A Proof-of-Concept Analysis of Plasma-Derived Exosomal microRNAs in Interstitial Pulmonary Fibrosis Secondary to Antisynthetase Syndrome
Abstract
1. Introduction
2. Results
2.1. Demographic and Clinical Features of Patients
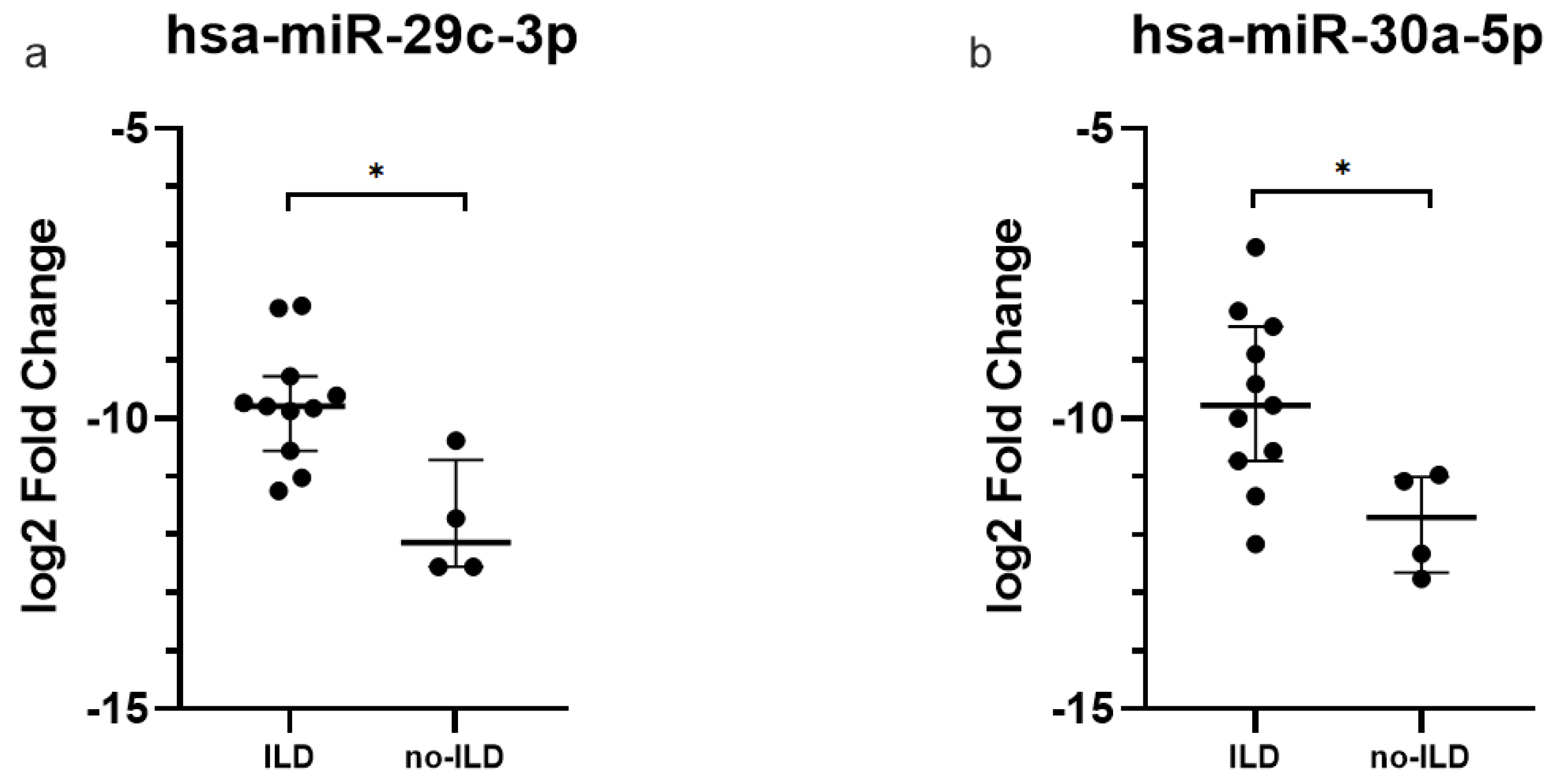
2.2. miRNAs Expression Levels in Plasma-Derived Exosomes
2.3. Signaling Pathway Prediction and Targets Analyses
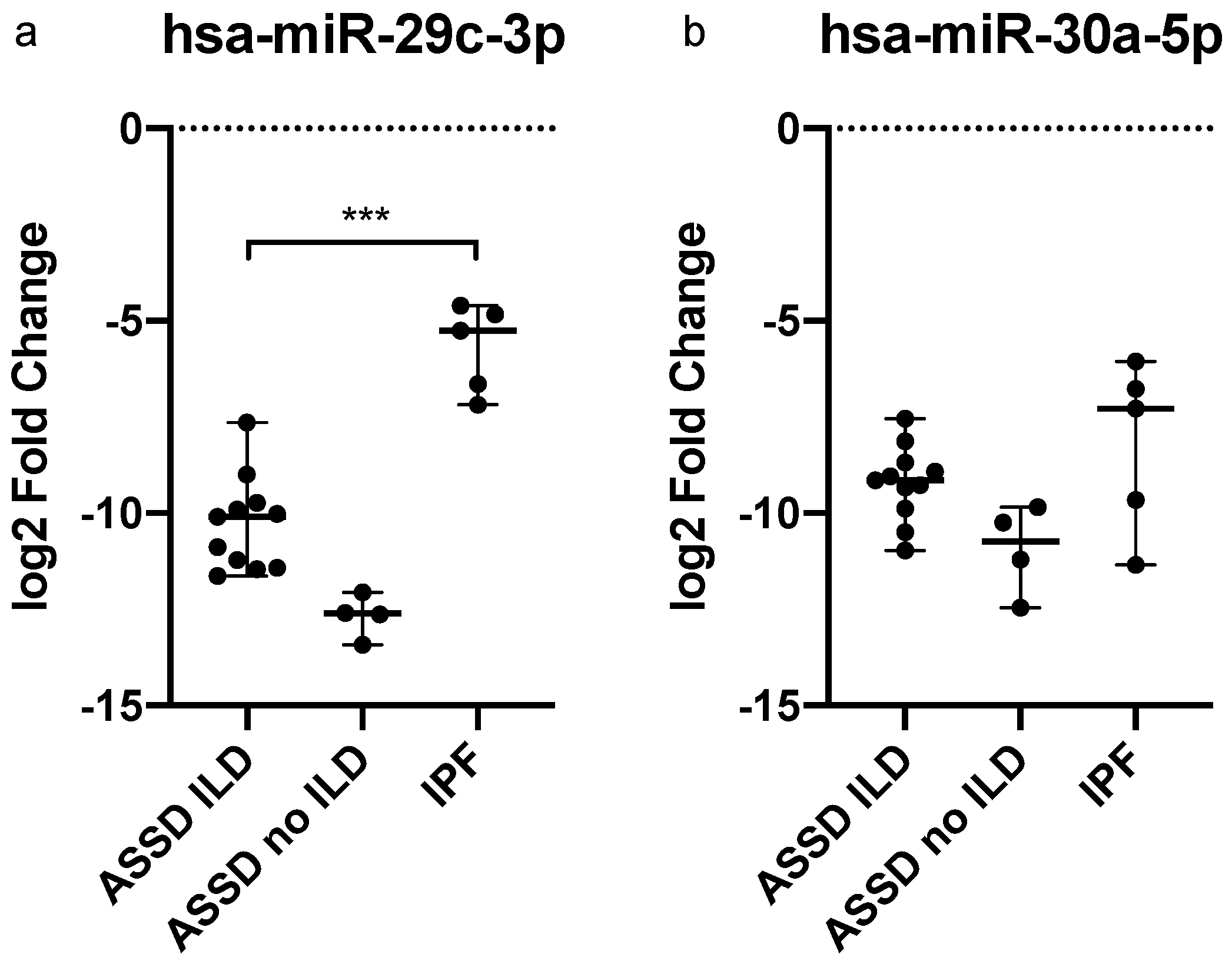
2.4. Dysregulated miRNAs in IPF Patients
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Exosomes and RNA Isolation
4.3. Quantitative Real-Time Reverse Transcription PCR
4.4. miRNAs Target Prediction and Pathway Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Imbert-Masseau, A.; Hamidou, M.; Agard, C.; Grolleau, J.Y.; Chérin, P. Antisynthetase syndrome. Jt. Bone Spine 2003, 70, 161–168. [Google Scholar] [CrossRef]
- Cavagna, L.; Nuño, L.; Scirè, C.A.; Govoni, M.; Longo, F.J.L.; Franceschini, F.; Neri, R.; Castañeda, S.; Giraldo, W.; Caporali, R.; et al. Clinical Spectrum Time Course in Anti Jo-1 Positive Antisynthetase Syndrome: Results from an International Retrospective Multicenter Study. Medicine 2015, 94, e1144. [Google Scholar] [CrossRef]
- Cavagna, L.; Trallero-Araguás, E.; Meloni, F.; Cavazzana, I.; Rojas-Serrano, J.; Feist, E.; Zanframundo, G.; Morandi, V.; Meyer, A.; Pereira da Silva, J.A.; et al. Influence of Antisynthetase Antibodies Specificities on Antisynthetase Syndrome Clinical Spectrum Time Course. J. Clin. Med. 2019, 8, 2013. [Google Scholar] [CrossRef]
- Cavagna, L.; Castañeda, S.; Sciré, C.; Gonzalez-Gay, M.A.; AENEAS Collaborative Group Members. Antisynthetase syndrome or what else? Different perspectives indicate the need for new classification criteria. Ann. Rheum Dis. 2018, 77, e50. [Google Scholar] [CrossRef]
- Monti, S.; Montecucco, C.; Cavagna, L. Clinical spectrum of anti-Jo-1-associated disease. Curr. Opin. Rheumatol. 2017, 29, 612–617. [Google Scholar] [CrossRef]
- Cavagna, L.; Monti, S.; Caporali, R.; Gatto, M.; Iaccarino, L.; Doria, A. How I treat idiopathic patients with inflammatory myopathies in the clinical practice. Autoimmun. Rev. 2017, 16, 999–1007. [Google Scholar] [CrossRef]
- Vlachos, I.S.; Kostoulas, N.; Vergoulis, T.; Georgakilas, G.; Reczko, M.; Maragkakis, M.; Paraskevopoulou, M.D.; Prionidis, K.; Dalamagas, T.; Hatzigeorgiou, A.G. DIANA miRPath v.2.0: Investigating the combinatorial effect of microRNAs in pathways. Nucleic Acids Res. 2012, 40, W498–W504. [Google Scholar] [CrossRef]
- Li, L.; Zuo, X.; Liu, D.; Luo, H.; Zhang, H.; Peng, Q.; Wang, G.; Zhu, H. Plasma exosomal RNAs has potential as both clinical biomarkers and therapeutic targets of dermatomyositis. Rheumatology 2021, 61, 2672–2681. [Google Scholar] [CrossRef]
- Zhong, D.; Wu, C.; Xu, D.; Bai, J.; Wang, Q.; Zeng, X. Plasma-Derived Exosomal hsa-miR-4488 and hsa-miR-1228-5p: Novel Biomarkers for Dermatomyositis-Associated Interstitial Lung Disease with Anti-Melanoma Differentiation-Associated Protein 5 Antibody-Positive Subset. BioMed Res. Int. 2021, 2021, 6676107. [Google Scholar] [CrossRef]
- Kumari, R.; Roy, U.; Desai, S.; Nilavar, N.M.; Van Nieuwenhuijze, A.; Paranjape, A.; Radha, G.; Bawa, P.; Srivastava, M.; Nambiar, M.; et al. MicroRNA miR-29c regulates RAG1 expression and modulates V(D)J recombination during B cell development. Cell Rep. 2021, 36, 109390. [Google Scholar] [CrossRef]
- Georgantas, R.W.; Streicher, K.; Greenberg, S.A.; Greenlees, L.M.; Zhu, W.; Brohawn, P.Z.; Higgs, B.W.; Czapiga, M.; Morehouse, C.A.; Amato, A.; et al. Inhibition of myogenic microRNAs 1, 133, and 206 by inflammatory cytokines links inflammation and muscle degeneration in adult inflammatory myopathies. Arthritis Rheumatol. 2014, 66, 1022–1033. [Google Scholar] [CrossRef]
- Yu, L.; Li, J.; Chen, Y.; Jiang, J.; Fang, Q.; Jiang, J.; Wang, D.; Liu, M. hsa-miR-7 Is a Potential Biomarker for Idiopathic Inflammatory Myopathies with Interstitial Lung Disease in Humans. Ann. Clin. Lab. Sci. 2018, 48, 764–769. [Google Scholar]
- Lee, C.C.; Ho, K.H.; Huang, T.W.; Shih, C.M.; Hsu, S.Y.; Liu, A.J.; Chen, K.C. A regulatory loop among CD276; miR-29c-3p; and Myc exists in cancer cells against natural killer cell cytotoxicity. Life Sci. 2021, 277, 119438. [Google Scholar] [CrossRef]
- Wang, C.M.; Wang, Y.; Fan, C.G.; Xu, F.F.; Sun, W.S.; Liu, Y.G.; Jia, J.H. miR-29c targets TNFAIP3; inhibits cell proliferation and induces apoptosis in hepatitis B virus-related hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2011, 411, 586–592. [Google Scholar] [CrossRef]
- Zhang, B.; Yu, L.; Sheng, Y. Clinical value and role of microRNA-29c-3p in sepsis-induced inflammation and cardiac dysfunction. Eur. J. Med. Res. 2021, 26, 90. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, R.; Zhao, Y.; Wang, J. MiRNA-29c-3p Promotes Intestinal Inflammation via Targeting Leukemia Inhibitory Factor in Ulcerative Colitis. J. Inflamm. Res. 2021, 14, 2031–2043. [Google Scholar] [CrossRef]
- Cushing, L.; Kuang, P.; Lü, J. The role of miR-29 in pulmonary fibrosis. Biochem. Cell Biol. 2015, 93, 109–118. [Google Scholar] [CrossRef]
- Sun, C.M.; Zhang, W.Y.; Wang, S.Y.; Qian, G.; Pei, D.L.; Zhang, G.M. Fer exacerbates renal fibrosis and can be targeted by miR-29c-3p. Open Med. Wars. Pol. 2021, 16, 1378–1385. [Google Scholar] [CrossRef]
- Liang, J.N.; Zou, X.; Fang, X.H.; Xu, J.D.; Xiao, Z.; Zhu, J.N.; Li, H.; Yang, J.; Zeng, N.; Yuan, S.J.; et al. The Smad3-miR-29b/miR-29c axis mediates the protective effect of macrophage migration inhibitory factor against cardiac fibrosis. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2441–2450. [Google Scholar] [CrossRef]
- Jiang, L.H.; Zhang, H.D.; Tang, J.H. MiR-30a: A Novel Biomarker and Potential Therapeutic Target for Cancer. J. Oncol. 2018, 2018, 5167829. [Google Scholar] [CrossRef]
- Caserta, S.; Mengozzi, M.; Kern, F.; Newbury, S.F.; Ghezzi, P.; Llewelyn, M.J. Severity of Systemic Inflammatory Response Syndrome Affects the Blood Levels of Circulating Inflammatory-Relevant MicroRNAs. Front. Immunol. 2017, 8, 1977. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yao, Y.; Ma, Y.; Chen, Y. MiR-30a-5p ameliorates LPS-induced inflammatory injury in human A549 cells and mice via targeting RUNX2. Innate Immun. 2021, 27, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.X.; Zhao, Z.Y. miR-30a-5p inhibits the proliferation and collagen formation of cardiac fibroblasts in diabetic cardiomyopathy. Can. J. Physiol. Pharmacol. 2022, 100, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cai, S.; Ding, X.; Lu, C.; Wu, R.; Wu, H.; Shang, Y.; Pang, M. MicroRNA-30a-5p silencing polarizes macrophages toward M2 phenotype to alleviate cardiac injury following viral myocarditis by targeting SOCS1. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H1348–H1360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, H.; Liu, Y.; Zhang, J.; Li, H.; Liu, W.; Cao, G.; Xv, P.; Zhang, J.; Lv, C.; et al. miR-30a as Potential Therapeutics by Targeting TET1 through Regulation of Drp-1 Promoter Hydroxymethylation in Idiopathic Pulmonary Fibrosis. IJMS 2017, 18, 633. [Google Scholar] [CrossRef]
- Liu, B.; Jiang, T.; Hu, X.; Liu, Z.; Zhao, L.; Liu, H.; Liu, Z.; Ma, L. Downregulation of microRNA-30a in bronchoalveolar lavage fluid from idiopathic pulmonary fibrosis patients. Mol. Med. Rep. 2018, 18, 5799–5806. [Google Scholar] [CrossRef]

| Patients | Sex | Age | ARS | Myositis | Arthritis | ILD | Type of ILD |
|---|---|---|---|---|---|---|---|
| 1 | Female | 76 years | Anti-Jo1 | − | + | + | NSIP |
| 2 | Female | 81 years | Anti-PL7 | − | + | + | NSIP |
| 3 | Female | 70 years | Anti-Jo1 | + | + | + | NSIP + OP |
| 4 | Female | 36 years | Anti-Jo1 | + | + | + | NSIP |
| 5 | Male | 47 years | Anti-Jo1 | + | + | − | - |
| 6 | Male | 54 years | Anti-PL7 | + | − | − | - |
| 7 | Female | 70 years | Anti-Jo1 | − | + | + | NSIP + OP |
| 8 | Female | 61 years | Anti-PL7 | + | − | + | NSIP |
| 9 | Female | 64 years | Anti-Jo1 | + | − | + | NSIP |
| 10 | Female | 61 years | Anti-Jo1 | + | + | + | OP |
| 11 | Female | 59 years | Anti-Jo1 | − | − | + | NSIP + OP |
| 12 | Female | 39 years | Anti-Jo1 | − | − | + | NSIP |
| 13 | Female | 66 years | Anti-Jo1 | + | − | + | NSIP |
| 14 | Female | 70 years | Anti-Jo1 | + | + | − | - |
| 15 | Female | 74 years | Anti-Jo1 | + | + | − | - |
| KEGG Pathway | p-Value | Genes |
|---|---|---|
| Fatty acid biosynthesis | <1 × 10−5 | 5 |
| Fatty acid metabolism | 1.39 × 10−4 | 11 |
| Viral carcinogenesis | 3.52 × 10−3 | 52 |
| Lysine degradation | 2.19 × 10−3 | 18 |
| Proteoglycans in cancer | 2.94 × 10−2 | 56 |
| Colorectal cancer | 2.18 | 25 |
| p53 signaling pathway | 6.48 × 10 | 25 |
| Hippo signaling pathway | 0.001118592 | 36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bozzini, S.; Zanframundo, G.; Bagnera, C.; Bozza, E.; Lettieri, S.; Vertui, V.; Codullo, V.; Cuzzocrea, F.; Atienza-Mateo, B.; Remuzgo Martinez, S.; et al. A Proof-of-Concept Analysis of Plasma-Derived Exosomal microRNAs in Interstitial Pulmonary Fibrosis Secondary to Antisynthetase Syndrome. Int. J. Mol. Sci. 2022, 23, 14579. https://doi.org/10.3390/ijms232314579
Bozzini S, Zanframundo G, Bagnera C, Bozza E, Lettieri S, Vertui V, Codullo V, Cuzzocrea F, Atienza-Mateo B, Remuzgo Martinez S, et al. A Proof-of-Concept Analysis of Plasma-Derived Exosomal microRNAs in Interstitial Pulmonary Fibrosis Secondary to Antisynthetase Syndrome. International Journal of Molecular Sciences. 2022; 23(23):14579. https://doi.org/10.3390/ijms232314579
Chicago/Turabian StyleBozzini, Sara, Giovanni Zanframundo, Cecilia Bagnera, Eleonora Bozza, Sara Lettieri, Valentina Vertui, Veronica Codullo, Francesca Cuzzocrea, Belén Atienza-Mateo, Sara Remuzgo Martinez, and et al. 2022. "A Proof-of-Concept Analysis of Plasma-Derived Exosomal microRNAs in Interstitial Pulmonary Fibrosis Secondary to Antisynthetase Syndrome" International Journal of Molecular Sciences 23, no. 23: 14579. https://doi.org/10.3390/ijms232314579
APA StyleBozzini, S., Zanframundo, G., Bagnera, C., Bozza, E., Lettieri, S., Vertui, V., Codullo, V., Cuzzocrea, F., Atienza-Mateo, B., Remuzgo Martinez, S., Montecucco, C., González-Gay, M. A., Cavagna, L., & Meloni, F. (2022). A Proof-of-Concept Analysis of Plasma-Derived Exosomal microRNAs in Interstitial Pulmonary Fibrosis Secondary to Antisynthetase Syndrome. International Journal of Molecular Sciences, 23(23), 14579. https://doi.org/10.3390/ijms232314579







