Lipid Nanoparticles Delivering Constitutively Active STING mRNA to Stimulate Antitumor Immunity
Abstract
:1. Introduction
2. Results
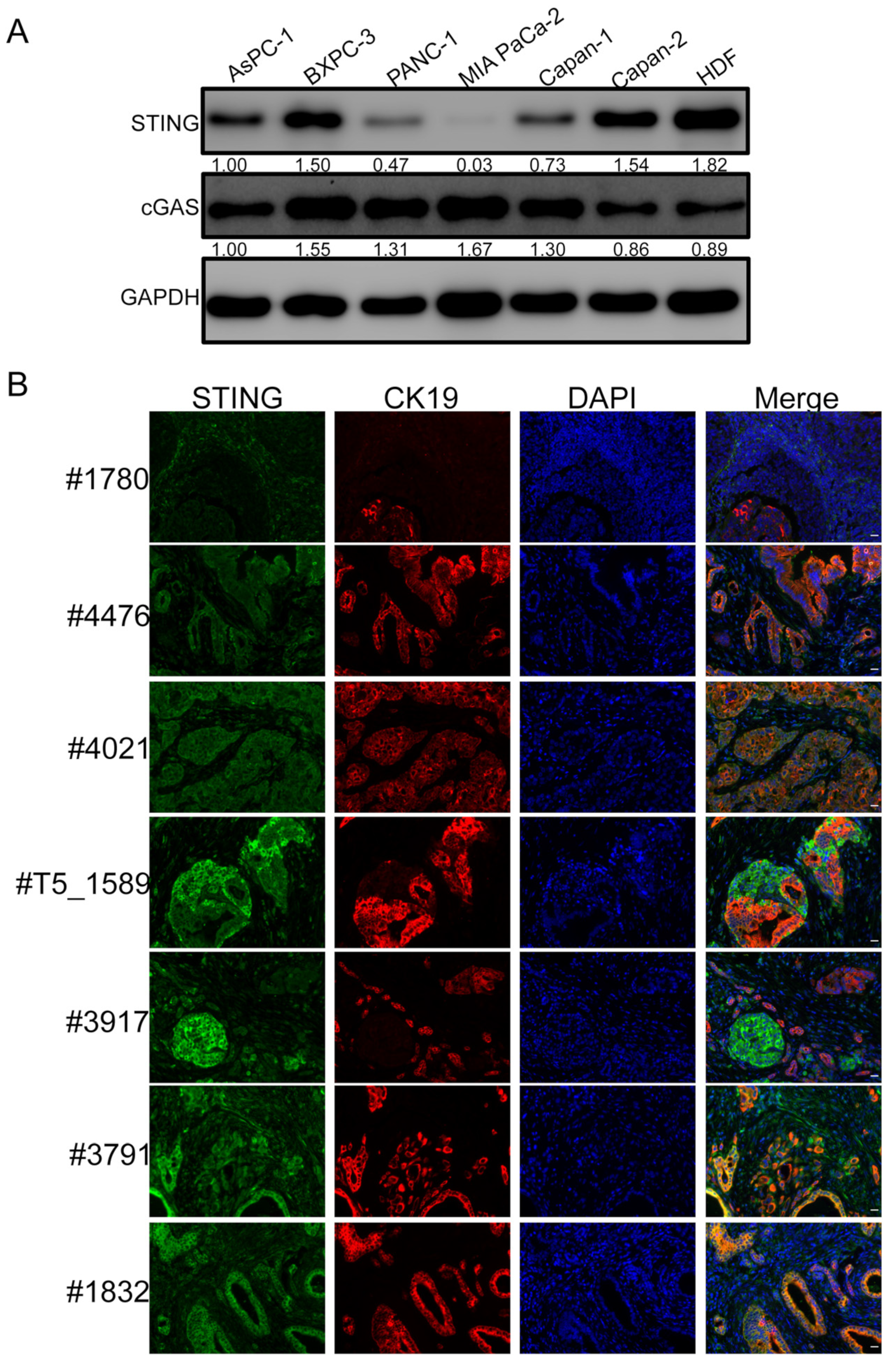
2.1. STING Is Downregulated in Some PDAC Lesions
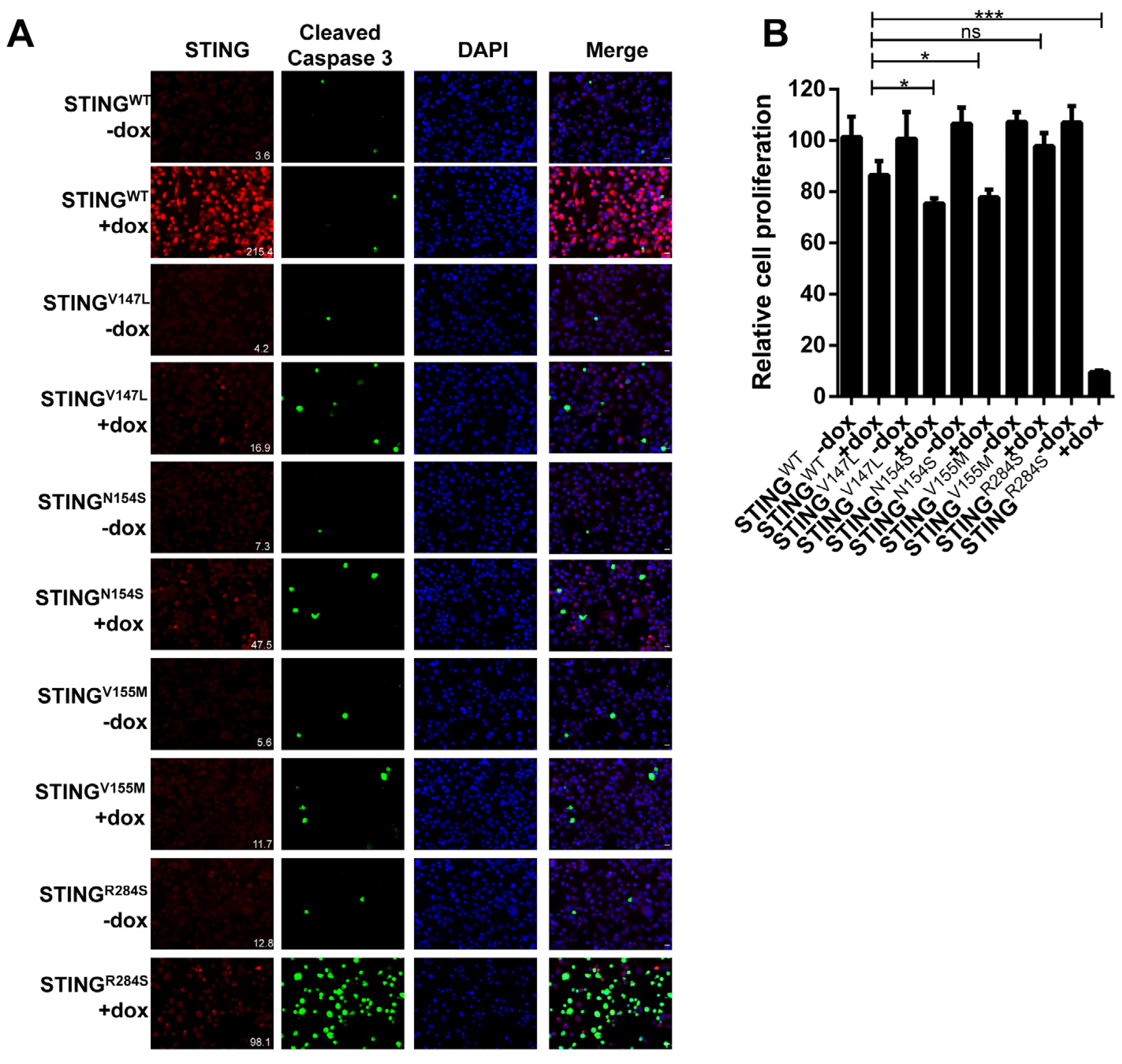
2.2. Identification of a Highly Active STING Gain-of-Function Mutant
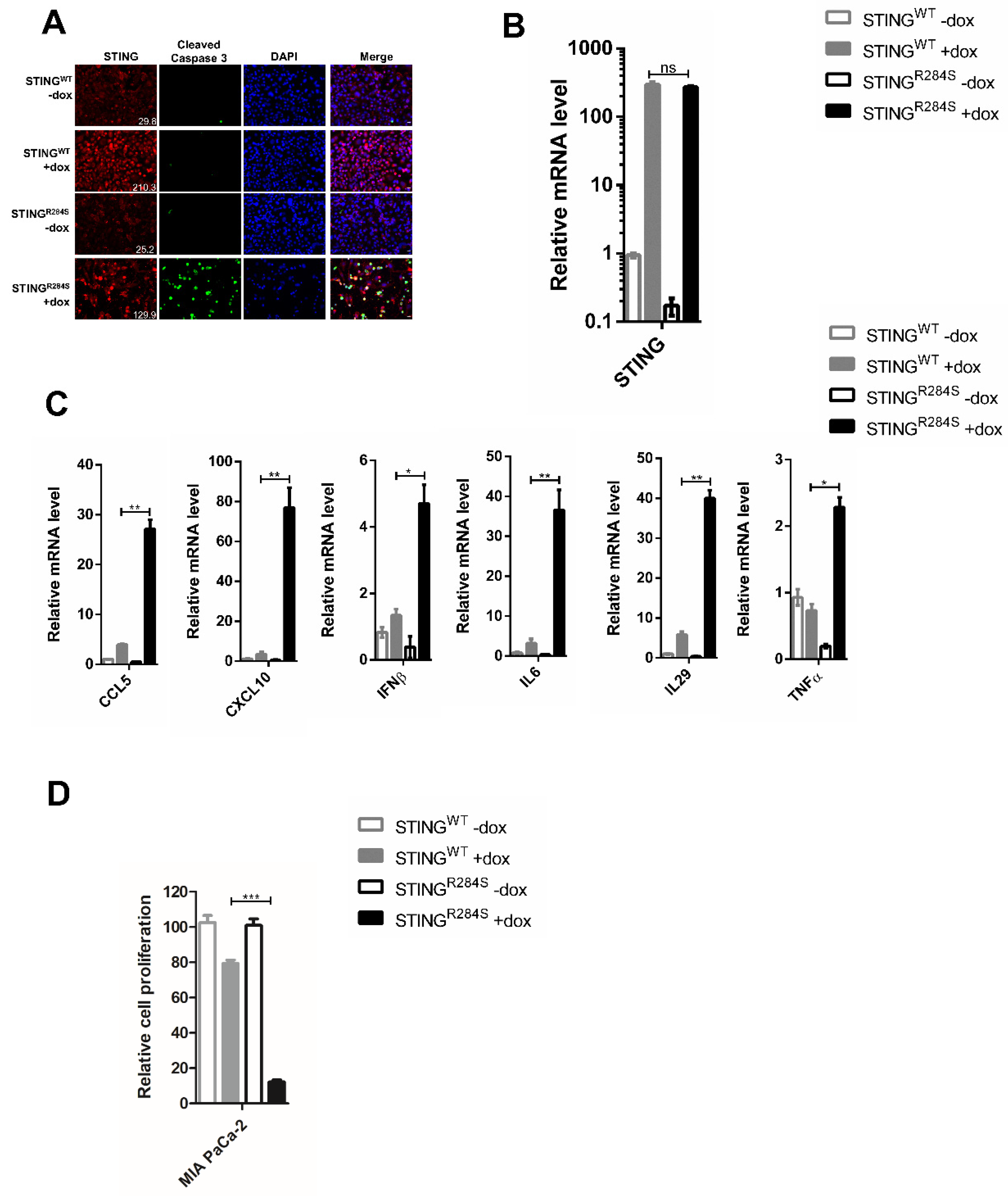
2.3. Ectopic Expression of Dox-Inducible STINGR284S Induces Key Anti-Tumor Cytokine Production and Cell Death in PDAC Cells
2.4. A Novel Approach to Reactivate the STING Signaling Pathway
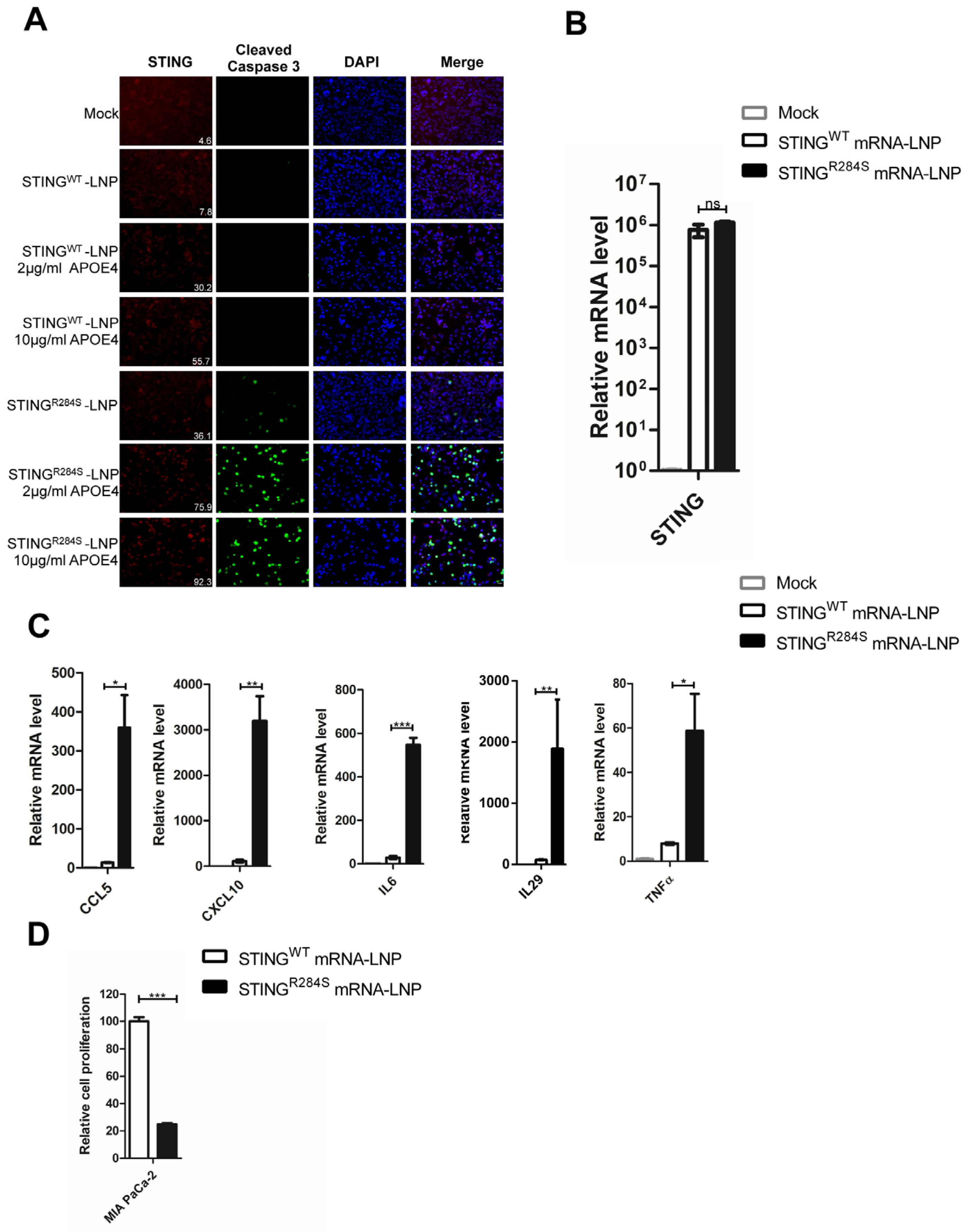
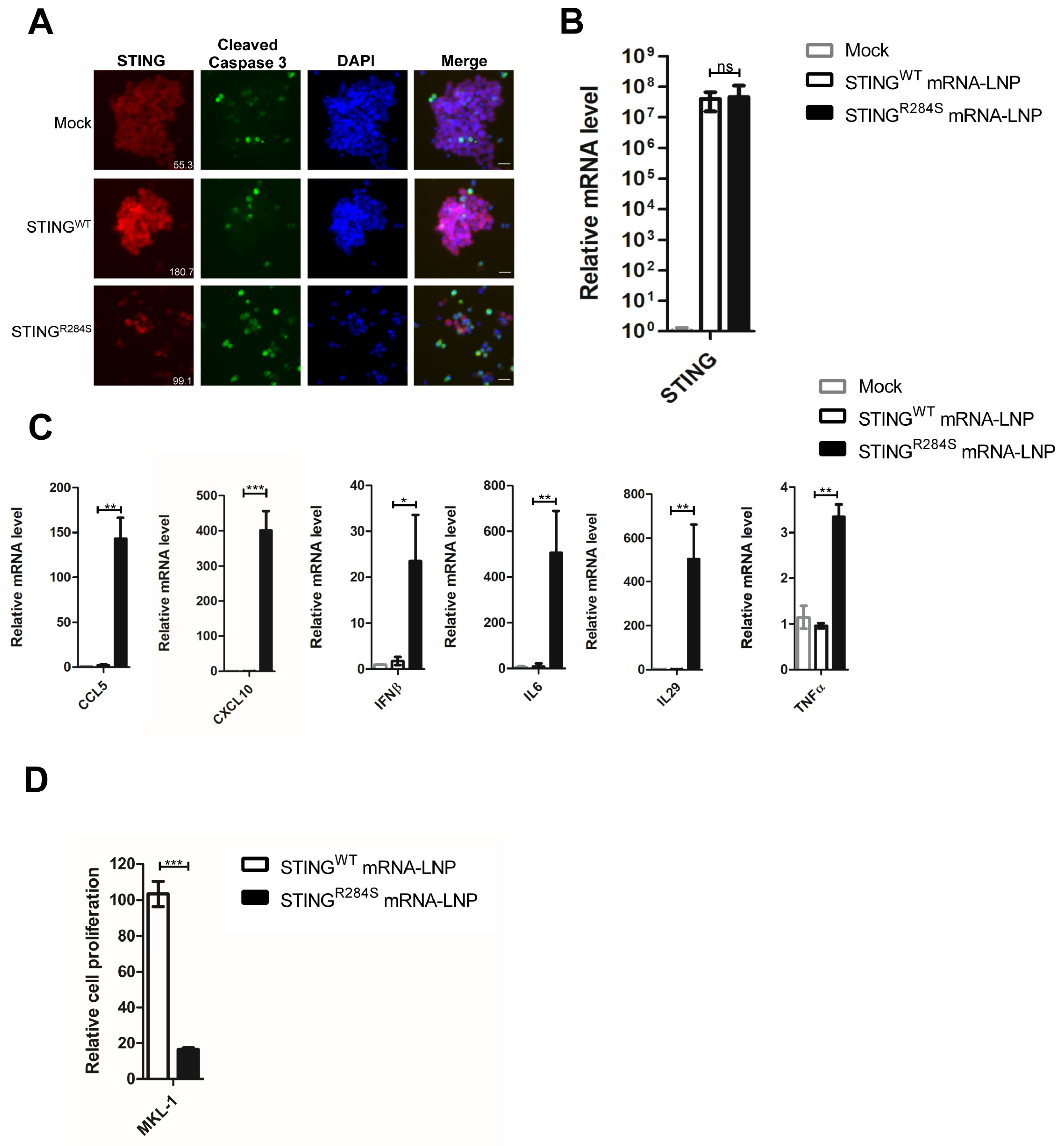
2.5. STINGR284S Expression Delivered by mRNA-LNP Activates Vital Antitumor Cytokines and Induces PDAC Cell Death
2.6. STINGR284S mRNA-LNP Also Triggers Vital Anti-Tumor Cytokine Production and Cell Death in MCC Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Cancer Lesions
4.2. Macrophage Differentiation
4.3. Western Blot Analysis
4.4. Cell Proliferation Assay
4.5. Reverse Transcription and Quantitative Real-Time PCR
4.6. Immunofluorescent Staining
4.7. Recombinant Plasmid Construction
4.8. mRNA Production
4.9. mRNA Transfection
4.10. LNP Encapsulation of the mRNA
4.11. Mutagenesis Primers
4.12. Statistical Analyses
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of pancreatic cancer: Global trends, etiology and risk factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Looi, C.-K.; Chung, F.F.-L.; Leong, C.-O.; Wong, S.-F.; Rosli, R.; Mai, C.-W. Therapeutic challenges and current immunomodulatory strategies in targeting the immunosuppressive pancreatic tumor microenvironment. J. Exp. Clin. Cancer Res. 2019, 38, 162. [Google Scholar] [CrossRef] [PubMed]
- Karamitopoulou, E. Tumour microenvironment of pancreatic cancer: Immune landscape is dictated by molecular and histopathological features. Br. J. Cancer 2019, 121, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Adel, N. Current treatment landscape and emerging therapies for pancreatic cancer. Am. J. Manag. Care 2019, 25, S3–S10. [Google Scholar] [PubMed]
- Feng, M.; Xiong, G.; Cao, Z.; Yang, G.; Zheng, S.; Song, X.; You, L.; Zheng, L.; Zhang, T.; Zhao, Y. Pd-1/pd-l1 and immunotherapy for pancreatic cancer. Cancer Lett. 2017, 407, 57–65. [Google Scholar] [CrossRef]
- Soares, K.C.; Rucki, A.A.; Wu, A.A.; Olino, K.; Xiao, Q.; Chai, Y.; Wamwea, A.; Bigelow, E.; Lutz, E.; Liu, L.; et al. Pd-1/pd-l1 blockade together with vaccine therapy facilitates effector t-cell infiltration into pancreatic tumors. J. Immunother. (Hagerstown Md. 1997) 2015, 38, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Liu, K. Epigenetic regulation of pd-l1 expression and pancreatic cancer response to checkpoint immunotherapy. Transl. Cancer Res. 2017, 6, S652–S654. [Google Scholar] [CrossRef]
- Chang, J.H.; Jiang, Y.; Pillarisetty, V.G. Role of immune cells in pancreatic cancer from bench to clinical application: An updated review. Medicine 2016, 95, e5541. [Google Scholar] [CrossRef]
- Upadhrasta, S.; Zheng, L. Strategies in developing immunotherapy for pancreatic cancer: Recognizing and correcting multiple immune "defects" in the tumor microenvironment. J. Clin. Med. 2019, 8, 1472. [Google Scholar] [CrossRef] [Green Version]
- Bazzichetto, C.; Conciatori, F.; Luchini, C.; Simionato, F.; Santoro, R.; Vaccaro, V.; Corbo, V.; Falcone, I.; Ferretti, G.; Cognetti, F.; et al. From genetic alterations to tumor microenvironment: The ariadne’s string in pancreatic cancer. Cells 2020, 9, 309. [Google Scholar] [CrossRef]
- Liu, W.; Kim, G.B.; Krump, N.A.; Zhou, Y.; Riley, J.L.; You, J. Selective reactivation of sting signaling to target merkel cell carcinoma. Proc. Natl. Acad. Sci. USA 2020, 117, 13730–13739. [Google Scholar] [CrossRef]
- Harms, P.W.; Harms, K.L.; Moore, P.S.; DeCaprio, J.A.; Nghiem, P.; Wong, M.K.K.; Brownell, I.; International Workshop on Merkel Cell Carcinoma Research Working Group. The biology and treatment of merkel cell carcinoma: Current understanding and research priorities. Nat. Rev. Clin. Oncol. 2018, 15, 763–776. [Google Scholar] [CrossRef] [Green Version]
- Becker, J.C.; Stang, A.; DeCaprio, J.A.; Cerroni, L.; Lebbe, C.; Veness, M.; Nghiem, P. Merkel cell carcinoma. Nat. Rev. Dis. Prim. 2017, 3, 17077. [Google Scholar] [CrossRef]
- Barber, G.N. Sting: Infection, inflammation and cancer. Nat. Rev. Immunol. 2015, 15, 760–770. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.; Kanne, D.B.; Leong, M.; Glickman, L.H.; McWhirter, S.M.; Lemmens, E.; Mechette, K.; Leong, J.J.; Lauer, P.; Liu, W.; et al. Sting agonist formulated cancer vaccines can cure established tumors resistant to pd-1 blockade. Sci. Transl. Med. 2015, 7, 283ra252. [Google Scholar] [CrossRef] [Green Version]
- Foote, J.B.; Kok, M.; Leatherman, J.M.; Armstrong, T.D.; Marcinkowski, B.C.; Ojalvo, L.S.; Kanne, D.B.; Jaffee, E.M.; Dubensky, T.W., Jr.; Emens, L.A. A sting agonist given with ox40 receptor and pd-l1 modulators primes immunity and reduces tumor growth in tolerized mice. Cancer Immunol. Res. 2017, 5, 468–479. [Google Scholar] [CrossRef] [Green Version]
- Xia, T.; Konno, H.; Ahn, J.; Barber, G.N. Deregulation of sting signaling in colorectal carcinoma constrains DNA damage responses and correlates with tumorigenesis. Cell Rep. 2016, 14, 282–297. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Sun, L.; Chen, Z.J. Regulation and function of the cgas-sting pathway of cytosolic DNA sensing. Nat. Immunol. 2016, 17, 1142–1149. [Google Scholar] [CrossRef]
- Cai, X.; Chiu, Y.H.; Chen, Z.J. The cgas-cgamp-sting pathway of cytosolic DNA sensing and signaling. Mol. Cell 2014, 54, 289–296. [Google Scholar] [CrossRef] [Green Version]
- Hubert, M.; Gobbini, E.; Couillault, C.; Manh, T.V.; Doffin, A.C.; Berthet, J.; Rodriguez, C.; Ollion, V.; Kielbassa, J.; Sajous, C.; et al. Ifn-iii is selectively produced by cdc1 and predicts good clinical outcome in breast cancer. Sci. Immunol. 2020, 5, eaav3942. [Google Scholar] [CrossRef]
- Diamond, M.S.; Kinder, M.; Matsushita, H.; Mashayekhi, M.; Dunn, G.P.; Archambault, J.M.; Lee, H.; Arthur, C.D.; White, J.M.; Kalinke, U.; et al. Type i interferon is selectively required by dendritic cells for immune rejection of tumors. J. Exp. Med. 2011, 208, 1989–2003. [Google Scholar] [CrossRef] [PubMed]
- Fuertes, M.B.; Kacha, A.K.; Kline, J.; Woo, S.R.; Kranz, D.M.; Murphy, K.M.; Gajewski, T.F. Host type i ifn signals are required for antitumor cd8+ t cell responses through cd8{alpha}+ dendritic cells. J. Exp. Med. 2011, 208, 2005–2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zumwalt, T.J.; Arnold, M.; Goel, A.; Boland, C.R. Active secretion of cxcl10 and ccl5 from colorectal cancer microenvironments associates with granzymeb+ cd8+ t-cell infiltration. Oncotarget 2015, 6, 2981–2991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mowat, C.; Mosley, S.R.; Namdar, A.; Schiller, D.; Baker, K. Anti-tumor immunity in mismatch repair-deficient colorectal cancers requires type i ifn-driven ccl5 and cxcl10. J. Exp. Med. 2021, 218, e20210108. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.R.; Fuertes, M.B.; Corrales, L.; Spranger, S.; Furdyna, M.J.; Leung, M.Y.; Duggan, R.; Wang, Y.; Barber, G.N.; Fitzgerald, K.A.; et al. Sting-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity 2014, 41, 830–842. [Google Scholar] [CrossRef] [Green Version]
- Corrales, L.; Glickman, L.H.; McWhirter, S.M.; Kanne, D.B.; Sivick, K.E.; Katibah, G.E.; Woo, S.R.; Lemmens, E.; Banda, T.; Leong, J.J.; et al. Direct activation of sting in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep. 2015, 11, 1018–1030. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Miao, L.; Gao, W.; Chen, Z.; McHugh, K.J.; Sun, Y.; Tochka, Z.; Tomasic, S.; Sadtler, K.; Hyacinthe, A.; et al. Engineered plga microparticles for long-term, pulsatile release of sting agonist for cancer immunotherapy. Sci. Transl. Med. 2020, 12, eaaz6606. [Google Scholar] [CrossRef]
- Demaria, O.; De Gassart, A.; Coso, S.; Gestermann, N.; Di Domizio, J.; Flatz, L.; Gaide, O.; Michielin, O.; Hwu, P.; Petrova, T.V.; et al. Sting activation of tumor endothelial cells initiates spontaneous and therapeutic antitumor immunity. Proc. Natl. Acad. Sci. USA 2015, 112, 15408–15413. [Google Scholar] [CrossRef] [Green Version]
- Jing, W.; McAllister, D.; Vonderhaar, E.P.; Palen, K.; Riese, M.J.; Gershan, J.; Johnson, B.D.; Dwinell, M.B. Sting agonist inflames the pancreatic cancer immune microenvironment and reduces tumor burden in mouse models. J. Immunother. Cancer 2019, 7, 115. [Google Scholar] [CrossRef]
- Wang-Bishop, L.; Wehbe, M.; Shae, D.; James, J.; Hacker, B.C.; Garland, K.; Chistov, P.P.; Rafat, M.; Balko, J.M.; Wilson, J.T. Potent sting activation stimulates immunogenic cell death to enhance antitumor immunity in neuroblastoma. J. Immunother. Cancer 2020, 8, e000282. [Google Scholar] [CrossRef]
- Pan, B.S.; Perera, S.A.; Piesvaux, J.A.; Presland, J.P.; Schroeder, G.K.; Cumming, J.N.; Trotter, B.W.; Altman, M.D.; Buevich, A.V.; Cash, B.; et al. An orally available non-nucleotide sting agonist with antitumor activity. Science 2020, 369, eaba6098. [Google Scholar] [CrossRef]
- Ramanjulu, J.M.; Pesiridis, G.S.; Yang, J.; Concha, N.; Singhaus, R.; Zhang, S.Y.; Tran, J.L.; Moore, P.; Lehmann, S.; Eberl, H.C.; et al. Design of amidobenzimidazole sting receptor agonists with systemic activity. Nature 2018, 564, 439–443. [Google Scholar] [CrossRef]
- Sivick, K.E.; Desbien, A.L.; Glickman, L.H.; Reiner, G.L.; Corrales, L.; Surh, N.H.; Hudson, T.E.; Vu, U.T.; Francica, B.J.; Banda, T.; et al. Magnitude of therapeutic sting activation determines cd8(+) t cell-mediated anti-tumor immunity. Cell Rep. 2018, 25, 3074–3085.e5. [Google Scholar] [CrossRef] [Green Version]
- Harrington, K.J.; Brody, J.; Ingham, M.; Strauss, J.; Cemerski, S.; Wang, M.; Tse, A.; Khilnani, A.; Marabelle, A.; Golan, T. Preliminary results of the first-in-human (fih) study of mk-1454, an agonist of stimulator of interferon genes (sting), as monotherapy or in combination with pembrolizumab (pembro) in patients with advanced solid tumors or lymphomas. Ann. Oncol. 2018, 29, 712. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Sandhu, S.K.; Hamid, O.; Spreafico, A.; Kasper, S.; Dummer, R.; Shimizu, T.; Steeghs, N.; Lewis, N.; Talluto, C.C.; et al. Phase ib study of miw815 (adu-s100) in combination with spartalizumab (pdr001) in patients (pts) with advanced/metastatic solid tumors or lymphomas. J. Clin. Oncol. 2019, 37, 2507. [Google Scholar] [CrossRef]
- Chin, E.N.; Yu, C.; Vartabedian, V.F.; Jia, Y.; Kumar, M.; Gamo, A.M.; Vernier, W.; Ali, S.H.; Kissai, M.; Lazar, D.C.; et al. Antitumor activity of a systemic sting-activating non-nucleotide cgamp mimetic. Science 2020, 369, 993–999. [Google Scholar] [CrossRef]
- Gajewski, T.F.; Higgs, E.F. Immunotherapy with a sting. Science 2020, 369, 921–922. [Google Scholar] [CrossRef]
- Luksch, H.; Stinson, W.A.; Platt, D.J.; Qian, W.; Kalugotla, G.; Miner, C.A.; Bennion, B.G.; Gerbaulet, A.; Rösen-Wolff, A.; Miner, J.J. Sting-associated lung disease in mice relies on t cells but not type i interferon. J. Allergy Clin. Immunol. 2019, 144, 254–266.e258. [Google Scholar] [CrossRef] [Green Version]
- Konno, H.; Chinn, I.K.; Hong, D.; Orange, J.S.; Lupski, J.R.; Mendoza, A.; Pedroza, L.A.; Barber, G.N. Pro-inflammation associated with a gain-of-function mutation (r284s) in the innate immune sensor sting. Cell Rep. 2018, 23, 1112–1123. [Google Scholar] [CrossRef] [Green Version]
- Jeremiah, N.; Neven, B.; Gentili, M.; Callebaut, I.; Maschalidi, S.; Stolzenberg, M.C.; Goudin, N.; Frémond, M.L.; Nitschke, P.; Molina, T.J.; et al. Inherited sting-activating mutation underlies a familial inflammatory syndrome with lupus-like manifestations. J. Clin. Investig. 2014, 124, 5516–5520. [Google Scholar] [CrossRef]
- Liu, Y.; Jesus, A.A.; Marrero, B.; Yang, D.; Ramsey, S.E.; Sanchez, G.A.M.; Tenbrock, K.; Wittkowski, H.; Jones, O.Y.; Kuehn, H.S.; et al. Activated sting in a vascular and pulmonary syndrome. N. Engl. J. Med. 2014, 371, 507–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- König, N.; Fiehn, C.; Wolf, C.; Schuster, M.; Cura Costa, E.; Tüngler, V.; Alvarez, H.A.; Chara, O.; Engel, K.; Goldbach-Mansky, R.; et al. Familial chilblain lupus due to a gain-of-function mutation in sting. Ann. Rheum. Dis. 2017, 76, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Melki, I.; Rose, Y.; Uggenti, C.; Van Eyck, L.; Frémond, M.L.; Kitabayashi, N.; Rice, G.I.; Jenkinson, E.M.; Boulai, A.; Jeremiah, N.; et al. Disease-associated mutations identify a novel region in human sting necessary for the control of type i interferon signaling. J. Allergy Clin. Immunol. 2017, 140, 543–552.e545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobbs, N.; Burnaevskiy, N.; Chen, D.; Gonugunta, V.K.; Alto, N.M.; Yan, N. Sting activation by translocation from the er is associated with infection and autoinflammatory disease. Cell Host Microbe 2015, 18, 157–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keskitalo, S.; Haapaniemi, E.; Einarsdottir, E.; Rajamäki, K.; Heikkilä, H.; Ilander, M.; Pöyhönen, M.; Morgunova, E.; Hokynar, K.; Lagström, S.; et al. Novel tmem173 mutation and the role of disease modifying alleles. Front. Immunol. 2019, 10, 2770. [Google Scholar] [CrossRef] [Green Version]
- Munoz, J.; Rodière, M.; Jeremiah, N.; Rieux-Laucat, F.; Oojageer, A.; Rice, G.I.; Rozenberg, F.; Crow, Y.J.; Bessis, D. Stimulator of interferon genes-associated vasculopathy with onset in infancy: A mimic of childhood granulomatosis with polyangiitis. JAMA Dermatol. 2015, 151, 872–877. [Google Scholar] [CrossRef]
- Patel, S.; Jin, L. Tmem173 variants and potential importance to human biology and disease. Genes Immun. 2019, 20, 82–89. [Google Scholar] [CrossRef] [Green Version]
- Tang, E.D.; Wang, C.-Y. Single amino acid change in sting leads to constitutive active signaling. PLoS ONE 2015, 10, e0120090. [Google Scholar] [CrossRef]
- Gonugunta, V.K.; Sakai, T.; Pokatayev, V.; Yang, K.; Wu, J.; Dobbs, N.; Yan, N. Trafficking-mediated sting degradation requires sorting to acidified endolysosomes and can be targeted to enhance anti-tumor response. Cell Rep. 2017, 21, 3234–3242. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Reyes, H.M.; Yang, J.F.; Li, Y.; Stewart, K.M.; Basil, M.C.; Lin, S.M.; Katzen, J.; Morrisey, E.E.; Weiss, S.R.; et al. Activation of sting signaling pathway effectively blocks human coronavirus infection. J. Virol. 2021, 95, e00490-21. [Google Scholar] [CrossRef]
- Prabakaran, T.; Bodda, C.; Krapp, C.; Zhang, B.C.; Christensen, M.H.; Sun, C.; Reinert, L.; Cai, Y.; Jensen, S.B.; Skouboe, M.K.; et al. Attenuation of cgas-sting signaling is mediated by a p62/sqstm1-dependent autophagy pathway activated by tbk1. EMBO J. 2018, 37, e97858. [Google Scholar] [CrossRef] [Green Version]
- Yum, S.; Li, M.; Chen, Z.J. Old dogs, new trick: Classic cancer therapies activate cgas. Cell Res. 2020, 30, 639–648. [Google Scholar] [CrossRef]
- Yamashiro, L.H.; Wilson, S.C.; Morrison, H.M.; Karalis, V.; Chung, J.J.; Chen, K.J.; Bateup, H.S.; Szpara, M.L.; Lee, A.Y.; Cox, J.S.; et al. Interferon-independent sting signaling promotes resistance to hsv-1 in vivo. Nat. Commun. 2020, 11, 3382. [Google Scholar] [CrossRef]
- Gao, D.; Wu, J.; Wu, Y.T.; Du, F.; Aroh, C.; Yan, N.; Sun, L.; Chen, Z.J. Cyclic gmp-amp synthase is an innate immune sensor of hiv and other retroviruses. Science 2013, 341, 903–906. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Alameh, M.G.; Tombacz, I.; Bettini, E.; Lederer, K.; Sittplangkoon, C.; Wilmore, J.R.; Gaudette, B.T.; Soliman, O.Y.; Pine, M.; Hicks, P.; et al. Lipid nanoparticles enhance the efficacy of mrna and protein subunit vaccines by inducing robust t follicular helper cell and humoral responses. Immunity 2021, 54, 2877–2892.e7. [Google Scholar] [CrossRef]
- Kariko, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of rna recognition by toll-like receptors: The impact of nucleoside modification and the evolutionary origin of rna. Immunity 2005, 23, 165–175. [Google Scholar] [CrossRef] [Green Version]
- Kariko, K.; Weissman, D. Naturally occurring nucleoside modifications suppress the immunostimulatory activity of rna: Implication for therapeutic rna development. Curr. Opin. Drug Discov. Dev. 2007, 10, 523–532. [Google Scholar]
- Kariko, K.; Muramatsu, H.; Welsh, F.A.; Ludwig, J.; Kato, H.; Akira, S.; Weissman, D. Incorporation of pseudouridine into mrna yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 2008, 16, 1833–1840. [Google Scholar] [CrossRef]
- Anderson, B.R.; Muramatsu, H.; Nallagatla, S.R.; Bevilacqua, P.C.; Sansing, L.H.; Weissman, D.; Kariko, K. Incorporation of pseudouridine into mrna enhances translation by diminishing pkr activation. Nucleic Acids Res. 2010, 38, 5884–5892. [Google Scholar] [CrossRef] [Green Version]
- Kariko, K.; Muramatsu, H.; Ludwig, J.; Weissman, D. Generating the optimal mrna for therapy: Hplc purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mrna. Nucleic Acids Res. 2011, 39, e142. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.; Weissman, D.; Whitehead, K.A. Mrna vaccines for infectious diseases: Principles, delivery and clinical translation. Nat. Rev. Drug Discov. 2021, 20, 817–838. [Google Scholar] [CrossRef] [PubMed]
- Akinc, A.; Querbes, W.; De, S.; Qin, J.; Frank-Kamenetsky, M.; Jayaprakash, K.N.; Jayaraman, M.; Rajeev, K.G.; Cantley, W.L.; Dorkin, J.R.; et al. Targeted delivery of rnai therapeutics with endogenous and exogenous ligand-based mechanisms. Mol. Ther. 2010, 18, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, F.; Yanez Arteta, M.; Lerche, M.; Porcar, L.; Lang, C.; Bragg, R.A.; Elmore, C.S.; Krishnamurthy, V.R.; Russell, R.A.; Darwish, T.; et al. Apolipoprotein e binding drives structural and compositional rearrangement of mrna-containing lipid nanoparticles. ACS Nano 2021, 15, 6709–6722. [Google Scholar] [CrossRef] [PubMed]
- Gheysarzadeh, A.; Ansari, A.; Emami, M.H.; Razavi, A.E.; Mofid, M.R. Over-expression of low-density lipoprotein receptor-related protein-1 is associated with poor prognosis and invasion in pancreatic ductal adenocarcinoma. Pancreatology 2019, 19, 429–435. [Google Scholar] [CrossRef]
- Larkin, B.; Ilyukha, V.; Sorokin, M.; Buzdin, A.; Vannier, E.; Poltorak, A. Cutting edge: Activation of sting in t cells induces type i ifn responses and cell death. J. Immunol. 2017, 199, 397–402. [Google Scholar] [CrossRef] [Green Version]
- Gulen, M.F.; Koch, U.; Haag, S.M.; Schuler, F.; Apetoh, L.; Villunger, A.; Radtke, F.; Ablasser, A. Signalling strength determines proapoptotic functions of sting. Nat. Commun. 2017, 8, 427. [Google Scholar] [CrossRef] [Green Version]
- Cerboni, S.; Jeremiah, N.; Gentili, M.; Gehrmann, U.; Conrad, C.; Stolzenberg, M.C.; Picard, C.; Neven, B.; Fischer, A.; Amigorena, S.; et al. Intrinsic antiproliferative activity of the innate sensor sting in t lymphocytes. J. Exp. Med. 2017, 214, 1769–1785. [Google Scholar] [CrossRef] [Green Version]
- Gaidt, M.M.; Ebert, T.S.; Chauhan, D.; Ramshorn, K.; Pinci, F.; Zuber, S.; O’Duill, F.; Schmid-Burgk, J.L.; Hoss, F.; Buhmann, R.; et al. The DNA inflammasome in human myeloid cells is initiated by a sting-cell death program upstream of nlrp3. Cell 2017, 171, 1110–1124.e1118. [Google Scholar] [CrossRef]
- Ahn, J.; Xia, T.; Konno, H.; Konno, K.; Ruiz, P.; Barber, G.N. Inflammation-driven carcinogenesis is mediated through sting. Nat. Commun. 2014, 5, 5166. [Google Scholar] [CrossRef] [Green Version]
- Santos-Juanes, J.; Fernandez-Vega, I.; Fuentes, N.; Galache, C.; Coto-Segura, P.; Vivanco, B.; Astudillo, A.; Martinez-Camblor, P. Merkel cell carcinoma and merkel cell polyomavirus: A systematic review and meta-analysis. Br. J. Dermatol. 2015, 173, 42–49. [Google Scholar] [CrossRef]
- Park, D.E.; Cheng, J.; McGrath, J.P.; Lim, M.Y.; Cushman, C.; Swanson, S.K.; Tillgren, M.L.; Paulo, J.A.; Gokhale, P.C.; Florens, L.; et al. Merkel cell polyomavirus activates lsd1-mediated blockade of non-canonical baf to regulate transformation and tumorigenesis. Nat. Cell Biol. 2020, 22, 603–615. [Google Scholar] [CrossRef]
- Cheng, J.; Park, D.E.; Berrios, C.; White, E.A.; Arora, R.; Yoon, R.; Branigan, T.; Xiao, T.; Westerling, T.; Federation, A.; et al. Merkel cell polyomavirus recruits mycl to the ep400 complex to promote oncogenesis. PLoS Pathog. 2017, 13, e1006668. [Google Scholar] [CrossRef]
- O’Reilly, E.M.; Oh, D.Y.; Dhani, N.; Renouf, D.J.; Lee, M.A.; Sun, W.; Fisher, G.; Hezel, A.; Chang, S.C.; Vlahovic, G.; et al. Durvalumab with or without tremelimumab for patients with metastatic pancreatic ductal adenocarcinoma: A phase 2 randomized clinical trial. JAMA Oncol. 2019, 5, 1431–1438. [Google Scholar] [CrossRef]
- Nghiem, P.; Bhatia, S.; Lipson, E.J.; Sharfman, W.H.; Kudchadkar, R.R.; Brohl, A.S.; Friedlander, P.A.; Daud, A.; Kluger, H.M.; Reddy, S.A.; et al. Three-year survival, correlates and salvage therapies in patients receiving first-line pembrolizumab for advanced merkel cell carcinoma. J. Immunother. Cancer 2021, 9, e002478. [Google Scholar] [CrossRef]
- Carstens, J.L.; Correa de Sampaio, P.; Yang, D.; Barua, S.; Wang, H.; Rao, A.; Allison, J.P.; LeBleu, V.S.; Kalluri, R. Spatial computation of intratumoral t cells correlates with survival of patients with pancreatic cancer. Nat. Commun. 2017, 8, 15095. [Google Scholar] [CrossRef] [Green Version]
- Clark, C.E.; Hingorani, S.R.; Mick, R.; Combs, C.; Tuveson, D.A.; Vonderheide, R.H. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007, 67, 9518–9527. [Google Scholar] [CrossRef] [Green Version]
- Karamitopoulou, E. The tumor microenvironment of pancreatic cancer. Cancers 2020, 12, 3076. [Google Scholar] [CrossRef]
- Xia, T.; Konno, H.; Barber, G.N. Recurrent loss of sting signaling in melanoma correlates with susceptibility to viral oncolysis. Cancer Res. 2016, 76, 6747–6759. [Google Scholar] [CrossRef] [Green Version]
- Dall’Olio, F.G.; Marabelle, A.; Caramella, C.; Garcia, C.; Aldea, M.; Chaput, N.; Robert, C.; Besse, B. Tumour burden and efficacy of immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2021, 19, 75–90. [Google Scholar] [CrossRef]
- Huang, A.C.; Postow, M.A.; Orlowski, R.J.; Mick, R.; Bengsch, B.; Manne, S.; Xu, W.; Harmon, S.; Giles, J.R.; Wenz, B.; et al. T-cell invigoration to tumour burden ratio associated with anti-pd-1 response. Nature 2017, 545, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Knudson, C.J.; Alves-Peixoto, P.; Muramatsu, H.; Stotesbury, C.; Tang, L.; Lin, P.J.C.; Tam, Y.K.; Weissman, D.; Pardi, N.; Sigal, L.J. Lipid-nanoparticle-encapsulated mrna vaccines induce protective memory cd8 t cells against a lethal viral infection. Mol. Ther. 2021, 29, 2769–2781. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Oehm, P.; Derhovanessian, E.; Jabulowsky, R.A.; Vormehr, M.; Gold, M.; Maurus, D.; Schwarck-Kokarakis, D.; Kuhn, A.N.; Omokoko, T.; et al. An rna vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature 2020, 585, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, S.; Hook, L.M.; Pardi, N.; Wang, F.; Myles, A.; Cancro, M.P.; Cohen, G.H.; Weissman, D.; Friedman, H.M. Nucleoside-modified mrna encoding hsv-2 glycoproteins c, d, and e prevents clinical and subclinical genital herpes. Sci. Immunol. 2019, 4, eaaw7083. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, S.; Knox, J.J.; Desmond, A.; Alameh, M.G.; Gaudette, B.T.; Lubinski, J.M.; Naughton, A.; Hook, L.M.; Egan, K.P.; Tam, Y.K.; et al. Trivalent nucleoside-modified mrna vaccine yields durable memory b cell protection against genital herpes in preclinical models. J. Clin. Investig. 2021, 131, e152310. [Google Scholar] [CrossRef]
- LaTourette, P.C., 2nd; Awasthi, S.; Desmond, A.; Pardi, N.; Cohen, G.H.; Weissman, D.; Friedman, H.M. Protection against herpes simplex virus type 2 infection in a neonatal murine model using a trivalent nucleoside-modified mrna in lipid nanoparticle vaccine. Vaccine 2020, 38, 7409–7413. [Google Scholar] [CrossRef]
- Sahin, U.; Derhovanessian, E.; Miller, M.; Kloke, B.P.; Simon, P.; Lower, M.; Bukur, V.; Tadmor, A.D.; Luxemburger, U.; Schrors, B.; et al. Personalized rna mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 2017, 547, 222–226. [Google Scholar] [CrossRef]
- Munakata, L.; Tanimoto, Y.; Osa, A.; Meng, J.; Haseda, Y.; Naito, Y.; Machiyama, H.; Kumanogoh, A.; Omata, D.; Maruyama, K.; et al. Lipid nanoparticles of type-a cpg d35 suppress tumor growth by changing tumor immune-microenvironment and activate cd8 t cells in mice. J. Control. Release 2019, 313, 106–119. [Google Scholar] [CrossRef]
- Yanagi, T.; Tachikawa, K.; Wilkie-Grantham, R.; Hishiki, A.; Nagai, K.; Toyonaga, E.; Chivukula, P.; Matsuzawa, S. Lipid nanoparticle-mediated sirna transfer against pctaire1/pctk1/cdk16 inhibits in vivo cancer growth. Mol. Ther. Nucleic Acids 2016, 5, e327. [Google Scholar] [CrossRef] [Green Version]
- Hewitt, S.L.; Bai, A.; Bailey, D.; Ichikawa, K.; Zielinski, J.; Karp, R.; Apte, A.; Arnold, K.; Zacharek, S.J.; Iliou, M.S.; et al. Durable anticancer immunity from intratumoral administration of il-23, il-36gamma, and ox40l mrnas. Sci. Transl. Med. 2019, 11, eaat9143. [Google Scholar] [CrossRef]
- Hewitt, S.L.; Bailey, D.; Zielinski, J.; Apte, A.; Musenge, F.; Karp, R.; Burke, S.; Garcon, F.; Mishra, A.; Gurumurthy, S.; et al. Intratumoral il12 mrna therapy promotes th1 transformation of the tumor microenvironment. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 6284–6298. [Google Scholar] [CrossRef]
- Tombacz, I.; Laczko, D.; Shahnawaz, H.; Muramatsu, H.; Natesan, A.; Yadegari, A.; Papp, T.E.; Alameh, M.G.; Shuvaev, V.; Mui, B.L.; et al. Highly efficient cd4+ t cell targeting and genetic recombination using engineered cd4+ cell-homing mrna-lnps. Mol. Ther. 2021, 29, 3293–3304. [Google Scholar] [CrossRef]
- Liu, W.; Yang, R.; Payne, A.S.; Schowalter, R.M.; Spurgeon, M.E.; Lambert, P.F.; Xu, X.; Buck, C.B.; You, J. Identifying the target cells and mechanisms of merkel cell polyomavirus infection. Cell Host Microbe 2016, 19, 775–787. [Google Scholar] [CrossRef] [Green Version]
- Casson, C.N.; Yu, J.; Reyes, V.M.; Taschuk, F.O.; Yadav, A.; Copenhaver, A.M.; Nguyen, H.T.; Collman, R.G.; Shin, S. Human caspase-4 mediates noncanonical inflammasome activation against gram-negative bacterial pathogens. Proc. Natl. Acad. Sci. USA 2015, 112, 6688–6693. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Krump, N.A.; Herlyn, M.; You, J. Combining DNA damage induction with bcl-2 inhibition to enhance merkel cell carcinoma cytotoxicity. Biology 2020, 9, 35. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Stein, P.; Cheng, X.; Yang, W.; Shao, N.Y.; Morrisey, E.E.; Schultz, R.M.; You, J. Brd4 regulates nanog expression in mouse embryonic stem cells and preimplantation embryos. Cell Death Differ. 2014, 21, 1950–1960. [Google Scholar] [CrossRef] [Green Version]
- Pardi, N.; Hogan, M.J.; Pelc, R.S.; Muramatsu, H.; Andersen, H.; DeMaso, C.R.; Dowd, K.A.; Sutherland, L.L.; Scearce, R.M.; Parks, R.; et al. Zika virus protection by a single low-dose nucleoside-modified mrna vaccination. Nature 2017, 543, 248–251. [Google Scholar] [CrossRef] [Green Version]
- Pardi, N.; Muramatsu, H.; Weissman, D.; Kariko, K. Synthetic Messenger RNA and Cell Metabolism Modulation. Methods in Molecular Biology (Methods and Protocols); Rabinovich, P., Ed.; Humana Press: Totowa, NJ, USA, 2013; Volume 969, pp. 29–42. [Google Scholar]
- Corrales, L.; Gajewski, T.F. Molecular pathways: Targeting the stimulator of interferon genes (sting) in the immunotherapy of cancer. Clin. Cancer Res. 2015, 21, 4774–4779. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Alameh, M.-G.; Yang, J.F.; Xu, J.R.; Lin, P.J.C.; Tam, Y.K.; Weissman, D.; You, J. Lipid Nanoparticles Delivering Constitutively Active STING mRNA to Stimulate Antitumor Immunity. Int. J. Mol. Sci. 2022, 23, 14504. https://doi.org/10.3390/ijms232314504
Liu W, Alameh M-G, Yang JF, Xu JR, Lin PJC, Tam YK, Weissman D, You J. Lipid Nanoparticles Delivering Constitutively Active STING mRNA to Stimulate Antitumor Immunity. International Journal of Molecular Sciences. 2022; 23(23):14504. https://doi.org/10.3390/ijms232314504
Chicago/Turabian StyleLiu, Wei, Mohamad-Gabriel Alameh, June F. Yang, Jonathan R. Xu, Paulo J. C. Lin, Ying K. Tam, Drew Weissman, and Jianxin You. 2022. "Lipid Nanoparticles Delivering Constitutively Active STING mRNA to Stimulate Antitumor Immunity" International Journal of Molecular Sciences 23, no. 23: 14504. https://doi.org/10.3390/ijms232314504
APA StyleLiu, W., Alameh, M.-G., Yang, J. F., Xu, J. R., Lin, P. J. C., Tam, Y. K., Weissman, D., & You, J. (2022). Lipid Nanoparticles Delivering Constitutively Active STING mRNA to Stimulate Antitumor Immunity. International Journal of Molecular Sciences, 23(23), 14504. https://doi.org/10.3390/ijms232314504





