Abstract
Functional gastrointestinal diseases (FGID) are worldwide prevalent conditions. Pharmacological treatments can be ineffective, leading the population to turn to herbal or traditional remedies. Helichrysum stoechas (L.) Moench is a medicinal plant traditionally used in the Iberian Peninsula to treat digestive disorders, but its effects on gastrointestinal motility have not been scientifically demonstrated. The aim of this work was to evaluate the antispasmodic effect of a polyphenolic extract of H. stoechas (HSM), its mechanism of action and its antioxidant activity. Isometric myography studies were performed in rat ileum, and malondialdehyde (MDA) and 4-hydroxyalkenals (4-HDA) levels were measured in rat jejunum. HSM reduced the integrated mechanical activity of spontaneous contractions. In Ca2+-free medium, HSM reduced the concentration–response curve of CaCl2 similarly to verapamil. Pre-incubation with the extract blocked the contraction induced by Bay K8644, KCl and carbachol. L-NAME, ODQ, Rp-8-Br-PET-cGMPS, KT-5823, apamin, TRAM-34 and charybdotoxin reduced the relaxant effect of the extract on spontaneous contractions. MDA+4-HDA levels in LPS-treated tissue were reduced by the extract, showing antioxidant activity. In conclusion, HSM showed antispasmodic activity through inhibition of Ca2+ influx, activation of the NO/PKG/cGMP pathway and opening of Ca2+-activated K+ channels. The results suggest that H. stoechas could help in the prevention or treatment of FGIDs.
1. Introduction
Digestive disorders with symptoms without a clear etiology, as abdominal pain, motility alteration or nausea, are included as functional gastrointestinal diseases (FGID), affecting a large part of the population [1,2]. Irritable bowel syndrome, functional constipation and functional abdominal pain, among others, are included as FGID, which pathophysiology is characterized by alterations in the gut microbiota, mucosal immune function, visceral hypersensitivity and dysmotility [1,3]. In a large-scale multinational study, it was described that the worldwide prevalence of FGID is approximately 40% [4]. The treatment of these pathologies can be complex and sometimes ineffective, and that is why a large part of the population turns to complementary and alternative medicine (CAM), especially herbal medicine, to complement or replace conventional treatment [2,5].
CAM is all practices and products that are not considered part of modern medicine. CAM includes not only herbs and other plant treatments but also non-botanical supplements and mind–body therapies [6]. According to the World Health Organization (WHO) strategy 2014–2023, more than 100 million Europeans currently use CAM, with a much higher number of users in other continents such as Africa, Asia, Australia and North America [7]. CAMs have been and are currently used for the treatment of a wide variety of syndromes [6].
In the FGID treatment, phytotherapy plays an important therapeutic role [2,8,9]. Different online compendia of scientific bodies on medicinal plants as Committee on Herbal Medicinal Products (HMPC) or European Medicines Agency (EMA) reported, in 2017, a total number of 141 medicinal plants, of which 34% are used for the treatment of gastrointestinal diseases [8]. In this sense, the herbal tea form of Helichrysum arenarium, for instance, has been approved by the WHO and the EMA for the treatment of digestive problems such as fullness and bloating [10].
The genus Helichrysum (Asteraceae), from the Greek “helios” (ἥλιος, sun) and “chrysos” (χρῡσός, gold) by the intense yellow color of its flowers, has approximately 600 species, which has been widely used in traditional medicine throughout the world [11]. Helichrysum stoechas (L.) Moench is one of the least known species of its genus. This species, also known as everlasting flower, is distributed in the Iberian Peninsula where it has cooking and culinary uses in beverages, dishes or desserts [12]. The infusion or decoction of its flowers have been traditionally used over the years to improve some problems of the gastrointestinal (e.g., fullness, floating, hepatic and pancreatic disorders), cardiovascular (e.g., hypertension) and respiratory systems (e.g., influenza, flu, common cold), or as a diuretic, suggesting a possible role of this plant for preventing and treating urolithiasis [11,13,14,15,16]. This wide range of therapeutic applications may be due to its anti-inflammatory, antioxidant, antidiabetic, anti-tyrosinase, anti-acetylcholinesterase and antimicrobial activity demonstrated in vitro [17,18,19,20,21,22,23]. To our knowledge, it is only a clinical trial that used H. stoechas in combination with other plants in syrup to treat persisting cough in children, showing a reduction in the severity and duration of this symptom [24]. H. stoechas has also shown a neuroprotective effect in both in vitro and in vivo assays [18,25] and analgesic effect in vivo [26]. It also has anti-tumorinegic actions as suggested by its inhibition of the proliferation of HeLa cells [18]. Recently, it has been shown to have a hypotensive effect by inducing vascular smooth muscle relaxation through endothelium-independent and endothelium-dependent mechanisms [27].
Reported phytochemicals of different extracts of H. stoechas have demonstrated that this species is rich in polyphenols, such as flavonoids and phenolic acids [20,21,22]. In the same way, the phytochemical analysis of this methanolic extract of H. stoechas, by the Folin–Ciocalteu method, presented a high content of polyphenols with a strong antioxidant capacity. Furthermore, the main compounds were isolated and identified by chromatography and NMR [18]. These bioactive compounds could explain its range of biological activities. Moreover, various studies reported the potential beneficial effects of polyphenols or their metabolites in several gastrointestinal diseases as inflammatory bowel disease, colitis-associated cancer or FGID [28,29,30,31], although more research is needed in this field. Despite the knowledge about H. stoechas is increasing and its flowers are still taken as infusions to treat stomach and intestinal diseases, there is no scientific evidence of the effect of H. stoechas on digestive tract. Therefore, the aim of this study was to evaluate the possible intestinal antispasmodic effect of a methanolic extract of H. stoechas and its mechanism of action.
2. Results and Discussion
2.1. Effect of H. stoechas Extract on Spontaneous Contractions
Figure 1a,c show the effect of H. stoechas (0.01–3 mg/mL) on the spontaneous contractions in the longitudinal smooth muscle of rat ileum. The H. stoechas extract reduced the area under the curve (AUC) in a concentration-dependent manner, with an EC50 value of 0.33 mg/mL (0.39–0.28, 95% CI). The amplitude of spontaneous contractions, but not the frequency, was significantly reduced (Table 1). Although methanol was removed from the extract before experiments, the solvent of H. stoechas, did not modify the AUC, the amplitude or the frequency of spontaneous contractions of the ileum (Figure 1c and Table 2).
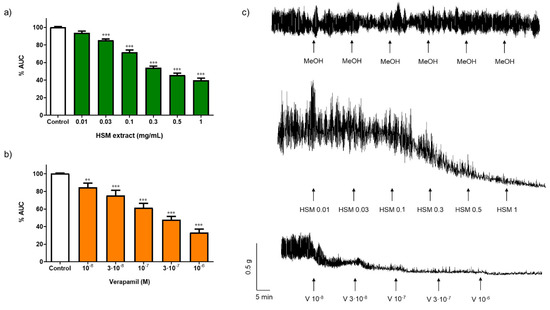
Figure 1.
Effect of different concentrations of (a) H. stoechas extract (HSM) and (b) verapamil (V) on the spontaneous contractions in segments of the longitudinal smooth muscle of rat ileum. Data points are mean ± SEM (n = 6–8). ** p < 0.01 and *** p < 0.001 vs. control (basal spontaneous contractions). (c) Representative recordings of spontaneous contractions from cumulative concentration response curves of methanol (MeOH), HSM (0.01–3 mg/mL) and V (10−8–10−6 M). AUC: area under the curve.

Table 1.
Effect of H. stoechas (HSM, mg/mL) extract and verapamil (V, M) on the amplitude and frequency of spontaneous contractions of longitudinal smooth muscle of rat ileum. Data are expressed as the percentage of the amplitude and frequency of spontaneous contractions from those of the control period ± SEM (n = 6–8). *** p < 0.001 vs. control (basal spontaneous contractions).

Table 2.
Effect of methanol (MeOH) on the AUC, amplitude and frequency of spontaneous contractions of longitudinal smooth muscle of rat ileum. Data are expressed as the percentage of the amplitude and frequency of spontaneous contractions from those of the control period ± SEM (n = 4). AUC: area under the curve.
The result reports for the first time the antispasmodic activity of a methanolic extract of H. stoechas. Extracts from other Helichrysum species also exert antispasmodic activity by reducing the spontaneous motility in intestinal smooth muscle strips. Thus, extracts of H. plicatum [32], H. arenarium [33,34] and H. italicum [35] inhibit the spontaneous contractions in rat ileum, rat and rabbit intestine and mouse ileum, respectively. Furthermore, other plants belonging to the Asteraceae family, such as Jasonia glutinosa [36] and Chrysactinia mexicana [37], decrease the amplitude of spontaneous contractions without modifying their frequency in the rat duodenum and rabbit ileum, respectively.
The spontaneous rhythmic contractions of the intestinal smooth muscle are necessary for the maintenance of the physiological functions of the intestine. These contractions, initiated in interstitial cells of Cajal, are due to changes in the membrane potential. Ca2+ is responsible for gastrointestinal motility. Thus, it has an important role in smooth muscle depolarization and repolarization participating in maintaining the tone, amplitude and frequency of spontaneous contractions and contractile response. The increase in intracellular Ca2+ may be due to its entry from the extracellular medium and/or the release of Ca2+ from intracellular stores [38].
In our study, verapamil, an antagonist of L-type Ca2+ channels, used as positive control, reduced the AUC in a concentration-dependent manner, with an EC50 value of 0.17 µM (0.12–0.25, 95% CI). Verapamil also decreased the amplitude, but it did not modify the frequency on spontaneous contractions (Figure 1b,c and Table 1). The decrease in the spontaneous motility induced by the H. stoechas extract in the study was similar to that induced by verapamil, an agent that prevents the entry of Ca2+ into the cell by blocking the voltage-dependent L-type Ca2+ channels (VDCCs), suggesting that H. stoechas extract could be acting through the same pathway.
2.2. Effect of H. stoechas Extract on Influx of Ca2+
To examine the effect of H. stoechas extract on influx of extracellular Ca2+, ileum segments were pre-incubated in a Ca2+-free K+-rich buffer with methanol (control), H. stoechas (0.3 or 1 mg/mL) or verapamil (10−6 M). After the incubation period, a cumulative Ca2+ curve was performed by adding increasing concentrations of CaCl2 (10−5–10−2 M). As shown in Figure 2, H. stoechas shifted the CaCl2 response curve down and to the right. H. stoechas at 0.3 and 1 mg/mL reduced the maximum response to CaCl2 by 27.3% and 56.5%, respectively. Verapamil 10−6 M produced a similar relaxation of 73.1% (Figure 2).
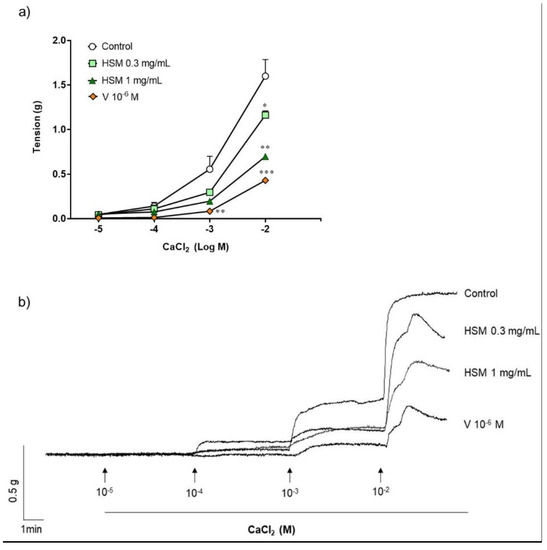
Figure 2.
(a) Effect of H. stoechas extract (HSM, 0.3 or 1 mg/mL) and verapamil (V, 10−6 M) on the contraction produced by CaCl2 in rat ileum. (b) Representative recordings of spontaneous contractions from cumulative concentration of CaCl2 in strips pre-incubated with methanol (control), H. stoechas or verapamil. Data points are mean ± SEM (n = 6). * p < 0.05, ** p < 0.01 and *** p < 0.001 vs. control.
2.3. Effect of H. stoechas Extract on the Contractions Induced by an Agonist of L-type Ca2+ Channels
Bay K8644 (10−6 M), an agonist of L-type Ca2+ channels, produced a contractile response on ileum (control). Pre-incubation with H. stoechas extract to 0.3 and 1 mg/mL reduced the Bay K8644-induced contractile response by 45.4% and 74.2%, respectively (Figure 3). Similar pre-incubation with the antagonist of L-type Ca2+ channels, verapamil, also induced an inhibition of 59.4% (Figure 3).
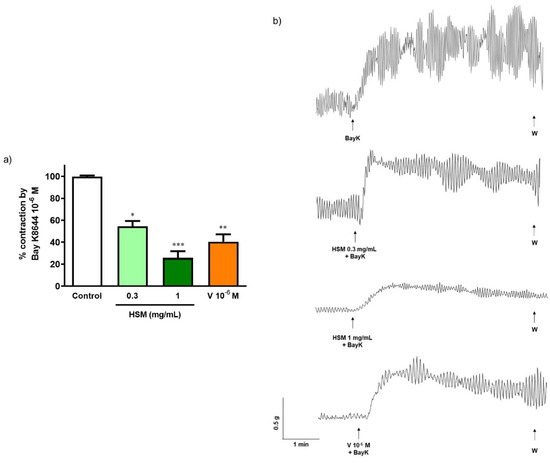
Figure 3.
(a) Effect of pre-incubation with H. stoechas extract (HSM, 0.3 and 1 mg/mL) and verapamil (V, 10−6 M) on the contractile response produced by Bay K8644 (10−6 M) on rat ileum segments. (b) Representative recordings of contractions produced by Bay K8644 alone or in the presence of HSM or verapamil. Data points are mean ± SEM (n = 6–8). * p < 0.05, ** p < 0.01 and *** p < 0.001 vs. control (Bay K8644 alone).
These results suggest that H. stoechas extract reduces rat spontaneous intestinal contractions by inhibiting Ca2+ influx into smooth muscle through the blockade of L-type Ca2+ channels.
2.4. Effect of H. stoechas Extract on the Contractions Induced by Other Contractile Agents
To assess the effect of H. stoechas extract on the response to classical intestinal smooth muscle contractile agents, either KCl (80 mM), a membrane depolarizing agent, or carbachol (10−6 M), a muscarinic cholinergic agonist, were added to the bath. Pre-incubation of the segments of longitudinal smooth muscle of the ileum for 20 min with the H. stoechas extract (1 mg/mL) significantly reduced the contractile response induced by KCl and carbachol by 63.0% and 65.6%, respectively (Figure 4).
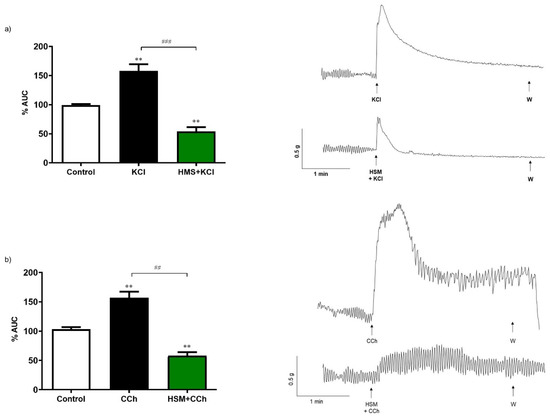
Figure 4.
Contractile effect of (a) KCl (80 mM) and (b) carbachol (CCh, 10−6 M) on spontaneous contractions and effect of pre-incubation of H. stoechas extract (HSM, 1 mg/mL) on KCl or CCh-evoked contractile responses. Their representative recordings are showed on the right. Data points are mean ± SEM (n = 6). ** p < 0.01 vs. control (basal spontaneous contractions) and ## p < 0.01, ### p < 0.001 vs. KCl or CCh. AUC: area under the curve.
KCl at high concentration can evoke a depolarization in the smooth muscle membrane and therefore produce the entry of extracellular Ca2+ through the opening of VDCC without any receptor stimulation. In contrast, the muscarinic agonist carbachol induces contraction through the activation of muscarinic cholinergic receptors. These receptors increase the influx of extracellular Ca2+ through of activation the non-selective cation channels (ROCCs) as well as the Ca2+ output from the sarcoplasmic reticulum through the opening of IP3-sentitive Ca2+ channels and ryanodine receptors [39]. These results suggest that, in addition to inhibiting extracellular Ca2+ influx through VDCC, the H. stoechas extract could also act by blocking ROCC and Ca2+ release from the sarcoplasmic reticulum.
Extracts from several plants of the Asteraceae family also reduce contractions induced by cholinergic agonists or high K+ concentrations, such as H. italicum in mouse ileum [35], H. plicatum in rat ileum [32], J. glutinosa in rat duodenum [36], Artemisia vulgaris in rabbit jejunum [40], Achillea millefolium in rat ileum [41] and Chrysactinia mexicana in rabbit ileum [37]. The results of these works show that, like H. stoechas, polyphenolic extracts of these plants inhibit both spontaneous and induced contractions by blocking the entry of Ca2+ ions into the smooth muscle cell, mainly through L-type Ca2+ channels.
2.5. Role of NO and cGMP on the Effect of H. stoechas Extract on Spontaneous Contractions
To study whether NO and cGMP were involved in the relaxant response of H. stoechas extract, the ileum segments were pre-incubated 20 min before the addition of the H. stoechas extract (1 mg/mL) with different substances such as L-NAME (10 µM), an inhibitor of NOS; ODQ (1 µM), a potent and selective inhibitor of soluble guanylyl cyclase; Rp-8-Br-PET-cGMPs (1 µM), an inhibitor of cGKI; and cGKII, KT-5823 (1 µM), a selective inhibitor of PKG. The incubation of all substances reduced the H. stoechas extract effect on the AUC of spontaneous contractions (Figure 5a). Inhibitors of the NO/PKG/cGMP pathway had no effect per se on spontaneous motility.
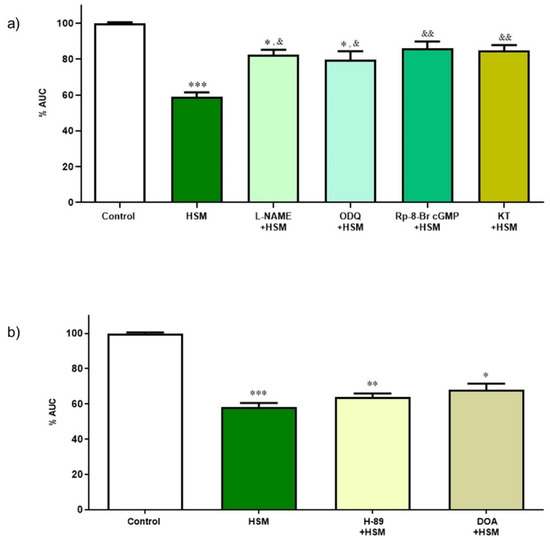
Figure 5.
(a) Effect of inhibitors of the NO/cGMP/PKG pathway on the relaxant response induced by H. stoechas extract (HSM, 1 mg/mL) on the longitudinal smooth muscle of the rat ileum. (b) Effect of inhibitors of the cAMP/PKA pathway on the relaxant response induced by the HSM extract on the longitudinal smooth muscle of the rat ileum. Data points are mean ± SEM (n = 6–8). * p < 0.05, ** p < 0.01, *** p < 0.001 vs. control (basal spontaneous contractions) and & p < 0.05, && p < 0.01, vs. HSM. AUC: area under the curve.
2.6. Role of cAMP on the Effect of H. stoechas Extract on Spontaneous Contractions
Like the previous assay, the effect of cAMP was investigated for the relaxant response produced by the H. stoechas extract (1 mg/mL) after pre-incubating the ileum segments with H-89 (1 µM), an inhibitor of the PKA, and 2,5-dideoxiadenosina (DOA, 1 mM), an inhibitor of adenylate cyclase. These substances had no effect per se on the spontaneous motility.
None of the substances tested modified the effect of H. stoechas extract on AUC of spontaneous contractions of the ileum muscle (H-89 + HSM vs. HSM, p = 0.198 and DOA + HSM vs. HSM, p = 0.129) (Figure 5b).
These results suggest that the spasmolytic effect evoked by the extract on the longitudinal smooth muscle of the rat ileum is mediated in part by the NO/PKG/cGMP pathway, but not by the PKA/cAMP one.
Under physiological conditions, NO has a very important role in the motility of the GI tract. Thus, an alteration in NO homeostasis produces gastrointestinal motor dysfunction [42,43,44]. NO is a non-adrenergic non-cholinergic neurotransmitter produced by NO synthases and it regulates the intestinal motility through an inhibitory action, relaxing smooth muscle through the intracellular messenger cGMP and PKG [42]. In the smooth muscle, PKG is able to inhibit the Ca2+ channels of the sarcoplasmic reticulum, to stimulate the closure of L-type Ca2+ channels, to promote the opening of K+ channels, to inhibit the RhoA factor and to activate the myosin light chain phosphatase. All of these actions lead to the reduction in cytosolic Ca2+, and to dephosphorylate the myosin light chain, thus converging in the relaxation of the smooth muscle cell [45]. In contrast to these results, spasmolytic activities evoked by H. italicum extracts in isolated mouse ileum [35] or by essential oil of Chrysactinia mexicana in rabbit ileum [37] are not mediated through the NO/cGMP pathway, whereas cAMP is involved in the effects induced by Chrysactinia mexicana. This difference may be due to their different phytochemical composition.
2.7. Role of K+ Channels on Response of H. stoechas Extract on Spontaneous Contractions
Figure 6 shows the participation of K+ channels in the relaxing response evoked by H. stoechas extract (1 mg/mL) after pre-incubation of the ileum segments with various inhibitors of K+ channels as apamin, a selective small-conductance Ca2+- and voltage-activated K+ channel (SKCa) inhibitor (AP, 1 µM); TRAM-34 (1-[(2-chlorophenyl) diphenylmethyl]-1H-pyrazole, a selective inhibitor of intermediate conductance Ca2+-activated K+ channels (IKCa) (1 µM); charybdotoxin, a specific inhibitor of intermediate- and large-conductance Ca2+-activated K+ channels (ChTx, 0.01 µM); glibenclamide, an inhibitor of ATP-sensitive K+ channels (KATP) (Glib, 10 µM); BaCl2, an inhibitor of the inward rectifier K+ channel (KIR) (BC, 30 mM); and quinine, an inhibitor of voltage-sensitive K+ channels (Qn, 10 µM).
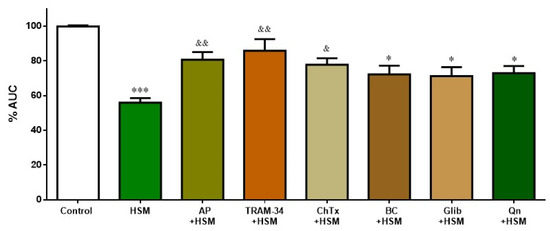
Figure 6.
Effect of K+ channel inhibitors on the inhibition induced by H. stoechas extract (HSM, 1 mg/mL) on the longitudinal smooth muscle of rat ileum: apamin (AP), TRAM-34, charybdotoxin (ChTx), BaCl2 (BC), glibenclamide (Glib) and quinine (Qn). Data points are mean ± SEM (n = 6–8). * p < 0.05, *** p < 0.001 vs. control (basal spontaneous contractions), & p < 0.05 and && p < 0.01 vs. HSM. AUC: area under the curve.
Apamin, TRAM-34 and charybdotoxin significantly reduced the effect of H. stoechas extract on the AUC from the ileum spontaneous contractions (by 24.7%, 30% and 21.4%, respectively). However, glibenclamide, BaCl2 and quinine, although they reduced this effect slightly (by approximately 15%), did not reach statistical significance (Figure 6). K+ channels inhibitors had no effect per se on the spontaneous motility.
K+ channels also have an important role in maintaining intestinal homeostasis. The activation of smooth muscle K+ channels produces cell hyperpolarization and, as a consequence, the closure of the VDCCs, the decrease in cytosolic Ca2+ and, finally, the relaxation of smooth muscle. In this study, the relaxant effect induced by the H. stoechas extract was significantly reduced by apamin, TRAM-34 and charybdotoxin, suggesting the role of small-, intermediate- and large-conductance Ca2+-activated K+ channels (IKCa, SKCa and BKCa), respectively. However, the fact that glibenclamide, BaCl2 and quinine did not modify the H. stoechas extract-induced relaxation showed that ATP-sensitive, inward rectifier or voltage-sensitive K+ channels are not involved. Thus, these findings suggest that the spasmolytic activity of H. stoechas is mediated through the opening of Ca2+-activated K+ channels. Similar results were obtained with essential oil of Chrysactinia mexicana in rabbit ileum [37].
2.8. Potential Additive Effects on Relaxation Induced by H. stoechas Extract
As the Ca2+-activated K+ channels can be opened by NO, the possible additive effects on the relaxant response produced by H. stoechas extract (1 mg/mL) were studied by the combination of TRAM-34 and apamin, as K+ channels inhibitors, and L-NAME, as NO inhibitor.
The combination of TRAM-34 and apamin did not modify the AUC of spontaneous contractions shown by the treatment with these K+ channel blockers alone. However, the addition of L-NAME with the K+ channel inhibitors showed an additive effect of the NOS inhibitor, reaching to an almost complete blockade of the effect of H. stoechas on the AUC from spontaneous contractions (Table 3). This result demonstrates an additive effect of NO release and hyperpolarization.

Table 3.
Effect of pre-incubation with L-NAME (10−5 M), TRAM-34 (10−6 M), apamin (AP, 10−6 M), and the combination of TRAM-34+AP and L-NAME+TRAM-34+AP on the reduction induced by H. stoechas extract (HSM, 1 mg/mL) on the longitudinal smooth muscle of rat ileum. Data points are mean ± SEM (n = 6–8). *** p < 0.001 vs. control (basal spontaneous contractions) and & p < 0.05, && p < 0.05, &&& p < 0.001 vs. HSM. AUC: area under the curve.
2.9. Role of the Rho-Kinase Pathway on the Effect of H. stoechas Extract on Spontaneous Contractions
After showing that the H. stoechas extract has clear impact on Ca2+-dependent contraction, it was studied whether the Ca2+-independent Rho-kinase pathway was involved in the effect of H. stoechas extract (1 mg/mL). Thus, ileum segments were pre-incubated with Y-27632 (10–6 M), a Rho-kinase inhibitor, and with the combination Y-27632 + extract.
As in the other assays, the extract significantly reduced spontaneous contractions compared to the control. This effect was not additive when extract was added with Y-27632. Y-27632 per se did not cause a statistically significant change on the AUC from spontaneous contractions of ileum (Figure 7).
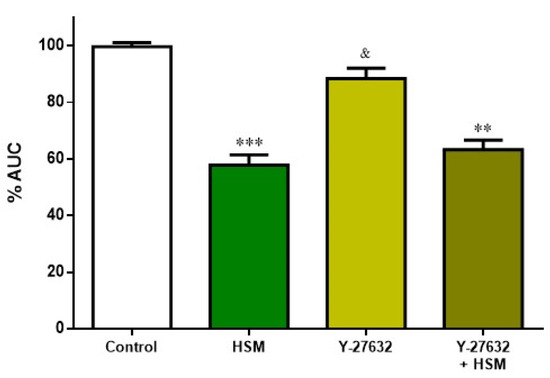
Figure 7.
Effect of pre-incubation with H. stoechas extract (HSM, 1 mg/mL), Y-27632 (10−6 M) and the combination of Y-27632+HSM on the AUC from spontaneous contractions of rat ileum. Data points are mean ± SEM (n = 6–8). ** p < 0.01, *** p < 0.001 vs. control (basal spontaneous contractions), and & p < 0.05 vs. HSM. AUC: area under the curve.
The fact that the inhibitor of Rho-kinase Y-27632 did not alter the response of H. stoechas extract on spontaneous contractions would indicate that the extract does not change the Ca2+ sensitization.
2.10. MDA+4-HDA Content
To investigate the role of H. stoechas in lipid peroxidation, the content of malondialdehyde (MDA) and 4-hydroxyalkenals (4-HDA) was determined. MDA+4-HDA is one product of polyunsaturated fatty acid peroxidation in the cells and it is produced by an increase in free radicals. Therefore, MDA+4-HDA level is commonly known as a marker of oxidative stress. As shown in Figure 8, H. stoechas extract to 0.1 and 1 mg/mL did not increase the level of MDA+4-HDA in tissue relative to MeOH (as control). However, LPS, 0.1 and 1 µg/mL, increased three and eight times the MDA+4-HDA content, respectively. Pre-incubation of H. stoechas extract 1 h before the addition of LPS reduced MDA+4-HDA content to a level similar to that of the control (Figure 8).
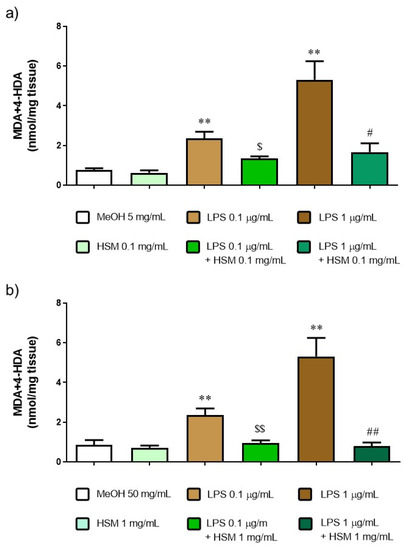
Figure 8.
MDA+4-HDA concentration after incubation with MeOH or H. stoechas extract (HSM) at either (a) low or (b) high concentrations, in presence or absence of lipid peroxidation evoked by LPS 0.1 or 1 µg/mL on rat jejunum. Data points are mean ± SEM (n = 5). ** p < 0.01 vs. MeOH (control) and HSM, $ p < 0.05, $$ p < 0.01 vs. LPS 0.1 µg/mL and # p < 0.05, ## p < 0.01 vs. LPS 1 µg/mL.
The NO plays an important role in the regulation of intestinal motility. The formation of reactive oxygen species would produce an alteration in the NO bioavailability, leading to alterations in the intestinal motility [43,44,46,47]. This study showed that H. stoechas extract inhibit the content of MDA+4-HDA produced in the tissue treated with LPS, demonstrating the antioxidant activity of the H. stoechas extract. Therefore, everlasting flower extract, which has an antioxidant effect, can improve the NO availability and preserve intestinal motor function. Different studies have shown that the administration of flavonoids prevents the structural and functional damage of a digestive tissue through its antioxidant activity by increasing the level of antioxidant enzymes, decreasing lipid peroxidation and modulating the NO level [48,49,50,51,52]. In addition to the phenolic compounds, arzanol, one of the most characteristic molecules of Helichrysum, could also explain the reduction in the level of MDA+4-HDA, since it has been shown to have anti-inflammatory and protective effects against lipid oxidation in the plasma membranes of Caco-2 and Vero cells [53].
We find among the spectrum of plants used in FGID those that are characterized by presenting flavonoids [8,54], compounds that have spasmolytic, antioxidant and anti-inflammatory activity, among others. Their inhibitory effects on intestinal motility have been demonstrated in in vitro and in vivo assays [54,55]. Previous studies of the authors showed that H. stoechas extracts present a high phenolic profile, with a particular presence of phloroglucinols, being the main compounds arzanol, helipyrone, p-hydroxybenzoic acids, caffeic acids, neochlorogenic acids, 5–7-dihydroxy-3,6,8-trimethoxyflavone, isoquercitrin and quercetagetin-7-O-glucopyranoside [18]. A review shows that the members of the Asteraceae family contain the highest number of antispasmodic compounds, with flavonoids being the group with the highest number of compounds with this activity [56].
Caffeic acid has been shown to have a relaxing effect on different smooth muscles, its most powerful effect being on the ileum. Thus, the spasmolytic effect of caffeic acid was due to the blockade of L-type Ca2+ channels and the inhibitory effect on muscarinic receptors [57]. Chlorogenic acid, which is hydrolyzed in the small intestine to caffeic acid, quercetin and other polyphenols, increases NO production by reducing nitrite, and thus produces a relaxing effect on smooth muscle [58]. This result would be in agreement with the relaxation produced by H. stoechas extract through the activation of the NO/PKG/cGMP pathway. Additionally, other studies reported that caffeic acid blocks small- and intermediate-conductance Ca2+-activated K+ channels [59]. These results suggest that caffeic acid may be, at least in part, responsible for the effects evoked by methanolic extract of H. stoechas.
Ethanolic extracts of Drosera madagascariensis and Drosera rotundifolia, rich in isoquercitrin, quercetin and hyperoside, reduce the contractions induced by charbacol and histamine in guinea-pig ileum [60,61]. In the same way, the methanolic extract of Psidium guajava showed a spasmolytic effect, which was mainly due to the aglycone quercetin and isoquercetin present in the extract [62]. Thus, the spasmolytic effect of H. stoechas extract could be due to some of its constituents, mainly those of phenolic nature.
3. Conclusions
The present study reports for the first time the antispasmodic activity of a methanolic extract of H. stoechas and its mechanism of action. Thus, the methanolic extract of H. stoechas relaxes the smooth muscle of the rat ileum through the reduction in intracellular Ca2+ level, the activation of the NO/PKG/cGMP pathway and the opening of Ca2+-activated K+ channels. The antispasmodic and antioxidant activities of H. stoechas extract could help to prevent tissue damage and to preserve intestinal motor function. This study provides new data supporting the traditional use of H. stoechas to treat digestive disorders and its use for the development of herbal medicines for the treatment or prevention of FGID.
4. Materials and Methods
4.1. Reagents, Chemicals and Plant Material
H. stoechas aerial parts were collected in July 2014 and a plant voucher was deposited at Universidad San Jorge herbarium (ref. 002-2014). The preparation of the methanolic extract of H. stoechas and the identification of its phenolic profile were previously described [18]. The solvent (methanol) was completely removed by rotatory evaporator under vacuum.
Acetylcholine (ACh), carbamoylcholine chloride (carbachol), Bay K8644, apamin (AP), charybdotoxin (ChTx), glibenclamide (Glib), quinine (Q), verapamil (V), H-89 dihydrochloride hydrate, KT-5823, (+)-(R)-trans-4- (1-aminoethyl)-N-(4-pyridyl) cyclohexanecarboxamide dihydrochloride monohydrate (Y-27632), Nω-Nitro-L-arginine methyl ester hydrochloride (L-NAME) and barium chloride dihydrate (BaCl2) were obtained from Sigma (Madrid, Spain). TRAM-34, 1H-[1,2,4]oxadiazolo [4,3-α]quinoxalin-1-one (ODQ) and Rp-8-Br-PET-cGMPS were purchased from Tocris (Madrid, Spain). Bay K8644 was dissolved in ethanol. Apamin was diluted in acetic acid. Gliblencamide, TRAM-34 and ODQ were prepared in dimethyl sulfoxide (DMSO). All other chemicals were dissolved in distilled water.
Tissues were incubated in Krebs buffer (in mM: NaCl 120, KCl 4.7, CaCl2 2.4, MgSO4 1.2, NaHCO3 24.5, KH2PO4 1, and glucose 5.6), calcium-free Krebs (NaCl 120, KCl 4.7, CaCl2 0, MgSO4 1.2, NaHCO3 24.5, KH2PO4 1, glucose 5.6, and ethyleneglycoltetraacetic acid (EGTA) 1) or Ca2+-free high K+ Krebs ([K+]o = 50 mM). The buffers were adjusted to pH 7.4. The compounds of the buffers were obtained from Sigma.
Lipopolysaccharide (LPS) from Escherichia coli O111:B4 was obtained from Sigma.
4.2. Animals
Male Wistar rats weighting 200–250 g were purchased from Janvier, LeGenest St. Isle, France. Animals were fed ad libitum with standard feed and free access to water.
The experimental protocols were approved by the Ethics Committee from University of Zaragoza under Project License PI66/17 (18 January 2018). Animal care and use of animals were conducted in accordance with the Spanish Policy for Animal Protection RD 53/2013, RD1386/2018 and RD118/2021, which meets the European Union Directive 2010/63 on the protection of animals used for experimental and other scientific purposes.
4.3. Preparation of Ileum Segments
After cervical dislocation, ileum was carefully removed, placed in ice-cold Krebs buffer, and cleaned of fat and adherent connective tissue. The ileum was cut into longitudinal smooth muscle segments (10 mm long) and each segment was individually connected to an isometric force transducer (Pioden UF1, Graham Bell House, Canterbury, UK) for tension measurement. The organ bath, with 5 mL Krebs buffer, was maintained at 37 °C and gassed with 95% O2 and 5% CO2. Mechanical activity was amplified with a range of 2 mV, recorded and digitalized using a data acquisition system (eDAQ, e-corder 410 (model ED410), Cibertec, Madrid, Spain). An initial tension of 1 g was applied to the preparations to achieve spontaneous contractions. The segments were allowed to equilibrate in Krebs solution for 60 min before use by changing the bath buffer every 20 min.
4.4. Experimental Protocols
After the stabilization period, the spontaneous contractions of longitudinal smooth muscle from ileum were recorded. To study the effect of H. stoechas extract, methanol (solvent of the extract) or verapamil, increasing concentrations of these compounds were added every 10 min and cumulative concentration–response curves were performed. The spontaneous contractions of the ileum recorded in Krebs solution before agents were considered as the control. The relaxant effect of H. stoechas was calculated as the percentage change from the control period (Krebs).
To examine the role of Ca2+ influx on the H. stoechas extract-evoked relaxation of longitudinal smooth muscle, after incubation with Krebs buffer, the segments were incubated with Ca2+-free Krebs solutions for 20 min and then with Ca2+-free high-K+ buffer. Subsequently, the segments were pre-incubated with methanol, H. stoechas extract (0.3 and 1 mg/mL) or verapamil (1 µM) for 15 min and cumulative concentration–response curves for CaCl2 (10−5–10−2 M) were performed by adding each CaCl2 concentration every 5 min. The responses to CaCl2 obtained in the presence of methanol served as control.
Furthermore, the effect of the H. stoechas extract on L-type Ca2+ channels was evaluated. Thus, ileum segments were incubated 15 min with H. stoechas extract (0.3 and 1 mg/mL) and verapamil (10−6 M) before the addition of Bay K8644. The contractile response of Bay K8644 obtained in presence of H. stoechas extract or verapamil was compared with the response obtained by Bay K8644 alone (control, 100%).
A similar protocol was used to examine the effects of H. stoechas extract (1 mg/mL) on the effects induced by the contractile agents KCl and CCh. The tissue was incubated for 15 min with the extract or the solvent prior to the addition of KCl (80 mM) or CCh (10−6 M). Contractions to KCl or CCh in the presence or absence of extract were compared to the control period.
To investigate the participation of AMP/protein kinase A (PKA) and nitric oxide (NO)/GMP/protein kinase G (PKG) pathways on the relaxant response evoked by H. stoechas extract (1 mg/mL) in spontaneous motility, segments were incubated 20 min before the extract with L-NAME (10 µM), an inhibitor of NO synthase (NOS); ODQ (1 µM), a potent and selective inhibitor of soluble guanylyl cyclase; Rp-8-Br-PET-cGMPs (1 µM), an inhibitor of cGKI and cGKII; KT-5823 (1 µM), a selective inhibitor of PKG, H-89 (200 nM), an inhibitor of the PKA; and 2,5-dideoxiadenosina (DOA, 1 mM), an inhibitor of the soluble adenylyl cyclase.
To assess whether K+ channels were involved in the response of the H. stoechas extract (1 mg/mL), 20 min before addition of extract, ileum segments were pre-incubated with apamin, a selective small-conductance Ca2+- and voltage-activated K+ channel (SKCa) inhibitor (AP, 1 µM); TRAM-34 (1-[(2-chlorophenyl) diphenylmethyl]-1H-pyrazole, a selective inhibitor of intermediate conductance Ca2+-activated K+ channels (IKCa) (1 µM); charybdotoxin, a specific inhibitor of intermediate- and large-conductance Ca2+-activated K+ channels (ChTx, 0.01 µM); glibenclamide, an inhibitor of ATP-sensitive K+ channel (KATP) (Glib, 10 µM) and BaCl2, an inhibitor of the inward rectifier K+ channel (KIR), (BC, 30 mM); and quinine, an inhibitor of voltage-sensitive K+ channels (Qn, 10 µM).
To study the possible simultaneous role of several pathways in the effects of the H. stoechas extract (1 mg/mL), two combinations of inhibitors were also incubated: TRAM-34+AP and L-NAME+TRAM-34+AP, and their effects on the H. stoechas-induced relaxation compared with those evoked by each inhibitor alone.
Using the same protocol, the role of the Ca2+-independent Rho-kinase pathway on H. stoechas extract (1 mg/mL) effect was studied with the Rho-kinase inhibitor Y-27632 (10–6 M). In this series of experiments, the response to each substance in the presence of H. stoechas extract was compared with the response obtained with the spontaneous contractions (control).
Segments that did not show spontaneous activity were discarded. Thus, each segment served as its own control. Each experimental protocol was performed on ileum segments of longitudinal smooth muscle from 6–8 animals.
4.5. Malondialdehyde (MDA) and 4-Hydroxyalkenals (4-HDA)
The tissue concentrations of MDA and 4-HDA (MDA+4-HDA) were used as an index of lipid peroxidation. After cervical dislocation, jejunum was extracted. Jejunum samples were incubated at room temperature in Krebs buffer divided into the following experimental groups: MeOH (as control), H. stoechas extract (0.1 or 1 mg/mL), lipopolysaccharide (LPS, 0.1 or 1 µg/mL) and H. stoechas+LPS (each at the two referred concentrations). After 1 h of incubation with H. stoechas extract or methanol, LPS or its solvent was added. After 2 h, the pieces of jejunum were frozen in dry ice for MDA+4-HDA analysis. The tissues were homogenized in ice-cold Tris buffer (50 mM, pH 7.4) and centrifuged at 3000× g for 10 min at 4 °C. In the assay, MDA+4HDA reacts with N-methyl-2-phenylindole to yield a chromophore with maximal absorbance at 586 nm; 1,1,3,3- tetramethoxypropane was used as a standard. Results were expressed as nmol MDA+4-HDA per milligram of tissue.
4.6. Analysis of Data
Amplitude and frequency of spontaneous contractions were calculated as the average of peak-to-peak differences and as the number of contractions per minute, respectively, during a 5 min recording period. The area under the curve (AUC) represents the integrated mechanical activity per second (g/s) and normalized to weight (g) of wet tissue. The AUC was calculated as the AUC during the first 3 min of response to the studied substance (AUC1) minus the AUC of the spontaneous contractions 3 min before adding the substance (control) (AUC0) (AUC = AUC1 − AUC0). Data were calculated as the percentage with respect to the mean value of the control period and are expressed as mean ± SEM. Normal distribution of the samples was assessed by the Shapiro–Wilk test. Statistical significance was analyzed using the Kruskal–Wallis test (non-parametric) followed by post hoc Dunn’s test. p values < 0.05 were considered statistically significant. The concentration of compound that inhibited 50% of the maximal contraction (EC50) was expressed as a geometric mean with 95% confidence intervals (IC) and calculated for the concentration–response curve. Statistical analyses and figures were carried out using GraphPad Prism 6.
Author Contributions
Conceptualization, M.S.V., V.L. and M.Á.P.; formal analysis, M.S.V., V.L. and M.Á.P.; funding acquisition, M.S.V., V.L., C.G.-R. and F.L.; investigation, M.S.V., V.L., M.C., C.G.-R., M.P.A., F.L. and M.Á.P.; methodology, M.S.V., M.C., M.P.A., V.L. and M.Á.P.; project administration, M.S.V., V.L. and M.Á.P.; resources, M.S.V. and V.L.; supervision, M.S.V. and V.L.; visualization, M.S.V., V.L. and M.Á.P.; writing—original draft, M.S.V. and M.Á.P.; writing—review and editing, M.S.V., V.L., M.C., C.G.-R., M.P.A., F.L. and M.Á.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Government of Aragon (financial support of the group B44_20D-Phyto-Pharm: Principios vegetales bioactivos y ciencias farmacéuticas), Universidad de Zaragoza (JIUZ-2018-BIO-09 and JIUZ-2021-BIO-08) and Universidad San Jorge (Internal Research Project Call 2021–2022, number 2122041).
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee for Animal Experiments of the University of Zaragoza (Spain) (reference: PI66/17, 18 January 2018).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
Authors would like to acknowledge the use of Servicio General de Apoyo a la Investigación—SAI, Universidad de Zaragoza.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript or in the decision to publish the results.
References
- Black, C.J.; Drossman, D.A.; Talley, N.J.; Ruddy, J.; Ford, A.C. Functional gastrointestinal disorders: Advances in understanding and management. Lancet 2020, 396, 1664–1674. [Google Scholar] [CrossRef]
- Holtmann, G.; Schrenk, D.; Madisch, A.; Allescher, H.D.; Ulrich-Merzenich, G.; Mearin, F.; Larrey, D.; Malfertheiner, P. Use of Evidence-Based Herbal Medicines for Patients with Functional Gastrointestinal Disorders: A Conceptional Framework for Risk-Benefit Assessment and Regulatory Approaches. Dig. Dis. 2020, 38, 269–279. [Google Scholar] [CrossRef]
- Nightingale, S.; Sharma, A. Functional gastrointestinal disorders in children: What is new? J. Paediatr. Child Health 2020, 56, 1724–1730. [Google Scholar] [CrossRef]
- Sperber, A.D.; Bangdiwala, S.I.; Drossman, D.A.; Ghoshal, U.C.; Simren, M.; Tack, J.; Whitehead, W.E.; Dumitrascu, D.L.; Fang, X.; Fukudo, S.; et al. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology 2021, 160, 99–114.e3. [Google Scholar] [CrossRef]
- Anheyer, D.; Frawley, J.; Koch, A.K.; Lauche, R.; Langhorst, J.; Dobos, G.; Cramer, H. Herbal Medicines for Gastrointestinal Disorders in Children and Adolescents: A Systematic Review. Pediatrics 2017, 139, e20170062. [Google Scholar] [CrossRef]
- Kraft, K. Complementary/Alternative Medicine in the context of prevention of disease and maintenance of health. Prev. Med. 2009, 49, 88–92. [Google Scholar] [CrossRef]
- World Health Organization. WHO Traditional Medicines Strategy 2014–2023; WHO: Geneva, Switzerland, 2013. [Google Scholar]
- Kelber, O.; Bauer, R.; Kubelka, W. Phytotherapy in Functional Gastrointestinal Disorders. Dig. Dis. 2017, 35, 36–42. [Google Scholar] [CrossRef]
- Fifi, A.C.; Axelrod, C.H.; Chakraborty, P.; Saps, M. Herbs and spices in the treatment of functional gastrointestinal disorders: A review of clinical trials. Nutrients 2018, 10, 1715. [Google Scholar] [CrossRef]
- Helichrysi Flos|European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/herbal/helichrysi-flos (accessed on 24 July 2022).
- Akaberi, M.; Sahebkar, A.; Azizi, N.; Emami, S.A. Everlasting flowers: Phytochemistry and pharmacology of the genus Helichrysum. Ind. Crops Prod. 2019, 138, 111471. [Google Scholar] [CrossRef]
- Garcia-Oliveira, P.; Barral, M.; Carpena, M.; Gullón, P.; Fraga-Corral, M.; Otero, P.; Prieto, M.A.; Simal-Gandara, J. Traditional plants from Asteraceae family as potential candidates for functional food industry. Food Funct. 2021, 12, 2850–2873. [Google Scholar] [CrossRef]
- Orhan, N.; Onaran, M.; Şen, I.; Işik Gönül, I.; Aslan, M. Preventive treatment of calcium oxalate crystal deposition with immortal flowers. J. Ethnopharmacol. 2015, 163, 60–67. [Google Scholar] [CrossRef]
- Onaran, M.; Orhan, N.; Farahvash, A.; Ekin, H.N.; Kocabıyık, M.; Gönül, İ.I.; Şen, İ.; Aslan, M. Successful treatment of sodium oxalate induced urolithiasis with Helichrysum flowers. J. Ethnopharmacol. 2016, 186, 322–328. [Google Scholar] [CrossRef]
- Benítez, G.; González-Tejero, M.R.; Molero-Mesa, J. Pharmaceutical ethnobotany in the western part of Granada province (southern Spain): Ethnopharmacological synthesis. J. Ethnopharmacol. 2010, 129, 87–105. [Google Scholar] [CrossRef]
- Carmona, M.D.; Llorach, R.; Obon, C.; Rivera, D. “Zahraa”, a Unani multicomponent herbal tea widely consumed in Syria: Components of drug mixtures and alleged medicinal properties. J. Ethnopharmacol. 2005, 102, 344–350. [Google Scholar] [CrossRef]
- Albayrak, S.; Aksoy, A.; Sagdic, O.; Hamzaoglu, E. Compositions, antioxidant and antimicrobial activities of Helichrysum (Asteraceae) species collected from Turkey. Food Chem. 2010, 119, 114–122. [Google Scholar] [CrossRef]
- Les, F.; Venditti, A.; Cásedas, G.; Frezza, C.; Guiso, M.; Sciubba, F.; Serafini, M.; Bianco, A.; Valero, M.S.; López, V. Everlasting flower (Helichrysum stoechas Moench) as a potential source of bioactive molecules with antiproliferative, antioxidant, antidiabetic and neuroprotective properties. Ind. Crops Prod. 2017, 108, 295–302. [Google Scholar] [CrossRef]
- Bremner, P.; Rivera, D.; Calzado, M.A.; Obón, C.; Inocencio, C.; Beckwith, C.; Fiebich, B.L.; Muñoz, E.; Heinrich, M. Assessing medicinal plants from South-Eastern Spain for potential anti-inflammatory effects targeting nuclear factor-Kappa B and other pro-inflammatory mediators. J. Ethnopharmacol. 2009, 124, 295–305. [Google Scholar] [CrossRef]
- Kherbache, A.; Senator, A.; Laouicha, S.; Al-Zoubi, R.M.; Bouriche, H. Phytochemical analysis, antioxidant and anti-inflammatory activities of Helichrysum stoechas (L.) Moench extracts. Biocatal. Agric. Biotechnol. 2020, 29, 101826. [Google Scholar] [CrossRef]
- Zengin, G.; Cvetanović, A.; Gašić, U.; Tešić, Ž.; Stupar, A.; Bulut, G.; Sinan, K.I.; Uysal, S.; Picot-Allain, M.C.N.; Mahomoodally, M.F. A comparative exploration of the phytochemical profiles and bio-pharmaceutical potential of Helichrysum stoechas subsp. barrelieri extracts obtained via five extraction techniques. Process Biochem. 2020, 91, 113–125. [Google Scholar] [CrossRef]
- Barroso, M.R.; Barros, L.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Fernandes, I.P.; Barreiro, M.F.; Ferreira, I.C.F.R. Exploring the antioxidant potential of Helichrysum stoechas (L.) Moench phenolic compounds for cosmetic applications: Chemical characterization, microencapsulation and incorporation into a moisturizer. Ind. Crops Prod. 2014, 53, 330–336. [Google Scholar] [CrossRef]
- Carini, M.; Aldini, G.; Furlanetto, S.; Stefani, R.; Facino, R.M. LC coupled to ion-trap MS for the rapid screening and detection of polyphenol antioxidants from Helichrysum stoechas. J. Pharm. Biomed. Anal. 2001, 24, 517–526. [Google Scholar] [CrossRef]
- Carnevali, I.; La Paglia, R.; Pauletto, L.; Raso, F.; Testa, M.; Mannucci, C.; Sorbara, E.E.; Calapai, G. Efficacy and safety of the syrup “KalobaTUSS®” as a treatment for cough in children: A randomized, double blind, placebo-controlled clinical trial. BMC Pediatr. 2021, 21, 9. [Google Scholar] [CrossRef]
- Borgonetti, V.; Les, F.; López, V.; Galeotti, N. Attenuation of Anxiety-Like Behavior by Helichrysum stoechas (L.) Moench Methanolic Extract through Up-Regulation of ERK Signaling Pathways in Noradrenergic Neurons. Pharmaceuticals 2020, 13, 472. [Google Scholar] [CrossRef]
- Hwisa, N.; Auzi, A.; Parvez, N. Antinociceptive effect of Helichrysum stoechas in experimental animals. IJPI’s J. Pharmacol. Toxicol. 2011, 1, 23–27. [Google Scholar]
- Valero, M.S.; Nuñez, S.; Les, F.; Castro, M.; Gómez-Rincón, C.; Arruebo, M.P.; Plaza, M.Á.; Köhler, R.; López, V. The Potential Role of Everlasting Flower (Helichrysum stoechas Moench) as an Antihypertensive Agent: Vasorelaxant Effects in the Rat Aorta. Antioxidants 2022, 11, 1092. [Google Scholar] [CrossRef]
- da Machado, A.P.F.; Geraldi, M.V.; do Nascimento, R.; Moya, A.M.T.M.; Vezza, T.; Diez-Echave, P.; Gálvez, J.J.; Cazarin, C.B.B.; Maróstica Júnior, M.R. Polyphenols from food by-products: An alternative or complementary therapy to IBD conventional treatments. Food Res. Int. 2021, 140, 110018. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, Q. Roles of the Polyphenol-Gut Microbiota Interaction in Alleviating Colitis and Preventing Colitis-Associated Colorectal Cancer. Adv. Nutr. 2021, 12, 546–565. [Google Scholar] [CrossRef]
- Hagan, M.; Hayee, B.H.; Rodriguez-Mateos, A. (Poly)phenols in Inflammatory Bowel Disease and Irritable Bowel Syndrome: A Review. Molecules 2021, 26, 1843. [Google Scholar] [CrossRef]
- Chiu, H.F.; Venkatakrishnan, K.; Golovinskaia, O.; Wang, C.K. Gastroprotective Effects of Polyphenols against Various Gastro-Intestinal Disorders: A Mini-Review with Special Focus on Clinical Evidence. Molecules 2021, 26, 2090. [Google Scholar] [CrossRef]
- Bigovic, D.; Brankovic, S.; Kitic, D.; Radenkovic, M.; Jankovic, T.; Savikin, K.; Zivanovic, S. Relaxant Effect of the Ethanol Extract of Helichrysum plicatum (Asteraceae) on Isolated Rat Ileum Contractions. Molecules 2010, 15, 3391–3401. [Google Scholar] [CrossRef]
- Pljevljakušić, D.; Bigović, D.; Janković, T.; Jelačić, S.; Šavikin, K. Sandy everlasting (Helichrysum arenarium (L.) Moench): Botanical, chemical and biological properties. Front Plant Sci. 2018, 9, 1123. [Google Scholar] [CrossRef]
- Szadowska, A. Pharmacological action of the galenicals and flavonoids isolated from Helichrysum arenarium. Acta Pol Pharm. 1962, 19, 465–479. [Google Scholar]
- Rigano, D.; Formisano, C.; Senatore, F.; Piacente, S.; Pagano, E.; Capasso, R.; Borrelli, F.; Izzo, A.A. Intestinal antispasmodic effects of Helichrysum italicum (Roth) Don ssp. italicum and chemical identification of the active ingredients. J. Ethnopharmacol. 2013, 150, 901–906. [Google Scholar] [CrossRef]
- Castro, M.; Ramón-Giménez, M.; Les, F.; Trejo, L.; Plaza, M.Á.; López, V.; Murillo, M.D.; Valero, M.S. Spasmolytic effect of Jasonia glutinosa on rodent intestine. Rev. Esp. Enferm. dig. 2016, 108, 785–789. [Google Scholar] [CrossRef]
- Zavala-Mendoza, D.; Grasa, L.; Zavala-Sánchez, M.Á.; Pérez-Gutiérrez, S.; Murillo, M.D. Antispasmodic effects and action mechanism of essential oil of Chrysactinia mexicana A. Gray on rabbit ileum. Molecules 2016, 21, 783. [Google Scholar] [CrossRef]
- McHale, N.G.; Hollywood, M.; Sergeant, G.; Thornbury, K. Origin of spontaneous rhythmicity in smooth muscle. J Physiol. 2006, 570, 23–28. [Google Scholar] [CrossRef]
- Brading, A.F.; Sneddon, P. Evidence for multiple sources of calcium for activation of the contractile mechanism of guinea-pig taenia coli on stimulation with carbachol. Br. J. Pharmacol. 1980, 70, 229–240. [Google Scholar] [CrossRef]
- Ullah Khan, A.; Gilani, A.H. Antispasmodic and bronchodilator activities of Artemisia vulgaris are mediated through dual blockade of muscarinic receptors and calcium influx. J. Ethnopharmacol. 2009, 126, 480–486. [Google Scholar] [CrossRef]
- Moradi, M.T.; Rafieian-Koupaei, M.; Imani-Rastabi, R.; Nasiri, J.; Shahrani, M.; Rabiei, Z.; Alibabaei, Z. Antispasmodic effects of yarrow (Achillea millefolium L.) extract in the isolated ileum of rat. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 499–503. [Google Scholar] [CrossRef]
- Idrizaj, E.; Traini, C.; Vannucchi, M.G.; Baccari, M.C. Nitric Oxide: From Gastric Motility to Gastric Dysmotility. Int. J. Mol. Sci. 2021, 22, 9990. [Google Scholar] [CrossRef]
- Sanders, K.M.; Ward, S.M. Nitric oxide and its role as a non-adrenergic, non-cholinergic inhibitory neurotransmitter in the gastrointestinal tract. Br. J. Pharmacol. 2019, 176, 212–227. [Google Scholar] [CrossRef] [PubMed]
- Parsons, S.P.; Huizinga, J.D. Nitric Oxide Is Essential for Generating the Minute Rhythm Contraction Pattern in the Small Intestine, Likely via ICC-DMP. Front. Neurosci. 2021, 14, 592664. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.H.; Busch, J.L.; Corbin, J.D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol. Rev. 2010, 62, 525–563. [Google Scholar] [CrossRef]
- Kalyanaraman, B. Teaching the basics of redox biology to medical and graduate students: Oxidants, antioxidants and disease mechanisms. Redox Biol. 2013, 1, 244–257. [Google Scholar] [CrossRef]
- Han, J.P.; Lee, J.H.; Lee, G.S.; Koo, O.J.; Yeom, S.C. Positive Correlation between nNOS and Stress-Activated Bowel Motility Is Confirmed by In Vivo HiBiT System. Cells. 2021, 10, 1028. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Feng, X.; Zhang, J.; Wei, Y.; Zhao, X. Preventive effect of anji white tea flavonoids on alcohol-induced gastric injury through their antioxidant effects in kunming mice. Biomolecules 2019, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, C.; Zhang, J.; Zhao, X. Wu shan shen cha (Malus asiatica nakai. leaves)-derived flavonoids alleviate alcohol-induced gastric injury in mice via an anti-oxidative mechanism. Biomolecules 2019, 9, 169. [Google Scholar] [CrossRef]
- Ramakrishnan, A.; Vijayakumar, N.; Renuka, M. Naringin regulates glutamate-nitric oxide cGMP pathway in ammonium chloride induced neurotoxicity. Biomed. Pharmacother. 2016, 84, 1717–1726. [Google Scholar] [CrossRef]
- Vieira-Frez, F.C.; Sehaber-Sierakowski, C.C.; Perles, J.V.C.M.; Bossolani, G.D.P.; Verri, W.A.; do Nascimento, R.C.; Guarnier, F.A.; Bordini, H.P.; Blegniski, F.P.; Martins, H.A.; et al. Anti- and pro-oxidant effects of quercetin stabilized by microencapsulation on interstitial cells of Cajal, nitrergic neurons and M2-like macrophages in the jejunum of diabetic rats. Neurotoxicology 2020, 77, 193–204. [Google Scholar] [CrossRef]
- Martins-Perles, J.V.C.; Bossolani, G.D.P.; Zignani, I.; de Souza, S.R.G.; Frez, F.C.V.; de Souza Melo, C.G.; Barili, E.; de Souza Neto, F.P.; Guarnier, F.A.; Armani, A.L.C.; et al. Quercetin increases bioavailability of nitric oxide in the jejunum of euglycemic and diabetic rats and induces neuronal plasticity in the myenteric plexus. Auton Neurosci. 2020, 227, 102675. [Google Scholar] [CrossRef]
- Rosa, A.; Pollastro, F.; Atzeri, A.; Appendino, G.; Melis, M.P.; Deiana, M.; Incani, A.; Loru, D.; Dess, M.A. Protective role of arzanol against lipid peroxidation in biological systems. Chem. Phys. Lipids 2011, 164, 24–32. [Google Scholar] [CrossRef]
- Rauf, A.; Akram, M.; Semwal, P.; Mujawah, A.A.H.; Muhammad, N.; Riaz, Z.; Munir, N.; Piotrovsky, D.; Vdovina, I.; Bouyahya, A.; et al. Antispasmodic Potential of Medicinal Plants: A Comprehensive Review. Oxidative Med. Cell. Longev. 2021, 2021, 4889719. [Google Scholar] [CrossRef]
- Gharzouli, K.; Holzer, P. Inhibition of guinea pig intestinal peristalsis by the flavonoids quercetin, naringenin, apigenin and genistein. Pharmacology 2004, 70, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pérez, E.F.; Juárez, Z.N.; Hernández, L.R.; Bach, H. Natural Antispasmodics: Source, Stereochemical Configuration, and Biological Activity. BioMed Res. Int. 2018, 2018, 3819714. [Google Scholar] [CrossRef]
- de Alencar Silva, A.; Pereira-de-Morais, L.; Rodrigues da Silva, R.E.; de Menezes Dantas, D.; Brito Milfont, C.G.; Gomes, M.F.; Araújo, I.M.; Kerntopf, M.R.; Alencar de Menezes, I.R.; Barbosa, R. Pharmacological screening of the phenolic compound caffeic acid using rat aorta, uterus and ileum smooth muscle. Chem. Biol. Interact. 2020, 332, 109269. [Google Scholar] [CrossRef] [PubMed]
- Rocha, B.S.; Gago, B.; Barbosa, R.M.; Laranjinha, J. Dietary polyphenols generate nitric oxide from nitrite in the stomach and induce smooth muscle relaxation. Toxicology 2009, 265, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Oliván-Viguera, A.; Valero, M.S.; Murillo, M.D.; Wulff, H.; García-Otín, Á.L.; Arbonés-Mainar, J.M.; Köhler, R. Novel Phenolic Inhibitors of Small/Intermediate-Conductance Ca2+-Activated K+ Channels, KCa3.1 and KCa2.3. PLoS ONE. 2013, 8, e58614. [Google Scholar] [CrossRef]
- Melzig, M.F.; Pertz, H.H.; Krenn, L. Anti-inflammatory and spasmolytic activity of extracts from Droserae herba. Phytomedicine 2001, 8, 225–229. [Google Scholar] [CrossRef]
- Krenn, L.; Beyer, G.; Pertz, H.H.; Karall, E.; Kremser, M.; Galambosi, B.; Melzig, M.F. In vitro antispasmodic and anti-inflammatory effects of Drosera rotundifolia. Arzneimittelforschung 2004, 54, 402–405. [Google Scholar] [CrossRef]
- Vier Lozoy, X.A.; Meckes, M.; Abou-zaid, M.; Tortoriello, J.; Ance Nozzolillo, C.; Thor Arnason, J. Quercetin Glycosides in Psidium guajava L. Leaves and Determination of a Spasmolytic Principie. Arch. Med. Res. 1994, 25, 11–15. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).