Chemical Element Profiling in the Sera and Brain of Bipolar Disorders Patients and Healthy Controls
Abstract
1. Introduction
2. Results
2.1. Chemical Elements in the Sera of BD Patients before and after Treatment
2.2. Element Concentrations in the PFC of BD Patients and Matched Controls
| Control (20) | Bipolar Patients (20) | |
|---|---|---|
| Age at death (years) | 43.12 ± 3.21 | 42.25 ± 3.23 |
| Cause of death (Suicide/other) | 0/20 | 15/5 |
| Brain weight (g) | 1382.75 ± 23.08 | 1511.75 ± 37.58 |
| Postmortem interval (h) | 30.70 ± 3.03 | 28.97 ± 3.94 |
| Brain tissue pH | 6.44 ± 0.05 | 6.34 ± 0.05 |
| Sex (Men/Women) | 14/6 | 14/6 |
| Alcohol history (none/positive) | 4/16 | 5/15 |
| Use of Mood stabilizers (none/positive) | 0/20 | 8/12 |
| Use of Psychoactive drugs (none/positive) | 14/6 | 0/20 |
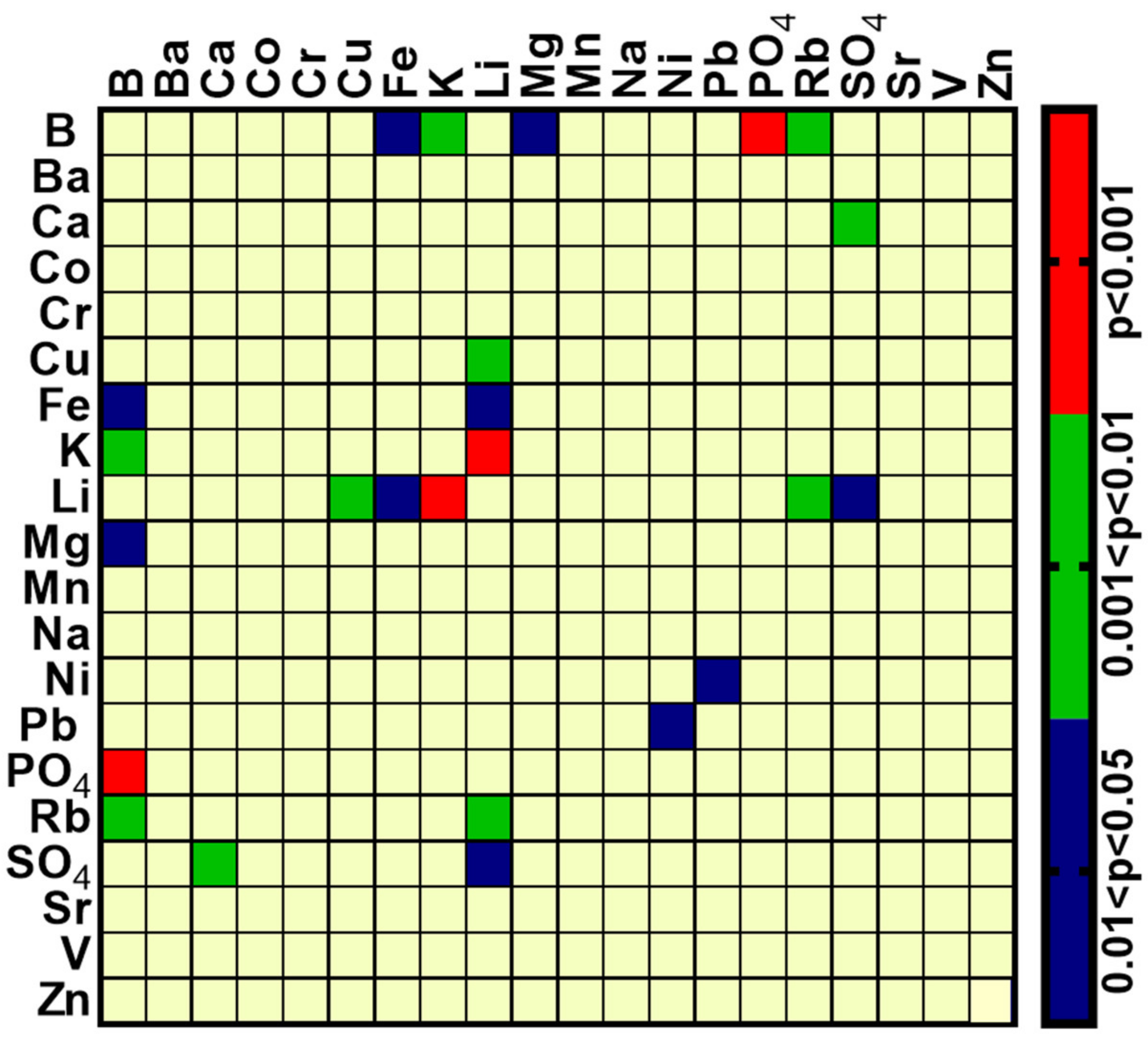
2.3. Inter-Element Correlations in Serum Samples
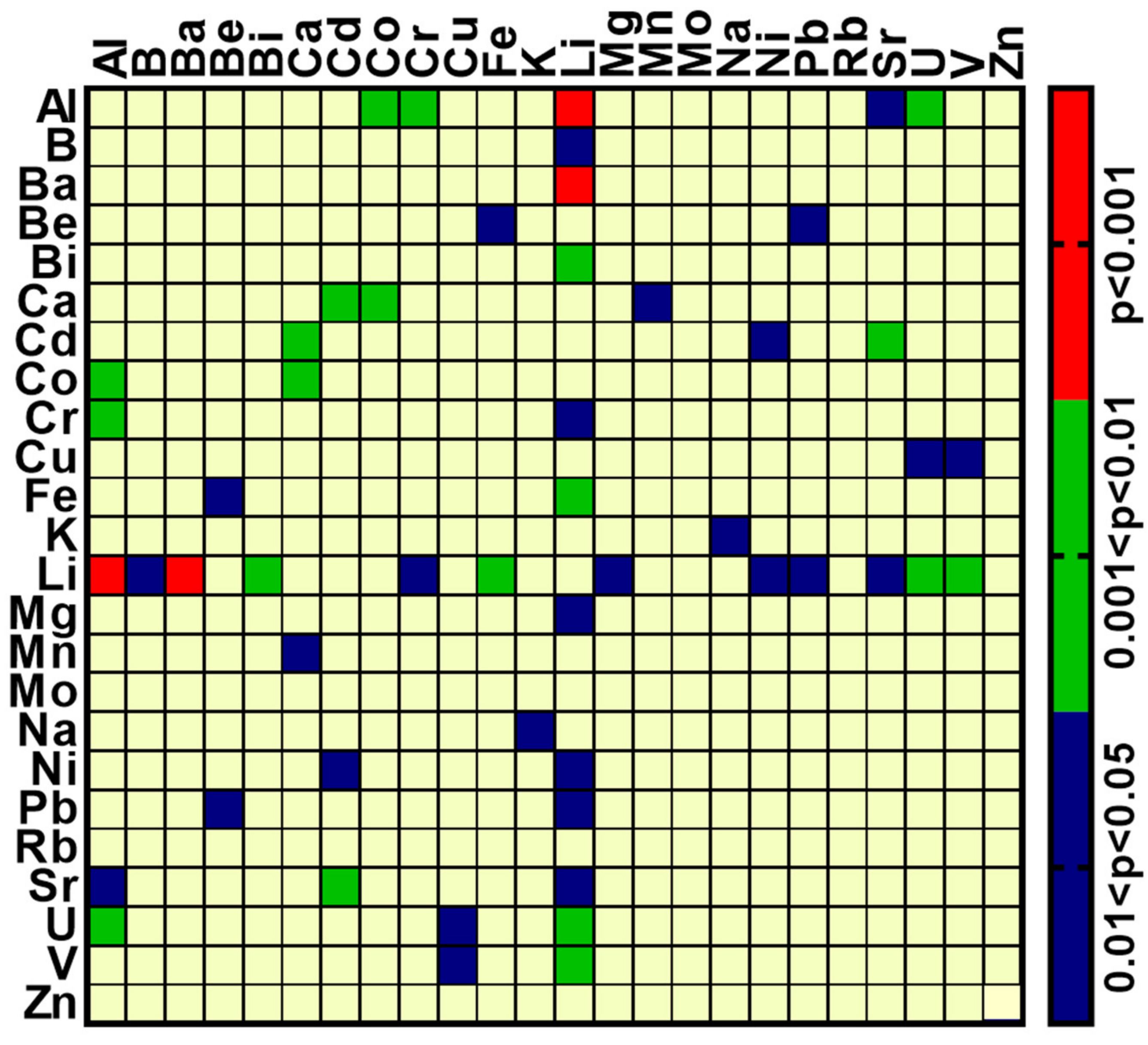
2.4. Inter-Element Correlations in Brain Samples from BD and Matched Control Samples
3. Discussion
3.1. Alterations in Element Concentrations in the Serum and Brain
3.2. Alterations in Inter-Elemental Correlations in Serum and Brain
4. Materials and Methods
4.1. Serum from BD Patients
4.2. Brain Postmortem Samples
4.3. Determination of Elements in Serum and Brain Tissue
4.4. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BD | Bipolar disease |
| PFC | Prefrontal cortex |
| ICP-MS | Inductively coupled plasma mass spectrometry |
| rs | Spearman’s correlation coefficient |
| Na+, K+-ATPase | Sodium, Potassium-activated Adenosine Triphosphatase |
References
- Taheri, S.; Asadi, S.; Nilashi, M.; Abumalloh, R.A.; Ghabban, N.M.; Yusuf, S.Y.M.; Supriyanto, E.; Samad, S. A literature review on beneficial role of vitamins and trace elements: Evidence from published clinical studies. J. Trace Elements Med. Biol. 2021, 67, 126789. [Google Scholar] [CrossRef] [PubMed]
- Mehri, A. Trace Elements in Human Nutrition (II)—An Update. Int. J. Prev. Med. 2020, 11, 2. [Google Scholar] [PubMed]
- Grochowski, C.; Blicharska, E.; Krukow, P.; Jonak, K.; Maciejewski, M.; Szczepanek, D.; Jonak, K.; Flieger, J.; Maciejewski, R. Analysis of Trace Elements in Human Brain: Its Aim, Methods, and Concentration Levels. Front. Chem. 2019, 7, 115. [Google Scholar] [CrossRef]
- Zheng, W.; Monnot, A.D. Regulation of brain iron and copper homeostasis by brain barrier systems: Implication in neurodegenerative diseases. Pharmacol. Ther. 2012, 133, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Strazielle, N.; Ghersi-Egea, J.-F. Physiology of Blood–Brain Interfaces in Relation to Brain Disposition of Small Compounds and Macromolecules. Mol. Pharm. 2013, 10, 1473–1491. [Google Scholar] [CrossRef]
- Szabo, S.T.; Harry, G.J.; Hayden, K.M.; Szabo, D.T.; Birnbaum, L. Comparison of Metal Levels between Postmortem Brain and Ventricular Fluid in Alzheimer’s Disease and Nondemented Elderly Controls. Toxicol. Sci. 2015, 150, 292–300. [Google Scholar] [CrossRef]
- Cicero, C.E.; Mostile, G.; Vasta, R.; Rapisarda, V.; Signorelli, S.S.; Ferrante, M.; Zappia, M.; Nicoletti, A. Metals and neurodegenerative diseases. A systematic review. Environ. Res. 2017, 159, 82–94. [Google Scholar] [CrossRef]
- Paglia, G.; Miedico, O.; Cristofano, A.; Vitale, M.; Angiolillo, A.; Chiaravalle, E.; Corso, G.; Di Costanzo, A. Distinctive Pattern of Serum Elements During the Progression of Alzheimer’s Disease. Sci. Rep. 2016, 6, 22769. [Google Scholar] [CrossRef]
- González-Estecha, M.; Trasobares, E.M.; Tajima, K.; Cano, S.; Fernández, C.; López, J.L.; Unzeta, B.; Arroyo, M.; Fuentenebro, F. Trace elements in bipolar disorder. J. Trace Elements Med. Biol. 2011, 25, S78–S83. [Google Scholar] [CrossRef]
- Dean, B.; Lam, L.Q.; Scarr, E.; Duce, J.A. Cortical biometals: Changed levels in suicide and with mood disorders. J. Affect. Disord. 2018, 243, 539–544. [Google Scholar] [CrossRef]
- Grande, I.; Berk, M.; Birmaher, B.; Vieta, E. Bipolar disorder. Lancet 2015, 387, 1561–1572. [Google Scholar] [CrossRef]
- Andreazza, A.C.; Young, L.T. The neurobiology of bipolar disorder: Identifying targets for specific agents and synergies for combination treatment. Int. J. Neuropsychopharmacol. 2013, 17, 1039–1052. [Google Scholar] [CrossRef] [PubMed]
- Sklar, P.; Smoller, J.W.; Fan, J.; Ferreira, M.; Perlis, R.H.; Chambert, K.; Nimgaonkar, V.L.; McQueen, M.B.; Faraone, S.; Kirby, A.; et al. Whole-genome association study of bipolar disorder. Mol. Psychiatry 2008, 13, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Belmaker, R. Bipolar Disorder. N. Engl. J. Med. 2004, 351, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Mullins, N.; Forstner, A.J.; O’Connell, K.S.; Coombes, B.; Coleman, J.R.I.; Qiao, Z.; Als, T.D.; Bigdeli, T.B.; Børte, S.; Bryois, J.; et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat. Genet. 2021, 53, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Cruz, E.C.S.; Madrid, K.C.; Arruda, M.A.; Sussulini, A. Association between trace elements in serum from bipolar disorder and schizophrenia patients considering treatment effects. J. Trace Elements Med. Biol. 2020, 59, 126467. [Google Scholar] [CrossRef]
- Millett, C.E.; Mukherjee, D.; Reider, A.; Can, A.; Groer, M.; Fuchs, D.; Postolache, T.T.; Kelleher, S.L.; Saunders, E.F. Peripheral zinc and neopterin concentrations are associated with mood severity in bipolar disorder in a gender-specific manner. Psychiatry Res. 2017, 255, 52–58. [Google Scholar] [CrossRef]
- Siwek, M.; Sowa-Kućma, M.; Styczeń, K.; Szewczyk, B.; Reczyński, W.; Misztak, P.; Topór-Mądry, R.; Nowak, G.; Dudek, D.; Rybakowski, J.K. Decreased serum zinc concentration during depressive episode in patients with bipolar disorder. J. Affect. Disord. 2015, 190, 272–277. [Google Scholar] [CrossRef]
- Goldstein, I.; Lax, E.; Gispan-Herman, I.; Ovadia, H.; Rosen, H.; Yadid, G.; Lichtstein, D. Neutralization of endogenous digitalis-like compounds alters catecholamines metabolism in the brain and elicits anti-depressive behavior. Eur. Neuropsychopharmacol. 2012, 22, 72–79. [Google Scholar] [CrossRef]
- Goldstein, I.; Lerer, E.; Laiba, E.; Mallet, J.; Mujaheed, M.; Laurent, C.; Rosen, H.; Ebstein, R.P.; Lichtstein, D. Association Between Sodium- and Potassium-Activated Adenosine Triphosphatase α Isoforms and Bipolar Disorders. Biol. Psychiatry 2009, 65, 985–991. [Google Scholar] [CrossRef]
- Hodes, A.; Lifschytz, T.; Rosen, H.; Ben-Ami, H.C.; Lichtstein, D. Reduction in endogenous cardiac steroids protects the brain from oxidative stress in a mouse model of mania induced by amphetamine. Brain Res. Bull. 2018, 137, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Hodes, A.; Rosen, H.; Deutsch, J.; Lifschytz, T.; Einat, H.; Lichtstein, D. Endogenous cardiac steroids in animal models of mania. Bipolar Disord. 2016, 18, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Fedorova, O.; Wei, W.; Rosen, H.; Horesh, N.; Ilani, A.; Lichtstein, D. Na+, K+-ATPase α Isoforms and Endogenous Cardiac Steroids in Prefrontal Cortex of Bipolar Patients and Controls. Int. J. Mol. Sci. 2020, 21, 5912. [Google Scholar] [CrossRef] [PubMed]
- Apiamu, A.; Osawaru, S.U.; Asagba, S.O.; Evuen, U.F.; Achuba, F.I. Exposure of African Catfish (Clarias gariepinus) to Lead and Zinc Modulates Membrane-Bound Transport Protein: A Plausible Effect on Na+/K+-ATPase Activity. Biol. Trace Element Res. 2021, 200, 4160–4170. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Zhao, H.Z.; Lu, G.H. Effects of selected metal oxide nanoparticles on multiple biomarkers in Carassius auratus. Biomed. Environ. Sci. 2013, 26. [Google Scholar] [CrossRef]
- Sousa, L.; Oliveira, M.M.; Pessôa, M.T.C.; Barbosa, L.A. Iron overload: Effects on cellular biochemistry. Clin. Chim. Acta 2020, 504, 180–189. [Google Scholar] [CrossRef]
- Ampath, V.P.; Singh, S.V.; Pelov, I.; Horesh, N.; Zannadeh, H.; Tirosh, O.; Erel, Y.; Lichtstein, D. Vanadium in Bipolar Disorders—Reviving an Old Hypothesis. Int. J. Mol. Sci. 2022, 23, 13901. [Google Scholar] [CrossRef]
- 2Dunn, O.J.; Clark, V. Correlation Coefficients Measured on the Same Individuals. J. Am. Stat. Assoc. 1969, 64, 366–377. [Google Scholar] [CrossRef]
- Silver, N.C.; Hittner, J.B.; May, K. Testing Dependent Correlations With Nonoverlapping Variables: A Monte Carlo Simulation. J. Exp. Educ. 2004, 73, 53–69. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Prentice-Hall: Hoboken, NJ, USA, 2010; p. 400. [Google Scholar]
- Wilcox, R.R. Comparing dependent robust correlations. Br. J. Math. Stat. Psychol. 2016, 69, 215–224. [Google Scholar] [CrossRef]
- Alda, M. Lithium in the treatment of bipolar disorder: Pharmacology and pharmacogenetics. Mol. Psychiatry 2015, 20, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Naylor, G.J.; Corrigan, F.M.; Smith, A.H.W.; Connelly, P.; Ward, N.I. Further Studies of Vanadium in Depressive Psychosis. Br. J. Psychiatry 1987, 150, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Conri, C.; Simonoff, M.; Fleury, B.; Moreau, F. Does vanadium play a role in depressive states? Biol. Psychiatry 1986, 21, 546–548. [Google Scholar] [CrossRef]
- Naylor, G.J.; Smith, A.H.W. Vanadium: A possible aetiological factor in manic depressive illness. Psychol. Med. 1981, 11, 249–256. [Google Scholar] [CrossRef]
- Maes, M.; De Vos, N.; Demedts, P.; Wauters, A.; Neels, H. Lower serum zinc in major depression in relation to changes in serum acute phase proteins. J. Affect. Disord. 1999, 56, 189–194. [Google Scholar] [CrossRef]
- Siwek, M.; Wróbel, A.; Dudek, D.; Nowak, G.; Zieba, A. The role of copper and magnesium in the pathogenesis and treatment of affective disorders. Psychiatr. Polska 2005, 39, 911–920. [Google Scholar]
- Świądro, M.; Ordon, K.; Herman, M.; Dudek, D.; Wietecha-Posłuszny, R. Copper and Zinc as Potential Biomarkers of Mood Disorders and Pandemic Syndrome. Molecules 2021, 27, 91. [Google Scholar] [CrossRef]
- Sun, C.; Wang, R.; Li, Z.; Zhang, D. Dietary magnesium intake and risk of depression. J. Affect. Disord. 2018, 246, 627–632. [Google Scholar] [CrossRef]
- Nakamura, M.; Miura, A.; Nagahata, T.; Shibata, Y.; Okada, E.; Ojima, T. Low Zinc, Copper, and Manganese Intake is Associated with Depression and Anxiety Symptoms in the Japanese Working Population: Findings from the Eating Habit and Well-Being Study. Nutrients 2019, 11, 847. [Google Scholar] [CrossRef]
- Koek, W.N.H.; Campos-Obando, N.; van der Eerden, B.C.J.; de Rijke, Y.B.; A Ikram, M.; Uitterlinden, A.G.; van Leeuwen, J.P.T.M.; Zillikens, M.C. Age-dependent sex differences in calcium and phosphate homeostasis. Endocr. Connect. 2021, 10, 273–282. [Google Scholar] [CrossRef]
- Yildirim, I.; Hur, E.; Magden, K.; Ilikhan, S.; Engin, H.; Can, M.; Yıldız, G.; Özer, I. Serum Sulphate Levels in Hemodialysis Patients. Int. J. Nephrol. 2019, 2019, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Scholefield, M.; Church, S.J.; Xu, J.; Kassab, S.; Gardiner, N.J.; Roncaroli, F.; Hooper, N.M.; Unwin, R.D.; Cooper, G.J.S. Evidence that levels of nine essential metals in post-mortem human-Alzheimer’s-brain andex vivorat-brain tissues are unaffected by differences in post-mortem delay, age, disease staging, and brain bank location. Metallomics 2020, 12, 952–962. [Google Scholar] [CrossRef]
- Białek, M.; Czauderna, M.; Krajewska, K.A.; Przybylski, W. Selected physiological effects of boron compounds for animals and humans. A review. J. Anim. Feed Sci. 2019, 28, 307–320. [Google Scholar] [CrossRef]
- Pizzorno, L. Nothing Boring About Boron. Integr. Med. (Encinitas Calif.) 2015, 14, 35–48. [Google Scholar]
- Penland, J.G. The importance of boron nutrition for brain and psychological function. Biol. Trace Element Res. 1998, 66, 299–317. [Google Scholar] [CrossRef]
- Krewski, D.; Yokel, R.A.; Nieboer, E.; Borchelt, D.; Cohen, J.; Harry, J.; Kacew, S.; Lindsay, J.; Mahfouz, A.M.; Rondeau, V. Human health risk assessment for aluminium, aluminium oxide, and aluminium hydroxide. J. Toxicol. Environ. Health B Crit. Rev. 2007, 10 (Suppl. S1), 1–269. [Google Scholar] [CrossRef]
- Alasfar, R.H.; Isaifan, R.J. Aluminum environmental pollution: The silent killer. Environ. Sci. Pollut. Res. 2021, 28, 44587–44597. [Google Scholar] [CrossRef] [PubMed]
- Dey, M.; Singh, R.K. Neurotoxic effects of aluminium exposure as a potential risk factor for Alzheimer’s disease. Pharmacol. Rep. 2022, 74, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Maya, S.; Prakash, T.; Das Madhu, K.; Goli, D. Multifaceted effects of aluminium in neurodegenerative diseases: A review. Biomed. Pharmacother. 2016, 83, 746–754. [Google Scholar] [CrossRef] [PubMed]
| Element | Element Concentration | ||||||
|---|---|---|---|---|---|---|---|
| Before Treatment | SD | After Treatment | SD | Sum of Positive Ranks | Sum of Negative Ranks | Statistics p | |
| B (µg/L) | 58 | 36 | 63 | 59 | 39 | −27 | 0.311 |
| Ba (µg/L) | 2.73 | 0.98 | 2.92 | 0.83 | 28.5 | −26.5 | 0.469 |
| Ca (mg/L) | 136 | 8.49 | 134 | 5.06 | 22 | −33 | 0.303 |
| Co (µg/L) | 0.65 | 0.32 | 0.63 | 0.34 | 12.5 | −15.5 | 0.492 |
| Cr (µg/L) | 6.01 | 4.73 | 3.93 | 2.20 | 17 | −49 | 0.087 |
| Cu (mg/L) | 1.61 | 0.17 | 1.51 | 0.25 | 6.5 | −29.5 | 0.054 |
| Fe (mg/L) | 6.6 | 5.5 | 14 | 24.83 | 29 | −26 | 0.460 |
| K (mg/L) | 764 | 479 | 898 | 562.4 | 35 | −31 | 0.449 |
| Li (mg/L) | 22 | 9.3 | 18 | 4.48 | 23.5 | −42.5 | 0.215 |
| Mg (mg/L) | 33 | 3.49 | 33 | 4.38 | 30.5 | −35.5 | 0.429 |
| Mn (µg/L) | 5.91 | 8.25 | 2.24 | 1.28 | 26 | −40 | 0.287 |
| Na (%) | 0.43 | 0.02 | 0.42 | 0.03 | 13.5 | −41.5 | 0.085 |
| Ni (µg/L) | 6.69 | 7.79 | 4.57 | 2.35 | 28 | −27 | 0.490 |
| Pb (µg/L) | 0.60 | 0.55 | 0.76 | 0.66 | 25 | −11 | 0.203 |
| PO4 (mg/L) | 718 | 310 | 794 | 288.2 | 44 | −22 | 0.182 |
| Rb (mg/L) | 0.77 | 0.49 | 0.89 | 0.54 | 34 | −32 | 0.482 |
| SO4 (%) | 0.50 | 0.05 | 0.49 | 0.03 | 20.5 | −34.5 | 0.254 |
| Sr (µg/L) | 47 | 11.22 | 49 | 11.29 | 36 | −9 | 0.062 |
| V (µg/L) | 1.69 | 2.46 | 0.63 | 0.36 | 9 | −46 | 0.034 * |
| Zn (mg/L) | 1.22 | 0.28 | 1.40 | 0.90 | 29.5 | −36.5 | 0.389 |
| Element | Before Treatment | After Treatment |
|---|---|---|
| B (μg/L) | −0.3196 | −0.1383 |
| Ba (μg/L) | −0.0804 | 0.2112 |
| Ca (mg/L) | −0.6870 * | −0.0848 |
| Co (μg/L) | −0.1552 | -0.2953 |
| Cr (μg/L) | −0.0206 | 0.0183 |
| Cu (mg/L) | −0.4792 | −0.1528 |
| Fe (mg/L) | −0.1690 | 0.4110 |
| K (mg/L) | −0.0938 | 0.4749 |
| Li (mg/L) | −0.1287 | −0.1190 |
| Mg (mg/L) | −0.1759 | 0.7425 * |
| Mn (μg/L) | 0.0756 | 0.2425 |
| Na (%) | −0.1721 | −0.2910 |
| Ni (μg/L) | 0.0655 | −0.1432 |
| Pb (μg/L) | 0.3569 | 0.1212 |
| PO4 (mg/L) | −0.5525 | 0.5845 |
| Rb (mg/L) | 0.0823 | 0.5332 |
| SO4 (%) | −0.7875 ** | −0.0277 |
| Sr (μg/L) | −0.3836 | −0.0274 |
| V (μg/L) | 0.0742 | 0.2268 |
| Zn (mg/L) | −0.0206 | 0.2151 |
| Metal | Control | Bipolar | Statistics p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (n) | SD | Median | Sum of Rank | Mean | (n) | SD | Median | Sum of Rank | |||
| Al (μg/g) | 10.91 | 19 | 20.29 | 1.84 | 443 | 1.95 | 20 | 2.91 | 0.93 | 337 | 0.039 * |
| B (ng/g) | 0.66 | 20 | 0.32 | 0.65 | 486 | 0.45 | 19 | 0.32 | 0.42 | 294 | 0.007 * |
| Ba (ng/g) | 0.09 | 20 | 0.09 | 0.05 | 410 | 0.07 | 20 | 0.06 | 0.06 | 410 | 0.500 |
| Be (μg/g) | 0.003 | 11 | 0.002 | 0.003 | 116 | 0.003 | 08 | 0.001 | 0.002 | 74 | 0.311 |
| Bi (ng/g) | 0.004 | 20 | 0.006 | 0.002 | 420 | 0.003 | 20 | 0.003 | 0.001 | 400 | 0.397 |
| Ca (μg/g) | 109.7 | 20 | 89.12 | 72 | 394.5 | 133.9 | 20 | 165.8 | 74.5 | 425.5 | 0.341 |
| Cd (ng/g) | 0.01 | 20 | 0.01 | 0.008 | 432.5 | 0.009 | 20 | 0.006 | 0.007 | 387.5 | 0.275 |
| Co (ng/g) | 0.01 | 20 | 0.01 | 0.006 | 376.5 | 0.011 | 20 | 0.011 | 0.007 | 443.5 | 0.183 |
| Cr (ng/g) | 0.08 | 19 | 0.08 | 0.03 | 373 | 0.05 | 20 | 0.04 | 0.038 | 407 | 0.425 |
| Cu (μg/g) | 4.38 | 20 | 0.90 | 4.07 | 491.5 | 3.60 | 20 | 1.01 | 3.735 | 328 | 0.013 * |
| Fe (μg/g) | 86.3 | 20 | 44.56 | 71 | 414.5 | 102.4 | 20 | 86.68 | 61 | 405.5 | 0.454 |
| K (μg/g) | 2612 | 20 | 335.3 | 2401 | 488 | 2359 | 20 | 318.7 | 2401 | 332 | 0.017 * |
| Li μg/g) | 0.02 | 15 | 0.03 | 0.01 | 249 | 0.17 | 15 | 0.36 | 0.007 | 216 | 0.252 |
| Mg (μg/g) | 113.4 | 20 | 16.4 | 109 | 499 | 100.9 | 20 | 15.22 | 100.5 | 321 | 0.007 * |
| Mn (ng/g) | 0.37 | 20 | 0.17 | 0.32 | 448 | 0.36 | 20 | 0.25 | 0.23 | 372 | 0.155 |
| Mo (ng/g) | 0.04 | 20 | 0.02 | 0.04 | 468 | 0.04 | 20 | 0.01 | 0.03 | 352 | 0.059 |
| Na (μg/g) | 1548 | 20 | 310.2 | 1613 | 437 | 1469 | 20 | 284.8 | 1414 | 383 | 0.238 |
| Ni (ng/g) | 0.13 | 20 | 0.12 | 0.07 | 465 | 0.09 | 20 | 0.10 | 0.07 | 355 | 0.069 |
| Pb (ng/g) | 0.06 | 20 | 0.12 | 0.02 | 433.5 | 0.05 | 20 | 0.09 | 0.01 | 386.5 | 0.266 |
| Rb (μg/g) | 2.25 | 20 | 0.51 | 2.18 | 425.5 | 2.21 | 20 | 0.67 | 2.12 | 394.5 | 0.341 |
| Sr (ng/g) | 0.08 | 20 | 0.09 | 0.04 | 426 | 0.08 | 20 | 0.13 | 0.04 | 394 | 0.339 |
| U (ng/g) | 0.0004 | 20 | 0.0002 | 0.0003 | 445.5 | 0.0003 | 20 | 0.0002 | 0.0002 | 374.5 | 0.172 |
| V (ng/g) | 0.04 | 20 | 0.11 | 0.006 | 478.5 | 0.005 | 20 | 0.003 | 0.0045 | 341.5 | 0.030 * |
| Zn (μg/g) | 16.27 | 20 | 11.18 | 14 | 451.5 | 14.75 | 20 | 8.47 | 12 | 368.5 | 0.132 |
| Element | Control | Bipolar Disorder |
|---|---|---|
| Al (μg/g) | −0.277 | −0.074 |
| B (ng/g) | −0.176 | 0.160 |
| Ba (ng/g) | −0.056 | 0.482 |
| Be (μg/g) | 0.106 | −0.027 |
| Bi (ng/g) | −0.520 | -0.117 |
| Ca (μg/g) | −0.113 | 0.241 |
| Cd (ng/g) | 0.458 | −0.128 |
| Co (ng/g) | −0.198 | −0.163 |
| Cr (ng/g) | −0.221 | 0.348 |
| Cu (μg/g) | −0.208 | 0.032 |
| Fe (μg/g) | −0.084 | −0.135 |
| K (μg/g) | −0.374 | 0.329 |
| Li (μg/g) | −0.138 | 0.022 |
| Mg (μg/g) | −0.101 | 0.338 |
| Mn (ng/g) | 0.089 | 0.067 |
| Mo (ng/g) | −0.195 | 0.039 |
| Na (μg/g) | 0.256 | −0.030 |
| Ni (ng/g) | 0.111 | −0.118 |
| Pb (ng/g) | −0.029 | −0.037 |
| Rb (μg/g) | 0.108 | 0.391 |
| Sr (ng/g) | −0.031 | 0.205 |
| U (ng/g) | −0.061 | 0.313 |
| V (ng/g) | −0.106 | 0.174 |
| Zn (μg/g) | 0.048 | 0.460 |
| Before Treatment | After Treatment | ||
|---|---|---|---|
| Element | Correlated with | Element | Correlated with |
| Fe | K, Li, Mg, Na, PO4, Rb | Fe | K, Mn, Na, Rb, Zn |
| K | Li, Mg, Na, PO4, Rb | K | Mg, Na, Ni, Rb, Zn |
| Li | Mg, Na, Rb, Zn | Li | SO4 |
| Mg | Na, PO4, Rb | Na | Rb |
| Ca | PO4, SO4 | Co | Zn |
| Na | Rb, Zn | Cu | Li |
| Ba | Mn | Mn | Zn |
| Co | Ni | B | Mn |
| Cr | V | ||
| Cu | SO4 | ||
| Mn | Zn | ||
| Ni | Pb | ||
| Control | Bipolar Disorder | ||
|---|---|---|---|
| Element | Correlated with | Element | Correlated with |
| Al | B, Ba, Ca, Co, Cr, Li, Ni, Pb, Sr, U, V | Al | V |
| Ca | Cd, Co, Cr, Ni, Pb, Sr, U, V, Zn | Ca | Ni, Pb, Sr, U |
| Co | Cr, Fe, Mn, Ni, Pb, Sr, U, V, Zn | Co | Mn |
| Ba | Co, Cr, Fe, Li, Mn, Sr, U, V | Ba | Ca, Sr, U |
| Cr | Li, Ni, Pb, Sr, U, V, Zn | Cr | V |
| B | Ca, Ni, Pb, Sr, U | B | Bi, Mg |
| Ni | Pb, Sr, U, V, Zn | Ni | Pb, Sr |
| Li | Ni, Sr, U, V | Li | Pb |
| Cu | Mg, Na, Rb | Cu | Mg, U, V |
| Mg | Na, Ni, Rb | Mg | Mn, Rb |
| Pb | Sr, U, V | Pb | Sr, V |
| Fe | Mn, Zn | Fe | K, Li, Mn |
| Cd | Ni, Sr | Cd | Mn |
| Sr | U, V | Sr | U |
| K | Rb | K | Mg, Mn, Na, Rb |
| Mn | Zn | Mn | Zn |
| Be | Pb | U | V |
| U | V | Bi | Li, Mo |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sampath, V.P.; Singh, S.V.; Pelov, I.; Tirosh, O.; Erel, Y.; Lichtstein, D. Chemical Element Profiling in the Sera and Brain of Bipolar Disorders Patients and Healthy Controls. Int. J. Mol. Sci. 2022, 23, 14362. https://doi.org/10.3390/ijms232214362
Sampath VP, Singh SV, Pelov I, Tirosh O, Erel Y, Lichtstein D. Chemical Element Profiling in the Sera and Brain of Bipolar Disorders Patients and Healthy Controls. International Journal of Molecular Sciences. 2022; 23(22):14362. https://doi.org/10.3390/ijms232214362
Chicago/Turabian StyleSampath, Vishnu Priya, Shiv Vardan Singh, Ilana Pelov, Ofir Tirosh, Yigal Erel, and David Lichtstein. 2022. "Chemical Element Profiling in the Sera and Brain of Bipolar Disorders Patients and Healthy Controls" International Journal of Molecular Sciences 23, no. 22: 14362. https://doi.org/10.3390/ijms232214362
APA StyleSampath, V. P., Singh, S. V., Pelov, I., Tirosh, O., Erel, Y., & Lichtstein, D. (2022). Chemical Element Profiling in the Sera and Brain of Bipolar Disorders Patients and Healthy Controls. International Journal of Molecular Sciences, 23(22), 14362. https://doi.org/10.3390/ijms232214362






