MIG-6 Is Critical for Progesterone Responsiveness in Human Complex Atypical Hyperplasia and Early-Stage Endometrial Cancer
Abstract
1. Introduction
2. Results
2.1. MIG-6 Levels Are Significantly Lower in Human CAH and Early-Stage EEC Compared to Controls
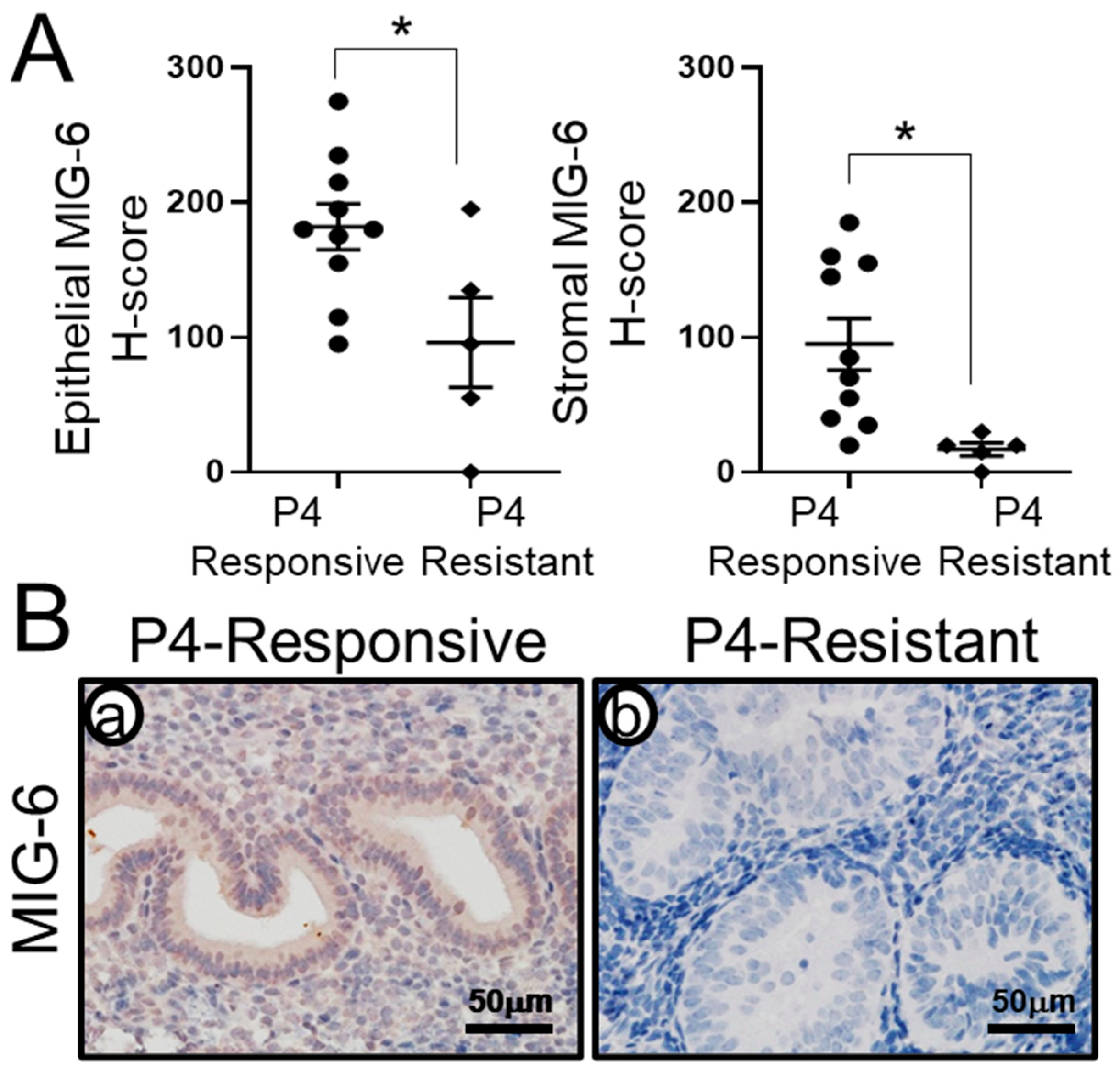
2.2. MIG-6 Levels Are Significantly Lower in P4-Resistant EEC Compared to P4-Responsive EEC Following P4 Treatment
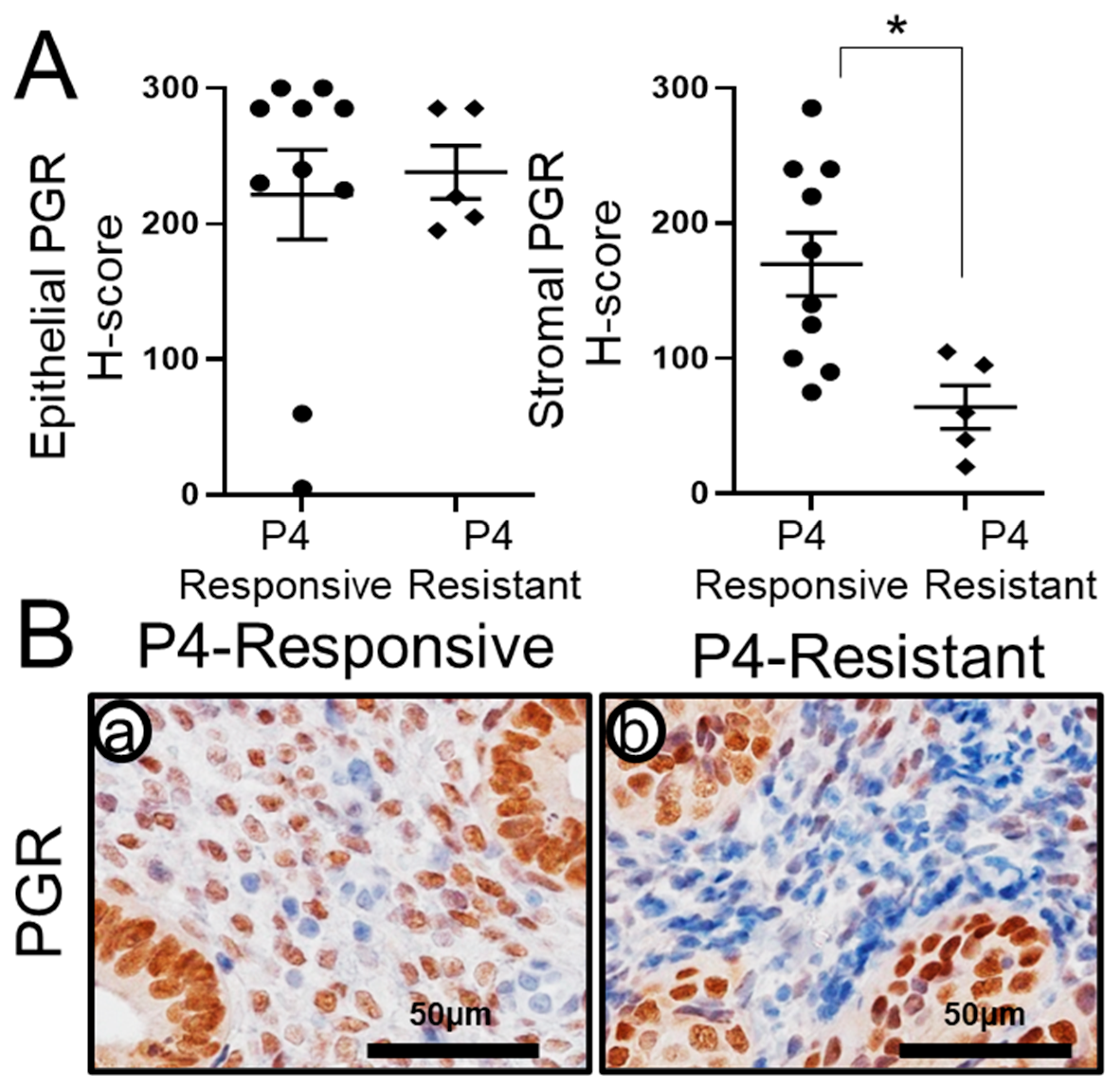
2.3. Recovery of Stromal PGR Levels in P4-Responsive Human CAH/EEC after P4 Treatment
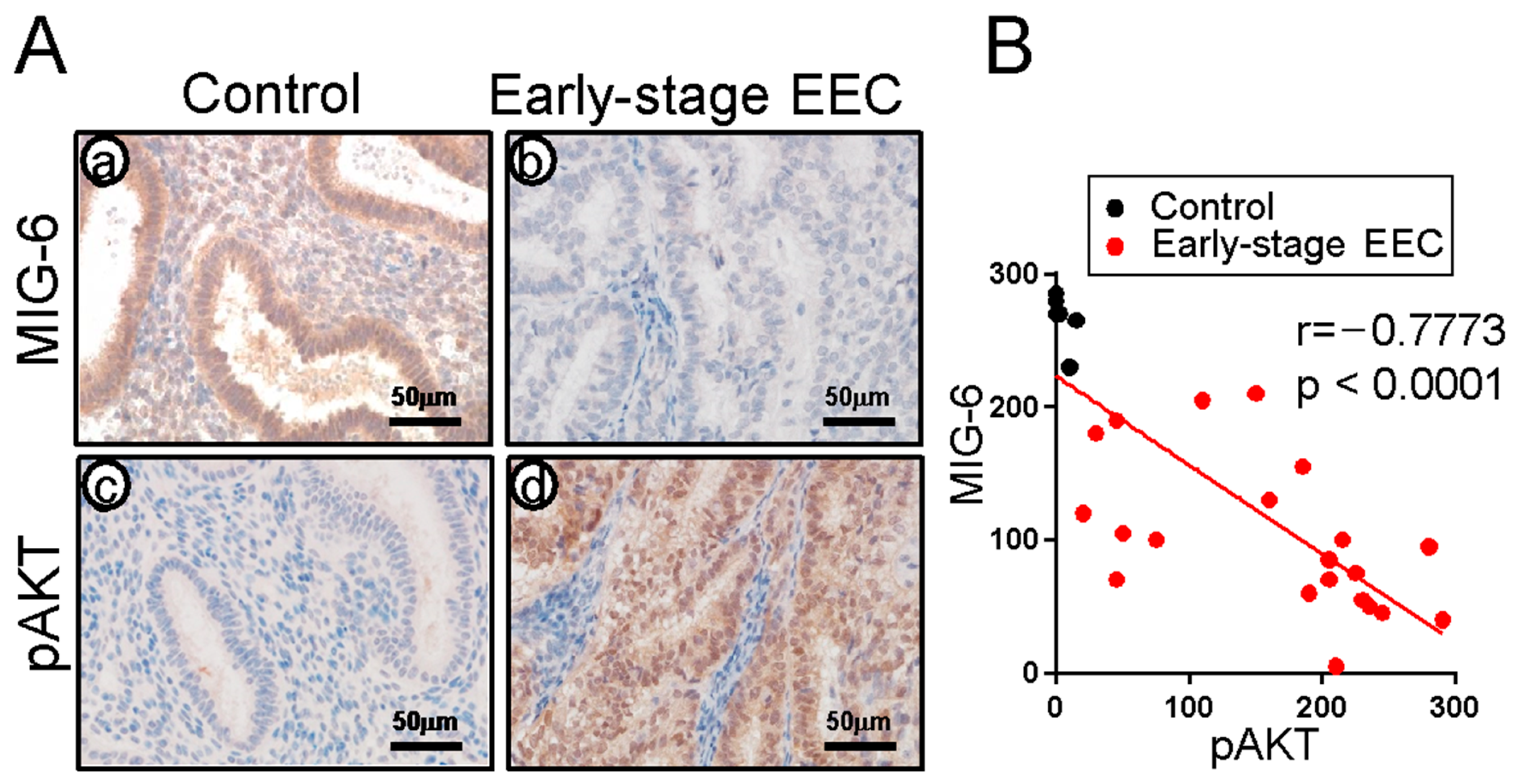
2.4. MIG-6 Levels Have a Reverse Correlation with pAKT in Human CAH/EEC
3. Discussion
4. Materials and Methods
4.1. Human Sample
4.2. Immunohistochemistry
4.3. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Chassot, P.G.; Delabays, A.; Spahn, D.R. Preoperative evaluation of patients with, or at risk of, coronary artery disease undergoing non-cardiac surgery. Br. J. Anaesth. 2002, 89, 747–759. [Google Scholar] [CrossRef]
- Charytan, D.M.; Li, S.; Liu, J.; Herzog, C.A. Risks of death and end-stage renal disease after surgical compared with percutaneous coronary revascularization in elderly patients with chronic kidney disease. Circulation 2012, 126, S164–S169. [Google Scholar] [CrossRef] [PubMed]
- Varon, J.; Marik, P.E. Perioperative hypertension management. Vasc. Health Risk Manag. 2008, 4, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Passarello, K.; Kurian, S.; Villanueva, V. Endometrial Cancer: An Overview of Pathophysiology, Management, and Care. Semin. Oncol. Nurs. 2019, 35, 157–165. [Google Scholar] [CrossRef]
- Mitamura, T.; Dong, P.; Ihira, K.; Kudo, M.; Watari, H. Molecular-targeted therapies and precision medicine for endometrial cancer. Jpn J. Clin. Oncol. 2019, 49, 108–120. [Google Scholar] [CrossRef]
- Ritterhouse, L.L.; Howitt, B.E. Molecular Pathology: Predictive, Prognostic, and Diagnostic Markers in Uterine Tumors. Surg. Pathol. Clin. 2016, 9, 405–426. [Google Scholar] [CrossRef]
- Moscarini, M.; Ricciardi, E.; Quarto, A.; Maniglio, P.; Caserta, D. Vaginal treatment of endometrial cancer: Role in the elderly. World J. Surg. Oncol. 2011, 9, 74. [Google Scholar] [CrossRef]
- Acharya, S.; Esthappan, J.; Badiyan, S.; DeWees, T.A.; Tanderup, K.; Schwarz, J.K.; Grigsby, P.W. Medically inoperable endometrial cancer in patients with a high body mass index (BMI): Patterns of failure after 3-D image-based high dose rate (HDR) brachytherapy. Radiother. Oncol. 2016, 118, 167–172. [Google Scholar] [CrossRef]
- Navarria, I.; Usel, M.; Rapiti, E.; Neyroud-Caspar, I.; Pelte, M.F.; Bouchardy, C.; Petignat, P. Young patients with endometrial cancer: How many could be eligible for fertility-sparing treatment? Gynecol. Oncol. 2009, 114, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Dursun, P.; Dogan, N.U.; Ayhan, A. Oncofertility for gynecologic and non-gynecologic cancers: Fertility sparing in young women of reproductive age. Crit. Rev. Oncol. Hematol. 2014, 92, 258–267. [Google Scholar] [CrossRef]
- Tock, S.; Jadoul, P.; Squifflet, J.L.; Marbaix, E.; Baurain, J.F.; Luyckx, M. Fertility Sparing Treatment in Patients With Early Stage Endometrial Cancer, Using a Combination of Surgery and GnRH Agonist: A Monocentric Retrospective Study and Review of the Literature. Front. Med. 2018, 5, 240. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.M.; Jeong, D.H.; Kim, Y.N.; Cho, E.B.; Cha, J.E.; Sung, M.S.; Lee, K.B.; Kim, K.T. Endometrial cancer arising from atypical complex hyperplasia: The significance in an endometrial biopsy and a diagnostic challenge. Obs. Gynecol. Sci. 2015, 58, 468–474. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saso, S.; Chatterjee, J.; Georgiou, E.; Ditri, A.M.; Smith, J.R.; Ghaem-Maghami, S. Endometrial cancer. BMJ 2011, 343, d3954. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M.E. Theories of endometrial carcinogenesis: A multidisciplinary approach. Mod. Pathol. 2000, 13, 295–308. [Google Scholar] [CrossRef]
- Doherty, M.T.; Sanni, O.B.; Coleman, H.G.; Cardwell, C.R.; McCluggage, W.G.; Quinn, D.; Wylie, J.; McMenamin, U.C. Concurrent and future risk of endometrial cancer in women with endometrial hyperplasia: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0232231. [Google Scholar] [CrossRef]
- Lax, S.F. Vorläuferläsionen der Endometriumkarzinome. Precursor lesions of endometrial carcinoma. Pathologe 2019, 40, 13–20. [Google Scholar] [CrossRef]
- Sanderson, P.A.; Critchley, H.O.; Williams, A.R.; Arends, M.J.; Saunders, P.T. New concepts for an old problem: The diagnosis of endometrial hyperplasia. Hum. Reprod. Update 2017, 23, 232–254. [Google Scholar] [CrossRef]
- Di Cristofano, A.; Ellenson, L.H. Endometrial Carcinoma. Annu. Rev. Pathol. 2007, 2, 57–85. [Google Scholar] [CrossRef]
- Amant, F.; Moerman, P.; Neven, P.; Timmerman, D.; Van Limbergen, E.; Vergote, I. Endometrial cancer. Lancet 2005, 366, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Binkowska, M.; Woron, J. Progestogens in menopausal hormone therapy. Prz. Menopauzalny 2015, 14, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.G.; Rossetti, D.; Tropea, A.; Biondi, A.; Lagana, A.S. Fertility sparing surgery for stage IA type I and G2 endometrial cancer in reproductive-aged patients: Evidence-based approach and future perspectives. Updates Surg. 2017, 69, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Stanczyk, F.Z.; Hapgood, J.P.; Winer, S.; Mishell, D.R., Jr. Progestogens used in postmenopausal hormone therapy: Differences in their pharmacological properties, intracellular actions, and clinical effects. Endocr. Rev. 2013, 34, 171–208. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Li, H.; Hu, R.; Liu, Y.; Liu, X.; Gu, L. Fertility-Preserving Treatment in Young Women With Grade 1 Presumed Stage IA Endometrial Adenocarcinoma: A Meta-Analysis. Int. J. Gynecol. Cancer 2018, 28, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Montz, F.J.; Bristow, R.E.; Bovicelli, A.; Tomacruz, R.; Kurman, R.J. Intrauterine progesterone treatment of early endometrial cancer. Am. J. Obs. Gynecol. 2002, 186, 651–657. [Google Scholar] [CrossRef]
- Chiva, L.; Lapuente, F.; Gonzalez-Cortijo, L.; Carballo, N.; Garcia, J.F.; Rojo, A.; Gonzalez-Martin, A. Sparing fertility in young patients with endometrial cancer. Gynecol. Oncol. 2008, 111, S101–S104. [Google Scholar] [CrossRef]
- Ushijima, K.; Yahata, H.; Yoshikawa, H.; Konishi, I.; Yasugi, T.; Saito, T.; Nakanishi, T.; Sasaki, H.; Saji, F.; Iwasaka, T.; et al. Multicenter phase II study of fertility-sparing treatment with medroxyprogesterone acetate for endometrial carcinoma and atypical hyperplasia in young women. J. Clin. Oncol. 2007, 25, 2798–2803. [Google Scholar] [CrossRef]
- Wheeler, D.T.; Bristow, R.E.; Kurman, R.J. Histologic alterations in endometrial hyperplasia and well-differentiated carcinoma treated with progestins. Am. J. Surg. Pathol. 2007, 31, 988–998. [Google Scholar] [CrossRef]
- Gunderson, C.C.; Fader, A.N.; Carson, K.A.; Bristow, R.E. Oncologic and reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 adenocarcinoma: A systematic review. Gynecol. Oncol. 2012, 125, 477–482. [Google Scholar] [CrossRef]
- Pal, N.; Broaddus, R.R.; Urbauer, D.L.; Balakrishnan, N.; Milbourne, A.; Schmeler, K.M.; Meyer, L.A.; Soliman, P.T.; Lu, K.H.; Ramirez, P.T.; et al. Treatment of Low-Risk Endometrial Cancer and Complex Atypical Hyperplasia With the Levonorgestrel-Releasing Intrauterine Device. Obs. Gynecol. 2018, 131, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Koskas, M.; Azria, E.; Walker, F.; Luton, D.; Madelenat, P.; Yazbeck, C. Progestin treatment of atypical hyperplasia and well-differentiated adenocarcinoma of the endometrium to preserve fertility. Anticancer. Res. 2012, 32, 1037–1043. [Google Scholar] [PubMed]
- Ramirez, P.T.; Frumovitz, M.; Bodurka, D.C.; Sun, C.C.; Levenback, C. Hormonal therapy for the management of grade 1 endometrial adenocarcinoma: A literature review. Gynecol. Oncol. 2004, 95, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Eftekhar, Z.; Izadi-Mood, N.; Yarandi, F.; Shojaei, H.; Rezaei, Z.; Mohagheghi, S. Efficacy of megestrol acetate (megace) in the treatment of patients with early endometrial adenocarcinoma: Our experiences with 21 patients. Int. J. Gynecol. Cancer 2009, 19, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Tangjitgamol, S.; Manusirivithaya, S.; Hanprasertpong, J. Fertility-sparing in endometrial cancer. Gynecol. Obs. Investig. 2009, 67, 250–268. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research, N.; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Chambliss, K.L.; Yuhanna, I.S.; Anderson, R.G.; Mendelsohn, M.E.; Shaul, P.W. ERbeta has nongenomic action in caveolae. Mol. Endocrinol. 2002, 16, 938–946. [Google Scholar]
- Engelman, J.A.; Luo, J.; Cantley, L.C. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 2006, 7, 606–619. [Google Scholar] [CrossRef]
- Lee, I.I.; Maniar, K.; Lydon, J.P.; Kim, J.J. Akt regulates progesterone receptor B-dependent transcription and angiogenesis in endometrial cancer cells. Oncogene 2016, 35, 5191–5201. [Google Scholar] [CrossRef]
- Wang, X.; Wu, S.P.; DeMayo, F.J. Hormone dependent uterine epithelial-stromal communication for pregnancy support. Placenta 2017, 60 (Suppl. 1), S20–S26. [Google Scholar] [CrossRef]
- Pineda, M.J.; Lu, Z.; Cao, D.; Kim, J.J. Influence of Cancer-Associated Endometrial Stromal Cells on Hormone-Driven Endometrial Tumor Growth. Horm. Cancer 2015, 6, 131–141. [Google Scholar] [CrossRef]
- Janzen, D.M.; Rosales, M.A.; Paik, D.Y.; Lee, D.S.; Smith, D.A.; Witte, O.N.; Iruela-Arispe, M.L.; Memarzadeh, S. Progesterone receptor signaling in the microenvironment of endometrial cancer influences its response to hormonal therapy. Cancer Res. 2013, 73, 4697–4710. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.; Elguero, S.; Thakore, S.; Dahoud, W.; Bedaiwy, M.; Mesiano, S. Role of nuclear progesterone receptor isoforms in uterine pathophysiology. Hum. Reprod. Update 2015, 21, 155–173. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Zhang, Z.; Yu, Y.; Liu, Y.; Zhao, F.; Yin, L.; Feng, Y.; Chen, X. Inhibiting the PI3K/Akt pathway reversed progestin resistance in endometrial cancer. Cancer Sci. 2011, 102, 557–564. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, L.; Zhang, X.; Cui, Z. PI3K/AKT/mTOR pathway promotes progestin resistance in endometrial cancer cells by inhibition of autophagy. Onco Targets Ther. 2017, 10, 2865–2871. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Yang, W.S.; Lee, J.H.; Kim, B.G.; Broaddus, R.R.; Lim, J.M.; Kim, T.H.; Jeong, J.W. MIG-6 negatively regulates STAT3 phosphorylation in uterine epithelial cells. Oncogene 2018, 37, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Kang, H.B.; Broaddus, R.R.; Risinger, J.I.; Choi, K.C.; Kim, T.H. MIG-6 suppresses endometrial epithelial cell proliferation by inhibiting phospho-AKT. BMC Cancer 2018, 18, 605. [Google Scholar] [CrossRef] [PubMed]
- Wick, M.; Burger, C.; Funk, M.; Muller, R. Identification of a novel mitogen-inducible gene (mig-6): Regulation during G1 progression and differentiation. Exp. Cell Res. 1995, 219, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhu, S.; Zhou, Y.; Jin, Y.; Dai, H.; Wang, X. Upregulation of mitogen-inducible gene 6 triggers antitumor effect and attenuates progesterone resistance in endometrial carcinoma cells. Cancer Gene Ther. 2015, 22, 536–541. [Google Scholar] [CrossRef]
- Jeong, J.W.; Lee, H.S.; Lee, K.Y.; White, L.D.; Broaddus, R.R.; Zhang, Y.W.; Vande Woude, G.F.; Giudice, L.C.; Young, S.L.; Lessey, B.A.; et al. Mig-6 modulates uterine steroid hormone responsiveness and exhibits altered expression in endometrial disease. Proc. Natl. Acad. Sci. USA 2009, 106, 8677–8682. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Yoo, J.Y.; Kim, H.I.; Gilbert, J.; Ku, B.J.; Li, J.; Mills, G.B.; Broaddus, R.R.; Lydon, J.P.; Lim, J.M.; et al. Mig-6 suppresses endometrial cancer associated with Pten deficiency and ERK activation. Cancer Res. 2014, 74, 7371–7382. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.W.; Staal, B.; Su, Y.; Swiatek, P.; Zhao, P.; Cao, B.; Resau, J.; Sigler, R.; Bronson, R.; Vande Woude, G.F. Evidence that MIG-6 is a tumor-suppressor gene. Oncogene 2007, 26, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.I.; Du, J.; Shen, W.T.; Whang, E.E.; Donner, D.B.; Griff, N.; He, F.; Moore, F.D., Jr.; Clark, O.H.; Ruan, D.T. Mitogen-inducible gene-6 is a multifunctional adaptor protein with tumor suppressor-like activity in papillary thyroid cancer. J. Clin. Endocrinol. Metab. 2011, 96, E554–E565. [Google Scholar] [CrossRef]
- Ferby, I.; Reschke, M.; Kudlacek, O.; Knyazev, P.; Pante, G.; Amann, K.; Sommergruber, W.; Kraut, N.; Ullrich, A.; Fassler, R.; et al. Mig6 is a negative regulator of EGF receptor-mediated skin morphogenesis and tumor formation. Nat. Med. 2006, 12, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Amatschek, S.; Koenig, U.; Auer, H.; Steinlein, P.; Pacher, M.; Gruenfelder, A.; Dekan, G.; Vogl, S.; Kubista, E.; Heider, K.H.; et al. Tissue-wide expression profiling using cDNA subtraction and microarrays to identify tumor-specific genes. Cancer Res. 2004, 64, 844–856. [Google Scholar] [CrossRef] [PubMed]
- Anastasi, S.; Sala, G.; Huiping, C.; Caprini, E.; Russo, G.; Iacovelli, S.; Lucini, F.; Ingvarsson, S.; Segatto, O. Loss of RALT/MIG-6 expression in ERBB2-amplified breast carcinomas enhances ErbB-2 oncogenic potency and favors resistance to Herceptin. Oncogene 2005, 24, 4540–4548. [Google Scholar] [CrossRef]
- Mentrikoski, M.J.; Shah, A.A.; Hanley, K.Z.; Atkins, K.A. Assessing endometrial hyperplasia and carcinoma treated with progestin therapy. Am. J. Clin. Pathol. 2012, 138, 524–534. [Google Scholar] [CrossRef]
- Chandra, V.; Kim, J.J.; Benbrook, D.M.; Dwivedi, A.; Rai, R. Therapeutic options for management of endometrial hyperplasia. J. Gynecol. Oncol. 2016, 27, e8. [Google Scholar] [CrossRef]
- Upson, K.; Allison, K.H.; Reed, S.D.; Jordan, C.D.; Newton, K.M.; Swisher, E.M.; Doherty, J.A.; Garcia, R.L. Biomarkers of progestin therapy resistance and endometrial hyperplasia progression. Am. J. Obs. Gynecol. 2012, 207, 36.e1–36.e8. [Google Scholar] [CrossRef]
- Kim, H.I.; Kim, T.H.; Lim, J.M.; Jeong, J.W. Steroid hormone intervenes in the endometrial tumorigenesis of pten ablation. J. Cancer Prev. 2013, 18, 313–321. [Google Scholar] [CrossRef][Green Version]
- Kim, T.H.; Lee, D.K.; Cho, S.N.; Orvis, G.D.; Behringer, R.R.; Lydon, J.P.; Ku, B.J.; McCampbell, A.S.; Broaddus, R.R.; Jeong, J.W. Critical tumor suppressor function mediated by epithelial Mig-6 in endometrial cancer. Cancer Res. 2013, 73, 5090–5099. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Yoo, J.Y.; Jeong, J.W. Mig-6 Mouse Model of Endometrial Cancer. Adv. Exp. Med. Biol. 2017, 943, 243–259. [Google Scholar] [CrossRef] [PubMed]
- West, R.B.; van de Rijn, M. Experimental approaches to the study of cancer-stroma interactions: Recent findings suggest a pivotal role for stroma in carcinogenesis. Lab. Investig. A J. Tech. Methods Pathol. 2007, 87, 967–970. [Google Scholar] [CrossRef]
- Arnold, J.T.; Kaufman, D.G.; Seppala, M.; Lessey, B.A. Endometrial stromal cells regulate epithelial cell growth in vitro: A new co-culture model. Hum. Reprod. 2001, 16, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Wetendorf, M.; DeMayo, F.J. Progesterone receptor signaling in the initiation of pregnancy and preservation of a healthy uterus. Int. J. Dev. Biol. 2014, 58, 95–106. [Google Scholar] [CrossRef]
- Dai, D.; Wolf, D.M.; Litman, E.S.; White, M.J.; Leslie, K.K. Progesterone inhibits human endometrial cancer cell growth and invasiveness: Down-regulation of cellular adhesion molecules through progesterone B receptors. Cancer Res. 2002, 62, 881–886. [Google Scholar] [PubMed]
- Dai, D.; Kumar, N.S.; Wolf, D.M.; Leslie, K.K. Molecular tools to reestablish progestin control of endometrial cancer cell proliferation. Am. J. Obs. Gynecol. 2001, 184, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Merlino, A.A.; Welsh, T.N.; Tan, H.; Yi, L.J.; Cannon, V.; Mercer, B.M.; Mesiano, S. Nuclear progesterone receptors in the human pregnancy myometrium: Evidence that parturition involves functional progesterone withdrawal mediated by increased expression of progesterone receptor-A. J. Clin. Endocrinol. Metab. 2007, 92, 1927–1933. [Google Scholar] [CrossRef] [PubMed]
- Condon, J.C.; Hardy, D.B.; Kovaric, K.; Mendelson, C.R. Up-regulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of nuclear factor-kappaB may contribute to the onset of labor through inhibition of PR function. Mol. Endocrinol. 2006, 20, 764–775. [Google Scholar] [CrossRef]
- Richer, J.K.; Jacobsen, B.M.; Manning, N.G.; Abel, M.G.; Wolf, D.M.; Horwitz, K.B. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J. Biol. Chem. 2002, 277, 5209–5218. [Google Scholar] [CrossRef] [PubMed]
- Pieber, D.; Allport, V.C.; Hills, F.; Johnson, M.; Bennett, P.R. Interactions between progesterone receptor isoforms in myometrial cells in human labour. Mol. Hum. Reprod. 2001, 7, 875–879. [Google Scholar] [CrossRef]
- Giangrande, P.H.; Kimbrel, E.A.; Edwards, D.P.; McDonnell, D.P. The opposing transcriptional activities of the two isoforms of the human progesterone receptor are due to differential cofactor binding. Mol. Cell Biol. 2000, 20, 3102–3115. [Google Scholar] [CrossRef] [PubMed]
- Giangrande, P.H.; Pollio, G.; McDonnell, D.P. Mapping and characterization of the functional domains responsible for the differential activity of the A and B isoforms of the human progesterone receptor. J. Biol. Chem. 1997, 272, 32889–32900. [Google Scholar] [CrossRef] [PubMed]
- Tung, L.; Mohamed, M.K.; Hoeffler, J.P.; Takimoto, G.S.; Horwitz, K.B. Antagonist-occupied human progesterone B-receptors activate transcription without binding to progesterone response elements and are dominantly inhibited by A-receptors. Mol. Endocrinol. 1993, 7, 1256–1265. [Google Scholar] [CrossRef]
- Arnett-Mansfield, R.L.; deFazio, A.; Wain, G.V.; Jaworski, R.C.; Byth, K.; Mote, P.A.; Clarke, C.L. Relative expression of progesterone receptors A and B in endometrioid cancers of the endometrium. Cancer Res. 2001, 61, 4576–4582. [Google Scholar]
- Pant, A.; Lee, I.I.; Lu, Z.; Rueda, B.R.; Schink, J.; Kim, J.J. Inhibition of AKT with the orally active allosteric AKT inhibitor, MK-2206, sensitizes endometrial cancer cells to progestin. PLoS ONE 2012, 7, e41593. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, P.; Deng, W.; Oesterreich, S.; Lu, Y.; Mills, G.B.; Lee, A.V. Insulin-like growth factor-I inhibits progesterone receptor expression in breast cancer cells via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin pathway: Progesterone receptor as a potential indicator of growth factor activity in breast cancer. Mol. Endocrinol. 2003, 17, 575–588. [Google Scholar] [CrossRef]
- Eaton, J.L.; Unno, K.; Caraveo, M.; Lu, Z.; Kim, J.J. Increased AKT or MEK1/2 activity influences progesterone receptor levels and localization in endometriosis. J. Clin. Endocrinol. Metab. 2013, 98, E1871–E1879. [Google Scholar] [CrossRef]
- Barra, F.; Evangelisti, G.; Ferro Desideri, L.; Di Domenico, S.; Ferraioli, D.; Vellone, V.G.; De Cian, F.; Ferrero, S. Investigational PI3K/AKT/mTOR inhibitors in development for endometrial cancer. Expert Opin. Investig. Drugs 2019, 28, 131–142. [Google Scholar] [CrossRef]
- Wu, S.P.; Li, R.; DeMayo, F.J. Progesterone Receptor Regulation of Uterine Adaptation for Pregnancy. Trends Endocrinol. Metab. 2018, 29, 481–491. [Google Scholar] [CrossRef]
- Neal, A.S.; Nunez, M.; Lai, T.; Tosevska, A.; Morselli, M.; Amneus, M.; Zakhour, M.; Moatamed, N.A.; Pellegrini, M.; Memarzadeh, S. Expression of Stromal Progesterone Receptor and Differential Methylation Patterns in the Endometrium May Correlate with Response to Progesterone Therapy in Endometrial Complex Atypical Hyperplasia. Reprod. Sci. 2020, 27, 1778–1790. [Google Scholar] [CrossRef] [PubMed]
- Pavlidou, A.; Vlahos, N.F. Molecular alterations of PI3K/Akt/mTOR pathway: A therapeutic target in endometrial cancer. Sci. World J. 2014, 2014, 709736. [Google Scholar] [CrossRef] [PubMed]
- Minaguchi, T.; Nakagawa, S.; Takazawa, Y.; Nei, T.; Horie, K.; Fujiwara, T.; Osuga, Y.; Yasugi, T.; Kugu, K.; Yano, T.; et al. Combined phospho-Akt and PTEN expressions associated with post-treatment hysterectomy after conservative progestin therapy in complex atypical hyperplasia and stage Ia, G1 adenocarcinoma of the endometrium. Cancer Lett. 2007, 248, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Raffone, A.; Travaglino, A.; Saccone, G.; Viggiani, M.; Giampaolino, P.; Insabato, L.; Mollo, A.; De Placido, G.; Zullo, F. PTEN expression in endometrial hyperplasia and risk of cancer: A systematic review and meta-analysis. Arch. Gynecol. Obs. 2019, 299, 1511–1524. [Google Scholar] [CrossRef]
- Bajwa, P.; Nielsen, S.; Lombard, J.M.; Rassam, L.; Nahar, P.; Rueda, B.R.; Wilkinson, J.E.; Miller, R.A.; Tanwar, P.S. Overactive mTOR signaling leads to endometrial hyperplasia in aged women and mice. Oncotarget 2017, 8, 7265–7275. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, B.E.; Daum, G.S.; Dunton, C.J. Endometrial hyperplasia: A review. Obs. Gynecol. Surv. 2004, 59, 368–378. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, O.; Broaddus, R.R.; Lessey, B.A.; Risinger, J.I.; Hunter, M.I.; Kim, T.H. MIG-6 Is Critical for Progesterone Responsiveness in Human Complex Atypical Hyperplasia and Early-Stage Endometrial Cancer. Int. J. Mol. Sci. 2022, 23, 14596. https://doi.org/10.3390/ijms232314596
Jeong O, Broaddus RR, Lessey BA, Risinger JI, Hunter MI, Kim TH. MIG-6 Is Critical for Progesterone Responsiveness in Human Complex Atypical Hyperplasia and Early-Stage Endometrial Cancer. International Journal of Molecular Sciences. 2022; 23(23):14596. https://doi.org/10.3390/ijms232314596
Chicago/Turabian StyleJeong, Olivia, Russell R. Broaddus, Bruce A. Lessey, John I. Risinger, Mark I. Hunter, and Tae Hoon Kim. 2022. "MIG-6 Is Critical for Progesterone Responsiveness in Human Complex Atypical Hyperplasia and Early-Stage Endometrial Cancer" International Journal of Molecular Sciences 23, no. 23: 14596. https://doi.org/10.3390/ijms232314596
APA StyleJeong, O., Broaddus, R. R., Lessey, B. A., Risinger, J. I., Hunter, M. I., & Kim, T. H. (2022). MIG-6 Is Critical for Progesterone Responsiveness in Human Complex Atypical Hyperplasia and Early-Stage Endometrial Cancer. International Journal of Molecular Sciences, 23(23), 14596. https://doi.org/10.3390/ijms232314596




