Abstract
Recent evidence links chronic consumption of large amounts of fructose (FRU) with several non-communicable disease. After ingestion, dietary FRU is absorbed into the intestinal tract by glucose transporter (GLUT) 5 and transported to the portal vein via GLUT2. GLUT2 is primarily localized on the basolateral membrane, but GLUT2 may be dislocated post-prandially from the basolateral membrane of intestinal cells to the apical one. Polyphenols (PP) are plant secondary metabolites that exert hypoglycemic properties by modulating intracellular insulin signaling pathways and by inhibiting intestinal enzymes and transporters. Post-prandially, PP may reach high concentrations in the gut lumen, making the inhibition of FRU absorption a prime target for exploring the effects of PP on FRU metabolism. Herein, we have systematically reviewed studies on the effect of PP and PP-rich products on FRU uptake and transport in intestinal cells. In spite of expectations, the very different experimental conditions in the various individual studies do not allow definitive conclusions to be drawn. Future investigations should rely on standardized conditions in order to obtain comparable results that allow a credible rating of polyphenols and polyphenol-rich products as inhibitors of fructose uptake.
1. Introduction
The use of high-fructose (FRU) corn syrup as a sweetener in beverages and processed foods (including sodas, fruit-flavored drinks, frozen desserts, and sports drinks) has increased by 33% worldwide during the last five decades [1,2]. FRU accounts for about 10% of total energy intake, or close to 50 g/day, in most affluent countries [3]. The World Health Organization recommends that FRU should not provide more than 10% of the total caloric intake but advises lowering this threshold to less than 5% [4].
Epidemiological studies have shown that excessive FRU intake induces adiposity and insulin resistance [5], which can lead to the development of type II diabetes mellitus (T2DM) [6] and non-alcoholic fatty liver disease [7]. In a recent cohort study, Yuan et al. reported that the excessive consumption of sugar-sweetened beverages and high total FRU intake was associated with an increased incidence and mortality of proximal colon cancer [8].
Dietary FRU is passively absorbed from the luminal side of the intestine by glucose transporter (GLUT) 5, one of the facilitative GLUT family members [9]. In addition, GLUT2, a low-affinity transporter that can also recognize glucose and galactose, may transport FRU at the apical epithelial intestine level, with a Km for FRU more than fivefold higher than that of GLUT5 [1]. After being taken up by intestinal epithelial cells, FRU is carried across the basolateral membrane by GLUT2 into the portal blood [10]. Unlike glucose, the liver almost entirely metabolizes the absorbed FRU by the sequential actions of fructokinase, aldolase B, and triose kinase. Although the liver metabolizes the majority of the ingested FRU, the intestine itself can metabolize up to 30% of an oral FRU load [11]. Due to the absence of feedback inhibition, almost all FRU absorbed is converted into hepatic triose-phosphate regardless of the cellular energy status [12]. Then, various pathways dispose of the resulting metabolites. Among these are oxidation, conversion into hepatic glycogen, or de novo lipogenesis [13]. Management and FRU absorption could provide a route for preventing post-prandial FRU-induced liver inundation and hence help to prevent some of the acute effects of FRU overload on liver metabolic processes.
A report dating to more than forty years ago indicated that the glycemic index in both normal and diabetic volunteers was inversely correlated with the polyphenol (PP) content of foods. Quite intriguingly, this effect of food PP does not relate to their antioxidant or redox properties [14]. Subsequent studies indicated that PP (and other phytochemicals) might show hypoglycemic properties by affecting different targets, including the modulation of intracellular insulin signaling pathways, increase in insulin secretion from β-cells, and the inhibition of intestinal enzymes and transporters [15,16]. Various clinical studies have shown that dietary PP consumption was able to prevent fructose-induced oxidative stress and insulin resistance in T2DM patients [17], as well as to attenuate hepatic insulin resistance in healthy men [18].
Post-prandially, the gut lumen has a concentration of PP roughly 10-fold higher than any other site in the body, making the gut lumen itself the first place where the effects of PP on sugar metabolism should be investigated and where an intervention to lower the threats associated with FRU overconsumption may be designed [19]. For example, one possible strategy to lower the risks stemming from the obesity/T2DM connection implies lowering the uptake of high-energy nutrients into enterocytes, thus acting before the energy overload caused by excessive FRU affects the body as a whole. In this framework, the inhibition of enzymes and transporters involved in the earliest steps of the FRU release/uptake sequence leads to increased concentrations of nutrients in distal sections of the small intestine, which—in turn—initiates the “ileal brake” [20].
Many studies have been carried out to investigate the effect of PP on glucose absorption in intestinal cells [21,22,23,24,25,26], as is also summarized in an authoritative review [27]. However, much less is known about the effects of PP on FRU absorption, and no comprehensive analysis of literature reports is available. Herein, we are trying to provide an overview of studies on the potential effect of PP and PP-rich products on FRU uptake and transport in cultured intestinal cells. Although human intervention studies remain the gold standard to evaluate the relationship between food components and health, the development of reliable in vitro/ex vivo models allow the investigation and identification of the cellular/molecular working mechanisms and represent a first—and undoubtedly necessary—step when investigating the health properties of food/food components.
This review also highlights the difficulties related to drawing coherent conclusions from studies carried out under highly variable experimental conditions and suggests ways of integrating the most appropriate methodologies and conditions into a possible unifying approach.
2. Methods
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA) [28]. The search was carried out using the PubMed database in March 2022 and was conducted using the following keywords: “polyphenol” OR “phytochemical” AND “fructose transport” OR “fructose uptake” AND “intestinal cell” NOT “review”. The initial search yielded 99 hits. During the screening process (reviewing titles), 80 records were excluded. After abstract analysis, another 6 articles were excluded. Altogether, 13 records were selected and included in the review. Chosen studies were published between 2007 and 2022. Exclusion criteria were: (i) titles irrelevant to the research topic, (ii) abstract inappropriate or not related to the research topic, and (iii) experimental models other than enterocytes or intestinal tissues (i.e., X. laevis oocytes [29]). Reviews, letters, abstracts, and articles without a complete text in the English language were also excluded. Two independent investigators (S.I. and M.D.N.) checked the titles and abstracts of studies, and disagreements among the two reviewers were resolved through a mediator (F.B.). The detailed selection process is presented in Figure 1.
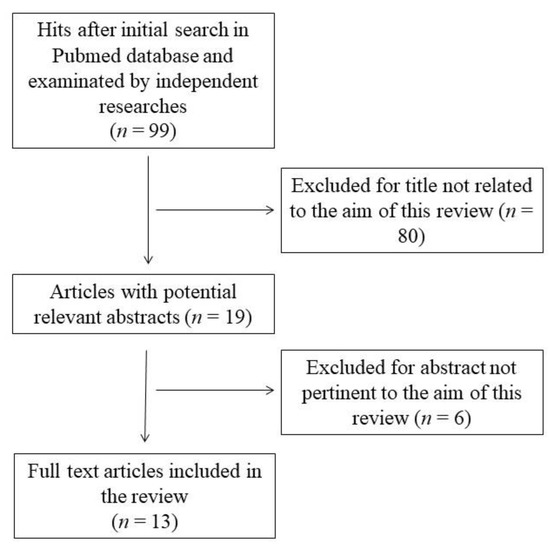
Figure 1.
Flow chart of papers included in the review.
3. Results and Discussion
3.1. Effect of Single PP on FRU Uptake and Transport in Intestinal Cells
Table 1 summarizes the data published on the effect of individual PP species on FRU uptake (i.e., the amount of FRU absorbed by and measured in the cells) and on FRU transport (i.e., the amount of FRU absorbed by the intestinal cells in the apical chamber and released to the basolateral one).

Table 1.
Summary of the information reported in the literature about the effect of phenolic compounds on intestinal FRU uptake and transport. FRU: fructose; NBDF: 1-deoxy-1-[(7-nitro-2,1,3-benzoxadiazol-4-yl)amino]-D-fructose; ↓: decrease; ↑: increase. Values between parentheses indicate the equivalent concentration expressed as molarity.
The number of available studies is limited to nine cases. Six of them used Caco-2 cells [30,32,34,35,36,37]; two relied on Caco-2/TC7 cells [31,33], and only one was performed on an ex-vivo rat jejunum mounted in an Ussing chamber [38]. Most of these studies did not consider the simultaneous presence of more than one PP species (a common occurrence in a PP-rich diet) and the possible occurrence of competition/synergy among individual PP species [39].
The studies listed above cover a wide variety of PP. However, comparing the reported results is difficult because essential experimental conditions varied greatly between individual labs. The PP concentrations spanned a range from approximately 1 µM [35] to roughly 800 µM [31], whereas the FRU concentration covered an even broader span: from 100 nM [32,35] to 130 mM [31]. In addition, the methods and the duration of supplementation were not consistent. Some studies involved the pretreatment of intestinal cells with PP [32,33,35] in contrast with co-supplementation with FRU [30,31,34,36,37], whereas exposure times ranged from 5 min [37] to 24 h [35]. Despite these limitations, PP supplementation studies generally resulted in a concentration-dependent decrease in FRU uptake [32,34,35,36,37] and transport [30,31,33,34,38]. Also noteworthy is that supplementation with quercetin, apigenin, and chrisyn was reportedly associated with decreased expression of GLUT2 and GLUT5 mRNA [32,35].
3.2. Effect of Polyphenol-Rich Products on FRU Uptake and Transport in Intestinal Cells
Table 2 summarizes the data available on the effect of PP-rich products on FRU uptake and transport in intestinal cells.

Table 2.
Summary of the literature information about the effect of PP rich products on intestinal FRU uptake and transport. FRU: fructose; GAE: gallic acid equivalent; NBDF: 1-deoxy-1-[(7-nitro-2,1,3-benzoxadiazol-4-yl)amino]-D-FRU; ↓: decrease; ↑: increase.
Similar to studies on individual PPs, there are only a limited number of studies (9) evaluating the effect of extracts [30,32,33,36,40,41,42] and of a phenolic-rich juice [31,43]. All of these studies were conducted in cell lines (six with Caco-2 cells and three with Caco-2/TC7 cells), and the experimental conditions differed considerably among the various studies. The total PP concentration ranged from approximately 0.01 mg/mL [32,41] to 10 mg/mL [32], whereas the FRU concentration varied from 54 nM [41] to 130 mM [31]. Similarly, the method and duration of supplementation varied from co-supplementation with FRU for only 10 min [36] to pretreatment with phenolic extract for 24 h and subsequent incubation with FRU for 3 h [40]. Even though the use of different approaches makes it difficult to draw general conclusions, most studies reported a pronounced effect of most PP-rich products on both FRU uptake [32,36,40,41,42] and transport [30,33,42,43]. However, sugarcane extract supplementation was determined to result in an upregulation of GLUT5 mRNA and a downregulation of GLUT2 mRNA expression [32].
Given the substantial evidence for a role of FRU in the development of metabolic diseases and the many positive effects of the consumption of plant bioactives, there is an apparent need for assessing the effect of plant-derived food bioactives on FRU uptake. PP are compounds naturally synthesized by the secondary metabolism of plants and have piqued the interest of the scientific community because of their protective or preventive effects [44]. A partial list of the health-promoting effects of PP includes: anti-radical scavenging activity, the induction of antioxidant enzymes, anti-inflammatory action, anti-cancer properties, and gut microbiota regulation [45,46,47,48,49,50]. There is also extensive evidence regarding the influence of PP on intestinal sugar metabolism, with reported effects on various glycohydrolases as well as on sugar transporters, possibly leading to the modulation of their function and the alteration of glucose absorption [27]. In addition, PP may ameliorate inflammatory-induced intestinal permeability [51], thus decreasing transcellular sugar absorption [52].
FRU transport is mediated by members of the GLUT family of facilitated sugar transporters encoded by the solute carrier family 2A (SLC2A) genes, which are essential for intestinal FRU uptake [53]. Intestinal GLUT5 mRNA levels and FRU transport rates are shallow pre-natally and rapidly increase with weaning, independently of the diet. However, transporter levels can increase further following weaning compared to diets containing FRU [54]. FRU transport from the intestine to the blood is mediated by GLUT2 (SLC2A2), a high-capacity, glucose-dependent fructose co-transporter. GLUT2 is primarily localized on the basolateral membrane of enterocytes, but after feeding, GLUT2 may be dislocated from the intestinal basolateral membrane to the apical one by a vesicular mediated mechanism [55]. Considering the mechanisms underlying intestinal FRU uptake and transport, the inhibitory effects mediated by PP may result in a decrease of GLUT5 and GLUT2 expression levels, the inhibition of their activity, or suppressing the post-prandial translocation of GLUT2 from the basolateral membrane to the apical one.
3.3. Effect of Polyphenols on GLUT Family Expression
Although various studies have explored the effect of PP on GLUT2 expression [21,56,57], only a limited number of selected studies investigated the effect of PP and PP-rich compound supplementation on GLUT5 expression level [32,35,40]. High-FRU feeding induces intestinal thioredoxin-interacting protein (TXNIP), which binds and regulates GLUT5-mediated intestinal FRU transport [58,59]. Consistent with this, carbohydrate-responsive element-binding protein (ChREBP), a transcription factor that responds to intracellular carbohydrate nutrients and a known transcriptional regulator of TXNIP [60], also regulates intestinal GLUT5 and GLUT2 expression [61] and is required for systemic FRU tolerance [11,62]. Very recently, Zakłos-Szyda et al. found evidence that Brassica juncea leaf extract suppresses TXNIP, ChREBP, and GLUT5 mRNA expression in Caco-2 cells [40]. Based on these findings, it is reasonable to suspect that their downregulation may be implicated in the mechanism responsible for decreased FRU uptake by PP. Contrary to the general trend on the effect of PP and PP-rich products on intestinal FRU transporters, Ji et al. [32] reported enhanced GLUT5 mRNA expression after supplementation with sugarcane extract in Caco-2 cells. Since sugarcane contains several sugars, it cannot be excluded that the increase in GLUT5 mRNA expression may be due to other types of residual sugar in the extract that promote transporter expression [63]. However, Schreck et al. [41] found that most plant extracts did not affect FRU uptake. Possibly this might be due to the displacement of the radioactive substrates by non-radioactive monosaccharides during the absorption processes.
GLUT2 and GLUT5 expression are also regulated by intracellular signaling mechanisms depending on several kinase proteins, including protein kinase C (PKC), protein kinase A (PKA), phosphoinositide 3-kinases (PI3K), and p38 mitogen-activated protein kinases (MAPK) [64]. Although PP affect several distinct intracellular signaling pathways [65], Andrade et al. found evidence that the inhibitory effects of quercetin, apigenin, and chrysin upon FRU uptake and GLUT2 and GLUT5 mRNA expression level did not involve interference with any of these signaling pathways [35].
3.4. Polyphenols and GLUT Family Interactions
For the final effect on FRU uptake by PP, one should consider not only the expression of membrane transporters but also the direct interaction between PP and proteins involved in the release and uptake of simple sugars. In this framework, molecular docking simulation techniques may offer an easy way to evaluate the affinity of various ligands, including potential substrates and potential inhibitors, and are used for the theoretical prediction of the binding affinity and/or an analysis of the interaction sites [66]. Zakłos-Szyda et al. [40] found that procyanidin C1 had the highest inhibitory activity on FRU uptake despite having the lowest binding affinity with GLUT5 among the PP tested in this study. Although the lowest value of binding affinity indicates the highest stability of the resulting PP/protein complex, it does not unequivocally mean that the interactions will result in the inhibitory effect, due to the peculiar interactions involving receptors’ residues and inhibitors. An inspection of the amino acid residues and hydrogen bonding interactions suggests that the complex between procyanidin C1 and GLUT5 does not involve hydrogen bond interactions or affect a specific Ile residue in the binding site, which was previously indicated as one of the determinants of GLUT5 specificity to FRU [67].
3.5. Effect of Polyphenols on GLUT2 Translocation
To prove the role of PP in inhibiting GLUT2 translocation, Cohen et al. [68] performed time-lapse microscopy in GLUT2 transiently transfected MDCK II cells by addressing the glucose-induced internalization and the glucose-removal-induced externalization following 30 min of pre-incubation with 1 mM phloretin, a PP found in apple trees’ leaves [69]. These authors found that both the glucose-induced internalization and the glucose-removal-induced externalization of GLUT2 were inhibited by phloretin, thus suggesting the ability of the PP to block the translocation of the transporter to the apical membrane.
A summary of the proposed mechanisms for PP inhibition on FRU uptake is illustrated in Figure 2.
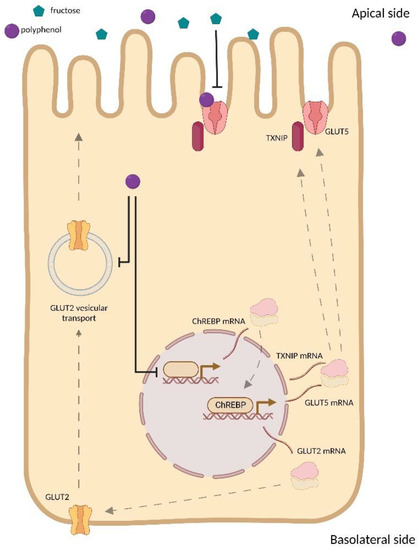
Figure 2.
Summary of the proposed mechanisms for PP inhibition on FRU uptake.
4. Conclusions
In spite of the promising and encouraging effects on the intestinal absorption of FRU by PP and PP-rich compounds, the different experimental conditions adopted by the reviewed studies did not allow definitive conclusions to be drawn. Some essential considerations must be considered in future studies and are listed herein. (I) Several of the reports analyzed herein did not report the phenolic composition of the extracts used, making it impossible to attribute a possible inhibitory effect to particular categories of compounds. The inhibitory effect is not attributable only to a class of compounds but to many of them, different by group, chemical structure, and food source. (II) Most of the available studies have evaluated the inhibitory effect on FRU absorption with supplementation times from a few minutes [37] to 24 h [35,40] without considering that, at the intestinal level, the chyme can reside in the duodenum from 3 to 6 h [70]. In addition, several studies have used a PP concentration of a few or tens of μM [32,34,36,37], which is in sharp contrast with the concentration in the intestine after a PP-rich meal, which may reach the mM range [19]. The selection of a physiological conditions also appears particularly important in light of the well-evidenced concentration-dependent effect of PP [32,34,35,36,37,42,43]. (III) Further room for improving the current experimental approaches relates to the fact that most in vitro studies have evaluated glucose inhibition, considering only marginally the effect on fructose and almost disregarding those on sucrose [33,42]. Additional studies that evaluate the effect on these carbohydrates (not only at the level of absorption but—in the case of sucrose—also at the level of the saccharase enzyme) by PP are fundamental. (IV) Most studies use radiolabeled FRU without considering that, inside cells, this can be converted into metabolic intermediates containing radionuclides that can then be excreted at the basolateral level [71], thus leading to an overestimate of the transport of sugar. (V) Hesperidin mildly inhibited FRU transport in Caco-2/TC-7 cells, but the inhibitory effect of hesperidin was abolished in the presence of glucose and sucrose [31]. Future studies may wish consider the simultaneous presence of several sugars and the interplay among them. (VI) All of the studies reviewed herein have evaluated either individual phenolic molecules or whole food extracts without considering a possible matrix effect on PP bioaccessibility [72]. Future studies considering the food as is—and changes occurring during digestion—will be helpful to assess the efficacy of bioactive compounds present in food in inhibiting FRU uptake and transport in intestinal cells.
Author Contributions
Conceptualization, M.D.N.; data curation, S.I., F.B. and M.D.N.; funding acquisition, M.D.N.; investigation, S.I., F.B. and M.D.N.; methodology, S.I., F.B. and M.D.N.; supervision, M.D.N.; validation, S.I., F.B. and M.D.N.; writing—original draft, M.D.N.; writing—review and editing S.I., F.B. and M.D.N. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by Italian Piano di Sostegno della Ricerca 2022-Azione A (Linea 2).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Lesley Currah for her skillful technical assistance in proofreading the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ferraris, R.P.; Choe, J.Y.; Patel, C.R. Intestinal Absorption of Fructose. Annu. Rev. Nutr. 2018, 38, 41–67. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, M.S.; Iossa, S.; Cigliano, L. Sweet but Bitter: Focus on Fructose Impact on Brain Function in Rodent Models. Nutrients 2021, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Tappy, L.; Rosset, R. Health outcomes of a high fructose intake: The importance of physical activity. J. Physiol. 2019, 597, 3561–3571. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Guidelines Approved by the Guidelines Review Committee. In Guideline: Sugars Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Busnatu, S.-S.; Salmen, T.; Pana, M.-A.; Rizzo, M.; Stallone, T.; Papanas, N.; Popovic, D.; Tanasescu, D.; Serban, D.; Stoian, A.P. The Role of Fructose as a Cardiovascular Risk Factor: An Update. Metabolites 2022, 12, 67. [Google Scholar] [CrossRef] [PubMed]
- Ang, B.; Yu, G. The Role of Fructose in Type 2 Diabetes and Other Metabolic Diseases. Nutr. Food Sci. 2017, 8, 1–4. [Google Scholar] [CrossRef]
- Federico, A.; Rosato, V.; Masarone, M.; Torre, P.; Dallio, M.; Romeo, M.; Persico, M. The Role of Fructose in Non-Alcoholic Steatohepatitis: Old Relationship and New Insights. Nutrients 2021, 13, 1314. [Google Scholar] [CrossRef]
- Yuan, C.; Joh, H.K.; Wang, Q.L.; Zhang, Y.; Smith-Warner, S.A.; Wang, M.; Song, M.; Cao, Y.; Zhang, X.; Zoltick, E.S.; et al. Sugar-sweetened beverage and sugar consumption and colorectal cancer incidence and mortality according to anatomic subsite. Am. J. Clin. Nutr. 2022, 115, 1481–1489. [Google Scholar] [CrossRef]
- Zwarts, I.; van Zutphen, T.; Kruit, J.K.; Liu, W.; Oosterveer, M.H.; Verkade, H.J.; Uhlenhaut, N.H.; Jonker, J.W. Identification of the fructose transporter GLUT5 (SLC2A5) as a novel target of nuclear receptor LXR. Sci. Rep. 2019, 9, 9299. [Google Scholar] [CrossRef]
- Schmidl, S.; Ursu, O.; Iancu, C.V.; Oreb, M.; Oprea, T.I.; Choe, J.Y. Identification of new GLUT2-selective inhibitors through in silico ligand screening and validation in eukaryotic expression systems. Sci. Rep. 2021, 11, 13751. [Google Scholar] [CrossRef]
- Hannou, S.A.; Haslam, D.E.; McKeown, N.M.; Herman, M.A. Fructose metabolism and metabolic disease. J. Clin. Investig. 2018, 128, 545–555. [Google Scholar] [CrossRef]
- Febbraio, M.A.; Karin, M. “Sweet death”: Fructose as a metabolic toxin that targets the gut-liver axis. Cell Metab. 2021, 33, 2316–2328. [Google Scholar] [CrossRef] [PubMed]
- Hengist, A.; Koumanov, F.; Gonzalez, J. Fructose and metabolic health: Governed by hepatic glycogen status? J. Physiol. 2019, 597, 3573–3585. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.U.; Yoon, J.H.; Jenkins, D.J.; Wolever, T.M.; Jenkins, A.L. Relationship between polyphenol intake and blood glucose response of normal and diabetic individuals. Am. J. Clin. Nutr. 1984, 39, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Sarker, M.M.R.; Sultana, T.N.; Chowdhury, M.N.R.; Rashid, M.A.; Chaity, N.I.; Zhao, C.; Xiao, J.; Hafez, E.E.; Khan, S.A.; et al. Antidiabetic Phytochemicals from Medicinal Plants: Prospective Candidates for New Drug Discovery and Development. Front. Endocrinol. 2022, 13, 800714. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Alarcón, K.; Victoriano, M.; Mardones, L.; Villagran, M.; Al-Harrasi, A.; Al-Rawahi, A.; Cruz-Martins, N.; Sharifi-Rad, J.; Martorell, M. Phytochemicals as Potential Epidrugs in Type 2 Diabetes Mellitus. Front. Endocrinol. 2021, 12, 656978. [Google Scholar] [CrossRef] [PubMed]
- Hokayem, M.; Blond, E.; Vidal, H.; Lambert, K.; Meugnier, E.; Feillet-Coudray, C.; Coudray, C.; Pesenti, S.; Luyton, C.; Lambert-Porcheron, S.; et al. Grape polyphenols prevent fructose-induced oxidative stress and insulin resistance in first-degree relatives of type 2 diabetic patients. Diabetes Care 2013, 36, 1454–1461. [Google Scholar] [CrossRef]
- Lecoultre, V.; Carrel, G.; Egli, L.; Binnert, C.; Boss, A.; MacMillan, E.L.; Kreis, R.; Boesch, C.; Darimont, C.; Tappy, L. Coffee consumption attenuates short-term fructose-induced liver insulin resistance in healthy men. Am. J. Clin. Nutr. 2014, 99, 268–275. [Google Scholar] [CrossRef]
- Williamson, G. Possible effects of dietary polyphenols on sugar absorption and digestion. Mol. Nutr. Food Res. 2013, 57, 48–57. [Google Scholar] [CrossRef]
- Santos-Hernández, M.; Miralles, B.; Amigo, L.; Recio, I. Intestinal Signaling of Proteins and Digestion-Derived Products Relevant to Satiety. J. Agric. Food Chem. 2018, 66, 10123–10131. [Google Scholar] [CrossRef]
- Alzaid, F.; Cheung, H.-M.; Preedy, V.R.; Sharp, P.A. Regulation of Glucose Transporter Expression in Human Intestinal Caco-2 Cells following Exposure to an Anthocyanin-Rich Berry Extract. PLoS ONE 2013, 8, e78932. [Google Scholar] [CrossRef]
- Cermak, R.; Landgraf, S.; Wolffram, S. Quercetin glucosides inhibit glucose uptake into brush-border-membrane vesicles of porcine jejunum. Br. J. Nutr. 2004, 91, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Farrell, T.L.; Ellam, S.L.; Forrelli, T.; Williamson, G. Attenuation of glucose transport across Caco-2 cell monolayers by a polyphenol-rich herbal extract: Interactions with SGLT1 and GLUT2 transporters. BioFactors 2013, 39, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Horita, M.; Nagai, H.; Nagatomo, A.; Nishida, N.; Matsuura, Y.; Nagaoka, S. Tiliroside, a glycosidic flavonoid, inhibits carbohydrate digestion and glucose absorption in the gastrointestinal tract. Mol. Nutr. Food Res. 2012, 56, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Ikumi, Y.; Kida, T.; Sakuma, S.; Yamashita, S.; Akashi, M. Polymer–phloridzin conjugates as an anti-diabetic drug that Inhibits glucose absorption through the Na+/glucose cotransporter (SGLT1) in the small intestine. J. Control. Release 2008, 125, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Terazono, Y.; Hirasaki, N.; Tatemichi, Y.; Kinoshita, E.; Obata, A.; Matsui, T. Inhibition of Glucose Transport by Tomatoside A, a Tomato Seed Steroidal Saponin, through the Suppression of GLUT2 Expression in Caco-2 Cells. J. Agric. Food Chem. 2018, 66, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Pico, J.; Martínez, M.M. Unraveling the Inhibition of Intestinal Glucose Transport by Dietary Phenolics: A Review. Curr. Pharm. Des. 2019, 25, 3418–3433. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Gauer, J.S.; Tumova, S.; Lippiat, J.D.; Kerimi, A.; Williamson, G. Differential patterns of inhibition of the sugar transporters GLUT2, GLUT5 and GLUT7 by flavonoids. Biochem. Pharmacol. 2018, 152, 11–20. [Google Scholar] [CrossRef]
- Sugimoto, K.; Amako, M.; Takeuchi, H.; Nakagawa, K.; Yoshimura, M.; Amakura, Y.; Fujita, T.; Takenaka, S.; Inui, H. Oenothein B in Eucalyptus Leaf Extract Suppresses Fructose Absorption in Caco-2 Cells. Molecules 2022, 27, 122. [Google Scholar] [CrossRef]
- Kerimi, A.; Gauer, J.S.; Crabbe, S.; Cheah, J.W.; Lau, J.; Walsh, R.; Cancalon, P.F.; Williamson, G. Effect of the flavonoid hesperidin on glucose and fructose transport, sucrase activity and glycaemic response to orange juice in a crossover trial on healthy volunteers. Br. J. Nutr. 2019, 121, 782–792. [Google Scholar] [CrossRef]
- Ji, J.; Yang, X.; Flavel, M.; Shields, Z.P.I.; Kitchen, B. Antioxidant and Anti-Diabetic Functions of a Polyphenol-Rich Sugarcane Extract. J. Am. Coll. Nutr. 2019, 38, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Villa-Rodriguez, J.A.; Kerimi, A.; Abranko, L.; Tumova, S.; Ford, L.; Blackburn, R.S.; Rayner, C.; Williamson, G. Acute metabolic actions of the major polyphenols in chamomile: An in vitro mechanistic study on their potential to attenuate postprandial hyperglycaemia. Sci. Rep. 2018, 8, 5471. [Google Scholar] [CrossRef] [PubMed]
- Satsu, H.; Awara, S.; Unno, T.; Shimizu, M. Suppressive effect of nobiletin and epicatechin gallate on fructose uptake in human intestinal epithelial Caco-2 cells. Biosci. Biotechnol. Biochem. 2018, 82, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Andrade, N.; Araújo, J.R.; Correia-Branco, A.; Carletti, J.V.; Martel, F. Effect of dietary polyphenols on fructose uptake by human intestinal epithelial (Caco-2) cells. J. Funct. Foods 2017, 36, 429–439. [Google Scholar] [CrossRef]
- Lee, Y.; Lim, Y.; Kwon, O. Selected Phytochemicals and Culinary Plant Extracts Inhibit Fructose Uptake in Caco-2 Cells. Molecules 2015, 20, 17393–17404. [Google Scholar] [CrossRef]
- Kwon, O.; Eck, P.; Chen, S.; Corpe, C.P.; Lee, J.-H.; Kruhlak, M.; Levine, M. Inhibition of the intestinal glucose transporter GLUT2 by flavonoids. FASEB J. 2007, 21, 366–377. [Google Scholar] [CrossRef]
- Andrade, N.; Andrade, S.; Silva, C.; Guimarães, J.T.; Keating, E.; Calhau, C.; Martel, F.; Rodrigues, I.; Marques, C. Effect of chrysin on changes in intestinal environment and microbiome induced by fructose-feeding in rats. Food Funct. 2019, 10, 4566–4576. [Google Scholar] [CrossRef]
- Mitra, S.; Tareq, A.; Das, R.; Emran, T.; Nainu, F.; Chakraborty, A.; Ahmad, I.; Tallei, T.; Idris, A.; Simal-Gandara, J. Polyphenols: A first evidence in the synergism and bioactivities. Food Rev. Int. 2022, 2022, 1–23. [Google Scholar] [CrossRef]
- Zakłos-Szyda, M.; Pietrzyk, N.; Kowalska-Baron, A.; Nowak, A.; Chałaśkiewicz, K.; Ratajewski, M.; Budryn, G.; Koziołkiewicz, M. Phenolics-Rich Extracts of Dietary Plants as Regulators of Fructose Uptake in Caco-2 Cells via GLUT5 Involvement. Molecules 2021, 26, 4745. [Google Scholar] [CrossRef]
- Schreck, K.; Melzig, M.F. Traditionally Used Plants in the Treatment of Diabetes Mellitus: Screening for Uptake Inhibition of Glucose and Fructose in the Caco2-Cell Model. Front. Pharm. 2021, 12, 692566. [Google Scholar] [CrossRef]
- Villa-Rodriguez, J.A.; Aydin, E.; Gauer, J.S.; Pyner, A.; Williamson, G.; Kerimi, A. Green and Chamomile Teas, but not Acarbose, Attenuate Glucose and Fructose Transport via Inhibition of GLUT2 and GLUT5. Mol. Nutr. Food Res. 2017, 61, 1700566. [Google Scholar] [CrossRef] [PubMed]
- Moser, S.; Lim, J.; Chegeni, M.; Wightman, J.D.; Hamaker, B.R.; Ferruzzi, M.G. Concord and Niagara Grape Juice and Their Phenolics Modify Intestinal Glucose Transport in a Coupled in Vitro Digestion/Caco-2 Human Intestinal Model. Nutrients 2016, 8, 414. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Polyphenols and their applications: An approach in food chemistry and innovation potential. Food Chem. 2021, 338, 127535. [Google Scholar] [CrossRef] [PubMed]
- Briguglio, G.; Costa, C.; Pollicino, M.; Giambò, F.; Catania, S.; Fenga, C. Polyphenols in cancer prevention: New insights (Review). Int. J. Funct. Nutr. 2020, 1, 9. [Google Scholar] [CrossRef]
- Di Nunzio, M.; Betoret, E.; Taccari, A.; Dalla Rosa, M.; Bordoni, A. Impact of processing on the nutritional and functional value of mandarin juice. J. Sci. Food Agric. 2020, 100, 4558–4564. [Google Scholar] [CrossRef]
- Di Nunzio, M.; Picone, G.; Pasini, F.; Chiarello, E.; Caboni, M.F.; Capozzi, F.; Gianotti, A.; Bordoni, A. Olive oil by-product as functional ingredient in bakery products. Influence of processing and evaluation of biological effects. Food Res. Int. 2020, 131, 108940. [Google Scholar] [CrossRef]
- Di Nunzio, M.; Toselli, M.; Verardo, V.; Caboni, M.F.; Bordoni, A. Counteraction of oxidative damage by pomegranate juice: Influence of the cultivar. J. Sci. Food Agric. 2013, 93, 3565–3573. [Google Scholar] [CrossRef]
- Nissen, L.; Casciano, F.; Chiarello, E.; Di Nunzio, M.; Bordoni, A.; Gianotti, A. Colonic In Vitro Model Assessment of the Prebiotic Potential of Bread Fortified with Polyphenols Rich Olive Fiber. Nutrients 2021, 13, 787. [Google Scholar] [CrossRef]
- Olszowy, M. What is responsible for antioxidant properties of polyphenolic compounds from plants? Plant Physiol. Biochem. 2019, 144, 135–143. [Google Scholar] [CrossRef]
- Bernardi, S.; Del Bo’, C.; Marino, M.; Gargari, G.; Cherubini, A.; Andrés-Lacueva, C.; Hidalgo-Liberona, N.; Peron, G.; González-Dominguez, R.; Kroon, P.; et al. Polyphenols and Intestinal Permeability: Rationale and Future Perspectives. J. Agric. Food Chem. 2020, 68, 1816–1829. [Google Scholar] [CrossRef]
- Farré, R.; Fiorani, M.; Abdu Rahiman, S.; Matteoli, G. Intestinal Permeability, Inflammation and the Role of Nutrients. Nutrients 2020, 12, 1185. [Google Scholar] [CrossRef] [PubMed]
- Lizák, B.; Szarka, A.; Kim, Y.; Choi, K.-s.; Németh, C.E.; Marcolongo, P.; Benedetti, A.; Bánhegyi, G.; Margittai, É. Glucose Transport and Transporters in the Endomembranes. Int. J. Mol. Sci. 2019, 20, 5898. [Google Scholar] [CrossRef] [PubMed]
- Mazzoli, A.; Gatto, C.; Crescenzo, R.; Spagnuolo, M.S.; Nazzaro, M.; Iossa, S.; Cigliano, L. Gut and liver metabolic responses to dietary fructose—Are they reversible or persistent after switching to a healthy diet? Food Funct. 2021, 12, 7557–7568. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tuo, B.; Dong, H. Regulation of Intestinal Glucose Absorption by Ion Channels and Transporters. Nutrients 2016, 8, 43. [Google Scholar] [CrossRef]
- Cao, H.; Hininger-Favier, I.; Kelly, M.A.; Benaraba, R.; Dawson, H.D.; Coves, S.; Roussel, A.M.; Anderson, R.A. Green Tea Polyphenol Extract Regulates the Expression of Genes Involved in Glucose Uptake and Insulin Signaling in Rats Fed a High Fructose Diet. J. Agric. Food Chem. 2007, 55, 6372–6378. [Google Scholar] [CrossRef] [PubMed]
- Fiesel, A.; Gessner, D.K.; Most, E.; Eder, K. Effects of dietary polyphenol-rich plant products from grape or hop on pro-inflammatory gene expression in the intestine, nutrient digestibility and faecal microbiota of weaned pigs. BMC Vet. Res. 2014, 10, 196. [Google Scholar] [CrossRef] [PubMed]
- Dotimas, J.R.; Lee, A.W.; Schmider, A.B.; Carroll, S.H.; Shah, A.; Bilen, J.; Elliott, K.R.; Myers, R.B.; Soberman, R.J.; Yoshioka, J.; et al. Diabetes regulates fructose absorption through thioredoxin-interacting protein. eLife 2016, 5, e18313. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Dagdeviren, S.; Lewandowski, J.P.; Schmider, A.B.; Ricci-Blair, E.M.; Natarajan, N.; Hundal, H.; Noh, H.L.; Friedline, R.H.; Vidoudez, C.; et al. Thioredoxin Interacting Protein Is Required for a Chronic Energy-Rich Diet to Promote Intestinal Fructose Absorption. iScience 2020, 23, 101521. [Google Scholar] [CrossRef]
- Iizuka, K.; Bruick, R.K.; Liang, G.; Horton, J.D.; Uyeda, K. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc. Natl. Acad. Sci. USA 2004, 101, 7281–7286. [Google Scholar] [CrossRef]
- Iizuka, K. The Role of Carbohydrate Response Element Binding Protein in Intestinal and Hepatic Fructose Metabolism. Nutrients 2017, 9, 181. [Google Scholar] [CrossRef]
- Kim, M.; Astapova, I.I.; Flier, S.N.; Hannou, S.A.; Doridot, L.; Sargsyan, A.; Kou, H.H.; Fowler, A.J.; Liang, G.; Herman, M.A. Intestinal, but not hepatic, ChREBP is required for fructose tolerance. JCI Insight 2017, 2, e96703. [Google Scholar] [CrossRef] [PubMed]
- Douard, V.; Ferraris, R.P. Regulation of the fructose transporter GLUT5 in health and disease. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E227–E237. [Google Scholar] [CrossRef]
- Gonçalves, A.S.; Andrade, N.; Martel, F. Intestinal fructose absorption: Modulation and relation to human diseases. PharmaNutrition 2020, 14, 100235. [Google Scholar] [CrossRef]
- Sun, L.R.; Zhou, W.; Zhang, H.M.; Guo, Q.S.; Yang, W.; Li, B.J.; Sun, Z.H.; Gao, S.H.; Cui, R.J. Modulation of Multiple Signaling Pathways of the Plant-Derived Natural Products in Cancer. Front. Oncol. 2019, 9, 1153. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Fu, A.; Zhang, L. Progress in molecular docking. Quant. Biol. 2019, 7, 83–89. [Google Scholar] [CrossRef]
- Ebert, K.; Ewers, M.; Geillinger-Kästle, K.; Schoberth, G.; Essenwanger, J.; Stolz, J.; Daniel, H.; Witt, H. Reassessment of GLUT7 and GLUT9 as Putative Fructose and Glucose Transporters. J. Membr. Biol. 2017, 250, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.; Kitsberg, D.; Tsytkin, S.; Shulman, M.; Aroeti, B.; Nahmias, Y. Live imaging of GLUT2 glucose-dependent trafficking and its inhibition in polarized epithelial cysts. Open Biol. 2014, 4, 140091. [Google Scholar] [CrossRef] [PubMed]
- Ben-Othman, S.; Kaldmäe, H.; Rätsep, R.; Bleive, U.; Aluvee, A.; Rinken, T. Optimization of Ultrasound-Assisted Extraction of Phloretin and Other Phenolic Compounds from Apple Tree Leaves (Malus domestica Borkh.) and Comparison of Different Cultivars from Estonia. Antioxidants 2021, 10, 189. [Google Scholar] [CrossRef]
- Szarka, L.A.; Camilleri, M. Methods for the assessment of small-bowel and colonic transit. Semin. Nucl. Med. 2012, 42, 113–123. [Google Scholar] [CrossRef]
- Chiarello, E.; Di Nunzio, M.; Picone, G.; Antonelli, G.; Capozzi, F.; Bordoni, A. Insight on Glucose and Fructose Absorption and Relevance in the Enterocyte Milieu. Nutrients 2022, 14, 517. [Google Scholar] [CrossRef]
- Antognoni, F.; Mandrioli, R.; Bordoni, A.; Di Nunzio, M.; Viadel, B.; Gallego, E.; Villalba, M.P.; Tomás-Cobos, L.; Taneyo Saa, D.L.; Gianotti, A. Integrated Evaluation of the Potential Health Benefits of Einkorn-Based Breads. Nutrients 2017, 9, 1232. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).