Comparative Transcriptomic Analysis of Inarching Invigorating Rootstock onto Incompatible Grafts in Citrus
Abstract
:1. Introduction
2. Results
2.1. Double-Root System Enhanced the Growth State of Incompatible Grafts
2.2. Effect of Inarched Rootstock on Transcript Levels of Different Grafted Plants
2.3. Identification of DEGs between Different Graft Combinations
2.4. Identification of DEGs between Single-Root and Double-Root Grafted Plants
2.5. Identification of WGCNA Modules Associated with Hormone Pathway
2.6. Validation of Candidate Genes by qRT-PCR Analysis
3. Discussion
3.1. Screening and Identification Candidate Graft Incompatibility Genes
3.2. Central Role of Hormonal Balance in Graft Compatibility
3.3. Underlying Mechanisms of Graft Compatibility in Citrus
4. Materials and Methods
4.1. Plant Materials and Treatment
4.2. Measurement of Carotenoids, Chlorophyll, Soluble Sugar and Starch
4.3. Measurement of Endogenous Phytohormones
4.4. Anatomical Observation
4.5. RNA Extraction, RNA-Seq Library Construction
4.6. Real-Time PCR Analysis
4.7. Statistical Analysis
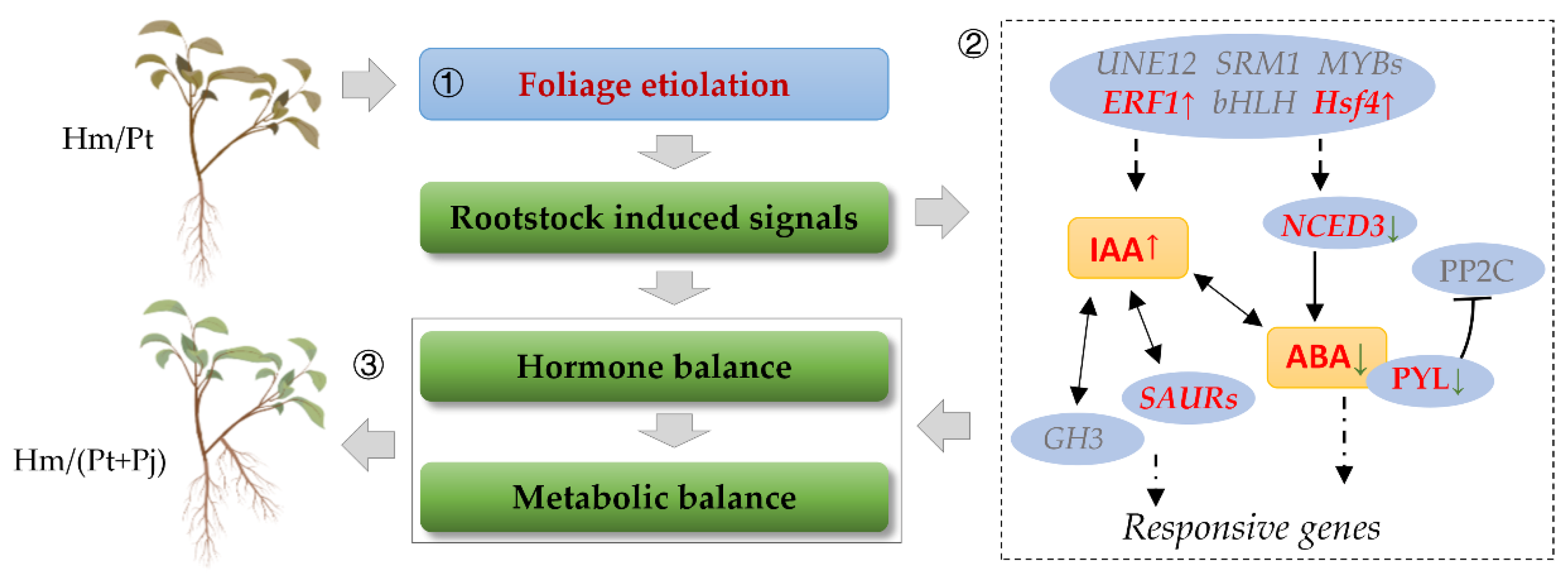
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adhikari, P.B.; Xu, Q.; Notaguchi, M. Compatible graft establishment in fruit trees and its potential markers. Agronomy 2022, 12, 1981. [Google Scholar] [CrossRef]
- Warschefsky, E.J.; Klein, L.L.; Frank, M.H.; Chitwood, D.H.; Londo, J.P.; von Wettberg, E.J.B.; Miller, A.J. Rootstocks: Diversity, domestication, and impacts on shoot phenotypes. Trends Plant Sci. 2016, 21, 418–437. [Google Scholar] [CrossRef] [PubMed]
- Zarrouk, O.; Gogorcena, Y.; Moreno, M.A.; Pinochet, J. Graft compatibility between peach cultivars and Prunus rootstocks. Hortscience 2006, 41, 1389–1394. [Google Scholar] [CrossRef]
- He, W.; Pan, H.L.; Pan, T.F.; Tang, H.R.; Wang, X.R.; Pan, D.M. Research progress on the interaction between scion and rootstock in fruit trees. Acta Hortic. Sinica. 2017, 44, 1645–1657, (In Chinese with English abstract). [Google Scholar]
- Gautier, A.T.; Chambaud, C.; Brocard, L.; Ollat, N.; Gambetta, G.A.; Delrot, S.; Cookson, S.J. Merging genotypes: Graft union formation and scion–rootstock interactions. J. Exp. Bot. 2019, 70, 747–755. [Google Scholar] [CrossRef]
- Dong, D.; Shi, Y.; Mou, Z.; Chen, S.; Zhao, D. Grafting: A potential method to reveal the differential accumulation mechanism of secondary metabolites. Hortic. Res. 2022, 9, c50. [Google Scholar] [CrossRef]
- Gaut, B.S.; Miller, A.J.; Seymour, D.K. Living with two genomes: Grafting and its implications for plant genome-to-genome interactions, phenotypic variation, and evolution. Annu. Rev. Genet. 2019, 53, 195–215. [Google Scholar] [CrossRef] [PubMed]
- Maizel, A.; Markmann, K.; Timmermans, M.; Wachter, A. To move or not to move: Roles and specificity of plant RNA mobility. Curr. Opin. Plant Biol. 2020, 57, 52–60. [Google Scholar] [CrossRef]
- Gong, Y.Q.; Qi, Y.H.; Fu, X.K.; Yang, R.; Chen, T.; Tang, H.R.; Wang, X.R. Graft compatibility evaluation of different rootstock-scion combinations of honey pomelo at seedling stage. J. Trop. Subtropic. Bot. 2016, 24, 287–295, (In Chinese with English abstract). [Google Scholar]
- He, W.; Wang, Y.; Chen, Q.; Sun, B.; Tang, H.; Pan, D.; Wang, X. Dissection of the mechanism for compatible and incompatible graft combinations of Citrus grandis (L.) Osbeck (‘Hongmian Miyou’). Int. J. Mol. Sci. 2018, 19, 505. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Xie, R.; Wang, Y.; Chen, Q.; Wang, H.; Yang, S.; Luo, Y.; Zhang, Y.; Tang, H.; Gmitter, F.G.; et al. Comparative transcriptomic analysis on compatible/incompatible grafts in Citrus. Hortic. Res. 2022, 9, uhab072. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wang, Y.; Chen, Q.; Xu, S.R.; Tang, H.R.; Pan, D.M.; Wang, X.R. Photosynthetic characteristics of etiolated seedling of Citrus grandis (L.) Osbeck. cv. ‘Hongmian Miyou’ grafted to Poncirus trifoliata and expression of CgSGR. Acta Bot. Boreal Occident. Sin. 2018, 38, 0808–0815, (In Chinese with English abstract). [Google Scholar]
- Qureshi, M.A.; Jaskani, M.J.; Khan, A.S.; Ahmad, R. Influence of endogenous plant hormones on physiological and growth attributes of kinnow mandarin grafted on nine rootstocks. J. Plant Growth Regul. 2022, 41, 1254–1264. [Google Scholar] [CrossRef]
- Kamboj, J.S.; Browning, G.; Blake, P.S.; Quinlan, J.D.; Baker, D.A. GC-MS-SIM analysis of abscisic acid and indole-3-acetic acid in shoot bark of apple rootstocks. Plant Growth Regul. 1999, 28, 21–27. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Imtiaz, M.; Kong, Q.; Cheng, F.; Ahmed, W.; Huang, Y.; Bie, Z. Grafting: A technique to modify ion accumulation in horticultural crops. Front. Plant Sci. 2016, 7, 1457. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Wei, Q.; Yan, T.; Pan, Z.; Liu, Y.; Peng, S.A. Improving the boron uptake of boron-deficient navel orange plants under low boron conditions by inarching boron-efficient rootstock. Sci. Hortic. 2016, 199, 49–55. [Google Scholar] [CrossRef]
- Du, W.; Hussain, S.B.; Jin, L.; Liu, X.; Li, R.; Han, Z.; Liu, Y.; Pan, Z.; Peng, S. Characteristics of boron distribution in the ’Newhall’ navel orange plant with two root systems. Plant Physiol. Biochem. 2021, 167, 42–48. [Google Scholar] [CrossRef]
- Li, X.; Zhou, H.; Cheng, L.; Ma, N.; Cui, B.; Wang, W.; Zhong, Y.; Liao, H. Shoot-to-root translocated GmNN1/FT2a triggers nodulation and regulates soybean nitrogen nutrition. PLoS. Biol. 2022, 20, e3001739. [Google Scholar] [CrossRef]
- Shabnam, R.; Tarek, M.H.; Iqbal, M.T. Understanding phosphorus dynamics in wheat plant and growth response in a split-root system in acidic soil. Agric. Nat. Resour. 2018, 52, 259–265. [Google Scholar] [CrossRef]
- Sun, F.; Ma, S.; Gao, L.; Qu, M.; Tian, Y. Enhancing root regeneration and nutrient absorption in double-rootcutting grafted seedlings by regulating light intensity and photoperiod. Sci. Hortic. 2020, 264, 109192. [Google Scholar] [CrossRef]
- Pulici, J.; Murata, M.M.; Johnson, E.G. Early physiological plant response and systemic effects of huanglongbing infection in split-root plants. Phytopathology 2022, 112, 1833–1843. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Huang, X.; Chen, T.; Zhang, J.; Wang, Y.; Chen, Q.; Lei, Q.; Tang, H.; Wang, X.R. A new citrus rootstock ‘Pujiang Xiangcheng’ (Citrus junos). J. Fruit Sci. 2017, 37, 917–920, (In Chinese with English abstract). [Google Scholar]
- Ernst, J.; Bar-Joseph, Z. STEM: A tool for the analysis of short time series gene expression data. BMC Bioinf. 2006, 7, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, B.; Liu, Q.; Wang, B.; Yuan, F. Roles of phytohormones and their signaling pathways in leaf development and stress responses. J. Agric. Food Chem. 2021, 69, 3566–3584. [Google Scholar] [CrossRef]
- Sun, X.; Wen, C.; Xu, J.; Wang, Y.; Zhu, J.; Zhang, Y. The apple columnar gene candidate MdCoL and the AP2/ERF factor MdDREB2 positively regulate ABA biosynthesis by activating the expression of MdNCED6/9. Tree Physiol. 2021, 41, 1065–1076. [Google Scholar] [CrossRef]
- Wu, C. Heat shock transcription factors: Structure and regulation. Annu. Rev. Cell Dev. Biol. 1995, 11, 441–469. [Google Scholar] [CrossRef]
- Canher, B.; Heyman, J.; Savina, M.; Devenan, A.; Eekhout, T.; Vercauteren, I.; Prinsen, E.; Matosevich, R.; Xu, J.; Mironova, V.; et al. Rocks in the auxin stream: Wound-induced auxin accumulation and ERF115 expression synergistically drive stem cell regeneration. Proc. Natl. Acad. Sci. USA 2020, 117, 16667–16677. [Google Scholar] [CrossRef]
- Ding, Z.; Li, S.; An, X.; Liu, X.; Qin, H.; Wang, D. Transgenic expression of MYB15 confers enhanced sensitivity to abscisic acid and improved drought tolerance in Arabidopsis thaliana. J. Genet. Genom. 2009, 36, 17–29. [Google Scholar] [CrossRef]
- Holl, J.; Vannozzi, A.; Czemmel, S.; D’Onofrio, C.; Walker, A.R.; Rausch, T.; Lucchin, M.; Boss, P.K.; Dry, I.B.; Bogs, J. The R2R3-MYB transcription factors MYB14 and MYB15 regulate stilbene biosynthesis in Vitis vinifera. Plant Cell 2013, 25, 4135–4149. [Google Scholar] [CrossRef] [Green Version]
- Kuromori, T.; Seo, M.; Shinozaki, K. ABA transport and plant water stress responses. Trends Plant Sci. 2018, 23, 513–522. [Google Scholar] [CrossRef]
- Zhai, L.; Wang, X.; Tang, D.; Qi, Q.; Yer, H.; Jiang, X.; Han, Z.; McAvoy, R.; Li, W.; Li, Y. Molecular and physiological characterization of the effects of auxin-enriched rootstock on grafting. Hortic. Res. 2021, 8, 184. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Underhill, S.J.R. Differential transcription pathways associated with rootstock-induced dwarfing in breadfruit (Artocarpus altilis) scions. BMC Plant Biol. 2021, 21, 261. [Google Scholar] [CrossRef] [PubMed]
- Sousa, M.C.; Marinho, C.S.; Silva, M.P.S.; Carvalho, W.S.G.; Amaral, B.D.; Assis-Gomes, M.M.; Rodrigues, W.P.; Campostrini, E. Effects of grafting and gradual rootstock substitution on gas exchanges of orange seedlings under high atmospheric evaporative demand. Sci. Hortic. 2019, 247, 67–74. [Google Scholar] [CrossRef]
- Li, W.R.; de Ollas, C.; Dodd, I.C. Long-distance ABA transport can mediate distal tissue responses by affecting local ABA concentrations. J. Integr. Plant Biol. 2018, 60, 16–33. [Google Scholar] [CrossRef]
- Primo-Capella, A.; Forner-Giner, M.A.; Martinez-Cuenca, M.R.; Terol, J. Comparative transcriptomic analyses of citrus cold-resistant vs. sensitive rootstocks might suggest a relevant role of ABA signaling in triggering cold scion adaption. BMC Plant Biol. 2022, 22, 209. [Google Scholar] [CrossRef]
- Zheng, X.D.; Zhao, Y.; Shan, D.Q.; Shi, K.; Wang, L.; Li, Q.T.; Wang, N.; Zhou, J.Z.; Yao, J.Z.; Xue, Y.; et al. MdWRKY9 overexpression confers intensive dwarfing in the M26 rootstock of apple by directly inhibiting brassinosteroid synthetase MdDWF4 expression. New Phytol. 2018, 217, 1086–1098. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.G.; Sun, H.H.; Tian, J.X.; Zhang, G.Y.; Gong, G.Y.; Ren, Y.; Zhang, J.; Li, M.Y.; Zhang, H.Y.; Li, H.Z.; et al. Grafting delays watermel on fruit ripening by altering gene expression of ABA centric phytohormone signaling. Front. Plant Sci. 2021, 12, 624319. [Google Scholar] [CrossRef]
- Himmelbach, A.; Yang, Y.; Grill, E. Relay and control of abscisic acid signaling. Curr. Opin. Plant Biol. 2003, 6, 470–479. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, F.; Friml, J.; Ding, Z. Auxin signaling: Research advances over the past 30 years. J. Integr. Plant Biol. 2022, 64, 371–392. [Google Scholar] [CrossRef]
- Wellburn, A.R.; Lichtenthaler, H. Formulae and Program to Determine Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents; Springer: Dordrecht, The Netherlands, 1984; pp. 9–12. [Google Scholar]
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013, 14, R36. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Xu, Y.; Zhang, S.; Cao, L.; Huang, Y.; Cheng, J.; Wu, G.; Tian, S.; Chen, C.; Liu, Y.; et al. Genomic analyses of primitive, wild and cultivated citrus provide insights into asexual reproduction. Nat. Genet. 2017, 49, 765–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinf. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, W.; Xie, R.; Luo, L.; Chai, J.; Wang, H.; Wang, Y.; Chen, Q.; Wu, Z.; Yang, S.; Li, M.; et al. Comparative Transcriptomic Analysis of Inarching Invigorating Rootstock onto Incompatible Grafts in Citrus. Int. J. Mol. Sci. 2022, 23, 14523. https://doi.org/10.3390/ijms232314523
He W, Xie R, Luo L, Chai J, Wang H, Wang Y, Chen Q, Wu Z, Yang S, Li M, et al. Comparative Transcriptomic Analysis of Inarching Invigorating Rootstock onto Incompatible Grafts in Citrus. International Journal of Molecular Sciences. 2022; 23(23):14523. https://doi.org/10.3390/ijms232314523
Chicago/Turabian StyleHe, Wen, Rui Xie, Liang Luo, Jiufeng Chai, Hao Wang, Yan Wang, Qing Chen, Zhiwei Wu, Shaofeng Yang, Mengyao Li, and et al. 2022. "Comparative Transcriptomic Analysis of Inarching Invigorating Rootstock onto Incompatible Grafts in Citrus" International Journal of Molecular Sciences 23, no. 23: 14523. https://doi.org/10.3390/ijms232314523
APA StyleHe, W., Xie, R., Luo, L., Chai, J., Wang, H., Wang, Y., Chen, Q., Wu, Z., Yang, S., Li, M., Lin, Y., Zhang, Y., Luo, Y., Zhang, Y., Tang, H., Gmitter, F. G., Jr., & Wang, X. (2022). Comparative Transcriptomic Analysis of Inarching Invigorating Rootstock onto Incompatible Grafts in Citrus. International Journal of Molecular Sciences, 23(23), 14523. https://doi.org/10.3390/ijms232314523







