IL-6 and IL-8: An Overview of Their Roles in Healthy and Pathological Pregnancies
Abstract
1. Introduction
2. IL-6 and IL-8 in a Healthy Pregnancy
2.1. Expression of IL-6 and IL-8 and Their Receptors at the Feto-Maternal Interface
2.2. Pregnancy Establishment
2.3. Parturition
2.4. Circulating IL-6 and IL-8 Levels in a Healthy Pregnancy
3. IL-6 and IL-8 in Selected Pregnancy Pathologies
3.1. Pregnancy Loss
| Pathology | Sample | Pathology-Related change | Cytokine | Reference |
|---|---|---|---|---|
| Pregnancy loss | Decidual macrophages and dNKs * | Decreased expression in SPL * | IL-6, IL-8 | [18] |
| Increased expression in RPL * | IL-8 | [124,125] | ||
| Decidua | Increased expression in RPL * | IL-6, IL-8 | [121,122,123] | |
| Serum | Increased concentration | IL-6 | [123,129,130] | |
| IL-8 | [133,136] | |||
| No change | IL-6 | [131,132] | ||
| IL-8 | [131] | |||
| Decreased concentration | IL-6 | [133,134,135,136] | ||
| IL-8 | [134] | |||
| Preeclampsia | Placenta | Increased expression | IL-6 | [147,148,149,150,151] |
| IL-8 | [149,152,153] | |||
| Serum | Increased concentration | IL-6 | [150,151,154,155,156,157,158] | |
| IL-8 | [151,153,156,159,160,161,162] | |||
| Gestational diabetes mellitus | Placenta | Increased expression | IL-6 | [115,163,164,165,166] |
| IL-8 | [115,167] | |||
| No change | IL-6 | [168,169] | ||
| IL-8 | [169,170] | |||
| Increased levels in extravillous and decreased in villi tissue | IL-6 | [167] | ||
| Sex-specific expression in STB * and EVTs * | IL-8 | [171] | ||
| Serum | Increased concentration | IL-6 | [113,115,172,173,174,175,176,177,178,179] | |
| IL-8 | [115,176,179,180] | |||
| No change | IL-6 | [180,181,182,183] | ||
| IL-8 | [167,184,185] | |||
| Decreased concentration in early pregnancy | IL-8 | [184] | ||
| Maternal immune activation | Placenta/trophoblast and fetal membranes | Increased expression in response to inflammatory stimuli | IL-6 | [186,187,188,189,190,191] |
| IL-8 | [189,190,191] | |||
| Amniotic fluid and cervicovaginal lavage | Increased concentration in API * | IL-6 | [192,193,194,195,196,197,198] | |
| IL-8 | [192,193,194,195,196,197,199,200] |
3.2. Preeclampsia
3.3. Gestational Diabetes Mellitus
3.4. Maternal Immune Activation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benirschke, K.; Burton, G.J.; Baergen, R.N. Pathology of the Human Placenta; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 978-3-642-23940-3. [Google Scholar]
- Saito, S. Cytokine Network at the Feto-Maternal Interface. J. Reprod. Immunol. 2000, 47, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Hirano, T. IL-6. Cytokine Reference; Oppenheim, J., Feldmann, M., Eds.; Academic Press: San Diego, CA, USA, 2000; Volume 1, pp. 537–563. [Google Scholar]
- Heinrich, P.C.; Behrmann, I.; Haan, S.; Hermanns, H.M.; Müller-Newen, G.; Schaper, F. Principles of Interleukin (IL)-6-Type Cytokine Signalling and Its Regulation. Biochem. J. 2003, 374, 1–20. [Google Scholar] [CrossRef]
- Naka, T.; Narazaki, M.; Hirata, M.; Matsumoto, T.; Minamoto, S.; Aono, A.; Nishimoto, N.; Kajita, T.; Taga, T.; Yoshizaki, K.; et al. Structure and Function of a New STAT-Induced STAT Inhibitor. Nature 1997, 387, 924–929. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.A.; Jenkins, B.J. Recent Insights into Targeting the IL-6 Cytokine Family in Inflammatory Diseases and Cancer. Nat. Rev. Immunol. 2018, 18, 773–789. [Google Scholar] [CrossRef] [PubMed]
- Rose-John, S. Interleukin-6 Signalling in Health and Disease. F1000Research 2020, 9, 1013. [Google Scholar] [CrossRef]
- Matsushima, K.; Yang, D.; Oppenheim, J.J. Interleukin-8: An Evolving Chemokine. Cytokine 2022, 153, 155828. [Google Scholar] [CrossRef]
- Corre, I.; Pineau, D.; Hermouet, S. Interleukin-8: An Autocrine/Paracrine Growth Factor for Human Hematopoietic Progenitors Acting in Synergy with Colony Stimulating Factor-1 to Promote Monocyte-Macrophage Growth and Differentiation. Exp. Hematol. 1999, 27, 28–36. [Google Scholar] [CrossRef]
- Li, A.; Dubey, S.; Varney, M.L.; Dave, B.J.; Singh, R.K. IL-8 Directly Enhanced Endothelial Cell Survival, Proliferation, and Matrix Metalloproteinases Production and Regulated Angiogenesis. J. Immunol. 2003, 170, 3369–3376. [Google Scholar] [CrossRef]
- Bréchard, S.; Bueb, J.-L.; Tschirhart, E.J. Interleukin-8 Primes Oxidative Burst in Neutrophil-like HL-60 through Changes in Cytosolic Calcium. Cell Calcium 2005, 37, 531–540. [Google Scholar] [CrossRef]
- Iizasa, H.; Matsushima, K. IL-8. Cytokine Reference; Oppenheim, J., Feldmann, M., Eds.; Academic Press: San Diego, CA, USA, 2000; Volume 1, pp. 1061–1067. [Google Scholar]
- Chan, L.-P.; Liu, C.; Chiang, F.-Y.; Wang, L.-F.; Lee, K.-W.; Chen, W.-T.; Kuo, P.-L.; Liang, C.-H. IL-8 Promotes Inflammatory Mediators and Stimulates Activation of P38 MAPK/ERK-NF-ΚB Pathway and Reduction of JNK in HNSCC. Oncotarget 2017, 8, 56375–56388. [Google Scholar] [CrossRef]
- Tabibzadeh, S.; Kong, Q.F.; Babaknia, A.; May, L.T. Progressive Rise in the Expression of Interleukin-6 in Human Endometrium during Menstrual Cycle Is Initiated during the Implantation Window. Hum. Reprod. 1995, 10, 2793–2799. [Google Scholar] [CrossRef] [PubMed]
- Arici, A.; Seli, E.; Senturk, L.M.; Gutierrez, L.S.; Oral, E.; Taylor, H.S. Interleukin-8 in the Human Endometrium. J. Clin. Endocrinol. Metab. 1998, 83, 1783–1787. [Google Scholar] [CrossRef] [PubMed]
- Mulayim, N.; Palter, S.F.; Kayisli, U.A.; Senturk, L.; Arici, A. Chemokine Receptor Expression in Human Endometrium. Biol. Reprod. 2003, 68, 1491–1495. [Google Scholar] [CrossRef] [PubMed]
- Jauniaux, E.; Gulbis, B.; Schandene, L.; Collette, J.; Hustin, J. Molecular Interactions during Pregnancy. MHR Basic Sci. Reprod. Med. 1996, 2, 239–243. [Google Scholar] [CrossRef]
- Pitman, H.; Innes, B.A.; Robson, S.C.; Bulmer, J.N.; Lash, G.E. Altered Expression of Interleukin-6, Interleukin-8 and Their Receptors in Decidua of Women with Sporadic Miscarriage. Hum. Reprod. 2013, 28, 2075–2086. [Google Scholar] [CrossRef]
- Pietro, L.; Bottcher-Luiz, F.; Velloso, L.A.; Morari, J.; Nomura, M.; Lucci De Angelo Andrade, L.A. Expression of Interleukin-6 (IL-6), Signal Transducer and Activator of Transcription-3 (STAT-3) and Telomerase in Choriocarcinomas. Surg. Exp. Pathol. 2020, 3, 28. [Google Scholar] [CrossRef]
- Champion, H.; Innes, B.A.; Robson, S.C.; Lash, G.E.; Bulmer, J.N. Effects of Interleukin-6 on Extravillous Trophoblast Invasion in Early Human Pregnancy. MHR Basic Sci. Reprod. Med. 2012, 18, 391–400. [Google Scholar] [CrossRef]
- Jovanović, M.; Vićovac, L. Interleukin-6 Stimulates Cell Migration, Invasion and Integrin Expression in HTR-8/SVneo Cell Line. Placenta 2009, 30, 320–328. [Google Scholar] [CrossRef]
- Saito, S.; Kasahara, T.; Sakakura, S.; Umekage, H.; Harada, N.; Ichijo, M. Detection and Localization of Interleukin-8 MRNA and Protein in Human Placenta and Decidual Tissues. J. Reprod. Immunol. 1994, 27, 161–172. [Google Scholar] [CrossRef]
- Hanna, J.; Goldman-Wohl, D.; Hamani, Y.; Avraham, I.; Greenfield, C.; Natanson-Yaron, S.; Prus, D.; Cohen-Daniel, L.; Arnon, T.I.; Manaster, I.; et al. Decidual NK Cells Regulate Key Developmental Processes at the Human Fetal-Maternal Interface. Nat. Med. 2006, 12, 1065–1074. [Google Scholar] [CrossRef]
- De Oliveira, L.G.; Lash, G.E.; Murray-Dunning, C.; Bulmer, J.N.; Innes, B.A.; Searle, R.F.; Sass, N.; Robson, S.C. Role of Interleukin 8 in Uterine Natural Killer Cell Regulation of Extravillous Trophoblast Cell Invasion. Placenta 2010, 31, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Shimoya, K.; Matsuzaki, N.; Taniguchi, T.; Kameda, T.; Koyama, M.; Neki, R.; Saji, F.; Tanizawa, O. Human Placenta Constitutively Produces Interleukin-8 during Pregnancy and Enhances Its Production in Intrauterine Infection. Biol. Reprod. 1992, 47, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, M.; Stefanoska, I.; Radojcić, L.; Vićovac, L. Interleukin-8 (CXCL8) Stimulates Trophoblast Cell Migration and Invasion by Increasing Levels of Matrix Metalloproteinase (MMP)2 and MMP9 and Integrins Alpha5 and Beta1. Reproduction 2010, 139, 789–798. [Google Scholar] [CrossRef]
- Makrigiannakis, A.; Minas, V. Mechanisms of Implantation. Reprod. Biomed. Online 2007, 14, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Xu, H.; Zhang, D.; Si, C.; Zhou, X.; Zhao, H.; Liu, Q.; Xu, B.; Zhang, A. Decreased PIBF1/IL6/p-STAT3 during the Mid-Secretory Phase Inhibits Human Endometrial Stromal Cell Proliferation and Decidualization. J. Adv. Res. 2021, 30, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, A.M.; Dellow, K.; Blayney, M.; Macnamee, M.; Charnock-Jones, S.; Smith, S.K. Stage-Specific Expression of Cytokine and Receptor Messenger Ribonucleic Acids in Human Preimplantation Embryos. Biol. Reprod. 1995, 53, 974–981. [Google Scholar] [CrossRef]
- Zolti, M.; Ben-Rafael, Z.; Meirom, R.; Shemesh, M.; Bider, D.; Mashiach, S.; Apte, R.N. Cytokine Involvement in Oocytes and Early Embryos. Fertil. Steril. 1991, 56, 265–272. [Google Scholar] [CrossRef]
- Zhong, H.; Sun, Q.; Chen, P.; Xiong, F.; Li, G.; Wan, C.; Yao, Z.; Zeng, Y. Detection of IL-6, IL-10, and TNF-α Level in Human Single-Blastocyst Conditioned Medium Using Ultrasensitive Single Molecule Array Platform and Its Relationship with Embryo Quality and Implantation: A Pilot Study. J. Assist. Reprod. Genet. 2020, 37, 1695–1702. [Google Scholar] [CrossRef]
- Plana-Carmona, M.; Stik, G.; Bulteau, R.; Segura-Morales, C.; Alcázar, N.; Wyatt, C.D.R.; Klonizakis, A.; de Andrés-Aguayo, L.; Gasnier, M.; Tian, T.V.; et al. The Trophectoderm Acts as a Niche for the Inner Cell Mass through C/EBPα-Regulated IL-6 Signaling. Stem Cell Rep. 2022, 17, 1991–2004. [Google Scholar] [CrossRef]
- Desai, N.; Scarrow, M.; Lawson, J.; Kinzer, D.; Goldfarb, J. Evaluation of the Effect of Interleukin-6 and Human Extracellullar Matrix on Embryonic Development. Hum. Reprod. 1999, 14, 1588–1592. [Google Scholar] [CrossRef][Green Version]
- Yang, J.; Yang, X.; Yang, H.; Bai, Y.; Zha, H.; Jiang, F.; Meng, Y. Interleukin 6 in Follicular Fluid Reduces Embryo Fragmentation and Improves the Clinical Pregnancy Rate. J. Assist. Reprod. Genet. 2020, 37, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.; O’Connell, A.; Ramsey, A. The Effect of Interleukin-6 Deficiency on Implantation, Fetal Development and Parturition in Mice. Proc. Aust. Soc. Reprod. Biol. 2000, 31, 97. [Google Scholar]
- Sakurai, T.; Takai, R.; Bürgin, H.; Ishihara, K.; Sakamoto, Y.; Amano, J.; Higuchi, Y.; Chiba, S.; Singer, T.; Kawamura, A.; et al. The Effects of Interleukin-6 Signal Blockade on Fertility, Embryo-Fetal Development, and Immunization In Vivo. Birth Defects Res. Part B-Dev. Reprod. Toxicol. 2012, 95, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Meisser, A.; Cameo, P.; Islami, D.; Campana, A.; Bischof, P. Effects of Interleukin-6 (IL-6) on Cytotrophoblastic Cells. Mol. Hum. Reprod. 1999, 5, 1055–1058. [Google Scholar] [CrossRef]
- Jovanovic, M.; Kovacevic, T.; Stefanoska, I.; Vicovac, L. The Effect of IL-6 on the Trophoblast Cell Line HTR-8/SVneo. Arch. Biol. Sci. 2010, 62, 531–538. [Google Scholar] [CrossRef]
- Damsky, C.H.; Fitzgerald, M.L.; Fisher, S.J. Distribution Patterns of Extracellular Matrix Components and Adhesion Receptors Are Intricately Modulated during First Trimester Cytotrophoblast Differentiation along the Invasive Pathway, in Vivo. J. Clin. Investig. 1992, 89, 210–222. [Google Scholar] [CrossRef]
- Vićovac, L.; Jones, C.J.; Aplin, J.D. Trophoblast Differentiation during Formation of Anchoring Villi in a Model of the Early Human Placenta in Vitro. Placenta 1995, 16, 41–56. [Google Scholar] [CrossRef]
- Lala, P.K.; Graham, C.H. Mechanisms of Trophoblast Invasiveness and Their Control: The Role of Proteases and Protease Inhibitors. Cancer Metastasis Rev. 1990, 9, 369–379. [Google Scholar] [CrossRef]
- Librach, C.L.; Werb, Z.; Fitzgerald, M.L.; Chiu, K.; Corwin, N.M.; Esteves, R.A.; Grobelny, D.; Galardy, R.; Damsky, C.H.; Fisher, S.J. 92-KD Type IV Collagenase Mediates Invasion of Human Cytotrophoblasts. J. Cell Biol. 1991, 113, 437–449. [Google Scholar] [CrossRef]
- Godbole, G.; Suman, P.; Malik, A.; Galvankar, M.; Joshi, N.; Fazleabas, A.; Gupta, S.K.; Modi, D. Decrease in Expression of HOXA10 in the Decidua After Embryo Implantation Promotes Trophoblast Invasion. Endocrinology 2017, 158, 2618–2633. [Google Scholar] [CrossRef]
- Dubinsky, V.; Poehlmann, T.G.; Suman, P.; Gentile, T.; Markert, U.R.; Gutierrez, G. Role of Regulatory and Angiogenic Cytokines in Invasion of Trophoblastic Cells. Am. J. Reprod. Immunol. 2010, 63, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, R.H.; Dunk, C.E.; Lye, S.J.; Aplin, J.D.; Harris, L.K.; Jones, R.L. Extravillous Trophoblast and Endothelial Cell Crosstalk Mediates Leukocyte Infiltration to the Early Remodeling Decidual Spiral Arteriole Wall. J. Immunol. 2017, 198, 4115–4128. [Google Scholar] [CrossRef] [PubMed]
- Nishino, E.; Matsuzaki, N.; Masuhiro, K.; Kameda, T.; Taniguchi, T.; Takagi, T.; Saji, F.; Tanizawa, O. Trophoblast-Derived Interleukin-6 (IL-6) Regulates Human Chorionic Gonadotropin Release through IL-6 Receptor on Human Trophoblasts. J. Clin. Endocrinol. Metab. 1990, 71, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Stephanou, A.; Handwerger, S. Interleukin-6 Stimulates Placental Lactogen Expression by Human Trophoblast Cells. Endocrinology 1994, 135, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Campo, P. Hormonal and Embryonic Regulation of Chemokines IL-8, MCP-1 and RANTES in the Human Endometrium during the Window of Implantation. Mol. Hum. Reprod. 2002, 8, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, F.; Galan, A.; Martin, J.J.L.; Remohi, J.; Pellicer, A.; Simón, C. Hormonal and Embryonic Regulation of Chemokine Receptors CXCR1, CXCR4, CCR5 and CCR2B in the Human Endometrium and the Human Blastocyst. Mol. Hum. Reprod. 2003, 9, 189–198. [Google Scholar] [CrossRef][Green Version]
- Sharma, S.; Godbole, G.; Modi, D. Decidual Control of Trophoblast Invasion. Am. J. Reprod. Immunol. 2016, 75, 341–350. [Google Scholar] [CrossRef]
- Huang, G.; Zhou, C.; Wei, C.-J.; Zhao, S.; Sun, F.; Zhou, H.; Xu, W.; Liu, J.; Yang, C.; Wu, L.; et al. Evaluation of In Vitro Fertilization Outcomes Using Interleukin-8 in Culture Medium of Human Preimplantation Embryos. Fertil. Steril. 2017, 107, 649–656. [Google Scholar] [CrossRef]
- Tsui, K.-H.; Chen, L.-Y.; Shieh, M.-L.; Chang, S.-P.; Yuan, C.-C.; Li, H.-Y. Interleukin-8 Can Stimulate Progesterone Secretion from a Human Trophoblast Cell Line, BeWo. In Vitro Cell. Dev. Biol. Anim. 2004, 40, 331–336. [Google Scholar] [CrossRef]
- Banerjee, P.; Malik, A.; Malhotra, S.S.; Gupta, S.K. Role of STAT Signaling and Autocrine Action of Chemokines during H2 O 2 Induced HTR-8/SVneo Trophoblastic Cells Invasion. J. Cell. Physiol. 2019, 234, 1380–1397. [Google Scholar] [CrossRef]
- Das, M.K.; Basak, S.; Ahmed, M.S.; Attramadal, H.; Duttaroy, A.K. Connective Tissue Growth Factor Induces Tube Formation and IL-8 Production in First Trimester Human Placental Trophoblast Cells. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 181, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, D.I.; Furaeva, K.N.; Stepanova, O.I.; Sel’kov, S.A. Proliferative and Migration Activity of JEG-3 Trophoblast Cell Line in the Presence of Cytokines. Bull. Exp. Biol. Med. 2015, 159, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Chim, S.S.C.; Wang, C.C.; Lau, C.S.L.; Leung, T.Y. Molecular Mechanism and Pathways of Normal Human Parturition in Different Gestational Tissues: A Systematic Review of Transcriptome Studies. Front. Physiol. 2021, 12, 730030. [Google Scholar] [CrossRef] [PubMed]
- Bollopragada, S.; Youssef, R.; Jordan, F.; Greer, I.; Norman, J.; Nelson, S. Term Labor Is Associated with a Core Inflammatory Response in Human Fetal Membranes, Myometrium, and Cervix. Am. J. Obstet. Gynecol. 2009, 200, 104.e1–104.e.11. [Google Scholar] [CrossRef] [PubMed]
- Kent, A.S.H.; Sullivan, M.H.F.; Sun, M.-Y.; Zosmer, A.; Elder, M.G. Effects of Interleukin-6 and Tumor Necrosis Factor-α on Prostaglandin Production by Cultured Human Fetal Membranes. Prostaglandins 1993, 46, 351–359. [Google Scholar] [CrossRef]
- Mitchell, M.D.; Dudley, D.J.; Edwin, S.S.; Schiller, S.L. Interleukin-6 Stimulates Prostaglandin Production by Human Amnion and Decidual Cells. Eur. J. Pharmacol. 1991, 192, 189–191. [Google Scholar] [CrossRef]
- Friebe-Hoffmann, U.; Chiao, J.P.; Rauk, P.N. Effect of IL-1beta and IL-6 on Oxytocin Secretion in Human Uterine Smooth Muscle Cells. Am. J. Reprod. Immunol. 2001, 46, 226–231. [Google Scholar] [CrossRef]
- Rauk, P.N.; Friebe-Hoffmann, U.; Winebrenner, L.D.; Chiao, J.P. Interleukin-6 up-Regulates the Oxytocin Receptor in Cultured Uterine Smooth Muscle Cells. Am. J. Reprod. Immunol. 2001, 45, 148–153. [Google Scholar] [CrossRef]
- Timmons, B.; Akins, M.; Mahendroo, M. Cervical Remodeling during Pregnancy and Parturition. Trends Endocrinol. Metab. 2010, 21, 353–361. [Google Scholar] [CrossRef]
- Strauss, J.F. Extracellular Matrix Dynamics and Fetal Membrane Rupture. Reprod. Sci. 2013, 20, 140–153. [Google Scholar] [CrossRef]
- Curry, A.E.; Vogel, I.; Skogstrand, K.; Drews, C.; Schendel, D.E.; Flanders, W.D.; Hougaard, D.M.; Thorsen, P. Maternal Plasma Cytokines in Early- and Mid-Gestation of Normal Human Pregnancy and Their Association with Maternal Factors. J. Reprod. Immunol. 2008, 77, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Blackmore, E.R.; Moynihan, J.A.; Rubinow, D.R.; Pressman, E.K.; Gilchrist, M.; O’Connor, T.G. Psychiatric Symptoms and Proinflammatory Cytokines in Pregnancy. Psychosom. Med. 2011, 73, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Björkander, S.; Bremme, K.; Persson, J.-O.; van Vollenhoven, R.F.; Sverremark-Ekström, E.; Holmlund, U. Pregnancy-Associated Inflammatory Markers Are Elevated in Pregnant Women with Systemic Lupus Erythematosus. Cytokine 2012, 59, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Tang, L.; Hu, M.; Xiang, Z.; Hu, Y. Changes of Serum Interleukin-6 in Healthy Pregnant Women and Establishment of Relevant Reference Intervals. Clin. Chim. Acta 2020, 502, 116–119. [Google Scholar] [CrossRef]
- Farah, N.; Hogan, A.E.; O’Connor, N.; Kennelly, M.M.; O’Shea, D.; Turner, M.J. Correlation between Maternal Inflammatory Markers and Fetomaternal Adiposity. Cytokine 2012, 60, 96–99. [Google Scholar] [CrossRef]
- Christian, L.M.; Porter, K. Longitudinal Changes in Serum Proinflammatory Markers across Pregnancy and Postpartum: Effects of Maternal Body Mass Index. Cytokine 2014, 70, 134–140. [Google Scholar] [CrossRef]
- Ross, K.M.; Miller, G.; Culhane, J.; Grobman, W.; Simhan, H.N.; Wadhwa, P.D.; Williamson, D.; McDade, T.; Buss, C.; Entringer, S.; et al. Patterns of Peripheral Cytokine Expression during Pregnancy in Two Cohorts and Associations with Inflammatory Markers in Cord Blood. Am. J. Reprod. Immunol. 2016, 76, 406–414. [Google Scholar] [CrossRef]
- Atta, D.S.; Girbash, E.F.; Abdelwahab, S.M.; Abdeldayem, H.M.; Tharwat, I.; Ghonaim, R. Maternal Cytokines and Disease Severity Influence Pregnancy Outcomes in Women with Rheumatoid Arthritis. J. Matern. Fetal. Neonatal Med. 2016, 29, 3358–3363. [Google Scholar] [CrossRef]
- Mitchell, A.M.; Porter, K.; Christian, L.M. Examination of the Role of Obesity in the Association between Childhood Trauma and Inflammation during Pregnancy. Health Psychol. 2018, 37, 114–124. [Google Scholar] [CrossRef]
- Doria, A.; Cutolo, M.; Ghirardello, A.; Zen, M.; Villalta, D.; Tincani, A.; Punzi, L.; Iaccarino, L.; Petri, M. Effect of Pregnancy on Serum Cytokines in SLE Patients. Arthritis Res. Ther. 2012, 14, R66. [Google Scholar] [CrossRef]
- Iaccarino, L.; Ghirardello, A.; Zen, M.; Villalta, D.; Tincani, A.; Punzi, L.; Doria, A. Polarization of TH2 Response Is Decreased during Pregnancy in Systemic Lupus Erythematosus. Reumatismo 2012, 64, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Jarmund, A.H.; Giskeødegård, G.F.; Ryssdal, M.; Steinkjer, B.; Stokkeland, L.M.T.; Madssen, T.S.; Stafne, S.N.; Stridsklev, S.; Moholdt, T.; Heimstad, R.; et al. Cytokine Patterns in Maternal Serum From First Trimester to Term and Beyond. Front. Immunol. 2021, 12, 752660. [Google Scholar] [CrossRef] [PubMed]
- Stokkeland, L.M.T.; Giskeødegård, G.F.; Stridsklev, S.; Ryan, L.; Steinkjer, B.; Tangerås, L.H.; Vanky, E.; Iversen, A.-C. Serum Cytokine Patterns in First Half of Pregnancy. Cytokine 2019, 119, 188–196. [Google Scholar] [CrossRef]
- Leimert, K.B.; Xu, W.; Princ, M.M.; Chemtob, S.; Olson, D.M. Inflammatory Amplification: A Central Tenet of Uterine Transition for Labor. Front. Cell. Infect. Microbiol. 2021, 11, 660983. [Google Scholar] [CrossRef] [PubMed]
- Talati, A.N.; Hackney, D.N.; Mesiano, S. Pathophysiology of Preterm Labor with Intact Membranes. Semin. Perinatol. 2017, 41, 420–426. [Google Scholar] [CrossRef]
- Keelan, J.A. Intrauterine Inflammatory Activation, Functional Progesterone Withdrawal, and the Timing of Term and Preterm Birth. J. Reprod. Immunol. 2018, 125, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Nadeau-Vallée, M.; Obari, D.; Palacios, J.; Brien, M.-È.; Duval, C.; Chemtob, S.; Girard, S. Sterile Inflammation and Pregnancy Complications: A Review. Reproduction 2016, 152, R277–R292. [Google Scholar] [CrossRef]
- Menon, R.; Bonney, E.A.; Condon, J.; Mesiano, S.; Taylor, R.N. Novel Concepts on Pregnancy Clocks and Alarms: Redundancy and Synergy in Human Parturition. Hum. Reprod. Update 2016, 22, 535–560. [Google Scholar] [CrossRef]
- Shynlova, O.; Nadeem, L.; Zhang, J.; Dunk, C.; Lye, S. Myometrial Activation: Novel Concepts Underlying Labor. Placenta 2020, 92, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Keelan, J.A.; Marvin, K.W.; Sato, T.A.; Coleman, M.; McCowan, L.M.E.; Mitchell, M.D. Cytokine Abundance in Placental Tissues: Evidence of Inflammatory Activation in Gestational Membranes with Term and Preterm Parturition. Am. J. Obstet. Gynecol. 1999, 181, 1530–1536. [Google Scholar] [CrossRef]
- Kemp, B.; Menon, R.; Fortunato, S.J.; Winkler, M.; Maul, H.; Rath, W. Quantitation and Localization of Inflammatory Cytokines Interleukin-6 and Interleukin-8 in the Lower Uterine Segment During Cervical Dilatation. J. Assist. Reprod. Genet. 2002, 19, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Osman, I.; Young, A.; Ledingham, M.A.; Thomson, A.J.; Jordan, F.; Greer, I.A.; Norman, J.E. Leukocyte Density and Pro-Inflammatory Cytokine Expression in Human Fetal Membranes, Decidua, Cervix and Myometrium before and during Labour at Term. Mol. Hum. Reprod. 2003, 9, 41–45. [Google Scholar] [CrossRef]
- Sennström, M.B.; Ekman, G.; Westergren-Thorsson, G.; Malmström, A.; Byström, B.; Endrésen, U.; Mlambo, N.; Norman, M.; Ståbi, B.; Brauner, A. Human Cervical Ripening, an Inflammatory Process Mediated by Cytokines. Mol. Hum. Reprod. 2000, 6, 375–381. [Google Scholar] [CrossRef]
- Singh, N.; Herbert, B.; Sooranna, G.; Shah, N.M.; Das, A.; Sooranna, S.R.; Johnson, M.R. Is There an Inflammatory Stimulus to Human Term Labour? PLoS ONE 2021, 16, e0256545. [Google Scholar] [CrossRef]
- Haddad, R.; Tromp, G.; Kuivaniemi, H.; Chaiworapongsa, T.; Kim, Y.M.; Mazor, M.; Romero, R. Human Spontaneous Labor without Histologic Chorioamnionitis Is Characterized by an Acute Inflammation Gene Expression Signature. Am. J. Obstet. Gynecol. 2006, 195, 394–405.e12. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Shim, S.H.; Kang, K.M.; Kang, J.H.; Park, D.Y.; Kim, S.H.; Farina, A.; Shim, S.S.; Cha, D.H. Global Gene Expression Changes Induced in the Human Placenta during Labor. Placenta 2010, 31, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Mittal, P.; Romero, R.; Tarca, A.L.; Gonzalez, J.; Draghici, S.; Xu, Y.; Dong, Z.; Nhan-Chang, C.-L.; Chaiworapongsa, T.; Lye, S.; et al. Characterization of the Myometrial Transcriptome and Biological Pathways of Spontaneous Human Labor at Term. J. Perinat. Med. 2010, 38, 617–643. [Google Scholar] [CrossRef]
- Rinaldi, S.F.; Makieva, S.; Saunders, P.T.; Rossi, A.G.; Norman, J.E. Immune Cell and Transcriptomic Analysis of the Human Decidua in Term and Preterm Parturition. MHR Basic Sci. Reprod. Med. 2017, 23, 708–724. [Google Scholar] [CrossRef]
- Stephen, G.L.; Lui, S.; Hamilton, S.A.; Tower, C.L.; Harris, L.K.; Stevens, A.; Jones, R.L. Transcriptomic Profiling of Human Choriodecidua During Term Labor: Inflammation as a Key Driver of Labor. Am. J. Reprod. Immunol. 2015, 73, 36–55. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; MacIntyre, D.A.; Firmino Da Silva, M.; Blanks, A.M.; Lee, Y.S.; Thornton, S.; Bennett, P.R.; Terzidou, V. Oxytocin Activates NF-ΚB-Mediated Inflammatory Pathways in Human Gestational Tissues. Mol. Cell. Endocrinol. 2015, 403, 64–77. [Google Scholar] [CrossRef]
- Adams Waldorf, K.M.; Singh, N.; Mohan, A.R.; Young, R.C.; Ngo, L.; Das, A.; Tsai, J.; Bansal, A.; Paolella, L.; Herbert, B.R.; et al. Uterine Overdistention Induces Preterm Labor Mediated by Inflammation: Observations in Pregnant Women and Nonhuman Primates. Am. J. Obstet. Gynecol. 2015, 213, 830.e1–830.e19. [Google Scholar] [CrossRef] [PubMed]
- Dajani, N.; Idriss, E.; Collins, P.L. Interleukin-6 Does Not Stimulate Rat Myometrial Contractions in an In Vitro Model. Am. J. Reprod. Immunol. 1994, 32, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K.; Hirsch, E. Interleukin-6 Is Neither Necessary Nor Sufficient for Preterm Labor in a Murine Infection Model. J. Soc. Gynecol. Investig. 2003, 10, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Osmers, R. Interleukin-8 Synthesis and the Onset of Labor. Obstet. Gynecol. 1995, 86, 223–229. [Google Scholar] [CrossRef]
- Winkler, M.; Fischer, D.C.; Hlubek, M.; van de Leur, E.; Haubeck, H.D.; Rath, W. Interleukin-1beta and Interleukin-8 Concentrations in the Lower Uterine Segment during Parturition at Term. Obstet. Gynecol. 1998, 91, 945–949. [Google Scholar] [CrossRef]
- el Maradny, E.; Kanayama, N.; Maehara, K.; Kobayashi, T.; Terao, T. Expression of Interleukin-8 Receptors in the Gestational Tissues before and after Initiation of Labor: Immunohistochemical Study. Acta Obstet. Gynecol. Scand. 1996, 75, 790–796. [Google Scholar] [CrossRef]
- Hamilton, S.A.; Tower, C.L.; Jones, R.L. Identification of Chemokines Associated with the Recruitment of Decidual Leukocytes in Human Labour: Potential Novel Targets for Preterm Labour. PLoS ONE 2013, 8, e56946. [Google Scholar] [CrossRef]
- Willems, J.; Joniau, M.; Cinque, S.; van Damme, J. Human Granulocyte Chemotactic Peptide (IL-8) as a Specific Neutrophil Degranulator: Comparison with Other Monokines. Immunology 1989, 67, 540–542. [Google Scholar]
- Pugin, J.; Widmer, M.C.; Kossodo, S.; Liang, C.M.; Preas, H.L., 2nd; Suffredini, A.F. Human Neutrophils Secrete Gelatinase B in Vitro and in Vivo in Response to Endotoxin and Proinflammatory Mediators. Am. J. Respir. Cell Mol. Biol. 1999, 20, 458–464. [Google Scholar] [CrossRef]
- Winkler, M.; Fischer, D.C.; Ruck, P.; Marx, T.; Kaiserling, E.; Oberpichler, A.; Tschesche, H.; Rath, W. Parturition at Term: Parallel Increases in Interleukin-8 and Proteinase Concentrations and Neutrophil Count in the Lower Uterine Segment. Hum. Reprod. 1999, 14, 1096–1100. [Google Scholar] [CrossRef]
- Ehsani, V.; Mortazavi, M.; Ghorban, K.; Dadmanesh, M.; Bahramabadi, R.; Rezayati, M.-T.; Javadi-Moghadam, E.; Rezaei, Z.; Sabzali, Z.; Fatemi, I.; et al. Role of Maternal Interleukin-8 (IL-8) in Normal-Term Birth in the Human. Reprod. Fertil. Dev. 2019, 31, 1049. [Google Scholar] [CrossRef] [PubMed]
- Shahshahan, Z.; Hashemi, L.; Rasouli, O. Maternal Serum Interleukin 6 and 8 and C-Reactive Protein in Predicting the Tocolytic Therapy in Preterm Labor. J. Res. Med. Sci. 2014, 19, 537–541. [Google Scholar] [PubMed]
- Rinaldi, S.F.; Hutchinson, J.L.; Rossi, A.G.; Norman, J.E. Anti-Inflammatory Mediators as Physiological and Pharmacological Regulators of Parturition. Expert Rev. Clin. Immunol. 2011, 7, 675–696. [Google Scholar] [CrossRef] [PubMed]
- Spence, T.; Allsopp, P.J.; Yeates, A.J.; Mulhern, M.S.; Strain, J.J.; McSorley, E.M. Maternal Serum Cytokine Concentrations in Healthy Pregnancy and Preeclampsia. J. Pregnancy 2021, 2021. [Google Scholar] [CrossRef]
- Enninga, E.A.L.; Nevala, W.K.; Creedon, D.J.; Markovic, S.N.; Holtan, S.G. Fetal Sex-Based Differences in Maternal Hormones, Angiogenic Factors, and Immune Mediators during Pregnancy and the Postpartum Period. Am. J. Reprod. Immunol. 2015, 73, 251–262. [Google Scholar] [CrossRef]
- Mitchell, A.M.; Palettas, M.; Christian, L.M. Fetal Sex Is Associated with Maternal Stimulated Cytokine Production, but Not Serum Cytokine Levels, in Human Pregnancy. Brain. Behav. Immun. 2017, 60, 32–37. [Google Scholar] [CrossRef]
- Ramiro-Cortijo, D.; de la Calle, M.; Böger, R.; Hannemann, J.; Lüneburg, N.; López-Giménez, M.R.; Rodríguez-Rodríguez, P.; Martín-Cabrejas, M.Á.; Benítez, V.; de Pablo, Á.L.L.; et al. Male Fetal Sex Is Associated with Low Maternal Plasma Anti-Inflammatory Cytokine Profile in the First Trimester of Healthy Pregnancies. Cytokine 2020, 136, 155290. [Google Scholar] [CrossRef] [PubMed]
- Burns, C.; Hall, S.T.; Smith, R.; Blackwell, C. Cytokine Levels in Late Pregnancy: Are Female Infants Better Protected Against Inflammation? Front. Immunol. 2015, 6, 318. [Google Scholar] [CrossRef]
- Straczkowski, M.; Dzienis-Straczkowska, S.; Stêpieñ, A.; Kowalska, I.; Szelachowska, M.; Kinalska, I. Plasma Interleukin-8 Concentrations Are Increased in Obese Subjects and Related to Fat Mass and Tumor Necrosis Factor-Alpha System. J. Clin. Endocrinol. Metab. 2002, 87, 4602–4606. [Google Scholar] [CrossRef]
- Yu, H.; Liu, Z.; Dong, S. Changes in Intestinal Flora, TNF-α, L-17, and IL-6 Levels in Patients with Gestational Diabetes Mellitus. Eur. J. Inflamm. 2018, 16, 205873921879355. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, J.; Shen, L.; Wang, A.; Wang, R. Correlation between Inflammatory Markers (Hs-CRP, TNF-α, IL-1β, IL-6, IL-18), Glucose Intolerance, and Gestational Diabetes Mellitus in Pregnant Women. Int. J. Clin. Exp. Med. 2018, 11, 8310–8316. [Google Scholar]
- Zhang, J.; Chi, H.; Xiao, H.; Tian, X.; Wang, Y.; Yun, X.; Xu, Y. Interleukin 6 (IL-6) and Tumor Necrosis Factor α (TNF-α) Single Nucleotide Polymorphisms (SNPs), Inflammation and Metabolism in Gestational Diabetes Mellitus in Inner Mongolia. Med. Sci. Monit. 2017, 23, 4149–4157. [Google Scholar] [CrossRef] [PubMed]
- Quenby, S.; Gallos, I.D.; Dhillon-Smith, R.K.; Podesek, M.; Stephenson, M.D.; Fisher, J.; Brosens, J.J.; Brewin, J.; Ramhorst, R.; Lucas, E.S.; et al. Miscarriage Matters: The Epidemiological, Physical, Psychological, and Economic Costs of Early Pregnancy Loss. Lancet 2021, 397, 1658–1667. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, C.; Wang, L.; Chen, D.; Guang, W.; French, J. Conception, Early Pregnancy Loss, and Time to Clinical Pregnancy: A Population-Based Prospective Study. Fertil. Steril. 2003, 79, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, A.J.; Weinberg, C.R.; O’Connor, J.F.; Baird, D.D.; Schlatterer, J.P.; Canfield, R.E.; Armstrong, E.G.; Nisula, B.C. Incidence of Early Loss of Pregnancy. N. Engl. J. Med. 1988, 319, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Regan, L. Recurrent Miscarriage. Lancet 2006, 368, 601–611. [Google Scholar] [CrossRef]
- The Practice Committee of the American Society for Reproductive Medicine. Evaluation and Treatment of Recurrent Pregnancy Loss: A Committee Opinion. Fertil. Steril. 2012, 98, 1103–1111. [Google Scholar] [CrossRef]
- Krieg, S.A.; Fan, X.; Hong, Y.; Sang, Q.-X.; Giaccia, A.; Westphal, L.M.; Lathi, R.B.; Krieg, A.J.; Nayak, N.R. Global Alteration in Gene Expression Profiles of Deciduas from Women with Idiopathic Recurrent Pregnancy Loss. MHR Basic Sci. Reprod. Med. 2012, 18, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Zhang, N.; Lin, J.; Wang, C.; Pan, X.; Chen, L.; Li, D.; Wang, L. Distinct Pattern of Th17/Treg Cells in Pregnant Women with a History of Unexplained Recurrent Spontaneous Abortion. Biosci. Trends 2018, 12, 157–167. [Google Scholar] [CrossRef]
- Zhao, L.; Han, L.; Hei, G.; Wei, R.; Zhang, Z.; Zhu, X.; Guo, Q.; Chu, C.; Fu, X.; Xu, K.; et al. Diminished MiR-374c-5p Negatively Regulates IL (Interleukin)-6 in Unexplained Recurrent Spontaneous Abortion. J. Mol. Med. 2022, 100, 1043–1056. [Google Scholar] [CrossRef]
- Chen, P.; Zhou, L.; Chen, J.; Lu, Y.; Cao, C.; Lv, S.; Wei, Z.; Wang, L.; Chen, J.; Hu, X.; et al. The Immune Atlas of Human Deciduas With Unexplained Recurrent Pregnancy Loss. Front. Immunol. 2021, 12, 689019. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Jia, W.; Fan, M.; Shao, X.; Li, Z.; Liu, Y.; Ma, Y.; Li, Y.-X.; Li, R.; Tu, Q.; et al. Single-Cell Immune Landscape of Human Recurrent Miscarriage. Genom. Proteom. Bioinform. 2021, 19, 208–222. [Google Scholar] [CrossRef] [PubMed]
- Madhappan, B.; Kempuraj, D.; Christodoulou, S.; Tsapikidis, S.; Boucher, W.; Karagiannis, V.; Athanassiou, A.; Theoharides, T.C. High Levels of Intrauterine Corticotropin-Releasing Hormone, Urocortin, Tryptase, and Interleukin-8 in Spontaneous Abortions. Endocrinology 2003, 144, 2285–2290. [Google Scholar] [CrossRef] [PubMed]
- Laisk, T.; Soares, A.L.G.; Ferreira, T.; Painter, J.N.; Censin, J.C.; Laber, S.; Bacelis, J.; Chen, C.-Y.; Lepamets, M.; Lin, K.; et al. The Genetic Architecture of Sporadic and Multiple Consecutive Miscarriage. Nat. Commun. 2020, 11, 5980. [Google Scholar] [CrossRef] [PubMed]
- Calleja-Agius, J.; Jauniaux, E.; Pizzey, A.R.; Muttukrishna, S. Investigation of Systemic Inflammatory Response in First Trimester Pregnancy Failure. Hum. Reprod. 2012, 27, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Drozdzik, M.; Szlarb, N.; Kurzawski, M. Interleukin-6 Level and Gene Polymorphism in Spontaneous Miscarriage. Tissue Antigens 2013, 82, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Thaker, R.; Oza, H.; Verma, V.; Gor, M.; Kumar, S. The Association of Circulatory Cytokines (IL-6 and IL-10) Level with Spontaneous Abortion—A Preliminary Observation. Reprod. Sci. 2021, 28, 857–864. [Google Scholar] [CrossRef]
- Hattori, Y.; Nakanishi, T.; Ozaki, Y.; Nozawa, K.; Sato, T.; Sugiura-Ogasawara, M. Uterine Cervical Inflammatory Cytokines, Interleukin-6 and -8, as Predictors of Miscarriage in Recurrent Cases. Am. J. Reprod. Immunol. 2007, 58, 350–357. [Google Scholar] [CrossRef]
- Luo, M.; Xiao, H.; Wang, L.; Zhao, J.; Gao, J.; Ma, W. The Expression and Clinical Significance of Three LncRNAs in Patients with a Missed Abortion. Exp. Ther. Med. 2020, 21, 8. [Google Scholar] [CrossRef]
- AlJameil, N.; Tabassum, H.; AlMayouf, H.; Alshenefy, A.; Almohizea, M.M.; Ali, M.N. Identification of Serum Cytokines as Markers in Women with Recurrent Pregnancy Loss or Miscarriage Using MILLIPLEX Analysis. Biomed. Res. 2018, 29, 3512–3517. [Google Scholar] [CrossRef]
- Koumantaki, Y.; Matalliotakis, I.; Sifakis, S.; Kyriakou, D.; Neonaki, M.; Goymenou, A.; Koumantakis, E. Detection of Interleukin-6, Interleukin-8, and Interleukin-11 in Plasma from Women with Spontaneous Abortion. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 98, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Kwiatek, M.; Gęca, T.; Kwaśniewska, A. Pro- and Anti-Inflammatory Cytokines in the First Trimester—Comparison of Missed Miscarriage and Normal Pregnancy. Int. J. Environ. Res. Public Health 2021, 18, 8538. [Google Scholar] [CrossRef]
- Tyagi, P.; Alharthi, N. Evaluation of Pro-Inflammatory Cytokine Level in Cases of Idiopathic Recurrent Spontaneous Miscarriage in Saudi Arabia. Biomed. Biotechnol. Res. J. 2020, 4, 225. [Google Scholar] [CrossRef]
- Ozkan, Z.S.; Devecı, D.; Sımsek, M.; Ilhan, F.; Rısvanlı, A.; Sapmaz, E. What Is the Impact of SOCS3, IL-35 and IL17 in Immune Pathogenesis of Recurrent Pregnancy Loss? J. Matern. Neonatal Med. 2015, 28, 324–328. [Google Scholar] [CrossRef]
- Arruvito, L.; Billordo, A.; Capucchio, M.; Prada, M.E.; Fainboim, L. IL-6 Trans-Signaling and the Frequency of CD4+FOXP3+ Cells in Women with Reproductive Failure. J. Reprod. Immunol. 2009, 82, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.; Waetzig, G.H.; Chalaris, A.; Reinheimer, T.M.; Wege, H.; Rose-John, S.; Garbers, C. Different Soluble Forms of the Interleukin-6 Family Signal Transducer Gp130 Fine-Tune the Blockade of Interleukin-6 Trans-Signaling. J. Biol. Chem. 2016, 291, 16186–16196. [Google Scholar] [CrossRef]
- Saifi, B.; Rezaee, S.A.; Tajik, N.; Ahmadpour, M.E.; Ashrafi, M.; Vakili, R.; SoleimaniAsl, S.; Aflatoonian, R.; Mehdizadeh, M. Th17 Cells and Related Cytokines in Unexplained Recurrent Spontaneous Miscarriage at the Implantation Window. Reprod. Biomed. Online 2014, 29, 481–489. [Google Scholar] [CrossRef]
- Ota, K.; Yamagishi, S.; Kim, M.; Dambaeva, S.; Gilman-Sachs, A.; Beaman, K.; Kwak-Kim, J. Elevation of Soluble Form of Receptor for Advanced Glycation End Products (SRAGE) in Recurrent Pregnancy Losses (RPL): Possible Participation of RAGE in RPL. Fertil. Steril. 2014, 102, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Li, L.; Du, M.; Xu, H.; Gao, M.; Liu, X.; Wei, X.; Zhong, X. Key Gene and Functional Pathways Identified in Unexplained Recurrent Spontaneous Abortion Using Targeted RNA Sequencing and Clinical Analysis. Front. Immunol. 2021, 12, 717832. [Google Scholar] [CrossRef]
- Jasper, M.J.; Tremellen, K.P.; Robertson, S.A. Reduced Expression of IL-6 and IL-1α MRNAs in Secretory Phase Endometrium of Women with Recurrent Miscarriage. J. Reprod. Immunol. 2007, 73, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.J.; Odukoya, O.A.; Ajjan, R.A.; Li, T.-C.; Weetman, A.P.; Cooke, I.D. The Role of T-Helper Cytokines in Human Reproduction. Fertil. Steril. 2000, 73, 136–142. [Google Scholar] [CrossRef] [PubMed]
- von Wolff, M. Regulated Expression of Cytokines in Human Endometrium throughout the Menstrual Cycle: Dysregulation in Habitual Abortion. Mol. Hum. Reprod. 2000, 6, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Jana, S.K.; Pasricha, P.; Ghosh, S.; Chakravarty, B.; Chaudhury, K. Proinflammatory Cytokines Induced Altered Expression of Cyclooxygenase-2 Gene Results in Unreceptive Endometrium in Women with Idiopathic Recurrent Spontaneous Miscarriage. Fertil. Steril. 2013, 99, 179–187.e2. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, C.J.; Yen, C.-F.; Basar, M.; Kayisli, U.A.; Martel, M.; Buhimschi, I.; Buhimschi, C.; Huang, S.J.; Krikun, G.; Schatz, F. Preeclampsia-Related Inflammatory Cytokines Regulate Interleukin-6 Expression in Human Decidual Cells. Am. J. Pathol. 2008, 172, 1571–1579. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, F.C.B.; Felisberto, F.; Vuolo, F.; Petronilho, F.; Souza, D.R.; Luciano, T.F.; de Souza, C.T.; Ritter, C.; Dal-Pizzol, F. Oxidative Damage, Inflammation, and Toll-like Receptor 4 Pathway Are Increased in Preeclamptic Patients: A Case-Control Study. Oxid. Med. Cell. Longev. 2012, 2012, 636419. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Muralimanoharan, S.; Maloyan, A.; Myatt, L. Evidence of Sexual Dimorphism in the Placental Function with Severe Preeclampsia. Placenta 2013, 34, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Jain, A.K.; Mittal, P.; Kohli, M.; Jawanjal, P.; Rath, G. Association of Pro- and Anti-Inflammatory Cytokines in Preeclampsia. J. Clin. Lab. Anal. 2019, 33, e22834. [Google Scholar] [CrossRef]
- Ma, Y.; Ye, Y.; Zhang, J.; Ruan, C.-C.; Gao, P.-J. Immune Imbalance Is Associated with the Development of Preeclampsia. Medicine 2019, 98, e15080. [Google Scholar] [CrossRef]
- Pang, Z.-J.; Xing, F.-Q. Comparative Study on the Expression of Cytokine—Receptor Genes in Normal and Preeclamptic Human Placentas Using DNA Microarrays. J. Perinat. Med. 2003, 31, 153–162. [Google Scholar] [CrossRef]
- Sun, L.; Mao, D.; Cai, Y.; Tan, W.; Hao, Y.; Li, L.; Liu, W. Association between Higher Expression of Interleukin-8 (IL-8) and Haplotype −353A/−251A/+678T of IL-8 Gene with Preeclampsia. Medicine 2016, 95, e5537. [Google Scholar] [CrossRef]
- Casart, Y.C.; Tarrazzi, K.; Camejo, M.I. Serum Levels of Interleukin-6, Interleukin-1β and Human Chorionic Gonadotropin in Pre-Eclamptic and Normal Pregnancy. Gynecol. Endocrinol. 2007, 23, 300–303. [Google Scholar] [CrossRef]
- Lau, S.Y.; Guild, S.-J.; Barrett, C.J.; Chen, Q.; McCowan, L.; Jordan, V.; Chamley, L.W. Tumor Necrosis Factor-Alpha, Interleukin-6, and Interleukin-10 Levels Are Altered in Preeclampsia: A Systematic Review and Meta-Analysis. Am. J. Reprod. Immunol. 2013, 70, 412–427. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, M.B.; Martins-Filho, O.A.; Mota, A.P.L.; Alpoim, P.N.; Godoi, L.C.; Silveira, A.C.O.; Teixeira-Carvalho, A.; Gomes, K.B.; Dusse, L.M. Severe Preeclampsia Goes along with a Cytokine Network Disturbance towards a Systemic Inflammatory State. Cytokine 2013, 62, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Krasnyi, A.M.; Gracheva, M.I.; Sadekova, A.A.; Vtorushina, V.V.; Balashov, I.S.; Kan, N.E.; Borovikov, P.I.; Krechetova, L.V.; Tyutyunnik, V.L. Complex Analysis of Total and Fetal DNA and Cytokines in Blood Plasma of Pregnant Women with Preeclampsia. Bull. Exp. Biol. Med. 2018, 164, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Negi, R.; Haritha, V.; Aziz, N.; Siddiqui, A.H. Biochemical Markers in the Pathogenesis of Preeclampsia: Novel Link between Placental Growth Factor and Interleukin-6. Mol. Cell. Biochem. 2022, 477, 1765–1774. [Google Scholar] [CrossRef] [PubMed]
- Kauma, S.; Takacs, P.; Scordalakes, C.; Walsh, S.; Green, K.; Peng, T. Increased Endothelial Monocyte Chemoattractant Protein-1 and Interleukin-8 in Preeclampsia. Obstet. Gynecol. 2002, 100, 706–714. [Google Scholar] [CrossRef]
- Molvarec, A.; Szarka, A.; Walentin, S.; Bekő, G.; Karádi, I.; Prohászka, Z.; Rigó, J. Serum Leptin Levels in Relation to Circulating Cytokines, Chemokines, Adhesion Molecules and Angiogenic Factors in Normal Pregnancy and Preeclampsia. Reprod. Biol. Endocrinol. 2011, 9, 124. [Google Scholar] [CrossRef]
- Moreno-Eutimio, M.A.; Tovar-Rodríguez, J.M.; Vargas-Avila, K.; Nieto-Velázquez, N.G.; Frías-De-León, M.G.; Sierra-Martinez, M.; Acosta-Altamirano, G. Increased Serum Levels of Inflammatory Mediators and Low Frequency of Regulatory T Cells in the Peripheral Blood of Preeclamptic Mexican Women. Biomed Res. Int. 2014, 2014, 413249. [Google Scholar] [CrossRef] [PubMed]
- Sahin, H.; Gunel, T.; Benian, A.; Onay Ucar, E.; Guralp, O.; Kilic, A. Genomic and Proteomic Investigation of Preeclampsia. Exp. Ther. Med. 2015, 10, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhou, Y.; Gui, J.; Li, A.-Z.; Su, X.-L.; Feng, L. Assessment of the Number and Function of Macrophages in the Placenta of Gestational Diabetes Mellitus Patients. J. Huazhong Univ. Sci. Technol. Med. Sci. 2013, 33, 725–729. [Google Scholar] [CrossRef]
- Bari, M.F.; Weickert, M.O.; Sivakumar, K.; James, S.G.; Snead, D.R.J.; Tan, B.K.; Randeva, H.S.; Bastie, C.C.; Vatish, M. Elevated Soluble CD163 in Gestational Diabetes Mellitus: Secretion from Human Placenta and Adipose Tissue. PLoS ONE 2014, 9, e101327. [Google Scholar] [CrossRef] [PubMed]
- Stirm, L.; Kovářová, M.; Perschbacher, S.; Michlmaier, R.; Fritsche, L.; Siegel-Axel, D.; Schleicher, E.; Peter, A.; Pauluschke-Fröhlich, J.; Brucker, S.; et al. BMI-Independent Effects of Gestational Diabetes on Human Placenta. J. Clin. Endocrinol. Metab. 2018, 103, 3299–3309. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, M.; Lizotte, F.; Hivert, M.-F.; Geraldes, P.; Perron, P. Calcifediol Decreases Interleukin-6 Secretion by Cultured Human Trophoblasts From GDM Pregnancies. J. Endocr. Soc. 2019, 3, 2165–2178. [Google Scholar] [CrossRef] [PubMed]
- Hara, C.d.C.P.; França, E.L.; Fagundes, D.L.G.; de Queiroz, A.A.; Rudge, M.V.C.; Honorio-França, A.C.; Calderon, I.d.M.P. Characterization of Natural Killer Cells and Cytokines in Maternal Placenta and Fetus of Diabetic Mothers. J. Immunol. Res. 2016, 2016, 7154524. [Google Scholar] [CrossRef]
- Mrizak, I.; Grissa, O.; Henault, B.; Fekih, M.; Bouslema, A.; Boumaiza, I.; Zaouali, M.; Tabka, Z.; Khan, N.A. Placental Infiltration of Inflammatory Markers in Gestational Diabetic Women. Gen. Physiol. Biophys. 2014, 33, 169–176. [Google Scholar] [CrossRef]
- Kleiblova, P.; Dostalova, I.; Bartlova, M.; Lacinova, Z.; Ticha, I.; Krejci, V.; Springer, D.; Kleibl, Z.; Haluzik, M. Expression of Adipokines and Estrogen Receptors in Adipose Tissue and Placenta of Patients with Gestational Diabetes Mellitus. Mol. Cell. Endocrinol. 2010, 314, 150–156. [Google Scholar] [CrossRef]
- Lappas, M.; Permezel, M.; Rice, G.E. Release of Proinflammatory Cytokines and 8-Isoprostane from Placenta, Adipose Tissue, and Skeletal Muscle from Normal Pregnant Women and Women with Gestational Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2004, 89, 5627–5633. [Google Scholar] [CrossRef]
- Keckstein, S.; Pritz, S.; Amann, N.; Meister, S.; Beyer, S.; Jegen, M.; Kuhn, C.; Hutter, S.; Knabl, J.; Mahner, S.; et al. Sex Specific Expression of Interleukin 7, 8 and 15 in Placentas of Women with Gestational Diabetes. Int. J. Mol. Sci. 2020, 21, 8026. [Google Scholar] [CrossRef]
- Kuzmicki, M.; Telejko, B.; Szamatowicz, J.; Zonenberg, A.; Nikolajuk, A.; Kretowski, A.; Gorska, M. High Resistin and Interleukin-6 Levels Are Associated with Gestational Diabetes Mellitus. Gynecol. Endocrinol. 2009, 25, 258–263. [Google Scholar] [CrossRef]
- Hassiakos, D.; Eleftheriades, M.; Papastefanou, I.; Lambrinoudaki, I.; Kappou, D.; Lavranos, D.; Akalestos, A.; Aravantinos, L.; Pervanidou, P.; Chrousos, G. Increased Maternal Serum Interleukin-6 Concentrations at 11 to 14 Weeks of Gestation in Low Risk Pregnancies Complicated with Gestational Diabetes Mellitus: Development of a Prediction Model. Horm. Metab. Res. 2016, 48, 35–41. [Google Scholar] [CrossRef]
- Morisset, A.-S.; Dubé, M.-C.; Côté, J.A.; Robitaille, J.; Weisnagel, S.J.; Tchernof, A. Circulating Interleukin-6 Concentrations during and after Gestational Diabetes Mellitus. Acta Obstet. Gynecol. Scand. 2011, 90, 524–530. [Google Scholar] [CrossRef]
- Siddiqui, S.; Waghdhare, S.; Goel, C.; Panda, M.; Soneja, H.; Sundar, J.; Banerjee, M.; Jha, S.; Dubey, S. Augmentation of IL-6 Production Contributes to Development of Gestational Diabetes Mellitus: An Indian Study. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Sudharshana Murthy, K.; Bhandiwada, A.; Chandan, S.; Gowda, S.; Sindhusree, G. Evaluation of Oxidative Stress and Proinflammatory Cytokines in Gestational Diabetes Mellitus and Their Correlation with Pregnancy Outcome. Indian J. Endocrinol. Metab. 2018, 22, 79. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, L.; Liu, B.; Li, Q.; Wang, Z.; Fan, S.; Wang, H.; Wang, L. Functional Defects of Regulatory T Cell Through Interleukin 10 Mediated Mechanism in the Induction of Gestational Diabetes Mellitus. DNA Cell Biol. 2018, 37, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Winzer, C.; Wagner, O.; Festa, A.; Schneider, B.; Roden, M.; Bancher-Todesca, D.; Pacini, G.; Funahashi, T.; Kautzky-Willer, A. Plasma Adiponectin, Insulin Sensitivity, and Subclinical Inflammation in Women with Prior Gestational Diabetes Mellitus. Diabetes Care 2004, 27, 1721–1727. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-X.; Long, D.-L.; Liu, J.; Qiu, D.; Wang, J.; Cheng, X.; Yang, X.; Li, R.-M.; Wang, G. Gestational Diabetes Mellitus in Women Increased the Risk of Neonatal Infection via Inflammation and Autophagy in the Placenta. Medicine 2020, 99, e22152. [Google Scholar] [CrossRef]
- Gümüş, P.; Özçaka, Ö.; Ceyhan-Öztürk, B.; Akcali, A.; Lappin, D.F.; Buduneli, N. Evaluation of Biochemical Parameters and Local and Systemic Levels of Osteoactive and B-Cell Stimulatory Factors in Gestational Diabetes in the Presence or Absence of Gingivitis. J. Periodontol. 2015, 86, 387–397. [Google Scholar] [CrossRef]
- Abell, S.K.; Shorakae, S.; Harrison, C.L.; Hiam, D.; Moreno-Asso, A.; Stepto, N.K.; De Courten, B.; Teede, H.J. The Association between Dysregulated Adipocytokines in Early Pregnancy and Development of Gestational Diabetes. Diabetes. Metab. Res. Rev. 2017, 33, e2926. [Google Scholar] [CrossRef]
- Braga, F.O.; Negrato, C.A.; Matta, M. de F.B. da; Carneiro, J.R.I.; Gomes, M.B. Relationship between Inflammatory Markers, Glycated Hemoglobin and Placental Weight on Fetal Outcomes in Women with Gestational Diabetes. Arch. Endocrinol. Metab. 2019, 63, 22–29. [Google Scholar] [CrossRef]
- Özyer, Ş.; Engin-Üstün, Y.; Uzunlar, Ö.; Katar, C.; Danışman, N. Inflammation and Glycemic Tolerance Status in Pregnancy: The Role of Maternal Adiposity. Gynecol. Obstet. Investig. 2014, 78, 53–58. [Google Scholar] [CrossRef]
- Tang, M.; Luo, M.; Lu, W.; Zhang, R.; Liang, W.; Gu, J.; Yu, X.; Zhang, X.; Hu, C. Nerve Growth Factor Is Closely Related to Glucose Metabolism, Insulin Sensitivity and Insulin Secretion in the Second Trimester: A Case-Control Study in Chinese. Nutr. Metab. 2020, 17, 98. [Google Scholar] [CrossRef]
- Tagoma, A.; Haller-Kikkatalo, K.; Oras, A.; Roos, K.; Kirss, A.; Uibo, R. Plasma Cytokines during Pregnancy Provide Insight into the Risk of Diabetes in the Gestational Diabetes Risk Group. J. Diabetes Investig. 2022, 13, 1596–1606. [Google Scholar] [CrossRef]
- Benyo, D.F.; Miles, T.M.; Conrad, K.P. Hypoxia Stimulates Cytokine Production by Villous Explants from the Human Placenta. J. Clin. Endocrinol. Metab. 1997, 82, 1582–1588. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, S.J.; Menon, R.P.; Swan, K.F.; Menon, R. Inflammatory Cytokine (Interleukins 1, 6, and 8 and Tumor Necrosis Factor-α) Release from Cultured Human Fetal Membranes in Response to Endotoxic Lipopolysaccharide Mirrors Amniotic Fluid Concentrations. Am. J. Obstet. Gynecol. 1996, 174, 1855–1862. [Google Scholar] [CrossRef]
- Anton, L.; Brown, A.G.; Parry, S.; Elovitz, M.A. Lipopolysaccharide Induces Cytokine Production and Decreases Extravillous Trophoblast Invasion through a Mitogen-Activated Protein Kinase-Mediated Pathway: Possible Mechanisms of First Trimester Placental Dysfunction. Hum. Reprod. 2012, 27, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, E.Y.; Patterson, P.H. Activation of the Maternal Immune System Induces Endocrine Changes in the Placenta via IL-6. Brain. Behav. Immun. 2011, 25, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic-Krivokuca, M.; Stefanoska, I.; Rabi, A.; Vilotic, A.; Petronijevic, M.; Vrzic-Petronijevic, S.; Radojcic, L.; Vicovac, L. MIF Is among the Proinflammatory Cytokines Increased by LPS in the Human Trophoblast Line. Arch. Biol. Sci. 2016, 68, 715–722. [Google Scholar] [CrossRef]
- Riewe, S.D.; Mans, J.J.; Hirano, T.; Katz, J.; Shiverick, K.T.; Brown, T.A.; Lamont, R.J. Human Trophoblast Responses to Porphyromonas Gingivalis Infection. Mol. Oral Microbiol. 2010, 25, 252–259. [Google Scholar] [CrossRef][Green Version]
- Romero, R.; Miranda, J.; Kusanovic, J.P.; Chaiworapongsa, T.; Chaemsaithong, P.; Martinez, A.; Gotsch, F.; Dong, Z.; Ahmed, A.I.; Shaman, M.; et al. Clinical Chorioamnionitis at Term I: Microbiology of the Amniotic Cavity Using Cultivation and Molecular Techniques. J. Perinat. Med. 2015, 43, 19–36. [Google Scholar] [CrossRef]
- Cherouny, P.H.; Pankuch, G.A.; Romero, R.; Botti, J.J.; Kuhn, D.C.; Demers, L.M.; Appelbaum, P.C. Neutrophil Attractant/Activating Peptide-1/Interleukin-8: Association with Histologic Chorioamnionitis, Preterm Delivery, and Bioactive Amniotic Fluid Leukoattractants. Am. J. Obstet. Gynecol. 1993, 169, 1299–1303. [Google Scholar] [CrossRef]
- Holst, R.-M.; Mattsby-Baltzer, I.; Wennerholm, U.-B.; Hagberg, H.; Jacobsson, B. Interleukin-6 and Interleukin-8 in Cervical Fluid in a Population of Swedish Women in Preterm Labor: Relationship to Microbial Invasion of the Amniotic Fluid, Intra-Amniotic Inflammation, and Preterm Delivery. Acta Obstet. Gynecol. Scand. 2005, 84, 551–557. [Google Scholar] [CrossRef]
- Gomez-Lopez, N.; Hernandez-Santiago, S.; Lobb, A.P.; Olson, D.M.; Vadillo-Ortega, F. Normal and Premature Rupture of Fetal Membranes at Term Delivery Differ in Regional Chemotactic Activity and Related Chemokine/Cytokine Production. Reprod. Sci. 2013, 20, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, V.; Hirsch, E. Intrauterine Infection and Preterm Labor. Semin. Fetal Neonatal Med. 2012, 17, 12–19. [Google Scholar] [CrossRef]
- Leaños-Miranda, A.; Nolasco-Leaños, A.G.; Carrillo-Juárez, R.I.; Molina-Pérez, C.J.; Isordia-Salas, I.; Ramírez-Valenzuela, K.L. Interleukin-6 in Amniotic Fluid: A Reliable Marker for Adverse Outcomes in Women in Preterm Labor and Intact Membranes. Fetal Diagn. Ther. 2021, 48, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Kacerovsky, M.; Drahosova, M.; Hornychova, H.; Pliskova, L.; Bolehovska, R.; Forstl, M.; Tosner, J.; Andrys, C. Value of Amniotic Fluid Interleukin-8 for the Prediction of Histological Chorioamnionitis in Preterm Premature Rupture of Membranes. Neuro Endocrinol. Lett. 2009, 30, 733–738. [Google Scholar]
- Yoon, B.H.; Romero, R.; Moon, J.B.; Shim, S.S.; Kim, M.; Kim, G.; Jun, J.K. Clinical Significance of Intra-Amniotic Inflammation in Patients with Preterm Labor and Intact Membranes. Am. J. Obstet. Gynecol. 2001, 185, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, S.; Shiozaki, A.; Ito, M.; Yoneda, N.; Inada, K.; Yonezawa, R.; Kigawa, M.; Saito, S. Accurate Prediction of the Stage of Histological Chorioamnionitis before Delivery by Amniotic Fluid IL-8 Level. Am. J. Reprod. Immunol. 2015, 73, 568–576. [Google Scholar] [CrossRef]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S. Hypertensive Disorders of Pregnancy. Hypertension 2018, 72, 24–43. [Google Scholar] [CrossRef]
- Karrar, S.A.; Hong, P.L. Preeclampsia; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Aneman, I.; Pienaar, D.; Suvakov, S.; Simic, T.P.; Garovic, V.D.; McClements, L. Mechanisms of Key Innate Immune Cells in Early- and Late-Onset Preeclampsia. Front. Immunol. 2020, 11, 01864. [Google Scholar] [CrossRef]
- Masini, G.; Foo, L.F.; Tay, J.; Wilkinson, I.B.; Valensise, H.; Gyselaers, W.; Lees, C.C. Preeclampsia Has Two Phenotypes Which Require Different Treatment Strategies. Am. J. Obstet. Gynecol. 2022, 226, S1006–S1018. [Google Scholar] [CrossRef]
- Valencia-Ortega, J.; Zárate, A.; Saucedo, R.; Hernández-Valencia, M.; Cruz, J.G.; Puello, E. Placental Proinflammatory State and Maternal Endothelial Dysfunction in Preeclampsia. Gynecol. Obstet. Investig. 2019, 84, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Geldenhuys, J.; Rossouw, T.M.; Lombaard, H.A.; Ehlers, M.M.; Kock, M.M. Disruption in the Regulation of Immune Responses in the Placental Subtype of Preeclampsia. Front. Immunol. 2018, 9, 01659. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef]
- Opichka, M.A.; Rappelt, M.W.; Gutterman, D.D.; Grobe, J.L.; McIntosh, J.J. Vascular Dysfunction in Preeclampsia. Cells 2021, 10, 3055. [Google Scholar] [CrossRef]
- Wang, Y.; Lewis, D.F.; Gu, Y.; Zhao, S.; Groome, L.J. Elevated Maternal Soluble Gp130 and IL-6 Levels and Reduced Gp130 and SOCS-3 Expressions in Women Complicated with Preeclampsia. Hypertension 2011, 57, 336–342. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, Y.; Alexander, J.S.; Lewis, D.F. Preeclampsia Status Controls Interleukin-6 and Soluble IL-6 Receptor Release from Neutrophils and Endothelial Cells: Relevance to Increased Inflammatory Responses. Pathophysiology 2021, 28, 202–211. [Google Scholar] [CrossRef]
- Kuźmicki, M.; Telejko, B.; Lipińska, D.; Pliszka, J.; Wilk, J.; Wawrusiewicz-Kurylonek, N.; Zielińska, A.; Sobota, A.; Krętowski, A.; Górska, M.; et al. Stężenie Interleukiny-6, Receptora Dla Interleukiny-6 i Glikoproteiny 130 Oraz Cytokin Zależnych Od Limfocytów Th17 u Pacjentek z Cukrzycą Ciążową. Endokrynol. Pol. 2014, 65, 169–175. [Google Scholar] [CrossRef]
- Rebouissou, C.; Wijdenes, J.; Autissier, P.; Tarte, K.; Costes, V.; Liautard, J.; Rossi, J.F.; Brochier, J.; Klein, B. A Gp130 Interleukin-6 Transducer-Dependent SCID Model of Human Multiple Myeloma. Blood 1998, 91, 4727–4737. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.M.; Liu, B.; Zhao, H.B.; Stone, P.; Chen, Q.; Chamley, L. IL-6, TNFalpha and TGFbeta Promote Nonapoptotic Trophoblast Deportation and Subsequently Causes Endothelial Cell Activation. Placenta 2010, 31, 75–80. [Google Scholar] [CrossRef]
- Faas, M.M.; Spaans, F.; De Vos, P. Monocytes and Macrophages in Pregnancy and Pre-Eclampsia. Front. Immunol. 2014, 5, 298. [Google Scholar] [CrossRef]
- Ning, F.; Liu, H.; Lash, G.E. The Role of Decidual Macrophages During Normal and Pathological Pregnancy. Am. J. Reprod. Immunol. 2016, 75, 298–309. [Google Scholar] [CrossRef]
- Michalczyk, M.; Celewicz, A.; Celewicz, M.; Woźniakowska-Gondek, P.; Rzepka, R. The Role of Inflammation in the Pathogenesis of Preeclampsia. Mediat. Inflamm. 2020, 2020, 3864941. [Google Scholar] [CrossRef]
- Kimura, A.; Kishimoto, T. IL-6: Regulator of Treg/Th17 Balance. Eur. J. Immunol. 2010, 40, 1830–1835. [Google Scholar] [CrossRef]
- Vargas-Rojas, M.I.; Solleiro-Villavicencio, H.; Soto-Vega, E. Th1, Th2, Th17 and Treg Levels in Umbilical Cord Blood in Preeclampsia. J. Matern. Fetal. Neonatal Med. 2016, 29, 1642–1645. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Dolati, S.; Hashemi, V.; Abdollahpour-Alitappeh, M.; Yousefi, M. Regulatory T and T Helper 17 Cells: Their Roles in Preeclampsia. J. Cell. Physiol. 2018, 233, 6561–6573. [Google Scholar] [CrossRef] [PubMed]
- Hoeltzenbein, M.; Beck, E.; Rajwanshi, R.; Gøtestam Skorpen, C.; Berber, E.; Schaefer, C.; Østensen, M. Tocilizumab Use in Pregnancy: Analysis of a Global Safety Database Including Data from Clinical Trials and Post-Marketing Data. Semin. Arthritis Rheum. 2016, 46, 238–245. [Google Scholar] [CrossRef]
- Jorgensen, S.C.J.; Lapinsky, S.E. Tocilizumab for Coronavirus Disease 2019 in Pregnancy and Lactation: A Narrative Review. Clin. Microbiol. Infect. 2022, 28, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Bellos, I.; Karageorgiou, V.; Kapnias, D.; Karamanli, K.-E.; Siristatidis, C. The Role of Interleukins in Preeclampsia: A Comprehensive Review. Am. J. Reprod. Immunol. 2018, 80, e13055. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, C.; Vatish, M. Impact of Haemostatic Mechanisms on Pathophysiology of Preeclampsia. Thromb. Res. 2017, 151, S48–S52. [Google Scholar] [CrossRef] [PubMed]
- Giaglis, S.; Stoikou, M.; Grimolizzi, F.; Subramanian, B.Y.; van Breda, S.V.; Hoesli, I.; Lapaire, O.; Hasler, P.; Than, N.G.; Hahn, S. Neutrophil Migration into the Placenta: Good, Bad or Deadly? Cell Adh. Migr. 2016, 10, 208–225. [Google Scholar] [CrossRef]
- Broere-Brown, Z.A.; Schalekamp-Timmermans, S.; Hofman, A.; Jaddoe, V.; Steegers, E. Fetal Sex Dependency of Maternal Vascular Adaptation to Pregnancy: A Prospective Population-Based Cohort Study. BJOG 2016, 123, 1087–1095. [Google Scholar] [CrossRef]
- Jaskolka, D.; Retnakaran, R.; Zinman, B.; Kramer, C.K. Fetal Sex and Maternal Risk of Pre-Eclampsia/Eclampsia: A Systematic Review and Meta-Analysis. BJOG 2017, 124, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Braun, A.E.; Muench, K.L.; Robinson, B.G.; Wang, A.; Palmer, T.D.; Winn, V.D. Examining Sex Differences in the Human Placental Transcriptome During the First Fetal Androgen Peak. Reprod. Sci. 2021, 28, 801–818. [Google Scholar] [CrossRef]
- Ellis, J.; Wennerholm, U.B.; Bengtsson, A.; Lilja, H.; Pettersson, A.; Sultan, B.; Wennergren, M.; Hagberg, H. Levels of Dimethylarginines and Cytokines in Mild and Severe Preeclampsia. Acta Obstet. Gynecol. Scand. 2001, 80, 602–608. [Google Scholar] [PubMed]
- Ouyang, Y.-Q.; Li, S.-J.; Zhang, Q.; Cai, H.-B.; Chen, H.-P. Interactions between Inflammatory and Oxidative Stress in Preeclampsia. Hypertens. Pregnancy 2009, 28, 56–62. [Google Scholar] [CrossRef]
- Guven, M.A.; Coskun, A.; Ertas, I.E.; Aral, M.; Zencırcı, B.; Oksuz, H. Association of Maternal Serum CRP, IL-6, TNF-α, Homocysteine, Folic Acid and Vitamin B12 Levels with the Severity of Preeclampsia and Fetal Birth Weight. Hypertens. Pregnancy 2009, 28, 190–200. [Google Scholar] [CrossRef]
- Tosun, M.; Celik, H.; Avci, B.; Yavuz, E.; Alper, T.; Malatyalioğlu, E. Maternal and Umbilical Serum Levels of Interleukin-6, Interleukin-8, and Tumor Necrosis Factor-α in Normal Pregnancies and in Pregnancies Complicated by Preeclampsia. J. Matern. Neonatal Med. 2010, 23, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.P.; Yin, Y.X.; Gao, Y.F.; Lau, S.; Shen, F.; Zhao, M.; Chen, Q. The Increased Maternal Serum Levels of IL-6 Are Associated with the Severity and Onset of Preeclampsia. Cytokine 2012, 60, 856–860. [Google Scholar] [CrossRef]
- Ozler, A.; Turgut, A.; Sak, M.E.; Evsen, M.S.; Soydinc, H.E.; Evliyaoglu, O.; Gul, T. Serum Levels of Neopterin, Tumor Necrosis Factor-Alpha and Interleukin-6 in Preeclampsia: Relationship with Disease Severity. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 1707–1712. [Google Scholar]
- Ovayolu, A.; Turksoy, V.A.; Ovayolu, G.; Ozek, M.A.; Dogan, I.; Karaman, E. Analyses of Interleukin-6, Presepsin and Pentraxin-3 in the Diagnosis and Severity of Late-Onset Preeclampsia. J. Matern. Neonatal Med. 2022, 35, 299–307. [Google Scholar] [CrossRef]
- Küçük, M.; Sezer, S.D.; Yenisey, Ç.; Yüksel, H.; Odabaşı, A.R. Comparison of Interleukin-6 Levels in Maternal and Umbilical Cord Blood in Early- and Late-Onset Preeclampsia. Gynecol. Endocrinol. 2012, 28, 640–643. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, D.; Chen, L. Lipid Profile and Cytokines in Hypertension of Pregnancy: A Comparison of Preeclampsia Therapies. J. Clin. Hypertens. 2018, 20, 394–399. [Google Scholar] [CrossRef]
- El-Mikkawy, D.M.E.; EL-Sadek, M.A.; EL-Badawy, M.A.; Samaha, D. Circulating Level of Interleukin-6 in Relation to Body Mass Indices and Lipid Profile in Egyptian Adults with Overweight and Obesity. Egypt. Rheumatol. Rehabil. 2020, 47, 7. [Google Scholar] [CrossRef]
- Kim, O.Y.; Chae, J.S.; Paik, J.K.; Seo, H.S.; Jang, Y.; Cavaillon, J.-M.; Lee, J.H. Effects of Aging and Menopause on Serum Interleukin-6 Levels and Peripheral Blood Mononuclear Cell Cytokine Production in Healthy Nonobese Women. Age 2012, 34, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Naugler, W.E.; Karin, M. The Wolf in Sheep’s Clothing: The Role of Interleukin-6 in Immunity, Inflammation and Cancer. Trends Mol. Med. 2008, 14, 109–119. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef] [PubMed]
- Angueira, A.R.; Ludvik, A.E.; Reddy, T.E.; Wicksteed, B.; Lowe, W.L.; Layden, B.T. New Insights Into Gestational Glucose Metabolism: Lessons Learned From 21st Century Approaches. Diabetes 2015, 64, 327–334. [Google Scholar] [CrossRef]
- Goldstein, R.F.; Abell, S.K.; Ranasinha, S.; Misso, M.; Boyle, J.A.; Black, M.H.; Li, N.; Hu, G.; Corrado, F.; Rode, L.; et al. Association of Gestational Weight Gain with Maternal and Infant Outcomes. JAMA 2017, 317, 2207. [Google Scholar] [CrossRef]
- Immanuel, J.; Simmons, D. Screening and Treatment for Early-Onset Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Curr. Diabetes Rep. 2017, 17, 115. [Google Scholar] [CrossRef]
- Moon, J.H.; Jang, H.C. Gestational Diabetes Mellitus: Diagnostic Approaches and Maternal-Offspring Complications. Diabetes Metab. J. 2022, 46, 3–14. [Google Scholar] [CrossRef]
- Kc, K.; Shakya, S.; Zhang, H. Gestational Diabetes Mellitus and Macrosomia: A Literature Review. Ann. Nutr. Metab. 2015, 66 (Suppl. 2), 14–20. [Google Scholar] [CrossRef] [PubMed]
- Durnwald, C. Gestational Diabetes: Linking Epidemiology, Excessive Gestational Weight Gain, Adverse Pregnancy Outcomes, and Future Metabolic Syndrome. Semin. Perinatol. 2015, 39, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Jenum, A.K.; Mørkrid, K.; Sletner, L.; Vangen, S.; Vange, S.; Torper, J.L.; Nakstad, B.; Voldner, N.; Rognerud-Jensen, O.H.; Berntsen, S.; et al. Impact of Ethnicity on Gestational Diabetes Identified with the WHO and the Modified International Association of Diabetes and Pregnancy Study Groups Criteria: A Population-Based Cohort Study. Eur. J. Endocrinol. 2012, 166, 317–324. [Google Scholar] [CrossRef]
- Anghebem-Oliveira, M.I.; Martins, B.R.; Alberton, D.; Ramos, E.A. de S.; Picheth, G.; Rego, F.G. de M. Type 2 Diabetes-Associated Genetic Variants of FTO, LEPR, PPARg, and TCF7L2 in Gestational Diabetes in a Brazilian Population. Arch. Endocrinol. Metab. 2017, 61, 238–248. [Google Scholar] [CrossRef]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef] [PubMed]
- Di Cianni, G.; Miccoli, R.; Volpe, L.; Lencioni, C.; Del Prato, S. Intermediate Metabolism in Normal Pregnancy and in Gestational Diabetes. Diabetes. Metab. Res. Rev. 2003, 19, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Mirghani Dirar, A.; Doupis, J. Gestational Diabetes from A to Z. World J. Diabetes 2017, 8, 489–511. [Google Scholar] [CrossRef]
- Buchanan, T.A. Pancreatic B-Cell Defects in Gestational Diabetes: Implications for the Pathogenesis and Prevention of Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2001, 86, 989–993. [Google Scholar] [CrossRef]
- Cerf, M.E. Beta Cell Dysfunction and Insulin Resistance. Front. Endocrinol. 2013, 4, 37. [Google Scholar] [CrossRef]
- Pantham, P.; Aye, I.L.M.H.; Powell, T.L. Inflammation in Maternal Obesity and Gestational Diabetes Mellitus. Placenta 2015, 36, 709–715. [Google Scholar] [CrossRef]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory Mechanisms in Obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef]
- Kim, J.-H.; Bachmann, R.A.; Chen, J. Interleukin-6 and Insulin Resistance. Vitam. Horm. 2009, 80, 613–633. [Google Scholar] [CrossRef] [PubMed]
- Amirian, A.; Mahani, M.B.; Abdi, F. Role of Interleukin-6 (IL-6) in Predicting Gestational Diabetes Mellitus. Obstet. Gynecol. Sci. 2020, 63, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Nikolajuk, A.; Kowalska, I.; Karczewska-Kupczewska, M.; Adamska, A.; Otziomek, E.; Wolczynski, S.; Kinalska, I.; Gorska, M.; Straczkowski, M. Serum Soluble Glycoprotein 130 Concentration Is Inversely Related to Insulin Sensitivity in Women with Polycystic Ovary Syndrome. Diabetes 2010, 59, 1026–1029. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zuliani, G.; Galvani, M.; Maggio, M.; Volpato, S.; Bandinelli, S.; Corsi, A.M.; Lauretani, F.; Cherubini, A.; Guralnik, J.M.; Fellin, R.; et al. Plasma Soluble Gp130 Levels Are Increased in Older Subjects with Metabolic Syndrome. The Role of Insulin Resistance. Atherosclerosis 2010, 213, 319–324. [Google Scholar] [CrossRef][Green Version]
- Lanton, T.; Levkovitch-Siany, O.; Udi, S.; Tam, J.; Abramovitch, R.; Perles, S.; Williams, E.; Rachmilewitz, J.; Mor, U.; Elinav, E.; et al. Peripheral Sgp130-Mediated Trans-Signaling Blockade Induces Obesity and Insulin Resistance in Mice via PPARα Suppression. bioRxiv 2020. [Google Scholar] [CrossRef]
- Liu, H.; Liu, A.; Kaminga, A.C.; McDonald, J.; Wen, S.W.; Pan, X. Chemokines in Gestational Diabetes Mellitus. Front. Immunol. 2022, 13, 705852. [Google Scholar] [CrossRef]
- Michailidou, Z.; Gomez-Salazar, M.; Alexaki, V.I. Innate Immune Cells in the Adipose Tissue in Health and Metabolic Disease. J. Innate Immun. 2022, 14, 4–30. [Google Scholar] [CrossRef]
- Richardson, A.C.; Carpenter, M.W. Inflammatory Mediators in Gestational Diabetes Mellitus. Obstet. Gynecol. Clin. N. Am. 2007, 34, 213–224. [Google Scholar] [CrossRef]
- Waki, H.; Tontonoz, P. Endocrine Functions of Adipose Tissue. Annu. Rev. Pathol. 2007, 2, 31–56. [Google Scholar] [CrossRef]
- Mohamed-Ali, V.; Goodrick, S.; Rawesh, A.; Katz, D.R.; Miles, J.M.; Yudkin, J.S.; Klein, S.; Coppack, S.W. Subcutaneous Adipose Tissue Releases Interleukin-6, but Not Tumor Necrosis Factor-Alpha, in Vivo. J. Clin. Endocrinol. Metab. 1997, 82, 4196–4200. [Google Scholar] [CrossRef]
- Rehman, K.; Akash, M.S.H.; Liaqat, A.; Kamal, S.; Qadir, M.I.; Rasul, A. Role of Interleukin-6 in Development of Insulin Resistance and Type 2 Diabetes Mellitus. Crit. Rev. Eukaryot. Gene Expr. 2017, 27, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, D.; Hochwald, S.; Brennan, M.F.; Burt, M. Interleukin-6 Stimulates Gluconeogenesis in Primary Cultures of Rat Hepatocytes. Metabolism 1995, 44, 145–146. [Google Scholar] [CrossRef]
- Piya, M.K.; McTernan, P.G.; Kumar, S. Adipokine Inflammation and Insulin Resistance: The Role of Glucose, Lipids and Endotoxin. J. Endocrinol. 2013, 216, T1–T15. [Google Scholar] [CrossRef]
- Kobashi, C.; Asamizu, S.; Ishiki, M.; Iwata, M.; Usui, I.; Yamazaki, K.; Tobe, K.; Kobayashi, M.; Urakaze, M. Inhibitory Effect of IL-8 on Insulin Action in Human Adipocytes via MAP Kinase Pathway. J. Inflamm. 2009, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.A.; Qiu, C.; Muy-Rivera, M.; Vadachkoria, S.; Song, T.; Luthy, D.A. Plasma Adiponectin Concentrations in Early Pregnancy and Subsequent Risk of Gestational Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2004, 89, 2306–2311. [Google Scholar] [CrossRef] [PubMed]
- Tretjakovs, P.; Jurka, A.; Bormane, I.; Mackevics, V.; Mikelsone, I.; Balode, L.; Reihmane, D.; Stukena, I.; Bahs, G.; Aivars, J.I.; et al. Relation of Inflammatory Chemokines to Insulin Resistance and Hypoadiponectinemia in Coronary Artery Disease Patients. Eur. J. Intern. Med. 2009, 20, 712–717. [Google Scholar] [CrossRef]
- Urakaze, M.; Temaru, R.; Satou, A.; Yamazaki, K.; Hamazaki, T.; Kobayashi, M. The IL-8 Production in Endothelial Cells Is Stimulated by High Glucose. Horm. Metab. Res. 1996, 28, 400–401. [Google Scholar] [CrossRef]
- Kuzmicki, M.; Telejko, B.; Wawrusiewicz-Kurylonek, N.; Citko, A.; Lipinska, D.; Pliszka, J.; Wilk, J.; Kalejta, K.; Lemancewicz, A.; Grabiec, M.; et al. The Expression of Suppressor of Cytokine Signaling 1 and 3 in Fat and Placental Tissue from Women with Gestational Diabetes. Gynecol. Endocrinol. 2012, 28, 841–844. [Google Scholar] [CrossRef]
- Bowen, J.M.; Chamley, L.; Mitchell, M.D.; Keelan, J.A. Cytokines of the Placenta and Extra-Placental Membranes: Biosynthesis, Secretion and Roles in Establishment of Pregnancy in Women. Placenta 2002, 23, 239–256. [Google Scholar] [CrossRef]
- Radaelli, T.; Uvena-Celebrezze, J.; Minium, J.; Huston-Presley, L.; Catalano, P.; Hauguel-de Mouzon, S. Maternal Interleukin-6: Marker of Fetal Growth and Adiposity. J. Soc. Gynecol. Investig. 2006, 13, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Desoye, G.; Hauguel-de Mouzon, S. The Human Placenta in Gestational Diabetes Mellitus. Diabetes Care 2007, 30, S120–S126. [Google Scholar] [CrossRef] [PubMed]
- Fetita, L.-S.; Sobngwi, E.; Serradas, P.; Calvo, F.; Gautier, J.-F. Consequences of Fetal Exposure to Maternal Diabetes in Offspring. J. Clin. Endocrinol. Metab. 2006, 91, 3718–3724. [Google Scholar] [CrossRef]
- Egan, A.M.; Dunne, F.P. Epidemiology of Gestational and Pregestational Diabetes Mellitus; Karger Publishers: Basel, Switzerland, 2020; pp. 1–10. [Google Scholar]
- Song, C.; Li, J.; Leng, J.; Ma, R.C.; Yang, X. Lifestyle Intervention Can Reduce the Risk of Gestational Diabetes: A Meta-Analysis of Randomized Controlled Trials. Obes. Rev. 2016, 17, 960–969. [Google Scholar] [CrossRef]
- Brown, A.S. The Environment and Susceptibility to Schizophrenia. Prog. Neurobiol. 2011, 93, 23–58. [Google Scholar] [CrossRef]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota Modulate Behavioral and Physiological Abnormalities Associated with Neurodevelopmental Disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef]
- Moretti, S.; Franchi, S.; Castelli, M.; Amodeo, G.; Somaini, L.; Panerai, A.; Sacerdote, P. Exposure of Adolescent Mice to Delta-9-Tetrahydrocannabinol Induces Long-Lasting Modulation of Pro- and Anti-Inflammatory Cytokines in Hypothalamus and Hippocampus Similar to That Observed for Peripheral Macrophages. J. Neuroimmune Pharmacol. 2015, 10, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Debost, J.-C.P.G.; Larsen, J.T.; Munk-Olsen, T.; Mortensen, P.B.; Meyer, U.; Petersen, L. Joint Effects of Exposure to Prenatal Infection and Peripubertal Psychological Trauma in Schizophrenia. Schizophr. Bull. 2017, 43, 171–179. [Google Scholar] [CrossRef]
- Money, K.M.; Barke, T.L.; Serezani, A.; Gannon, M.; Garbett, K.A.; Aronoff, D.M.; Mirnics, K. Gestational Diabetes Exacerbates Maternal Immune Activation Effects in the Developing Brain. Mol. Psychiatry 2018, 23, 1920–1928. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Mattei, D.; Pietrobelli, A. Micronutrients and Brain Development. Curr. Nutr. Rep. 2019, 8, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Han, V.X.; Patel, S.; Jones, H.F.; Nielsen, T.C.; Mohammad, S.S.; Hofer, M.J.; Gold, W.; Brilot, F.; Lain, S.J.; Nassar, N.; et al. Maternal Acute and Chronic Inflammation in Pregnancy Is Associated with Common Neurodevelopmental Disorders: A Systematic Review. Transl. Psychiatry 2021, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Gruys, E.; Toussaint, M.J.M.; Niewold, T.A.; Koopmans, S.J. Acute Phase Reaction and Acute Phase Proteins. J. Zhejiang Univ. Sci. 2005, 6, 1045–1056. [Google Scholar] [CrossRef]
- Patterson, P.H. Maternal Infection and Immune Involvement in Autism. Trends Mol. Med. 2011, 17, 389–394. [Google Scholar] [CrossRef]
- Atladóttir, H.Ó.; Thorsen, P.; Østergaard, L.; Schendel, D.E.; Lemcke, S.; Abdallah, M.; Parner, E.T. Maternal Infection Requiring Hospitalization During Pregnancy and Autism Spectrum Disorders. J. Autism Dev. Disord. 2010, 40, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.S.; Derkits, E.J. Prenatal Infection and Schizophrenia: A Review of Epidemiologic and Translational Studies. Am. J. Psychiatry 2010, 167, 261–280. [Google Scholar] [CrossRef]
- Brown, A.S.; Patterson, P.H. Maternal Infection and Schizophrenia: Implications for Prevention. Schizophr. Bull. 2011, 37, 284–290. [Google Scholar] [CrossRef]
- Lins, B. Maternal Immune Activation as a Risk Factor for Psychiatric Illness in the Context of the SARS-CoV-2 Pandemic. Brain, Behav. Immun.-Health 2021, 16, 100297. [Google Scholar] [CrossRef]
- Brown, A.S.; Schaefer, C.A.; Wyatt, R.J.; Goetz, R.; Begg, M.D.; Gorman, J.M.; Susser, E.S. Maternal Exposure to Respiratory Infections and Adult Schizophrenia Spectrum Disorders: A Prospective Birth Cohort Study. Schizophr. Bull. 2000, 26, 287–295. [Google Scholar] [CrossRef]
- McGrath, J.J.; Murray, R.M. Risk Factors for Schizophrenia: From Conception to Birth. In Schizophrenia; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2003; pp. 232–250. ISBN 9780470987353. [Google Scholar]
- Brown, A.S.; Begg, M.D.; Gravenstein, S.; Schaefer, C.A.; Wyatt, R.J.; Bresnahan, M.; Babulas, V.P.; Susser, E.S. Serologic Evidence of Prenatal Influenza in the Etiology of Schizophrenia. Arch. Gen. Psychiatry 2004, 61, 774. [Google Scholar] [CrossRef]
- Brown, A.S.; Schaefer, C.A.; Quesenberry, C.P.; Liu, L.; Babulas, V.P.; Susser, E.S. Maternal Exposure to Toxoplasmosis and Risk of Schizophrenia in Adult Offspring. Am. J. Psychiatry 2005, 162, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, P.B.; Norgaard-Pedersen, B.; Waltoft, B.L.; Sorensen, T.L.; Hougaard, D.; Yolken, R.H. Early Infections of Toxoplasma Gondii and the Later Development of Schizophrenia. Schizophr. Bull. 2007, 33, 741–744. [Google Scholar] [CrossRef] [PubMed]
- Ellman, L.M.; Yolken, R.H.; Buka, S.L.; Torrey, E.F.; Cannon, T.D. Cognitive Functioning Prior to the Onset of Psychosis: The Role of Fetal Exposure to Serologically Determined Influenza Infection. Biol. Psychiatry 2009, 65, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Zawadzka, A.; Cieślik, M.; Adamczyk, A. The Role of Maternal Immune Activation in the Pathogenesis of Autism: A Review of the Evidence, Proposed Mechanisms and Implications for Treatment. Int. J. Mol. Sci. 2021, 22, 11516. [Google Scholar] [CrossRef]
- Samuelsson, A.-M.; Jennische, E.; Hansson, H.-A.; Holmäng, A. Prenatal Exposure to Interleukin-6 Results in Inflammatory Neurodegeneration in Hippocampus with NMDA/GABA A Dysregulation and Impaired Spatial Learning. Am. J. Physiol. Integr. Comp. Physiol. 2006, 290, R1345–R1356. [Google Scholar] [CrossRef]
- Prins, J.R.; Gomez-Lopez, N.; Robertson, S.A. Interleukin-6 in Pregnancy and Gestational Disorders. J. Reprod. Immunol. 2012, 95, 1–14. [Google Scholar] [CrossRef]
- Wu, W.-L.; Hsiao, E.Y.; Yan, Z.; Mazmanian, S.K.; Patterson, P.H. The Placental Interleukin-6 Signaling Controls Fetal Brain Development and Behavior. Brain. Behav. Immun. 2017, 62, 11–23. [Google Scholar] [CrossRef]
- Mirabella, F.; Desiato, G.; Mancinelli, S.; Fossati, G.; Rasile, M.; Morini, R.; Markicevic, M.; Grimm, C.; Amegandjin, C.; Termanini, A.; et al. Prenatal Interleukin 6 Elevation Increases Glutamatergic Synapse Density and Disrupts Hippocampal Connectivity in Offspring. Immunity 2021, 54, 2611–2631.e8. [Google Scholar] [CrossRef] [PubMed]
- Khong, T.Y.; Mooney, E.E.; Ariel, I.; Balmus, N.C.M.; Boyd, T.K.; Brundler, M.-A.; Derricott, H.; Evans, M.J.; Faye-Petersen, O.M.; Gillan, J.E.; et al. Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch. Pathol. Lab. Med. 2016, 140, 698–713. [Google Scholar] [CrossRef]
- Smulian, J.C.; Vintzileos, A.M.; Lai, Y.L.; Santiago, J.; Shen-Schwarz, S.; Campbell, W.A. Maternal Chorioamnionitis and Umbilical Vein Interleukin-6 Levels for Identifying Early Neonatal Sepsis. J Matern Fetal Med 1999, 3, 88–94. [Google Scholar]
- Roberts, D.J.; Celi, A.C.; Riley, L.E.; Onderdonk, A.B.; Boyd, T.K.; Johnson, L.C.; Lieberman, E. Acute Histologic Chorioamnionitis at Term: Nearly Always Noninfectious. PLoS ONE 2012, 7, e31819. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Kim, Y.M.; Pacora, P.; Kim, C.J.; Benshalom-Tirosh, N.; Jaiman, S.; Bhatti, G.; Kim, J.-S.; Qureshi, F.; Jacques, S.M.; et al. The Frequency and Type of Placental Histologic Lesions in Term Pregnancies with Normal Outcome. J. Perinat. Med. 2018, 46, 613–630. [Google Scholar] [CrossRef] [PubMed]
- Kamity, R.; Patel, H.; Younis, S.; Nasim, M.; Miller, E.; Ahmed, M. Inhibition of Cxcr 1 and 2 Delays Preterm Delivery and Reduces Neonatal Mortality in a Mouse Model of Chorioamnionitis. Eur. J. Inflamm. 2014, 12, 447–457. [Google Scholar] [CrossRef]
- Reisenberger, K.; Egarter, C.; Vogl, S.; Sternberger, B.; Kiss, H.; Husslein, P. The Transfer of Interleukin-8 across the Human Placenta Perfused in Vitro. Obstet. Gynecol. 1996, 87, 613–616. [Google Scholar] [CrossRef]
- Aaltonen, R.; Heikkinen, T.; Hakala, K.; Laine, K.; Alanen, A. Transfer of Proinflammatory Cytokines Across Term Placenta. Obstet. Gynecol. 2005, 106, 802–807. [Google Scholar] [CrossRef]
- Ito, A.; Nakamura, T.; Uchiyama, T.; Hirose, K.; Hirakawa, S.; Sasaguri, Y.; Mori, Y. Stimulation of the Biosynthesis of Interleukin 8 by Interleukin 1 and Tumor Necrosis Factor Alpha in Cultured Human Chorionic Cells. Biol. Pharm. Bull. 1994, 17, 1463–1467. [Google Scholar] [CrossRef]
- Fortunato, S.J.; Menon, R.; Swan, K.F. Amniochorion: A Source of Interleukin-8. Am. J. Reprod. Immunol. 1995, 34, 156–162. [Google Scholar] [CrossRef]
- Hsu, C.-D.; Meaddough, E.; Aversa, K.; Hong, S.-F.; Lu, L.-C.; Jones, D.C.; Copel, J.A. Elevated Amniotic Fluid Levels of Leukemia Inhibitory Factor, Interleukin 6, and Interleukin 8 in Intra-Amniotic Infection. Am. J. Obstet. Gynecol. 1998, 179, 1267–1270. [Google Scholar] [CrossRef]
- Gomez, R.; Romero, R.; Ghezzi, F.; Yoon, B.H.; Mazor, M.; Berry, S.M. The Fetal Inflammatory Response Syndrome. Am. J. Obstet. Gynecol. 1998, 179, 194–202. [Google Scholar] [CrossRef]
- Yoon, B.H.; Romero, R.; Moon, J.; Chaiworapongsa, T.; Espinoza, J.; Kim, Y.M.; Edwin, S.; Kim, J.C.; Camacho, N.; Bujold, E.; et al. Differences in the Fetal Interleukin-6 Response to Microbial Invasion of the Amniotic Cavity between Term and Preterm Gestation. J. Matern. Neonatal Med. 2003, 13, 32–38. [Google Scholar] [CrossRef]
- Romero, R.; Gomez, R.; Ghezzi, F.; Yoon, B.H.; Mazor, M.; Edwin, S.S.; Berry, S.M. A Fetal Systemic Inflammatory Response Is Followed by the Spontaneous Onset of Preterm Parturition. Am. J. Obstet. Gynecol. 1998, 179, 186–193. [Google Scholar] [CrossRef]
- Saji, F.; Samejima, Y.; Kamiura, S.; Sawai, K.; Shimoya, K.; Kimura, T. Cytokine Production in Chorioamnionitis. J. Reprod. Immunol. 2000, 47, 185–196. [Google Scholar] [CrossRef]
- Oh, K.J.; Lee, J.; Romero, R.; Park, H.S.; Hong, J.-S.; Yoon, B.H. A New Rapid Bedside Test to Diagnose and Monitor Intraamniotic Inflammation in Preterm PROM Using Transcervically Collected Fluid. Am. J. Obstet. Gynecol. 2020, 223, 423.e1–423.e15. [Google Scholar] [CrossRef]
- Goldstein, J.M.; Cohen, J.E.; Mareckova, K.; Holsen, L.; Whitfield-Gabrieli, S.; Gilman, S.E.; Buka, S.L.; Hornig, M. Impact of Prenatal Maternal Cytokine Exposure on Sex Differences in Brain Circuitry Regulating Stress in Offspring 45 Years Later. Proc. Natl. Acad. Sci. USA 2021, 118, e2014464118. [Google Scholar] [CrossRef]
- Allswede, D.M.; Buka, S.L.; Yolken, R.H.; Torrey, E.F.; Cannon, T.D. Elevated Maternal Cytokine Levels at Birth and Risk for Psychosis in Adult Offspring. Schizophr. Res. 2016, 172, 41–45. [Google Scholar] [CrossRef]
- Mac Giollabhui, N.; Breen, E.C.; Murphy, S.K.; Maxwell, S.D.; Cohn, B.A.; Krigbaum, N.Y.; Cirillo, P.M.; Perez, C.; Alloy, L.B.; Drabick, D.A.G.; et al. Maternal Inflammation during Pregnancy and Offspring Psychiatric Symptoms in Childhood: Timing and Sex Matter. J. Psychiatr. Res. 2019, 111, 96–103. [Google Scholar] [CrossRef]
- Brown, A.S.; Hooton, J.; Schaefer, C.A.; Zhang, H.; Petkova, E.; Babulas, V.; Perrin, M.; Gorman, J.M.; Susser, E.S. Elevated Maternal Interleukin-8 Levels and Risk of Schizophrenia in Adult Offspring. Am. J. Psychiatry 2004, 161, 889–895. [Google Scholar] [CrossRef]
- Ellman, L.M.; Deicken, R.F.; Vinogradov, S.; Kremen, W.S.; Poole, J.H.; Kern, D.M.; Tsai, W.Y.; Schaefer, C.A.; Brown, A.S. Structural Brain Alterations in Schizophrenia Following Fetal Exposure to the Inflammatory Cytokine Interleukin-8. Schizophr. Res. 2010, 121, 46–54. [Google Scholar] [CrossRef]
- Aguilar-Valles, A.; Rodrigue, B.; Matta-Camacho, E. Maternal Immune Activation and the Development of Dopaminergic Neurotransmission of the Offspring: Relevance for Schizophrenia and Other Psychoses. Front. Psychiatry 2020, 11, 852. [Google Scholar] [CrossRef]
- Nist, M.D.; Pickler, R.H. An Integrative Review of Cytokine/Chemokine Predictors of Neurodevelopment in Preterm Infants. Biol. Res. Nurs. 2019, 21, 366–376. [Google Scholar] [CrossRef]
- Nisticò, R.; Salter, E.; Nicolas, C.; Feligioni, M.; Mango, D.; Bortolotto, Z.A.; Gressens, P.; Collingridge, G.L.; Peineau, S. Synaptoimmunology—Roles in Health and Disease. Mol. Brain 2017, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, J.H.; Fredrik Jarskog, L.; Vadlamudi, S.; Lauder, J.M. Prenatal Infection and Risk for Schizophrenia: IL-1β, IL-6, and TNFα Inhibit Cortical Neuron Dendrite Development. Neuropsychopharmacology 2004, 29, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Deverman, B.E.; Patterson, P.H. Cytokines and CNS Development. Neuron 2009, 64, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Parker-Athill, E.C.; Tan, J. Maternal Immune Activation and Autism Spectrum Disorder: Interleukin-6 Signaling as a Key Mechanistic Pathway. Neurosignals 2010, 18, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Desai, T.R.; Leeper, N.J.; Hynes, K.L.; Gewertz, B.L. Interleukin-6 Causes Endothelial Barrier Dysfunction via the Protein Kinase C Pathway. J. Surg. Res. 2002, 104, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Kendall, G.; Peebles, D. Acute Fetal Hypoxia: The Modulating Effect of Infection. Early Hum. Dev. 2005, 81, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.N.; Jansson, T.; Powell, T.L. IL-6 Stimulates System A Amino Acid Transporter Activity in Trophoblast Cells through STAT3 and Increased Expression of SNAT2. Am. J. Physiol. Physiol. 2009, 297, C1228–C1235. [Google Scholar] [CrossRef]
- Sullivan, G.; Galdi, P.; Cabez, M.B.; Borbye-Lorenzen, N.; Stoye, D.Q.; Lamb, G.J.; Evans, M.J.; Quigley, A.J.; Thrippleton, M.J.; Skogstrand, K.; et al. Interleukin-8 Dysregulation Is Implicated in Brain Dysmaturation Following Preterm Birth. Brain. Behav. Immun. 2020, 90, 311–318. [Google Scholar] [CrossRef]
- Ravaccia, D.; Ghafourian, T. Critical Role of the Maternal Immune System in the Pathogenesis of Autism Spectrum Disorder. Biomedicines 2020, 8, 557. [Google Scholar] [CrossRef]
- Makinson, R.; Lloyd, K.; Rayasam, A.; McKee, S.; Brown, A.; Barila, G.; Grissom, N.; George, R.; Marini, M.; Fabry, Z.; et al. Intrauterine Inflammation Induces Sex-Specific Effects on Neuroinflammation, White Matter, and Behavior. Brain. Behav. Immun. 2017, 66, 277–288. [Google Scholar] [CrossRef]
- Barke, T.L.; Money, K.M.; Du, L.; Serezani, A.; Gannon, M.; Mirnics, K.; Aronoff, D.M. Sex Modifies Placental Gene Expression in Response to Metabolic and Inflammatory Stress. Placenta 2019, 78, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bale, T.L. The Placenta and Neurodevelopment: Sex Differences in Prenatal Vulnerability. Dialogues Clin. Neurosci. 2016, 18, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Kalish, B.T.; Kim, E.; Finander, B.; Duffy, E.E.; Kim, H.; Gilman, C.K.; Yim, Y.S.; Tong, L.; Kaufman, R.J.; Griffith, E.C.; et al. Maternal Immune Activation in Mice Disrupts Proteostasis in the Fetal Brain. Nat. Neurosci. 2021, 24, 204–213. [Google Scholar] [CrossRef]
- Werling, D.M.; Geschwind, D.H. Sex Differences in Autism Spectrum Disorders. Curr. Opin. Neurol. 2013, 26, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.A.; Aavani, T.; Pittman, Q.J. Sex Effects on Neurodevelopmental Outcomes of Innate Immune Activation during Prenatal and Neonatal Life. Horm. Behav. 2012, 62, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, P.A.; Haditsch, U.; Braun, A.E.; Cantu, A.V.; Moon, H.M.; Price, R.O.; Anderson, M.P.; Saravanapandian, V.; Ismail, K.; Rivera, M.; et al. Stereotypical Alterations in Cortical Patterning Are Associated with Maternal Illness-Induced Placental Dysfunction. J. Neurosci. 2013, 33, 16874–16888. [Google Scholar] [CrossRef] [PubMed]
- Xuan, I.C.Y.; Hampson, D.R. Gender-Dependent Effects of Maternal Immune Activation on the Behavior of Mouse Offspring. PLoS ONE 2014, 9, e104433. [Google Scholar] [CrossRef] [PubMed]
- Braun, A.E.; Carpentier, P.A.; Babineau, B.A.; Narayan, A.R.; Kielhold, M.L.; Moon, H.M.; Shankar, A.; Su, J.; Saravanapandian, V.; Haditsch, U.; et al. “Females Are Not Just ‘Protected’ Males”: Sex-Specific Vulnerabilities in Placenta and Brain after Prenatal Immune Disruption. eNeuro 6. [CrossRef]
- Selten, J.-P.; Morgan, V.A. Prenatal Exposure to Influenza and Major Affective Disorder. Bipolar Disord. 2010, 12, 753–754. [Google Scholar] [CrossRef]
- Estes, M.L.; McAllister, A.K. Maternal Immune Activation: Implications for Neuropsychiatric Disorders. Science 2016, 353, 772–777. [Google Scholar] [CrossRef]
- Kentner, A.C.; Bilbo, S.D.; Brown, A.S.; Hsiao, E.Y.; McAllister, A.K.; Meyer, U.; Pearce, B.D.; Pletnikov, M.V.; Yolken, R.H.; Bauman, M.D. Maternal Immune Activation: Reporting Guidelines to Improve the Rigor, Reproducibility, and Transparency of the Model. Neuropsychopharmacology 2019, 44, 245–258. [Google Scholar] [CrossRef]
- Stefanatos, G.A.; Grover, W.; Geller, E. Case Study: Corticosteroid Treatment of Language Regression in Pervasive Developmental Disorder. J. Am. Acad. Child Adolesc. Psychiatry 1995, 34, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, S.; Arnold, S.; Chatila, T. Response to Steroid Therapy in Autism Secondary to Autoimmune Lymphoproliferative Syndrome. J. Pediatr. 2000, 136, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Zhang, Y.; Li, Q. Therapeutic Mechanisms of Ibuprofen, Prednisone and Betamethasone in Osteoarthritis. Mol. Med. Rep. 2017, 15, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Asadabadi, M.; Mohammadi, M.-R.; Ghanizadeh, A.; Modabbernia, A.; Ashrafi, M.; Hassanzadeh, E.; Forghani, S.; Akhondzadeh, S. Celecoxib as Adjunctive Treatment to Risperidone in Children with Autistic Disorder: A Randomized, Double-Blind, Placebo-Controlled Trial. Psychopharmacology 2013, 225, 51–59. [Google Scholar] [CrossRef]
- Katagiri, R.; Ishihara-Hattori, K.; Frings, W.; Amano, J.; Fuchs, A.; Chiba, S. Effects of SA237, a Humanized Anti-Interleukin-6 Receptor Monoclonal Antibody, on Pre- and Postnatal Development in Cynomolgus Monkey. Birth Defects Res. 2017, 109, 843–856. [Google Scholar] [CrossRef]
- Smith, S.E.P.; Li, J.; Garbett, K.; Mirnics, K.; Patterson, P.H. Maternal Immune Activation Alters Fetal Brain Development through Interleukin-6. J. Neurosci. 2007, 27, 10695–10702. [Google Scholar] [CrossRef]
- Pineda, E.; Shin, D.; You, S.J.; Auvin, S.; Sankar, R.; Mazarati, A. Maternal Immune Activation Promotes Hippocampal Kindling Epileptogenesis in Mice. Ann. Neurol. 2013, 74, 11–19. [Google Scholar] [CrossRef]
- Tanaka, T.; Ogata, A.; Narazaki, M. Tocilizumab: An Updated Review of Its Use in the Treatment of Rheumatoid Arthritis and Its Application for Other Immune-Mediated Diseases. Clin. Med. Insights Ther. 2013, 5, CMT.S9282. [Google Scholar] [CrossRef]
- Dördelmann, M.; Kerk, J.; Dressler, F.; Brinkhaus, M.-J.; Bartels, D.; Dammann, C.; Dörk, T.; Dammann, O. Interleukin-10 High Producer Allele and Ultrasound-Defined Periventricular White Matter Abnormalities in Preterm Infants: A Preliminary Study. Neuropediatrics 2006, 37, 130–136. [Google Scholar] [CrossRef]
- Zanno, A.E.; Romer, M.A.; Fox, L.; Golden, T.; Jaeckle-Santos, L.; Simmons, R.A.; Grinspan, J.B. Reducing Th2 Inflammation through Neutralizing IL-4 Antibody Rescues Myelination in IUGR Rat Brain. J. Neurodev. Disord. 2019, 11, 34. [Google Scholar] [CrossRef]
- Basil, P.; Li, Q.; Gui, H.; Hui, T.C.K.; Ling, V.H.M.; Wong, C.C.Y.; Mill, J.; McAlonan, G.M.; Sham, P.-C. Prenatal Immune Activation Alters the Adult Neural Epigenome but Can Be Partly Stabilised by a N-3 Polyunsaturated Fatty Acid Diet. Transl. Psychiatry 2018, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- Hennigar, S.R.; McClung, J.P.; Pasiakos, S.M. Nutritional Interventions and the IL-6 Response to Exercise. FASEB J. 2017, 31, 3719–3728. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, Z.; Guo, Y.; Xie, H.; Zhang, Z.; Sun, D.; Li, H.; Chen, L. Diterpenoids from the Seeds of Euphorbia Lathyris and Their Anti-Inflammatory Activity. Bioorg. Chem. 2021, 112, 104944. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, J.; Zhang, H.; Yu, J.; Yao, Z. Oral Probiotic Administration during Pregnancy Prevents Autism-related Behaviors in Offspring Induced by Maternal Immune Activation via Anti-inflammation in Mice. Autism Res. 2019, 12, 576–588. [Google Scholar] [CrossRef]
- Custodero, C.; Mankowski, R.T.; Lee, S.A.; Chen, Z.; Wu, S.; Manini, T.M.; Hincapie Echeverri, J.; Sabbà, C.; Beavers, D.P.; Cauley, J.A.; et al. Evidence-Based Nutritional and Pharmacological Interventions Targeting Chronic Low-Grade Inflammation in Middle-Age and Older Adults: A Systematic Review and Meta-Analysis. Ageing Res. Rev. 2018, 46, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Luan, W.; Hammond, L.A.; Vuillermot, S.; Meyer, U.; Eyles, D.W. Maternal Vitamin D Prevents Abnormal Dopaminergic Development and Function in a Mouse Model of Prenatal Immune Activation. Sci. Rep. 2018, 8, 9741. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-W.; Adams, J.B.; Coleman, D.M.; Pollard, E.L.; Maldonado, J.; McDonough-Means, S.; Caporaso, J.G.; Krajmalnik-Brown, R. Long-Term Benefit of Microbiota Transfer Therapy on Autism Symptoms and Gut Microbiota. Sci. Rep. 2019, 9, 5821. [Google Scholar] [CrossRef]
- Janssen, N.M.; Genta, M.S. The Effects of Immunosuppressive and Anti-Inflammatory Medications on Fertility, Pregnancy, and Lactation. Arch. Intern. Med. 2000, 160, 610–619. [Google Scholar] [CrossRef]
- Morse, J.E.; Calvert, S.B.; Jurkowski, C.; Tassinari, M.; Sewell, C.A.; Myers, E.R. Evidence-Based Pregnancy Testing in Clinical Trials: Recommendations from a Multi-Stakeholder Development Process. PLoS ONE 2018, 13, e0202474. [Google Scholar] [CrossRef]
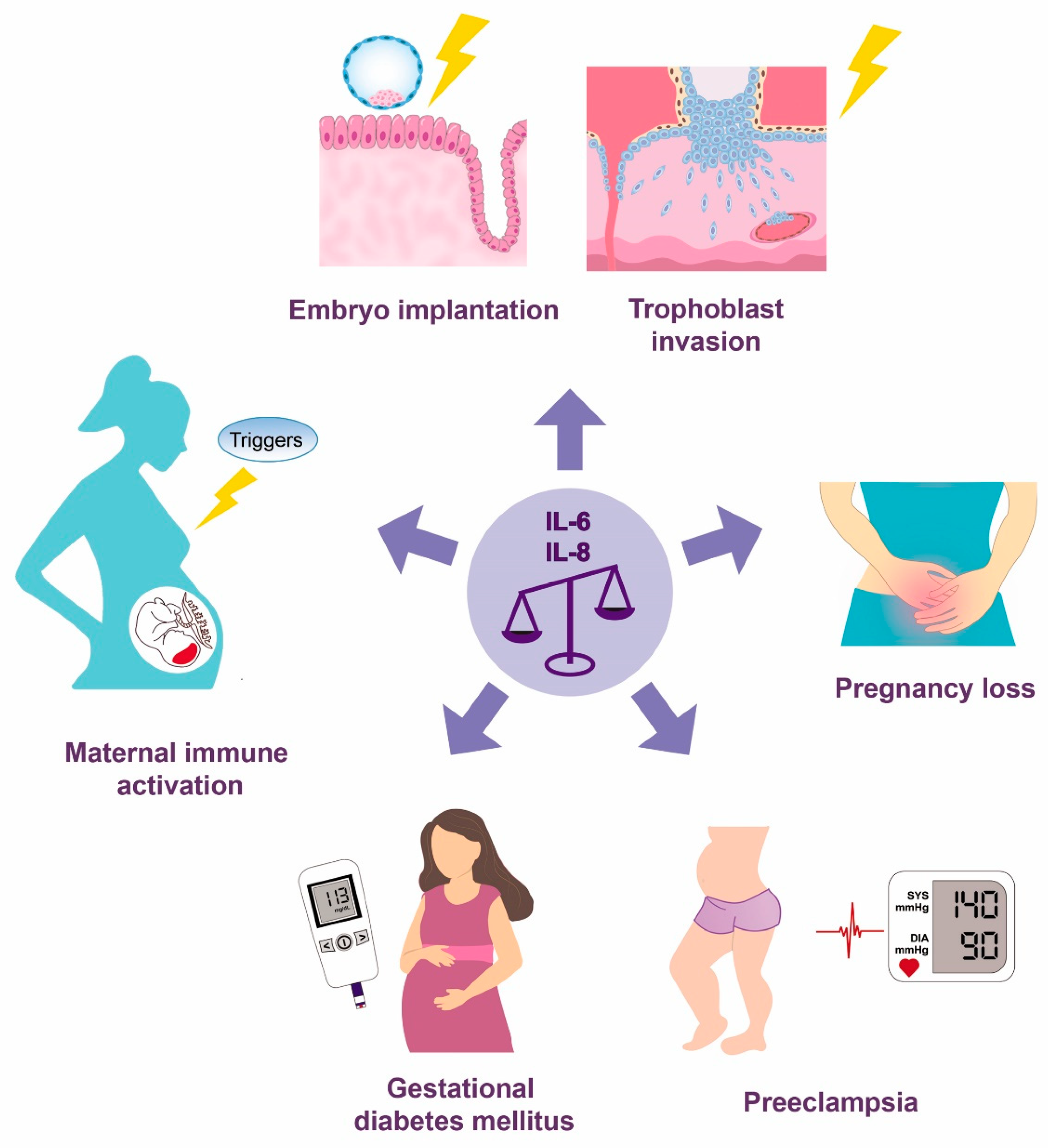
| Process | Role | Cytokine | Reference |
|---|---|---|---|
| Pregnancy establishment | Endometrial receptivity and implantation | IL-6 | [14,28] |
| IL-8 | [48,49] | ||
| Stimulation of human embryo blastulation and blastocyst hatching in vitro | IL-6 | [33] | |
| Potential indicator of human embryo developmental potential in ARTs * | IL-8 | [51] | |
| Regulation of trophoblast invasion and migration in vitro | IL-6 | [21,43,44] | |
| IL-8 | [23,24,26,53,55] | ||
| Spiral artery remodeling | IL-6 | [45] | |
| IL-8 | [45,54] | ||
| Parturition | Key gene triggering specific mechanisms in different gestational tissues leading to labor onset | IL6 | [56] |
| Most upregulated gene in the myometrium and cervix with the onset of labor | CXCL8 | [57] | |
| Stimulation of prostaglandin synthesis by decidual cells and chorioamniotic membranes | IL-6 | [58,59] | |
| Stimulation of oxytocin secretion and oxytocin receptors’ expression on MSMCs * | IL-6 | [60,61] | |
| Cervical remodeling and rupture of the gestational membranes | IL-8 | [62,63] | |
| Circulating concentrations | |||
| Pregnancy | Increased over the course of pregnancy | IL-6 | [64,65,66,67] |
| No difference between trimesters | IL-6 | [68,69,70,71,72] | |
| Decreased over the course of pregnancy | IL-6 | [73,74] | |
| IL-8 | [75] | ||
| U shape (decreased between the 1st and 2nd trimesters and increased between the 2nd and 3rd) | IL-8 | [68,69,70,76] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilotić, A.; Nacka-Aleksić, M.; Pirković, A.; Bojić-Trbojević, Ž.; Dekanski, D.; Jovanović Krivokuća, M. IL-6 and IL-8: An Overview of Their Roles in Healthy and Pathological Pregnancies. Int. J. Mol. Sci. 2022, 23, 14574. https://doi.org/10.3390/ijms232314574
Vilotić A, Nacka-Aleksić M, Pirković A, Bojić-Trbojević Ž, Dekanski D, Jovanović Krivokuća M. IL-6 and IL-8: An Overview of Their Roles in Healthy and Pathological Pregnancies. International Journal of Molecular Sciences. 2022; 23(23):14574. https://doi.org/10.3390/ijms232314574
Chicago/Turabian StyleVilotić, Aleksandra, Mirjana Nacka-Aleksić, Andrea Pirković, Žanka Bojić-Trbojević, Dragana Dekanski, and Milica Jovanović Krivokuća. 2022. "IL-6 and IL-8: An Overview of Their Roles in Healthy and Pathological Pregnancies" International Journal of Molecular Sciences 23, no. 23: 14574. https://doi.org/10.3390/ijms232314574
APA StyleVilotić, A., Nacka-Aleksić, M., Pirković, A., Bojić-Trbojević, Ž., Dekanski, D., & Jovanović Krivokuća, M. (2022). IL-6 and IL-8: An Overview of Their Roles in Healthy and Pathological Pregnancies. International Journal of Molecular Sciences, 23(23), 14574. https://doi.org/10.3390/ijms232314574







