Microgreens Biometric and Fluorescence Response to Iron (Fe) Biofortification
Abstract
:1. Introduction
2. Results
2.1. Growth Parameters
2.2. Minerals Content
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Composition of the Medium
4.3. Measurement and Collection of Growth Parameter Data
4.4. Analysis of Mineral Content
4.5. Experiment Design and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vasto, S.; Sabatino, L.; Santalucia, C.; Sciara, A.; Baldassano, S. Could bio-fortification of vegetables with iodine represent a tool to boost the immune system? A pilot study on human health. Biol. Life Sci. Forum 2022, 12, 11. [Google Scholar] [CrossRef]
- Bailey, R.L.; West, K.P.; Black, R.E. The epidemiology of global micronutrient deficiencies. Ann. Nutr. Metab. 2015, 66 (Suppl. S2), 22–33. [Google Scholar] [CrossRef] [PubMed]
- Beal, T.; Massiot, E.; Arsenault, J.E.; Smith, M.R.; Hijmans, R.J. Global trends in dietary micronutrient supplies and estimated prevalence of inadequate intakes. PLoS ONE 2017, 12, e0175554. [Google Scholar] [CrossRef] [Green Version]
- Shaw, J.G.; Friedman, J.F. Iron deficiency anemia: Focus on infectious diseases in lesser developed countries. Anemia 2011, 2011, 260380. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Jin, Y.; Li, Y.; Zhai, F.; Kok, F.; Jacobsen, E.; Yang, X. Iron and zinc deficiencies in China: What is a feasible and cost-effective strategy? Public Health Nutr. 2008, 11, 632–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, J.; Liao, P.; Wang, M. The role of emerging micro-scale vegetables in human diet and health benefits—An updated review based on microgreens. Food Funct. 2021, 12, 1914–1932. [Google Scholar] [CrossRef]
- Joosten, E. Iron deficiency anemia in older adults: A review. Geriatr. Gerontol. Int. 2018, 18, 373–379. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Palve, A.; Joshi, C.; Srivastava, R.K.; Rukhsar. Crop biofortification for iron (Fe), zinc (Zn) and vitamin A with transgenic approaches. Heliyon 2019, 5, e01914. [Google Scholar] [CrossRef] [Green Version]
- Aciksoz, S.B.; Yazici, A.; Ozturk, L.; Cakmak, I. Biofortification of wheat with iron through soil and foliar application of nitrogen and iron fertilizers. Plant Soil 2011, 349, 215–225. [Google Scholar] [CrossRef]
- He, W.; Shohag, M.J.; Wei, Y.; Feng, Y.; Yang, X. Iron concentration, bioavailability, and nutritional quality of polished rice affected by different forms of foliar iron fertilizer. Food Chem. 2013, 141, 4122–4126. [Google Scholar] [CrossRef] [PubMed]
- Kou, L.; Yang, T.; Luo, Y.; Liu, X.; Huang, L.; Codling, E. Pre-harvest calcium application increases biomass and delays senescence of broccoli microgreens. Postharvest Biol. Technol. 2014, 87, 70–78. [Google Scholar] [CrossRef]
- Fraga, C.G. Relevance, essentiality and toxicity of trace elements in human health. Mol. Asp. Med. 2005, 26, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Tavares Antunes, P.; Vaz-Tostes, M.d.G.; Tomáz Sant’Ana, C.; Araújo de Faria, R.; Lopes Toledo, R.C.; Brunoro Costa, N.M. Bioavailability of iron and the influence of vitamin A in biofortified foods. Agronomy 2019, 9, 777. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Galera, S.; Rojas, E.; Sudhakar, D.; Zhu, C.; Pelacho, A.M.; Capell, T.; Christou, P. Critical evaluation of strategies for mineral fortification of staple food crops. Transgenic Res. 2010, 19, 165–180. [Google Scholar] [CrossRef]
- Rout, G.R.; Sahoo, S.K. Role of iron in plant growth and metabolism. Rev. Agric. Sci. 2015, 3, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Fernandez, A.; Garcia-Lavina, P.; Fidalgo, C.; Abadia, J.; Abadía, A. Foliar fertilization to control iron chlorosis in pear (Pyrus communis L.) trees. Plant Soil 2004, 263, 5–15. [Google Scholar] [CrossRef]
- Ning, P.; Wang, S.; Fei, P.; Zhang, X.; Dong, J.; Shi, J.; Tian, X. Enhancing zinc accumulation and bioavailability in wheat grains by integrated zinc and pesticide application. Agronomy 2019, 9, 530. [Google Scholar] [CrossRef] [Green Version]
- Niyigaba, E.; Twizerimana, A.; Mugenzi, I.; Ngnadong, W.A.; Ye, Y.P.; Wu, B.M.; Hai, J.B. Winter wheat grain quality, zinc and iron concentration affected by a combined foliar spray of zinc and iron fertilizers. Agronomy 2019, 9, 250. [Google Scholar] [CrossRef] [Green Version]
- Palmgren, M.G.; Clemens, S.; Williams, L.E.; Krämer, U.; Borg, S.; Schjørring, J.K.; Sanders, D. Zinc biofortification of cereals: Problems and solutions. Trends Plant Sci. 2008, 13, 464–473. [Google Scholar] [CrossRef]
- Plum, L.M.; Rink, L.; Haase, H. The Essential Toxin: Impact of Zinc on Human Health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef]
- Frei, M.; Tetteh, R.N.; Razafindrazaka, A.L.; Fuh, M.A.; Wu, L.-B.; Becker, M. Responses of rice to chronic and acute iron toxicity: Genotypic differences and biofortification aspects. Plant Soil 2016, 408, 149–161. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C. Enhancing quality of fresh vegetables through salinity eustress and biofortification applications facilitated by soilless cultivation. Front. Plant Sci. 2018, 9, 1254. [Google Scholar] [CrossRef] [PubMed]
- Szerement, J.; Szatanik-Kloc, A.; Mokrzycki, J.; Mierzwa-Hersztek, M. Agronomic biofortification with Se, Zn, and Fe: An effective strategy to enhance crop nutritional quality and stress defence—A review. J. Soil Sci. Plant Nutr. 2021, 22, 1129–1159. [Google Scholar] [CrossRef]
- Xiao, Z.; Lester, G.E.; Luo, Y.; Wang, Q. Assessment of vitamin and carotenoid concentrations of emerging food products: Edible microgreens. J. Agric. Food Chem. 2012, 60, 7644–7651. [Google Scholar] [CrossRef] [PubMed]
- Misra, G.; Gibson, K.E. Characterization of microgreen growing operations and associated food safety practices. Food Prot. Trends 2021, 41, 56–69. [Google Scholar] [CrossRef]
- Truzzi, F.; Whittaker, A.; Roncuzzi, C.; Saltari, A.; Levesque, M.P.; Dinelli, G. Microgreens: Functional food with antiproliferative cancer properties influenced by light. Foods 2021, 10, 1690. [Google Scholar] [CrossRef] [PubMed]
- Yadav, L.P.; Koley, T.K.; Tripathi, A.; Singh, S. Antioxidant potentiality and mineral content of summer season leafy greens: Comparison at mature and microgreen stages using chemometric. Agric. Res. 2019, 8, 165–175. [Google Scholar] [CrossRef]
- Lester, G.; Hallman, G.; Pérez, J.A. γ-Irradiation dose: Effects on baby-leaf spinach ascorbic acid, carotenoids, folate, alpha-tocopherol, and phylloquinone concentrations. J. Agric. Food Chem. 2010, 58, 4901–4906. [Google Scholar] [CrossRef] [PubMed]
- Ghoora, M.D.; Babu, D.R.; Srividya, N. Nutrient composition, oxalate content and nutritional ranking of ten culinary microgreens. J. Food Compos. Anal. 2020, 91, 103495. [Google Scholar] [CrossRef]
- Xiao, Z.; Codling, E.E.; Luo, Y.; Nou, X.; Lester, G.E.; Wang, Q. Microgreens of Brassicaceae: Mineral composition and content of 30 varieties. J. Food Compos. Anal. 2016, 49, 87–93. [Google Scholar] [CrossRef]
- Xiao, J.; Bai, W. Bioactive phytochemicals. Crit. Rev. Food Sci. Nutr. 2019, 59, 827–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomas, M.; Zhang, L.; Zengin, G.; Rocchetti, G.; Capanoglu, E.; Lucini, L. Metabolomic insight into the profile, in vitro bioaccessibility and bioactive properties of polyphenols and glucosinolates from four Brassicaceae microgreens. Food Res. Int. 2021, 140, 110039. [Google Scholar] [CrossRef] [PubMed]
- Vaštakaitė-Kairienė, V.; Brazaitytė, A.; Miliauskienė, J.; Laužikė, K.; Sutulienė, R.; Duchovskis, P.; Samuolienė, G. Iron biofortification of broccoli microgreens under different radiation spectrum and composition of nutrient solution. Acta Hortic. 2022, 1337, 187–194. [Google Scholar] [CrossRef]
- Di Gioia, F.; Petropoulos, S.A.; Ozores-Hampton, M.; Morgan, K.; Rosskopf, E.N. Zinc and iron agronomic biofortification of Brassicaceae microgreens. Agronomy 2019, 9, 677. [Google Scholar] [CrossRef] [Green Version]
- Przybysz, A.; Wrochna, M.; Małecka-Przybysz, M.; Gawrońska, H.; Gawroński, S.W. Vegetable sprouts enriched with iron: Effects on yield, ROS generation and antioxidative system. Sci. Hortic. 2016, 203, 110–117. [Google Scholar] [CrossRef]
- Thongbai, P.; Goodman, B.A. Free radical generation and post-anoxic injury in rice grown in an iron-toxic soil. J. Plant Nutr. 2000, 23, 1887–1900. [Google Scholar] [CrossRef]
- Hänsch, R.; Mendel, R.R. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef]
- Sun, Z.; Yue, Z.; Liu, H.; Ma, K.; Li, C. Microbial-assisted wheat iron biofortification using endophytic Bacillus altitudinis WR10. Front. Nutr. 2021, 8, 704030. [Google Scholar] [CrossRef]
- Kowitcharoen, L.; Phornvillay, S.; Lekkham, P.; Pongprasert, N.; Srilaong, V. Bioactive composition and nutritional profile of microgreens cultivated in Thailand. Appl. Sci. 2021, 11, 7981. [Google Scholar] [CrossRef]
- Song, S.J.; Hansen, U. Stress effects on photosynthesis of greenhouse plants as measured by the fluorescence method. Korean J. Environ. Agric. 1994, 13, 183–190. [Google Scholar]
- Hogewoning, S.W.; Wientjes, E.; Douwstra, P.; Trouwborst, G.; Ieperen, W.V.; Croce, R.; Harbinson, J. Photosynthetic quantum yield dynamics: From photosystems to leaves. Plant Cell 2012, 24, 1921–1935. [Google Scholar] [CrossRef]
- Dobosy, P.; Endrédi, A.; Sandil, S.; Vetési, V.; Rékási, M.; Takács, T.; Záray, G. Biofortification of potato and carrot with iodine by applying different soils and irrigation with iodine-containing water. Front. Plant Sci. 2020, 11, 593047. [Google Scholar] [CrossRef] [PubMed]
- Kavčič, A.; Budič, B.; Vogel-Mikuš, K. The effects of selenium biofortification on mercury bioavailability and toxicity in the lettuce-slug food chain. Food Chem. Toxicol. 2020, 135, 110939. [Google Scholar] [CrossRef] [PubMed]
- Lanquar, V.; Lelièvre, F.; Bolte, S.; Hamès, C.; Alcon, C.; Neumann, D.; Vansuyt, G.; Curie, C.; Schröder, A.; Krämer, U.; et al. Mobilization of vacuolar iron by AtNRAMP3 and AtNRAMP4 is essential for seed germination on low iron. EMBO J. 2005, 24, 4041–4051. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Cao, X.; Jia, X.; Liu, L.; Cao, H.; Qin, W.; Li, M. Iron deficiency leads to chlorosis through impacting chlorophyll synthesis and nitrogen metabolism in Areca catechu L. Front. Plant Sci. 2021, 12, 710093. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Stockwell, B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014, 10, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Roth, A.J.; Garrick, M.D. Iron interactions and biological interactions mediating the physiological and toxic actions of manganese. Biochem. Pharmacol. 2003, 66, 1–13. [Google Scholar] [CrossRef]
- Lombj, E.; Tearall, K.L.; Howarth, J.R.; Zhao, F.J.; Hawkesford, M.J.; McGrath, S.P. Influence of iron status on cadmium and zinc uptake by different ecotypes of the hyperaccumulator Thlaspi caerulescens. Plant Physiol. 2002, 128, 1359–1367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Dorlodot, S.; Lutts, S.; Bertin, P. Effects of ferrous iron toxicity on the growth and mineral composition of an interspecific rice. J. Plant Nutr. 2005, 28, 1–20. [Google Scholar] [CrossRef]
- Rietra, R.P.J.J.; Heinen, M.; Dimkpa, C.O.; Bindraban, P.S. Effects of nutrient antagonism and synergism on yield and fertilizer use efficiency. Commun. Soil Sci. Plant Anal. 2017, 48, 1895–1920. [Google Scholar] [CrossRef] [Green Version]
- Snowden, R.E.D.; Wheeler, D. Chemical changes in selected wetland plant species with increasing Fe supply, with specific reference to root precipitates and Fe tolerance. New Phytol. 1995, 131, 503–520. [Google Scholar] [CrossRef] [PubMed]
- Souza-Santos, P.; Ramos, R.S.; Ferreira, S.T.; Carvalho-Alves, P.C. Iron-induced oxidative damage of corn root plasma membrane H+-ATPase. Biochem. Biophys. Acta 2001, 1512, 357–366. [Google Scholar] [CrossRef] [Green Version]
- Jalal, A.; Shah, S.; Filho, M.C.M.T.; Khan, A.; Shah, T.; Ilyas, M.; Leonel Rosa, P.A. Agro-biofortification of zinc and iron in wheat grains. Gesunde Pflanz. 2020, 72, 227–236. [Google Scholar] [CrossRef]
- Abbas, G.; Khan, M.Q.; Jamil, M.; Tahir, M.; Hussain, F. Nutrient uptake, growth and yield of wheat (Triticum aestivum L.) as affected by zinc application rates. Int. Asian J. Agric. Biol. 2009, 11, 389–396. [Google Scholar]
- Fruit Products and Vegetable. The Preparation of Samples and Method of Physic Chemist Investigations: Marking the Content of Dry Mass with Weight Method; PN-90/A-75101/03; PKN: Warsaw, Poland, 1990. (In Polish) [Google Scholar]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]


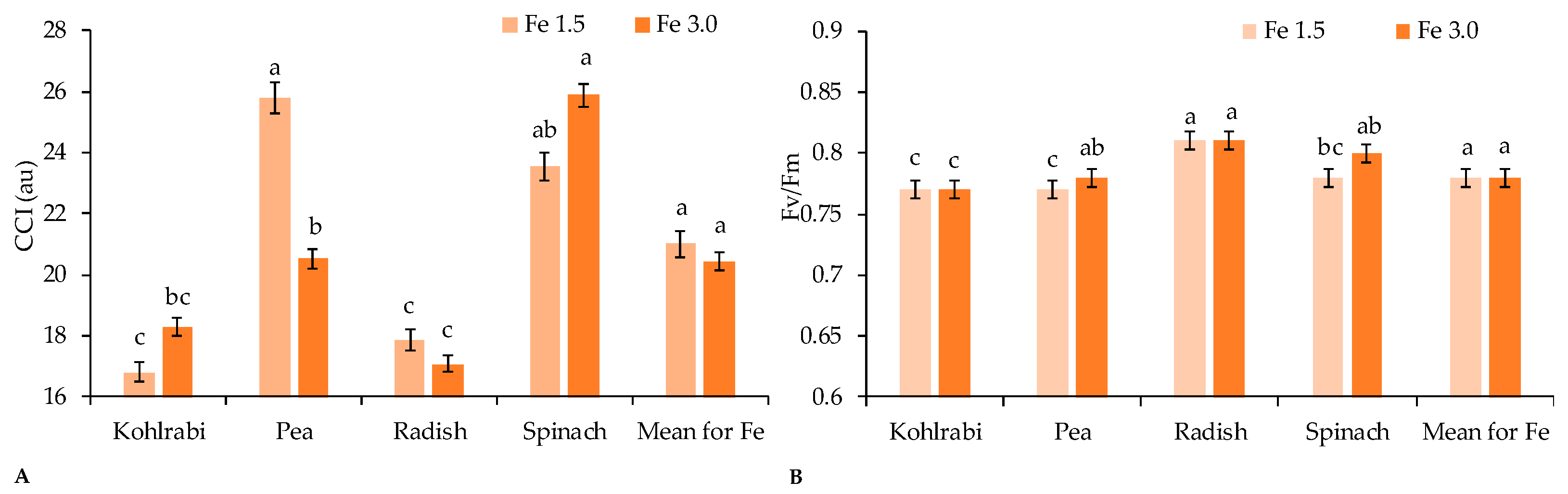

| Treatment | Species (g·Plant−1) | Mean | |||
|---|---|---|---|---|---|
| Kohlrabi | Pea | Radish | Spinach | ||
| Fe 1.5 | 0.0675 e * | 0.1438 a | 0.1138 b | 0.0813 d | 0.1016 a |
| Fe 3.0 | 0.0538 f | 0.1375 a | 0.0988 c | c 0.0700 de | 0.0900 b |
| Mean | c 0.0606 d | 0.1406 a | 0.1063 b | 0.0756 c | |
| Species | Treatment | Mineral Content (mg kg−1 dry Matter) | |||
|---|---|---|---|---|---|
| Cu | Fe | Mn | Zn | ||
| Kohlrabi | Fe 1.5 | 4.30 c * | 109.40 d | 72.80 b | 26.65 c |
| Fe 3.0 | 3.40 d | 125.10 c | 97.25 a | 23.71 d | |
| Pea | Fe 1.5 | 6.90 a | 174.00 ab | 27.45 d | 62.95 a |
| Fe 3.0 | 6.10 b | 180.05 a | 27.00 d | 55.25 b | |
| Radish | Fe 1.5 | 3.04 d | 122.55 c | 54.70 bc | 24.49 cd |
| Fe 3.0 | 2.25 e | 123.55 c | 62.70 b | 22.25 d | |
| Spinach | Fe 1.5 | 3.12 d | 123.10 c | 42.90 cd | 22.40 d |
| Fe 3.0 | 2.20 e | 167.30 b | 54.90 bc | 21.70 d | |
| Mean | Fe 1.5 | 4.34 a | 132.26 b | 49.46 b | 34.10 a |
| Fe 3.0 | 3.49 b | 149.00 a | 60.46 a | 30.73 b | |
| Treatment | Species (µg kg−1d.m) | Mean | |||
|---|---|---|---|---|---|
| Kohlrabi | Pea | Radish | Spinach | ||
| Fe | |||||
| Fe 1.5 | 7.3463 e * | 24.9875 a | 14.0188 b | 10.0675 d | 14.1050 a |
| Fe 3.0 | 6.7375 e | 24.7600 a | 12.3950 bc | 11.7500 cd | 13.9106 a |
| Mean | 7.0419 d | 24.8738 a | 13.2069 b | 10.9088 c | |
| Mn | |||||
| Fe 1.5 | 5.0738 b | 3.9563 c | 6.3375 a | 3.5088 c | 4.7191 a |
| Fe 3.0 | 5.2388 b | 3.7138 c | 6.2938 a | 3.8575 c | 4.7759 a |
| Mean | 5.1563 b | 3.8350 c | 6.3156 a | 3.6831 c | |
| Zn | |||||
| Fe 1.5 | 1.7925 de | 9.0313 a | 2.7863 c | 1.8313 de | 3.8603 a |
| Fe 3.0 | 1.2775 e | 7.6000 b | 2.2325 cd | 1.5238 de | 3.1584 b |
| Mean | 1.5350 c | 8.3156 a | 2.5094 b | 1.6775 c | |
| Cu | |||||
| Fe 1.5 | 0.2875 cd | 0.9950 a | 0.3500 c | 0.2575 de | 0.4725 a |
| Fe 3.0 | 0.1838 ef | 0.8388 b | 0.2275 def | 0.1538 f | 0.3509 b |
| Mean | 0.2356 bc | 0.9169 a | 0.2888 b | 0.2056 c | |
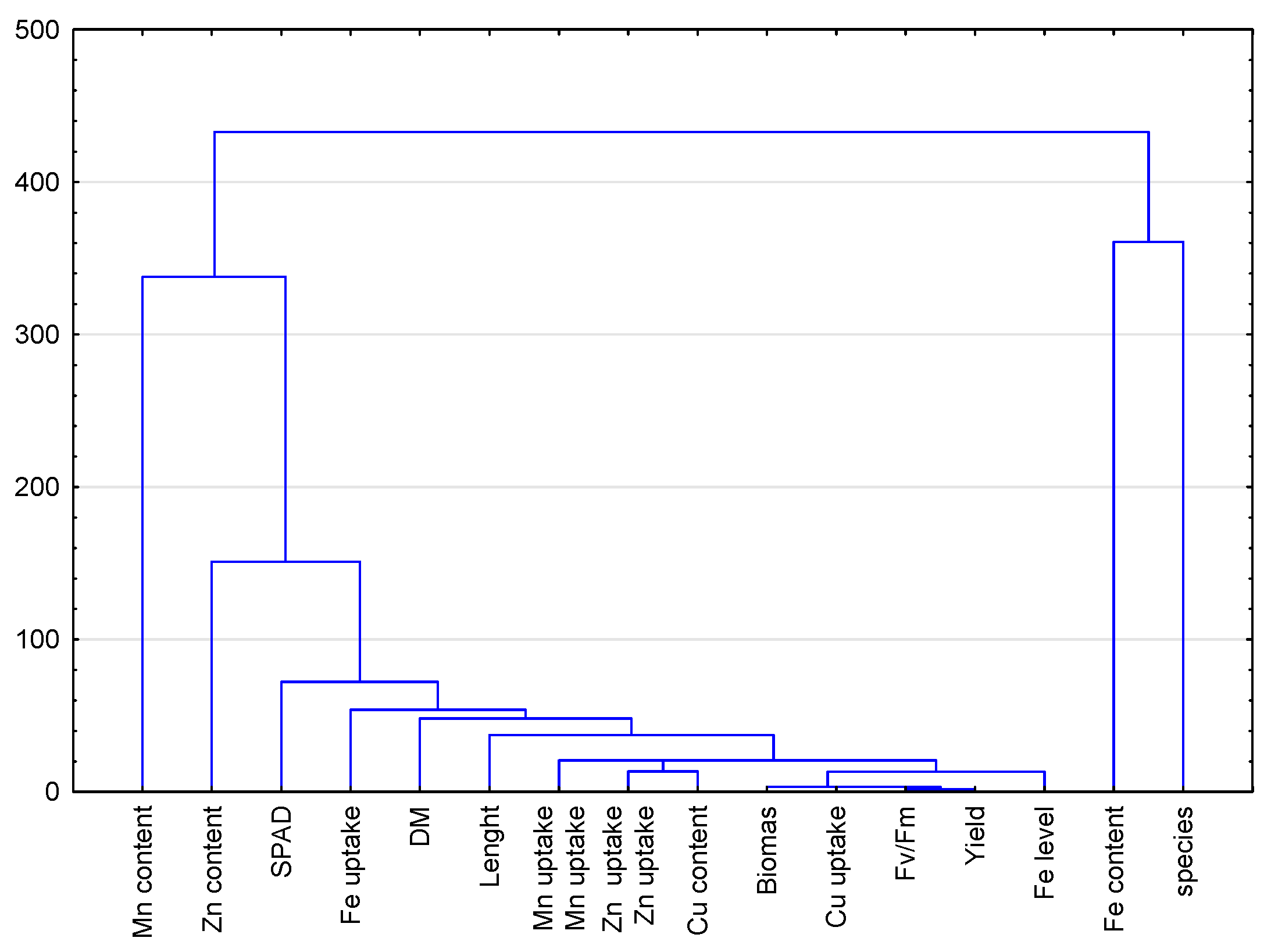
| Yield | DM (%) | Length | SPAD | FV/FM | Content | Biomass | Uptake | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe | Mn | Zn | Cu | Fe | Mn | Zn | Cu | |||||||
| Yield | 1.00 | |||||||||||||
| DM (%) | 0.05 | 1.00 | ||||||||||||
| Lenght | 0.81 | 0.15 | 1.00 | |||||||||||
| SPAD | 0.37 | −0.37 | 0.43 | 1.00 | ||||||||||
| FV/FM | 0.11 | −0.07 | −0.13 | 0.07 | 1.00 | |||||||||
| Fe content | 0.69 | −0.10 | 0.81 | 0.70 | 0.03 | 1.00 | ||||||||
| Mn content | −0.73 | −0.01 | −0.74 | −0.51 | −0.09 | −0.62 | 1.00 | |||||||
| Zn content | 0.67 | 0.24 | 0.95 | 0.41 | −0.20 | 0.74 | −0.67 | 1.00 | ||||||
| Cu content | 0.50 | 0.29 | 0.85 | 0.29 | −0.33 | 0.57 | −0.48 | 0.93 | 1.00 | |||||
| Biomass | 0.80 | 0.37 | 0.79 | 0.23 | 0.12 | 0.56 | −0.74 | 0.76 | 0.62 | 1.00 | ||||
| Fe uptake | 0.83 | 0.25 | 0.91 | 0.40 | 0.03 | 0.78 | −0.76 | 0.86 | 0.72 | 0.94 | 1.00 | |||
| Mn uptake | −0.22 | 0.36 | −0.42 | −0.58 | 0.19 | −0.47 | 0.57 | −0.40 | −0.31 | −0.01 | −0.20 | 1.00 | ||
| Zn uptake | 0.75 | 0.29 | 0.94 | 0.39 | −0.12 | 0.73 | −0.72 | 0.96 | 0.86 | 0.89 | 0.96 | −0.28 | 1.00 | |
| Cu uptake | 0.72 | 0.32 | 0.93 | 0.35 | −0.16 | 0.68 | −0.67 | 0.95 | 0.91 | 0.87 | 0.93 | −0.23 | 0.99 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frąszczak, B.; Kleiber, T. Microgreens Biometric and Fluorescence Response to Iron (Fe) Biofortification. Int. J. Mol. Sci. 2022, 23, 14553. https://doi.org/10.3390/ijms232314553
Frąszczak B, Kleiber T. Microgreens Biometric and Fluorescence Response to Iron (Fe) Biofortification. International Journal of Molecular Sciences. 2022; 23(23):14553. https://doi.org/10.3390/ijms232314553
Chicago/Turabian StyleFrąszczak, Barbara, and Tomasz Kleiber. 2022. "Microgreens Biometric and Fluorescence Response to Iron (Fe) Biofortification" International Journal of Molecular Sciences 23, no. 23: 14553. https://doi.org/10.3390/ijms232314553
APA StyleFrąszczak, B., & Kleiber, T. (2022). Microgreens Biometric and Fluorescence Response to Iron (Fe) Biofortification. International Journal of Molecular Sciences, 23(23), 14553. https://doi.org/10.3390/ijms232314553







