 Million Years of Vertebrate P450 Evolution. Arch. Biochem. Biophys. 2003, 409, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.S.; Helvig, C.; Taimi, M.; Ramshaw, H.A.; Collop, A.H.; Amad, M.; White, J.A.; Petkovich, M.; Jones, G.; Korczak, B. CYP2U1, a Novel Human Thymus- and Brain-Specific Cytochrome P450, Catalyzes Omega- and (Omega-1)-Hydroxylation of Fatty Acids. J. Biol. Chem. 2004, 279, 6305–6314. [Google Scholar] [CrossRef]
- Siller, M.; Goyal, S.; Yoshimoto, F.K.; Xiao, Y.; Wei, S.; Guengerich, F.P. Oxidation of Endogenous N-Arachidonoylserotonin by Human Cytochrome P450 2U1. J. Biol. Chem. 2014, 289, 10476–10487. [Google Scholar] [CrossRef] [PubMed]
- Ducassou, L.; Jonasson, G.; Dhers, L.; Pietrancosta, N.; Ramassamy, B.; Xu-Li, Y.; Loriot, M.A.; Beaune, P.; Bertho, G.; Lombard, M.; et al. Expression in Yeast, New Substrates, and Construction of a First 3D Model of Human Orphan Cytochrome P450 2U1: Interpretation of Substrate Hydroxylation Regioselectivity from Docking Studies. Biochim. Biophys. Acta 2015, 1850, 1426–1437. [Google Scholar] [CrossRef] [PubMed]
- Tesson, C.; Nawara, M.; Salih, M.A.; Rossignol, R.; Zaki, M.S.; Al Balwi, M.; Schule, R.; Mignot, C.; Obre, E.; Bouhouche, A.; et al. Alteration of Fatty-Acid-Metabolizing Enzymes Affects Mitochondrial Form and Function in Hereditary Spastic Paraplegia. Am. J. Human Genet. 2013, 91, 1051–1064. [Google Scholar] [CrossRef] [PubMed]
- Durand, C.M.; Dhers, L.; Tesson, C.; Tessa, A.; Fouillen, L.; Jacqueré, S.; Raymond, L.; Coupry, I.; Benard, G.; Darios, F.; et al. CYP2U1 Activity is Altered by Missense Mutations in Hereditary Spastic Paraplegia 56. Hum. Mutat. 2018, 39, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Pujol, C.; Legrand, A.; Parodi, L.; Thomas, P.; Mochel, F.; Saracino, D.; Coarelli, G.; Croon, M.; Popovic, M.; Valet, M.; et al. Implication of Folate Deficiency in CYP2U1 Loss of Function. J. Exp. Med. 2021, 218, e20210846. [Google Scholar] [CrossRef] [PubMed]
- Borgeat, P.; Samuelsson, B. Metabolism of Arachidonic Acid in Polymorphonuclear Leukocytes. Structural Analysis of Novel Hydroxylated Compounds. J. Biol. Chem. 1979, 254, 7865–7869. [Google Scholar] [CrossRef]
- Lindgren, J.A.; Hansson, G.; Samuelsson, B. Formation of Novel Hydroxylated Eicosatetraenoic Acids in Preparations of Human Polymorphonuclear Leukocytes. FEBS Lett. 1981, 128, 329–335. [Google Scholar] [CrossRef]
- Ford-Hutchinson, A.W.; Bray, M.A.; Doig, M.V.; Shipley, M.E.; Smith, M.J. Leukotriene B4, a Potent Chemokinetic and Aggregating Substance Released from Polymorphonuclear Leukocytes. Nature 1980, 286, 264–265. [Google Scholar] [CrossRef]
- Borgeat, P.; Naccache, P.H. Biosynthesis and Biological Activity of Leukotriene B4. Clin. Biochem. 1990, 23, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, J.; Kaisotra, A.; Turman, C.T.; Grill, R.J.; Dash, P.K.; Strobel, H.W. CYP4Fs Expression in Rat Brain Correlates with Changes in LTB4 Level after Traumatic Brain Injury. J. Neurotrauma 2008, 25, 1187–1194. [Google Scholar] [CrossRef]
- Hijioka, M.; Anan, J.; Ishibashi, H.; Kurauchi, Y.; Hisatsune, A.; Seki, T.; Koga, T.; Yokomizo, T.; Shimizu, T.; Katsuki, H. Inhibition of Leukotriene B4 Action Mitigates Intracerebral Hemorrhage-Associated Pathological Events in Mice. J. Pharm. Exp. Ther. 2017, 360, 399–408. [Google Scholar] [CrossRef]
- Jing Chan, S.; Ng, M.P.E.; Zhao, H.; Ng, G.J.L.; De Foo, C.; Wong, P.T.H.; Seet, R.C.S. Early and Sustained Increases in Leukotriene B4 Levels are Associated with Poor Clinical Outcome in Ischemia Stroke Patients. Neurotherapeutics 2020, 17, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Correia, M.A.; Ortiz de Montellano, P.R. Inhibition of Cytochrome P450 enzymes. In Cytochrome P450: Structure, Mechanism, and Biochemistry, 3rd ed.; Ortiz de Montellano, P.R., Ed.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2005; pp. 247–322. [Google Scholar]
- Wu, G.; Robertson, D.H.; Brooks, C.L.; Vieth, M. Detailed Analysis of Grid-Based Molecular Docking: A Case Study of CDOCKER-A CHARMm-Based MD Docking Algorithm. J. Compt. Chem. 2003, 24, 1549–1562. [Google Scholar] [CrossRef] [PubMed]
- Dhers, L.; Pietrancosta, N.; Ducassou, L.; Ramassamy, B.; Dairou, J.; Jaouen, M.; André, F.; Mansuy, D.; Boucher, J.L. Spectral and 3D Model Studies of the Interaction of Orphan Human Cytochrome P450 2U1 with Substrates and Ligands. Biochim. Biophys. Acta 2017, 1861, 3144–3153. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, V.; Winn, P.J.; Wade, R.C. The Ins and Outs of Cytochrome P450s. Biochim. Biophys. Acta 2007, 1770, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, V.; Balali-Mood, K.; Sansom, M.S.; Wade, R.C. Structure and Dynamics of the Membrane-Bound Cytochrome P450 2C9. PLoS Comput. Biol. 2011, 7, e1002152. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.; Lindgren, J.A.; Dahlen, S.E.; Hedqvist, P.; Samuelsson, B. Identification and Biological Activity of Novel Omega-Oxidized Metabolites of Leukotriene B4 from Human Leukocytes. FEBS Lett. 1981, 130, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Shak, S.; Goldstein, I.M. Omega-Oxidation is the Major Pathway for the Catabolism of Leukotriene B4 in Human Polymorphonuclear Leukocytes. J. Biol. Chem. 1984, 259, 10181–10187. [Google Scholar] [CrossRef]
- Shak, S.; Goldstein, I.M. Leukotriene B4 Omega-Hydroxylase in Human Polymorphonuclear Leukocytes. Partial Purification and Identification as a Cytochrome P-450. J. Clin. Investig. 1985, 76, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Kikuta, Y.; Kusunose, E.; Kusunose, M. Characterization of Human Liver Leukotriene B4 Omega-Hydroxylase P450 (CYP4F2). J. Biochem. 2000, 127, 1047–1052. [Google Scholar] [CrossRef]
- Harper, T.W.; Garrity, M.J.; Murphy, R.C. Metabolism of Leukotriene B4 in Isolated Hepatocytes: Identification of a Novel 18-Carboxy-19,20-dinor Leukotriene B4 Metabolite. J. Biol. Chem. 1986, 261, 5414–5418. [Google Scholar] [CrossRef]
- Le Merrer, Y.; Bonnet, A.; Depezay, J.C. Synthesis of 19-Hydroxy LTB4, an Assumed Metabolite of Leukotriene B4. Tetrahedron Lett. 1988, 29, 2647–2650. [Google Scholar] [CrossRef]
- LeMerrer, Y.; Gravier-Pelletier, C.; Micas-Languin, D.; Mestre, F.; Dureault, A.; Depezay, J.C. Total Synthesis of Leukotriene B4 [(+)-LTB4] and Homo-LTB4 from D-Mannitol. J. Org. Chem. 1989, 54, 2409–2416. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Omura, T.; Sato, R. The Carbon Monoxide-Binding Pigment of Liver Microsomes. I Evidence for Its Hemoprotein Nature. J. Biol. Chem. 1964, 239, 2370–2378. [Google Scholar] [CrossRef]
- Oda, A.; Yamaotsu, N.; Hirono, S. New AMBER Force Field Parameters of Heme Iron for Cytochrome P450s Determined by Quantum Chemical Calculations of Simplified Models. J. Comput. Chem. 2005, 26, 818–826. [Google Scholar] [CrossRef]
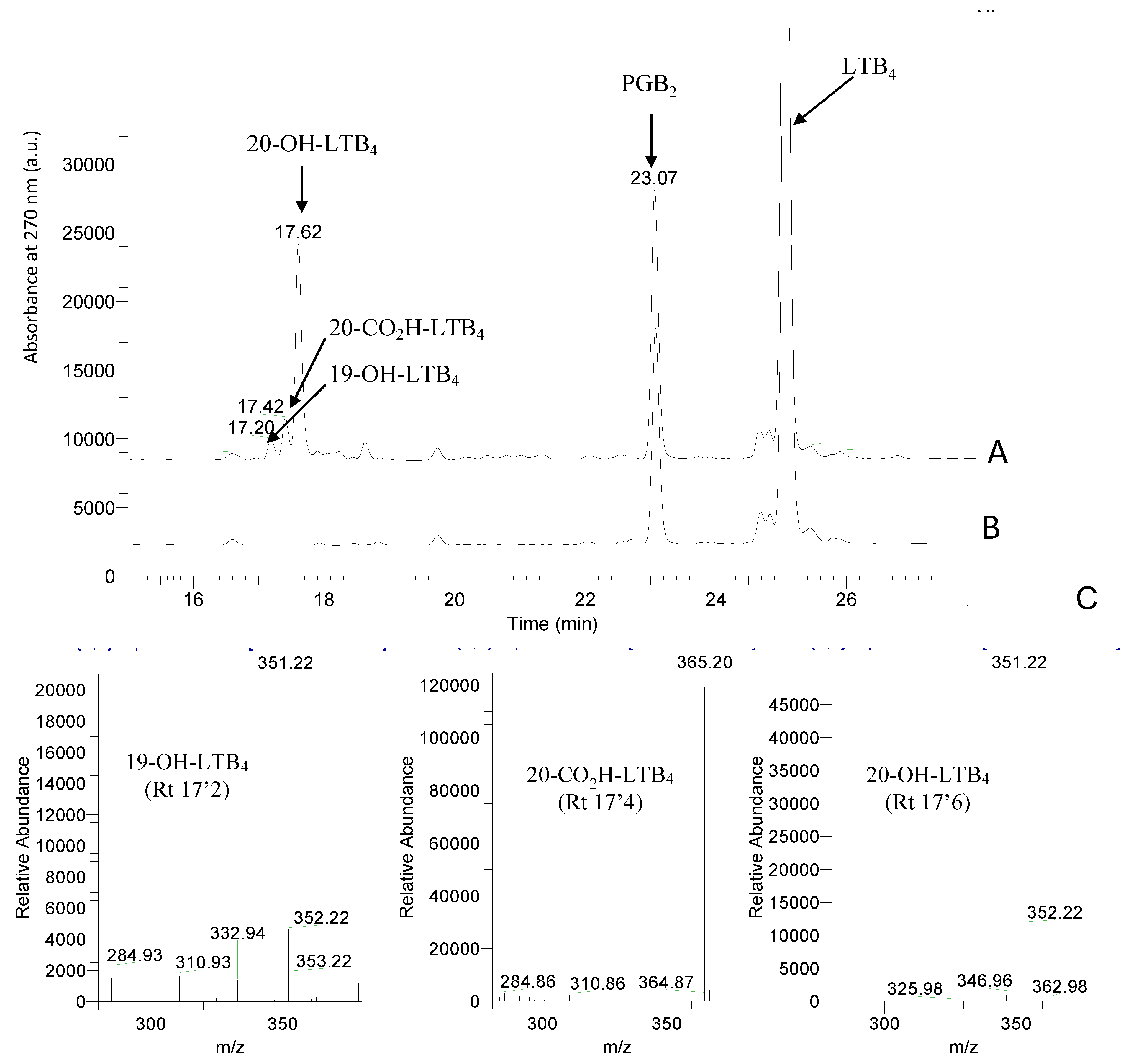

| Conditions a | Residual Activity b |
|---|---|
| Complete System | 100 |
| + 5-HETE 50 µM + 5-HETE 200 µM | 100 29 |
| + 12-HETE 50 µM + 12-HETE 200 µM | 41 9.5 |
| + LTD4 50 µM + LTD4 200 µM | 78 39 |
| + LTE4 50 µM + LTE4 200 µM | 100 27 |
| + Palm-Ser 50 µM + Palm-Ser 200 µM | 100 100 |
| + 1-Oleoyl-LPA 50 µM + 1-Oleoyl-LPA 200 µM | 42 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nouri, K.; Pietrancosta, N.; Le Corre, L.; Dansette, P.M.; Mansuy, D.; Boucher, J.-L. Human Orphan Cytochrome P450 2U1 Catalyzes the ω-Hydroxylation of Leukotriene B4. Int. J. Mol. Sci. 2022, 23, 14615. https://doi.org/10.3390/ijms232314615
Nouri K, Pietrancosta N, Le Corre L, Dansette PM, Mansuy D, Boucher J-L. Human Orphan Cytochrome P450 2U1 Catalyzes the ω-Hydroxylation of Leukotriene B4. International Journal of Molecular Sciences. 2022; 23(23):14615. https://doi.org/10.3390/ijms232314615
Chicago/Turabian StyleNouri, Khawla, Nicolas Pietrancosta, Laurent Le Corre, Patrick M. Dansette, Daniel Mansuy, and Jean-Luc Boucher. 2022. "Human Orphan Cytochrome P450 2U1 Catalyzes the ω-Hydroxylation of Leukotriene B4" International Journal of Molecular Sciences 23, no. 23: 14615. https://doi.org/10.3390/ijms232314615
APA StyleNouri, K., Pietrancosta, N., Le Corre, L., Dansette, P. M., Mansuy, D., & Boucher, J.-L. (2022). Human Orphan Cytochrome P450 2U1 Catalyzes the ω-Hydroxylation of Leukotriene B4. International Journal of Molecular Sciences, 23(23), 14615. https://doi.org/10.3390/ijms232314615






