Abiotic Stress Tolerance in Plants: Brassinosteroids Navigate Competently
Abstract
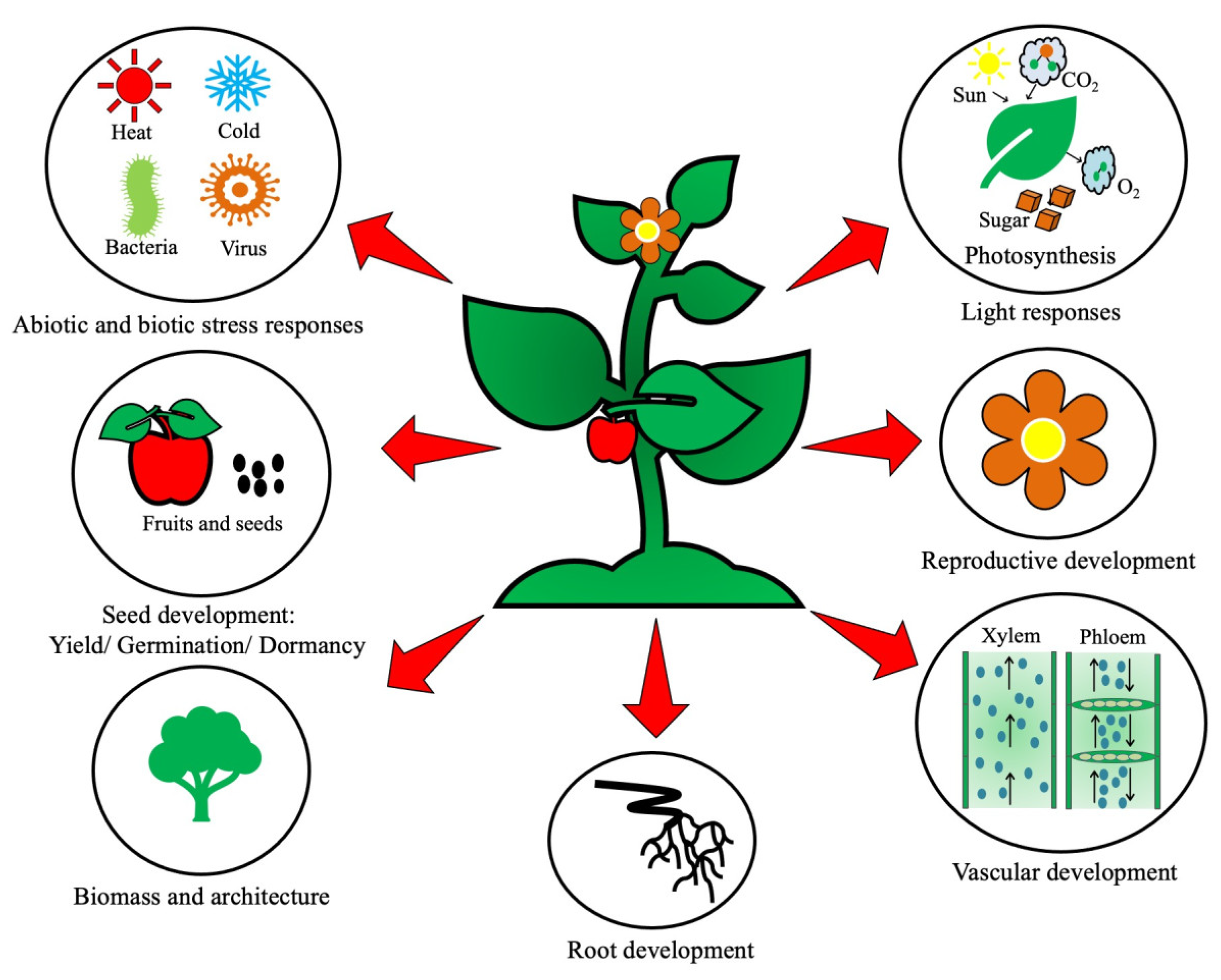
1. Introduction
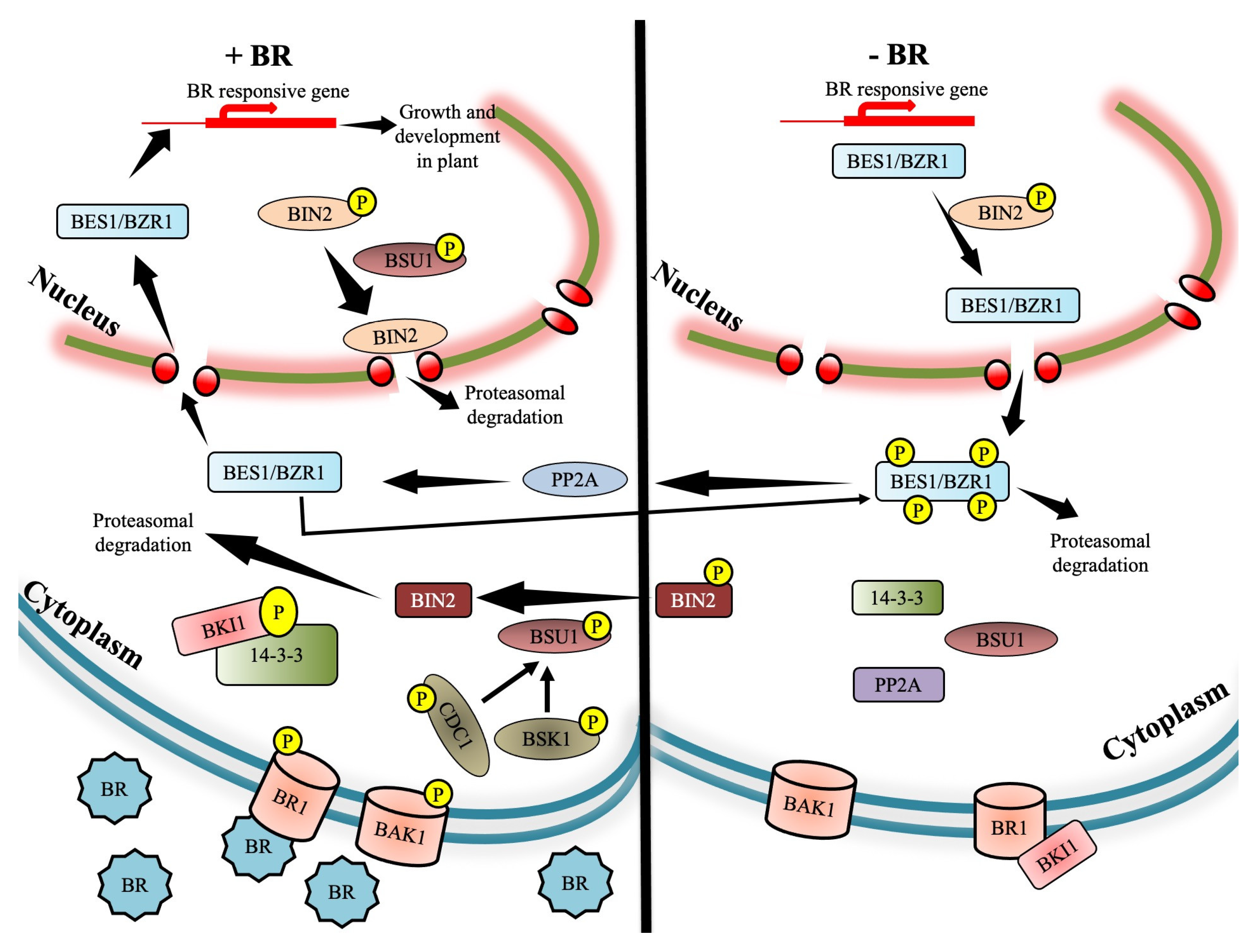
2. Brassinosteroid Signaling Cascade
2.1. Jump-Start of BRI1 Receptor Kinase by Brassinosteroid
2.2. Interaction of BRI1 with Receptor Complex Associates
2.3. Joining the Dots to Complete the BR Signaling Cascade
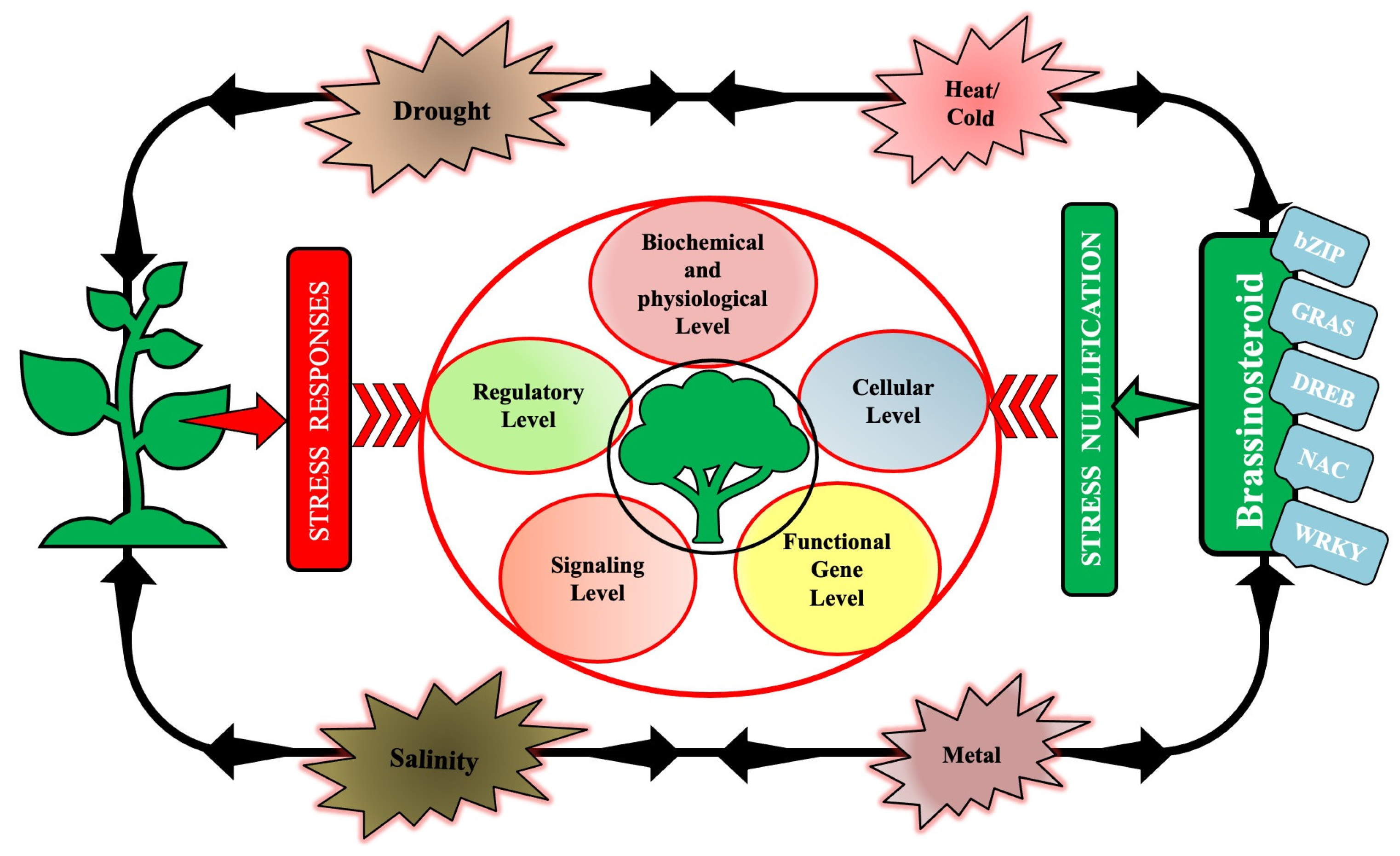
3. Signaling and Regulation by BRs (Endogenous and Exogenous) in Plants under Abiotic Stress
3.1. BRs in Mitigating Heat and Cold Stress
3.1.1. Heat Stress
3.1.2. Cold Stress
3.1.3. Drought Stress
3.1.4. Salinity Stress
3.1.5. Heavy Metal Stress
4. Future Prospects
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hasanuzzaman, M. Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives I: General Consequences and Plant Responses. In Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives I: General Consequences and Plant Responses; Springer Nature: Berlin/Heidelberg, Germany, 2020; pp. 1–859. ISBN 9789811521560. [Google Scholar]
- Saddiq, M.S.; Afzal, I.; Iqbal, S.; Hafeez, M.B.; Raza, A. Low Leaf Sodium Content Improves the Grain Yield and Physiological Performance of Wheat Genotypes in Saline-Sodic Soil. Pesqui. Agropecu. Trop. 2021, 51. [Google Scholar] [CrossRef]
- Zahra, N.; Mahmood, S.; Raza, Z.A. Salinity Stress on Various Physiological and Biochemical Attributes of Two Distinct Maize (Zea mays L.) Genotypes. J. Plant Nutr. 2018, 41, 1368–1380. [Google Scholar] [CrossRef]
- Zahra, N.; Wahid, A.; Shaukat, K.; Rasheed, T. Role of Seed Priming and Foliar Spray of Calcium in Improving Flag Leaf Growth, Grain Filling and Yield Characteristics in Wheat (Triticum aestivum)—A Field Appraisal. Int. J. Agric. Biol. 2020, 24, 1591–1600. [Google Scholar] [CrossRef]
- Wei, Z.; Li, J. Brassinosteroids Regulate Root Growth, Development, and Symbiosis. Mol. Plant 2016, 9, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Nazir, F.; Hussain, A.; Fariduddin, Q. Interactive Role of Epibrassinolide and Hydrogen Peroxide in Regulating Stomatal Physiology, Root Morphology, Photosynthetic and Growth Traits in Solanum lycopersicum L. under Nickel Stress. Environ. Exp. Bot. 2019, 162, 479–495. [Google Scholar] [CrossRef]
- Vardhini, B.V.; Anjum, N.A. Brassinosteroids Make Plant Life Easier under Abiotic Stresses Mainly by Modulating Major Components of Antioxidant Defense System. Front. Environ. Sci. 2015, 2, 67. [Google Scholar] [CrossRef]
- Hafeez, M.B.; Zahra, N.; Zahra, K.; Raza, A.; Khan, A.; Shaukat, K.; Khan, S. Brassinosteroids: Molecular and Physiological Responses in Plant Growth and Abiotic Stresses. Plant Stress 2021, 2, 100029. [Google Scholar] [CrossRef]
- Mubarik, M.S.; Khan, S.H.; Sajjad, M.; Raza, A.; Hafeez, M.B.; Yasmeen, T.; Rizwan, M.; Ali, S.; Ali, S.; Arif, M.S. A Manipulative Interplay between Positive and Negative Regulators of Phytohormones: A Way Forward for Improving Drought Tolerance in Plants. Physiol. Plant 2021, 172, 1269–1290. [Google Scholar] [CrossRef]
- Planas-Riverola, A.; Gupta, A.; Betegoń-Putze, I.; Bosch, N.; Ibañes, M.; Cano-Delgado, A.I. Brassinosteroid Signaling in Plant Development and Adaptation to Stress. Development 2019, 146, dev151894. [Google Scholar] [CrossRef]
- Kour, J.; Kohli, S.K.; Khanna, K.; Bakshi, P.; Sharma, P.; Singh, A.D.; Ibrahim, M.; Devi, K.; Sharma, N.; Ohri, P.; et al. Brassinosteroid Signaling, Crosstalk and, Physiological Functions in Plants under Heavy Metal Stress. Front. Plant Sci. 2021, 12, 608061. [Google Scholar] [CrossRef]
- Zhang, C.; Bai, M.; Chong, K. Brassinosteroid-Mediated Regulation of Agronomic Traits in Rice. Plant Cell Rep. 2014, 33, 683–696. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Mir, R.A.; Alyemeni, M.N.; Ahmad, P. Combined Effects of Brassinosteroid and Kinetin Mitigates Salinity Stress in Tomato through the Modulation of Antioxidant and Osmolyte Metabolism. Plant Physiol. Biochem. 2020, 147, 31–42. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Li, X.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Enhanced Photosynthetic Capacity and Antioxidant Potential Mediate Brassinosteriod-Induced Phenanthrene Stress Tolerance in Tomato. Environ. Pollut. 2015, 201, 58–66. [Google Scholar] [CrossRef]
- Divi, U.K.; Rahman, T.; Krishna, P. Gene Expression and Functional Analyses in Brassinosteroid-Mediated Stress Tolerance. Plant Biotechnol. J. 2016, 14, 419–432. [Google Scholar] [CrossRef]
- Tang, W.; Deng, Z.; Wang, Z.Y. Proteomics shed light on the brassinosteroid signaling mechanisms. Curr. Opin. Plant Biol. 2010, 13, 27–33. [Google Scholar] [CrossRef]
- Johnson, K.L.; Ingram, G.C. Sending the Right Signals: Regulating Receptor Kinase Activity. Curr. Opin. Plant Biol. 2005, 8, 648–656. [Google Scholar] [CrossRef]
- Vert, G.; Nemhauser, J.L.; Geldner, N.; Hong, F.; Chory, J. Molecular Mechanisms of Steroid Hormone Signaling in Plants. Annu. Rev. Cell Dev. Biol. 2005, 21, 177–201. [Google Scholar] [CrossRef]
- Tang, W.; Tae-Wuk, K.; Oses-Prieto, J.A.; Yu, S.; Deng, Z.; Zhu, S.; Wang, R.; Burlingame, A.L.; Wang, Z.-Y. BSKs Mediate Signal Transduction from the Receptor Kinase BRI1 in Arabidopsis. Science 2008, 321, 557–560. [Google Scholar] [CrossRef]
- Kim, T.W.; Guan, S.; Sun, Y.; Deng, Z.; Tang, W.; Shang, J.X.; Sun, Y.; Burlingame, A.L.; Wang, Z.Y. Brassinosteroid Signal Transduction from Cell-Surface Receptor Kinases to Nuclear Transcription Factors. Nat. Cell Biol. 2009, 11, 1254–1260. [Google Scholar] [CrossRef]
- Karlova, R.; Boeren, S.; Van Dongen, W.; Kwaaitaal, M.; Aker, J.; Vervoort, J.; De Vries, S. Identification of in Vitro Phosphorylation Sites in the Arabidopsis Thaliana Somatic Embryogenesis Receptor-like Kinases. Proteomics 2009, 9, 368–379. [Google Scholar] [CrossRef]
- Wang, X.; Kota, U.; He, K.; Blackburn, K.; Li, J.; Goshe, M.B.; Huber, S.C.; Clouse, S.D. Sequential Transphosphorylation of the BRI1/BAK1 Receptor Kinase Complex Impacts Early Events in Brassinosteroid Signaling. Dev. Cell 2008, 15, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Goshe, M.B.; Soderblom, E.J.; Phinney, B.S.; Kuchar, A.; Li, J.; Asami, T.; Yoshida, S.; Huber, S.C.; Clouse, S.D.; et al. Identification and Functional Analysis of in Vivo Phosphorylation Sites of the Arabidopsis BRASSINOSTEROID-INSENSITIVE1 Receptor Kinase. Plant Cell 2005, 17, 1685–1703. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Caño-Delgado, A.; Seto, H.; Hiranuma, S.; Fujioka, S.; Yoshida, S.; Chory, J. Binding of Brassinosteroids to the Extracellular Domain of Plant Receptor Kinase BRI1. Nature 2005, 433, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Meisenhelder, J.; Hunter, T.; Yoshida, S.; Asami, T.; Chory, J. Autoregulation and Homodimerization Are Involved in the Activation of the Plant Steroid Receptor BRI1. Dev. Cell 2005, 8, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chory, J. Brassinoteroids Regulate Dissociation of BKI1, a Negative Regulator of BRI1 Signaling, from the Plasma Membrane. Science 2006, 313, 1118–1122. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Seto, H.; Fujioka, S.; Yoshida, S.; Chory, J. BRI1 Is a Critical Component of a Plasma-Membrane Receptor for Plants Steroids. Nature 2001, 410, 380–383. [Google Scholar] [CrossRef]
- Nam, K.H.; Li, J. BRI1/BAK1, a Receptor Kinase Pair Mediating Brassinosteroid Signaling. Cell 2002, 110, 203–212. [Google Scholar] [CrossRef]
- Li, J.; Wen, J.; Lease, K.A.; Doke, J.T.; Tax, F.E.; Walker, J.C. BAK1, an Arabidopsis LRR Receptor-like Protein Kinase, Interacts with BRI1 and Modulates Brassinosteroid Signaling. Cell 2002, 110, 213–222. [Google Scholar] [CrossRef]
- He, J.X.; Gendron, J.M.; Yang, Y.; Li, J.; Wang, Z.Y. The GSK3-like Kinase BIN2 Phosphorylates and Destabilizes BZR1, a Positive Regulator of the Brassinosteroid Signaling Pathway in Arabidopsis. Proc. Natl. Acad. Sci. USA 2002, 99, 10185–10190. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, Z.Y.; Mora-Garcia, S.; Li, J.; Yoshida, S.; Asami, T.; Chory, J. BES1 Accumulates in the Nucleus in Response to Brassinosteroids to Regulate Gene Expression and Promote Stem Elongation. Cell 2002, 109, 181–191. [Google Scholar] [CrossRef]
- Vert, G.; Chory, J. Downstream Nuclear Events in Brassinosteroid Signalling. Nature 2006, 441, 96–100. [Google Scholar] [CrossRef]
- Gampala, S.S.; Kim, T.W.; He, J.X.; Tang, W.; Deng, Z.; Bai, M.Y.; Guan, S.; Lalonde, S.; Sun, Y.; Gendron, J.M.; et al. An Essential Role for 14-3-3 Proteins in Brassinosteroid Signal Transduction in Arabidopsis. Dev. Cell 2007, 13, 177–189. [Google Scholar] [CrossRef]
- Ryu, H.; Kim, K.; Cho, H.; Park, J.; Choe, S.; Hwang, I. Nucleocytoplasmic Shuttling of BZR1 Mediated by Phosphorylation Is Essential in Arabidopsis Brassinosteroid Signaling. Plant Cell 2007, 19, 2749–2762. [Google Scholar] [CrossRef]
- Mora-García, S.; Vert, G.; Yin, Y.; Caño-Delgado, A.; Cheong, H.; Chory, J. Nuclear Protein Phosphatases with Kelch-Repeat Domains Modulate the Response to Brassinosteroids in Arabidopsis. Genes Dev. 2004, 18, 448–460. [Google Scholar] [CrossRef]
- Anwar, A.; Liu, Y.; Dong, R.; Bai, L.; Yu, X.; Li, Y. The Physiological and Molecular Mechanism of Brassinosteroid in Response to Stress: A Review. Biol. Res. 2018, 51, 1–15. [Google Scholar] [CrossRef]
- Nolan, T.; Chen, J.; Yin, Y. Cross-Talk of Brassinosteroid Signaling in Controlling Growth and Stress Responses. Biochem. J. 2017, 474, 2461–2661. [Google Scholar] [CrossRef]
- Fàbregas, N.; Lozano-Elena, F.; Blasco-Escámez, D.; Tohge, T.; Martínez-Andújar, C.; Albacete, A.; Osorio, S.; Bustamante, M.; Riechmann, J.L.; Nomura, T.; et al. Overexpression of the Vascular Brassinosteroid Receptor BRL3 Confers Drought Resistance without Penalizing Plant Growth. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Zou, L.J.; Deng, X.G.; Zhang, L.E.; Zhu, T.; Tan, W.R.; Muhammad, A.; Zhu, L.J.; Zhang, C.; Zhang, D.W.; Lin, H.H. Nitric Oxide as a Signaling Molecule in Brassinosteroid-Mediated Virus Resistance to Cucumber Mosaic Virus in Arabidopsis thaliana. Physiol. Plant 2018, 163, 196–210. [Google Scholar] [CrossRef]
- Ye, H.; Liu, S.; Tang, B.; Chen, J.; Xie, Z.; Nolan, T.M.; Jiang, H.; Guo, H.; Lin, H.Y.; Li, L.; et al. RD26 Mediates Crosstalk between Drought and Brassinosteroid Signalling Pathways. Nat. Commun. 2017, 8, 14573. [Google Scholar] [CrossRef]
- Sharma, I.; Kaur, N.; Pati, P.K. Brassinosteroids: A Promising Option in Deciphering Remedial Strategies for Abiotic Stress Tolerance in Rice. Front. Plant Sci. 2017, 8, 2151. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, J.J.; Wang, K.X.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Yu, J.Q.; Zhoua, J. BZR1 Mediates Brassinosteroid-Induced Autophagy and Nitrogen Starvation in Tomato. Plant Physiol. 2019, 179, 671–685. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Li, X.; Liu, A.; Chen, S. Brassinosteroids in Plant Tolerance to Abiotic Stress. J. Plant Growth Regul. 2020, 39, 1451–1464. [Google Scholar] [CrossRef]
- Amraee, L.; Rahmani, F.; Abdollahi Mandoulakani, B. 24-Epibrassinolide Alters DNA Cytosine Methylation of Linum Usitatissimum L. under Salinity Stress. Plant Physiol. Biochem. 2019, 139, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Thakur, S.; Kumar, V.; Kanwar, M.K.; Kesavan, A.K.; Thukral, A.K.; Bhardwaj, R.; Alam, P.; Ahmad, P. Pre-Sowing Seed Treatment with 24-Epibrassinolide Ameliorates Pesticide Stress in Brassica juncea L. through the Modulation of Stress Markers. Front. Plant Sci. 2016, 7, 1569. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Yuan, H.; Kumar, V.; Ramakrishnan, M.; Kohli, S.K.; Kaur, R.; Thukral, A.K.; Bhardwaj, R.; Zheng, B. Castasterone Attenuates Insecticide Induced Phytotoxicity in Mustard. Ecotoxicol. Environ. Saf. 2019, 179, 50–61. [Google Scholar] [CrossRef]
- Yin, W.; Dong, N.; Niu, M.; Zhang, X.; Li, L.; Liu, J.; Liu, B.; Tong, H. Brassinosteroid-Regulated Plant Growth and Development and Gene Expression in Soybean. Crop J. 2019, 7, 411–418. [Google Scholar] [CrossRef]
- Yue, J.; You, Y.; Zhang, L.; Fu, Z.; Wang, J.; Zhang, J.; Guy, R.D. Exogenous 24-Epibrassinolide Alleviates Effects of Salt Stress on Chloroplasts and Photosynthesis in Robinia Pseudoacacia L. Seedlings. J. Plant Growth Regul. 2019, 38, 669–682. [Google Scholar] [CrossRef]
- Nolan, T.M.; Vukasinović, N.; Liu, D.; Russinova, E.; Yin, Y. Brassinosteroids: Multidimensional Regulators of Plant Growth, Development, and Stress Responses. Plant Cell 2020, 32, 298–318. [Google Scholar] [CrossRef]
- Martínez, C.; Espinosa-Ruíz, A.; Lucas, M.; Bernardo-García, S.; Franco-Zorrilla, J.M.; Prat, S. PIF 4-induced BR Synthesis Is Critical to Diurnal and Thermomorphogenic Growth. EMBO J. 2018, 37, e99552. [Google Scholar] [CrossRef]
- Martins, S.; Montiel-Jorda, A.; Cayrel, A.; Huguet, S.; Paysant-Le Roux, C.; Ljung, K.; Vert, G. Brassinosteroid Signaling-Dependent Root Responses to Prolonged Elevated Ambient Temperature. Nat. Commun. 2017, 8, 309. [Google Scholar] [CrossRef]
- Sadura, I.; Janeczko, A. Physiological and Molecular Mechanisms of Brassinosteroid-Induced Tolerance to High and Low Temperature in Plants. Biol. Plant 2018, 62, 601–616. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Yu, J.Q. Role of Hormones in Plant Adaptation to Heat Stress. In Plant Hormones under Challenging Environmental Factors; Springer: Dordrecht, The Netherlands, 2016; pp. 1–21. [Google Scholar] [CrossRef]
- Ogweno, J.O.; Song, X.S.; Shi, K.; Hu, W.H.; Mao, W.H.; Zhou, Y.H.; Yu, J.Q.; Nogués, S. Brassinosteroids Alleviate Heat-Induced Inhibition of Photosynthesis by Increasing Carboxylation Efficiency and Enhancing Antioxidant Systems in Lycopersicon Esculentum. J. Plant Growth Regul. 2008, 27, 49–57. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Zhu, X.H.; Ding, H.D.; Yang, S.J.; Chen, Y.Y. Foliar Application of 24-Epibrassinolide Alleviates High-Temperature-Induced Inhibition of Photosynthesis in Seedlings of Two Melon Cultivars. Photosynthetica 2013, 51, 341–349. [Google Scholar] [CrossRef]
- Wu, X.; Yao, X.; Chen, J.; Zhu, Z.; Zhang, H.; Zha, D. Brassinosteroids Protect Photosynthesis and Antioxidant System of Eggplant Seedlings from High-Temperature Stress. Acta Physiol. Plant 2014, 36, 251–261. [Google Scholar] [CrossRef]
- Hussain, M.; Khan, T.A.; Yusuf, M.; Fariduddin, Q. Silicon-Mediated Role of 24-Epibrassinolide in Wheat under High-Temperature Stress. Environ. Sci. Pollut. Res. 2019, 26, 17163–17172. [Google Scholar] [CrossRef]
- Sonjaroon, W.; Jutamanee, K.; Khamsuk, O.; Thussagunpanit, J.; Kaveeta, L.; Suksamrarn, A. Impact of Brassinosteroid Mimic on Photosynthesis, Carbohydrate Content and Rice Seed Set at Reproductive Stage under Heat Stress. Agric. Nat. Resour. 2018, 52, 234–240. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, J.; Li, X.; Xia, X.J.; Zhou, Y.H.; Shi, K.; Chen, Z.; Yu, J.Q. H2O2 Mediates the Crosstalk of Brassinosteroid and Abscisic Acid in Tomato Responses to Heat and Oxidative Stresses. J. Exp. Bot. 2014, 65, 4371–4383. [Google Scholar] [CrossRef]
- Dhaubhadel, S.; Browning, K.S.; Gallie, D.R.; Krishna, P. Brassinosteroid Functions to Protect the Translational Machinery and Heat-Shock Protein Synthesis Following Thermal Stress. Plant J. 2002, 29, 681–691. [Google Scholar] [CrossRef]
- Kagale, S.; Divi, U.K.; Krochko, J.E.; Keller, W.A.; Krishna, P. Brassinosteroid Confers Tolerance in Arabidopsis thaliana and Brassica Napus to a Range of Abiotic Stresses. Planta 2007, 225, 353–364. [Google Scholar] [CrossRef]
- Sadura, I.; Pociecha, E.; Dziurka, M.; Oklestkova, J.; Novak, O.; Gruszka, D.; Janeczko, A. Mutations in the HvDWARF, HvCPD and HvBRI1 Genes-Involved in Brassinosteroid Biosynthesis/Signalling: Altered Photosynthetic Efficiency, Hormonal Homeostasis and Tolerance to High/Low Temperatures in Barley. J. Plant Growth Regul. 2019, 38, 1062–1081. [Google Scholar] [CrossRef]
- Nie, S.; Huang, S.; Wang, S.; Mao, Y.; Liu, J.; Ma, R.; Wang, X. Enhanced Brassinosteroid Signaling Intensity via SlBRI1 Overexpression Negatively Regulates Drought Resistance in a Manner Opposite of That via Exogenous BR Application in Tomato. Plant Physiol. Biochem. 2019, 138, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wu, P.; Zhang, W.; Yang, Z.; Liu, H.; Ahammed, G.J.; Cui, J. Calcium Is Involved in Exogenous NO-Induced Enhancement of Photosynthesis in Cucumber (Cucumis sativus L.) Seedlings under Low Temperature. Sci. Hortic. 2019, 261, 108953. [Google Scholar] [CrossRef]
- Chen, S.; Jin, W.; Liu, A.; Zhang, S.; Liu, D.; Wang, F.; Lin, X.; He, C. Arbuscular Mycorrhizal Fungi (AMF) Increase Growth and Secondary Metabolism in Cucumber Subjected to Low Temperature Stress. Sci. Hortic. 2013, 160, 222–229. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Wang, Y.; Mao, Q.; Wu, M.; Yan, Y.; Ren, J.; Wang, X.; Liu, A.; Chen, S. Dopamine Alleviates Bisphenol A-Induced Phytotoxicity by Enhancing Antioxidant and Detoxification Potential in Cucumber. Environ. Pollut. 2020, 259, 113957. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Schumaker, K.S.; Zhu, J.K. Cell Signaling during Cold, Drought, and Salt Stress. Plant Cell 2002, 14, 165–183. [Google Scholar] [CrossRef]
- Xia, X.J.; Fang, P.P.; Guo, X.; Qian, X.J.; Zhou, J.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Brassinosteroid-Mediated Apoplastic H2O2-Glutaredoxin 12/14 Cascade Regulates Antioxidant Capacity in Response to Chilling in Tomato. Plant Cell Environ. 2018, 41, 1052–1064. [Google Scholar] [CrossRef]
- Jiang, Y.P.; Huang, L.F.; Cheng, F.; Zhou, Y.H.; Xia, X.J.; Mao, W.H.; Shi, K.; Yu, J.Q. Brassinosteroids Accelerate Recovery of Photosynthetic Apparatus from Cold Stress by Balancing the Electron Partitioning, Carboxylation and Redox Homeostasis in Cucumber. Physiol. Plant 2013, 148, 133–145. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Wang, Y.-T.; Pan, X.-B.; Xi, Z.-M. Amelioration of Cold-Induced Oxidative Stress by Exogenous 24-Epibrassinolide Treatment in Grapevine Seedlings: Toward Regulating the Ascorbate–Glutathione Cycle. Sci. Hortic. 2019, 244, 379–387. [Google Scholar] [CrossRef]
- Xi, Z.; Wang, Z.; Fang, Y.; Hu, Z.; Hu, Y.; Deng, M.; Zhang, Z. Effects of 24-Epibrassinolide on Antioxidation Defense and Osmoregulation Systems of Young Grapevines (V. vinifera L.) under Chilling Stress. Plant Growth Regul. 2013, 71, 57–65. [Google Scholar] [CrossRef]
- Fang, P.; Yan, M.; Chi, C.; Wang, M.; Zhou, Y.; Zhou, J.; Shi, K.; Xia, X.; Foyer, C.H.; Yu, J. Brassinosteroids Act as a Positive Regulator of Photoprotection in Response to Chilling Stress. Plant Physiol. 2019, 180, 2061–2076. [Google Scholar] [CrossRef]
- Eremina, M.; Unterholzner, S.J.; Rathnayake, A.I.; Castellanos, M.; Khan, M.; Kugler, K.G.; May, S.T.; Mayer, K.F.X.; Rozhon, W.; Poppenberger, B. Brassinosteroids Participate in the Control of Basal and Acquired Freezing Tolerance of Plants. Proc. Natl. Acad. Sci. USA 2016, 113, E5982–E5991. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ye, K.; Shi, Y.; Cheng, J.; Zhang, X.; Yang, S. BZR1 Positively Regulates Freezing Tolerance via CBF-Dependent and CBF-Independent Pathways in Arabidopsis. Mol. Plant 2017, 10, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Li, H.; Ding, Y.; Shi, Y.; Song, C.; Gong, Z.; Yang, S. Erratum: A Single-Nucleotide Polymorphism in the Promoter of a Hairpin RNA Contributes to Alternaria Alternata Leaf Spot Resistance in Apple (Malus 3 Domestica) (Plant Cell (2018) 30 (1924–1942). https://doi.org/10.1105/TPc.18.00042). Plant Cell 2019, 31, 2276. [Google Scholar] [CrossRef]
- Xiong, L.; Zhu, J.K. Molecular and Genetic Aspects of Plant Responses to Osmotic Stress. Plant Cell Environ. 2002, 25, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.T.; Chen, Z.Y.; Jiang, Y.; Duan, B.B.; Xi, Z.M. Involvement of ABA and Antioxidant System in Brassinosteroid-Induced Water Stress Tolerance of Grapevine (Vitis vinifera L.). Sci. Hortic. 2019, 256, 108596. [Google Scholar] [CrossRef]
- Yuan, L.; Yuan, Y.; Du, J.; Sun, J.; Guo, S. Effects of 24-Epibrassinolide on Nitrogen Metabolism in Cucumber Seedlings under Ca(NO3)2 Stress. Plant Physiol. Biochem. 2012, 61, 29–35. [Google Scholar] [CrossRef]
- Hu, W.H.; Yan, X.H.; Xiao, Y.A.; Zeng, J.J.; Qi, H.J.; Ogweno, J.O. 24-Epibrassinosteroid Alleviate Drought-Induced Inhibition of Photosynthesis in Capsicum Annuum. Sci. Hortic. 2013, 150, 232–237. [Google Scholar] [CrossRef]
- Fariduddin, Q.; Khanam, S.; Hasan, S.A.; Ali, B.; Hayat, S.; Ahmad, A. Effect of 28-Homobrassinolide on the Drought Stress-Induced Changes in Photosynthesis and Antioxidant System of Brassica juncea L. Acta Physiol. Plant 2009, 31, 889–897. [Google Scholar] [CrossRef]
- Yuan, G.F.; Jia, C.G.; Li, Z.; Sun, B.; Zhang, L.P.; Liu, N.; Wang, Q.M. Effect of Brassinosteroids on Drought Resistance and Abscisic Acid Concentration in Tomato under Water Stress. Sci. Hortic. 2010, 126, 103–108. [Google Scholar] [CrossRef]
- Castañeda-Murillo, C.C.; Rojas-Ortiz, J.G.; Sánchez-Reinoso, A.D.; Chávez-Arias, C.C.; Restrepo-Díaz, H. Foliar Brassinosteroid Analogue (DI-31) Sprays Increase Drought Tolerance by Improving Plant Growth and Photosynthetic Efficiency in Lulo Plants. Heliyon 2022, 8, e08977. [Google Scholar] [CrossRef]
- Northey, J.G.B.; Liang, S.; Jamshed, M.; Deb, S.; Foo, E.; Reid, J.B.; Mccourt, P.; Samuel, M.A. Farnesylation Mediates Brassinosteroid Biosynthesis to Regulate Abscisic Acid Responses. Nat. Plants 2016, 2, 16114. [Google Scholar] [CrossRef]
- Hayat, S.; Hasan, S.A.; Yusuf, M.; Hayat, Q.; Ahmad, A. Effect of 28-Homobrassinolide on Photosynthesis, Fluorescence and Antioxidant System in the Presence or Absence of Salinity and Temperature in Vigna Radiata. Environ. Exp. Bot. 2010, 69, 105–112. [Google Scholar] [CrossRef]
- Hayat, S.; Maheshwari, P.; Wani, A.S.; Irfan, M.; Alyemeni, M.N.; Ahmad, A. Comparative Effect of 28 Homobrassinolide and Salicylic Acid in the Amelioration of NaCl Stress in Brassica juncea L. Plant Physiol. Biochem. 2012, 53, 61–68. [Google Scholar] [CrossRef]
- Ali, B.; Hayat, S.; Fariduddin, Q.; Ahmad, A. 24-Epibrassinolide Protects against the Stress Generated by Salinity and Nickel in Brassica Juncea. Chemosphere 2008, 72, 1387–1392. [Google Scholar] [CrossRef]
- Zhao, Z.; Jin, R.; Fang, D.; Wang, H.; Dong, Y.; Xu, R.; Jiang, J. Paddy Cultivation Significantly Alters the Forms and Contents of Fe Oxides in an Oxisol and Increases Phosphate Mobility. Soil Tillage Res. 2018, 184, 176–180. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Wu, M.; Wang, Y.; Yan, Y.; Mao, Q.; Ren, J.; Ma, R.; Liu, A.; Chen, S. Melatonin Alleviates Iron Stress by Improving Iron Homeostasis, Antioxidant Defense and Secondary Metabolism in Cucumber. Sci. Hortic. 2020, 265, 109205. [Google Scholar] [CrossRef]
- Wang, J.; HuaLi, Z.; Cai, Z.; Wang, H.; Yang, Z.; Liu, Z. Puerarin Protects Rat Liver and Kidney against Cadmium-Induced Oxidative Stress. Indian J. Anim. Sci. 2019, 89, 927–931. [Google Scholar]
- Hasan, M.K.; Ahammed, G.J.; Sun, S.; Li, M.; Yin, H.; Zhou, J. Melatonin Inhibits Cadmium Translocation and Enhances Plant Tolerance by Regulating Sulfur Uptake and Assimilation in Solanum lycopersicum L. J. Agric. Food Chem. 2019, 67, 10563–10576. [Google Scholar] [CrossRef]
- Zhou, Y.; Huo, S.; Wang, L.; Meng, J.; Zhang, Z.; Xi, Z. Exogenous 24-Epibrassinolide Alleviates Oxidative Damage from Copper Stress in Grape (Vitis vinifera L.) Cuttings. Plant Physiol. Biochem. 2018, 130, 555–565. [Google Scholar] [CrossRef]
- Bücker-Neto, L.; Paiva, A.L.S.; Machado, R.D.; Arenhart, R.A.; Margis-Pinheiro, M. Interactions between Plant Hormones and Heavy Metals Responses. Genet. Mol. Biol. 2017, 40, 373–386. [Google Scholar] [CrossRef]
- Rajewska, I.; Talarek, M.; Bajguz, A. Brassinosteroids and Response of Plants to Heavy Metals Action. Front. Plant Sci. 2016, 7, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Choudhary, S.P.; Chen, S.; Xia, X.; Shi, K.; Zhou, Y.; Yu, J. Role of Brassinosteroids in Alleviation of Phenanthrene–Methylation and Chromatin Patterningcadmium Co-Contamination-Induced Photosynthetic Inhibition and Oxidative Stress in Tomato. J. Exp. Bot. 2012, 64, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Prasad, S.M. Effects of 28-Homobrassinoloid on Key Physiological Attributes of Solanum lycopersicum Seedlings under Cadmium Stress: Photosynthesis and Nitrogen Metabolism. Plant Growth Regul. 2017, 82, 161–173. [Google Scholar] [CrossRef]
- Hayat, S.; Alyemeni, M.N.; Hasan, S.A. Foliar Spray of Brassinosteroid Enhances Yield and Quality of Solanum lycopersicum under Cadmium Stress. Saudi J. Biol. Sci. 2012, 19, 325–335. [Google Scholar] [CrossRef]
- Hasan, S.A.; Hayat, S.; Ahmad, A. Brassinosteroids Protect Photosynthetic Machinery against the Cadmium Induced Oxidative Stress in Two Tomato Cultivars. Chemosphere 2011, 84, 1446–1451. [Google Scholar] [CrossRef]
- Hasan, S.A.; Hayat, S.; Ali, B.; Ahmad, A. 28-Homobrassinolide Protects Chickpea (Cicer arietinum) from Cadmium Toxicity by Stimulating Antioxidants. Environ. Pollut. 2008, 151, 60–66. [Google Scholar] [CrossRef]
- Bukhari, S.A.H.; Wang, R.; Wang, W.; Ahmed, I.M.; Zheng, W.; Cao, F. Genotype-Dependent Effect of Exogenous 24-Epibrassinolide on Chromium-Induced Changes in Ultrastructure and Physicochemical Traits in Tobacco Seedlings. Environ. Sci. Pollut. Res. 2016, 23, 18229–18238. [Google Scholar] [CrossRef]
- Song, Y.; Cui, J.; Zhang, H.; Wang, G.; Zhao, F.-J.; Shen, Z. Proteomic Analysis of Copper Stress Responses in the Roots of Two Rice (Oryza sativa L.) Varieties Differing in Cu Tolerance. Plant Soil 2013, 366, 647–658. [Google Scholar] [CrossRef]
- Kanwar, M.K.; Bhardwaj, R.; Arora, P.; Chowdhary, S.P.; Sharma, P.; Kumar, S. Plant Steroid Hormones Produced under Ni Stress Are Involved in the Regulation of Metal Uptake and Oxidative Stress in Brassica juncea L. Chemosphere 2012, 86, 41–49. [Google Scholar] [CrossRef]
- Li, M.; Ahammed, G.J.; Li, C.; Bao, X.; Yu, J.; Huang, C.; Yin, H.; Zhou, J. Brassinosteroid Ameliorates Zinc Oxide Nanoparticles-Induced Oxidative Stress by Improving Antioxidant Potential and Redox Homeostasis in Tomato Seedling. Front. Plant Sci. 2016, 7, 615. [Google Scholar] [CrossRef]
- Choudhary, S.P.; Yu, J.-Q.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.-S.P. Benefits of Brassinosteroid Crosstalk. Trends Plant Sci. 2012, 17, 594–605. [Google Scholar] [CrossRef]
- Ramakrishna, B.; Rao, S.S.R. Preliminary Studies on the Involvement of Glutathione Metabolism and Redox Status against Zinc Toxicity in Radish Seedlings by 28-Homobrassinolide. Environ. Exp. Bot. 2013, 96, 52–58. [Google Scholar] [CrossRef]
- Rady, M.M.; Osman, A.S. Response of Growth and Antioxidant System of Heavy Metal-Contaminated Tomato Plants to 24-Epibrassinolide. Afr. J. Agric. Res. 2012, 7, 3249–3254. [Google Scholar] [CrossRef][Green Version]
- Arora, P.; Bhardwaj, R.; Kanwar, M.K. 24-Epibrassinolide Induced Antioxidative Defense System of Brassica juncea L. under Zn Metal Stress. Physiol. Mol. Biol. Plants 2010, 16, 285–293. [Google Scholar] [CrossRef]
- Arora, N.; Bhardwaj, R.; Sharma, P.; Arora, H.K.; Arora, P. Amelioration of Zn Toxicity by 28-Homobrassinolide in Zea mays L. Can. J. Pure Appl. Sci. 2008, 2, 503–509. [Google Scholar]
- Yusuf, M.; Fariduddin, Q.; Ahmad, A. 28-Homobrassinolide Mitigates Boron Induced Toxicity through Enhanced Antioxidant System in Vigna Radiata Plants. Chemosphere 2011, 85, 1574–1584. [Google Scholar] [CrossRef]
- Arora, P.; Bhardwaj, R.; Kanwar, M.K. Effect of 24-Epibrassinolide on Growth, Protein Content and Antioxidative Defense System of Brassica juncea L. Subjected to Cobalt Ion Toxicity. Acta Physiol. Plant 2012, 34, 2007–2017. [Google Scholar] [CrossRef]
- Raghu, K.; Mahesh, K.; Divya Sri, N.; Rao, S. Effect of Brassinosteroids on Seed Germination and Seedling Growth of Radish (Raphanus sativus L.) under Arsenic Toxicity Stress. Int. J. Dev. Res. 2014, 4, 1929–1933. [Google Scholar]
- Wang, H.; Feng, T.; Peng, X.; Yan, M.; Zhou, P.; Tang, X. Ameliorative Effects of Brassinosteroid on Excess Manganese-Induced Oxidative Stress in Zea mays L. Leaves. Agric. Sci. China 2009, 8, 1063–1074. [Google Scholar] [CrossRef]
- Vragović, K.; Selaa, A.; Friedlander-Shani, L.; Fridman, Y.; Hacham, Y.; Holland, N.; Bartom, E.; Mockler, T.C.; Savaldi-Goldstein, S. Translatome Analyses Capture of Opposing Tissuespecific Brassinosteroid Signals Orchestrating Root Meristem Differentiation. Proc. Natl. Acad. Sci. USA 2015, 112, 923–928. [Google Scholar] [CrossRef]
- Vilarrasa-Blasi, J.; González-García, M.P.; Frigola, D.; Fàbregas, N.; Alexiou, K.G.; López-Bigas, N.; Rivas, S.; Jauneau, A.; Lohmann, J.U.; Benfey, P.N.; et al. Regulation of Plant Stem Cell Quiescence by a Brassinosteroid Signaling Module. Dev. Cell 2014, 30, 36–47. [Google Scholar] [CrossRef]
- Kothari, A.; Lachowiec, J. Roles of Brassinosteroids in Mitigating Heat Stress Damage in Cereal Crops. Int. J. Mol. Sci. 2021, 22, 2706. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaudhuri, A.; Halder, K.; Abdin, M.Z.; Majee, M.; Datta, A. Abiotic Stress Tolerance in Plants: Brassinosteroids Navigate Competently. Int. J. Mol. Sci. 2022, 23, 14577. https://doi.org/10.3390/ijms232314577
Chaudhuri A, Halder K, Abdin MZ, Majee M, Datta A. Abiotic Stress Tolerance in Plants: Brassinosteroids Navigate Competently. International Journal of Molecular Sciences. 2022; 23(23):14577. https://doi.org/10.3390/ijms232314577
Chicago/Turabian StyleChaudhuri, Abira, Koushik Halder, Malik Z. Abdin, Manoj Majee, and Asis Datta. 2022. "Abiotic Stress Tolerance in Plants: Brassinosteroids Navigate Competently" International Journal of Molecular Sciences 23, no. 23: 14577. https://doi.org/10.3390/ijms232314577
APA StyleChaudhuri, A., Halder, K., Abdin, M. Z., Majee, M., & Datta, A. (2022). Abiotic Stress Tolerance in Plants: Brassinosteroids Navigate Competently. International Journal of Molecular Sciences, 23(23), 14577. https://doi.org/10.3390/ijms232314577





