Polymer Electrolyte Membranes Containing Functionalized Organic/Inorganic Composite for Polymer Electrolyte Membrane Fuel Cell Applications
Abstract
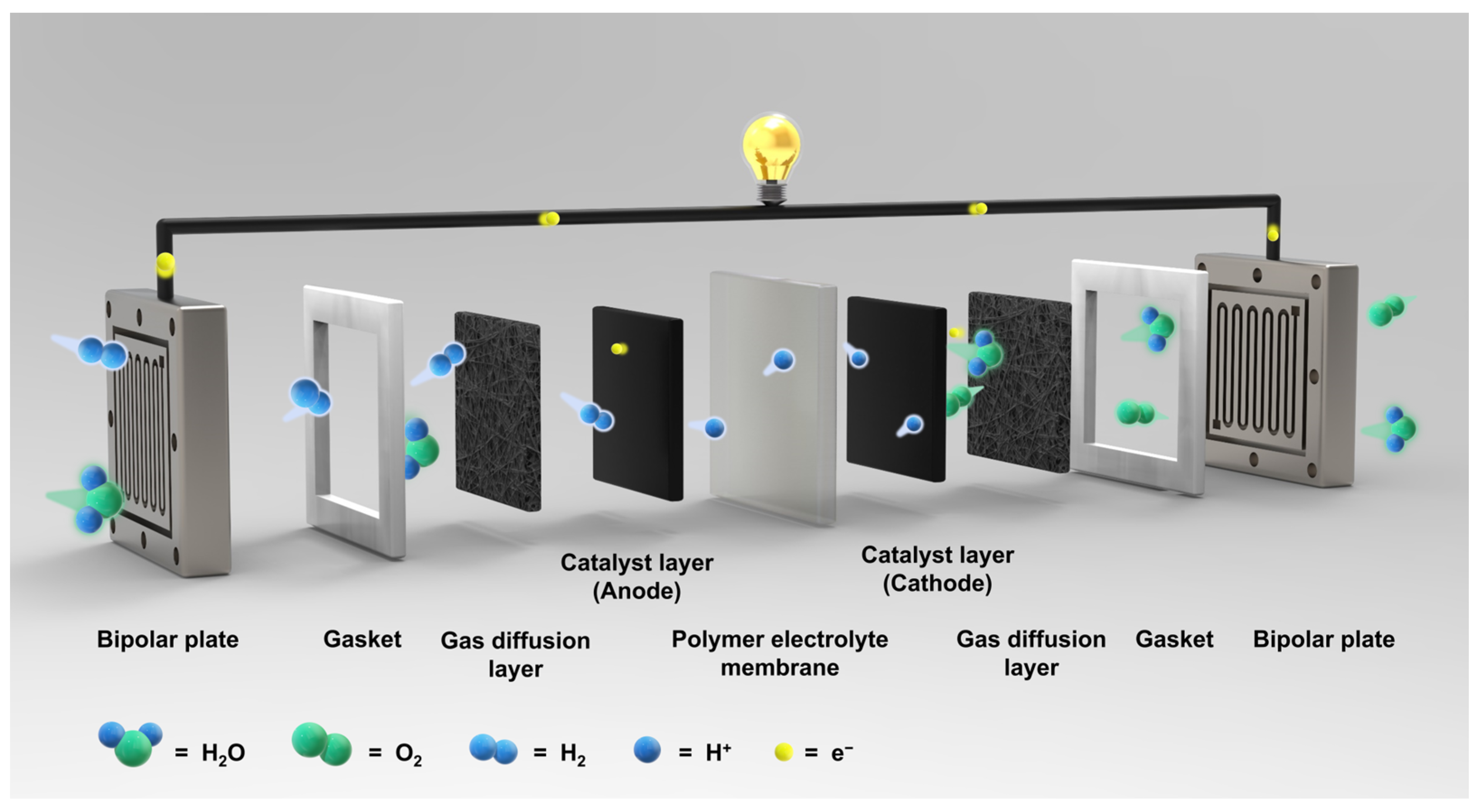
1. Introduction
2. Composite Membranes Used in PEMFCs
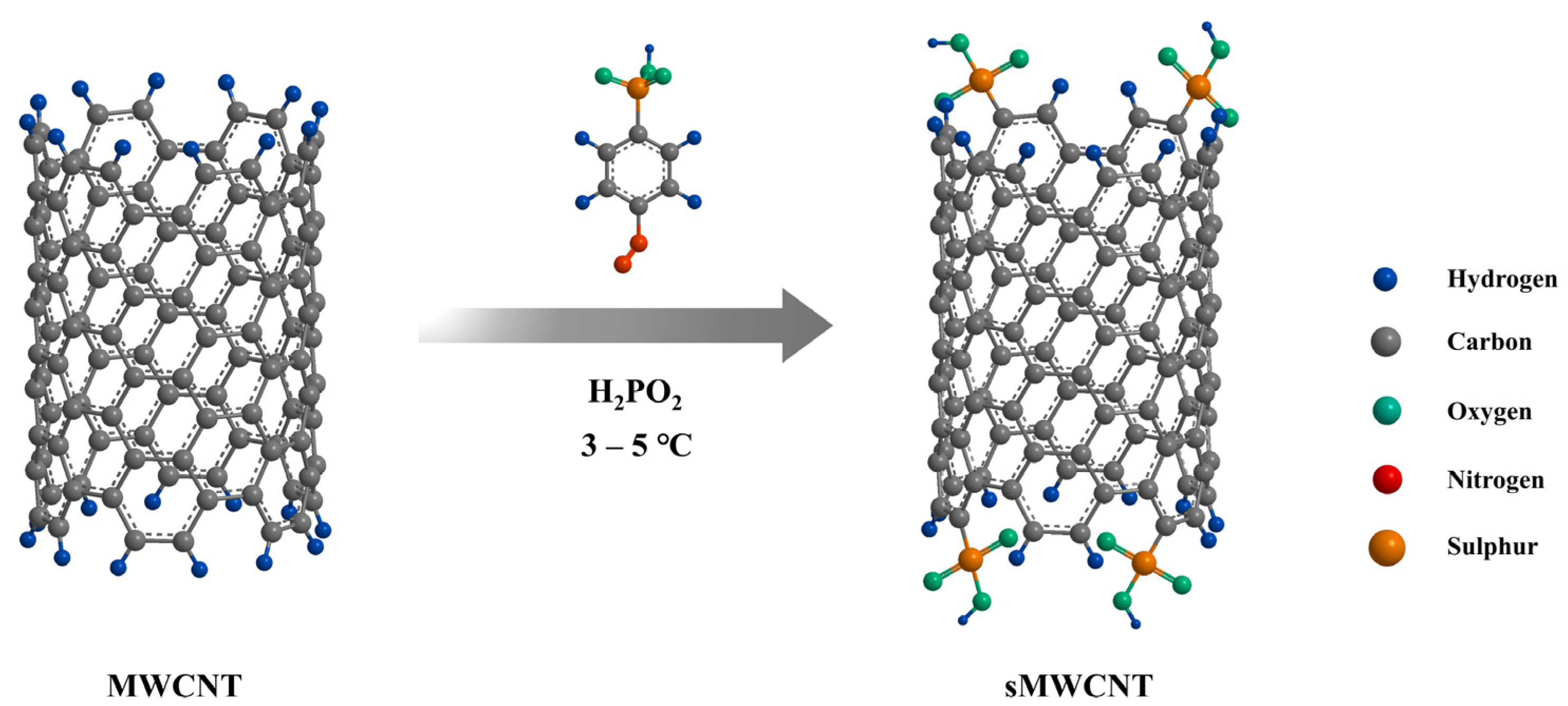
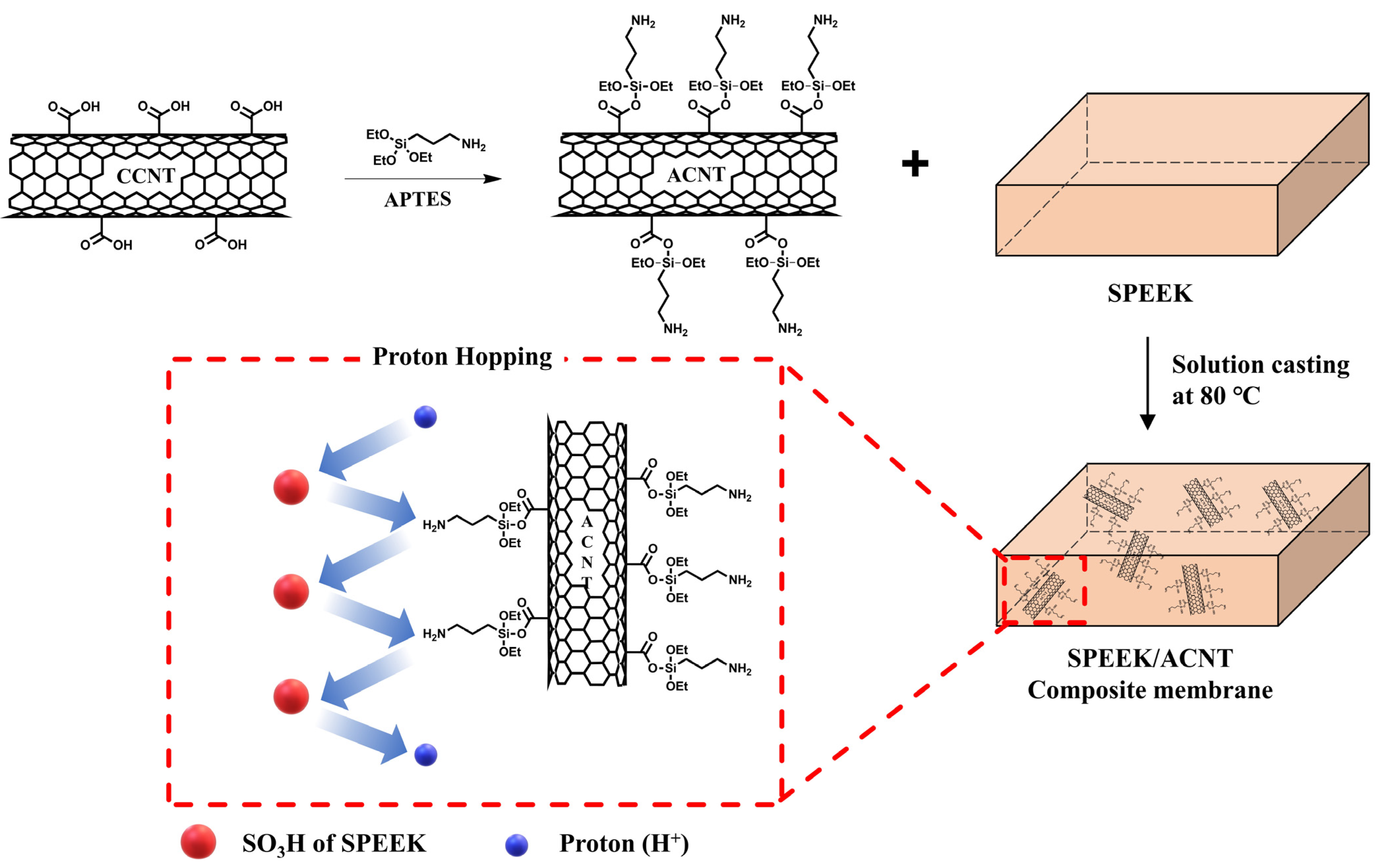
2.1. Composite Membranes with Functionalized Carbon Nanotubes
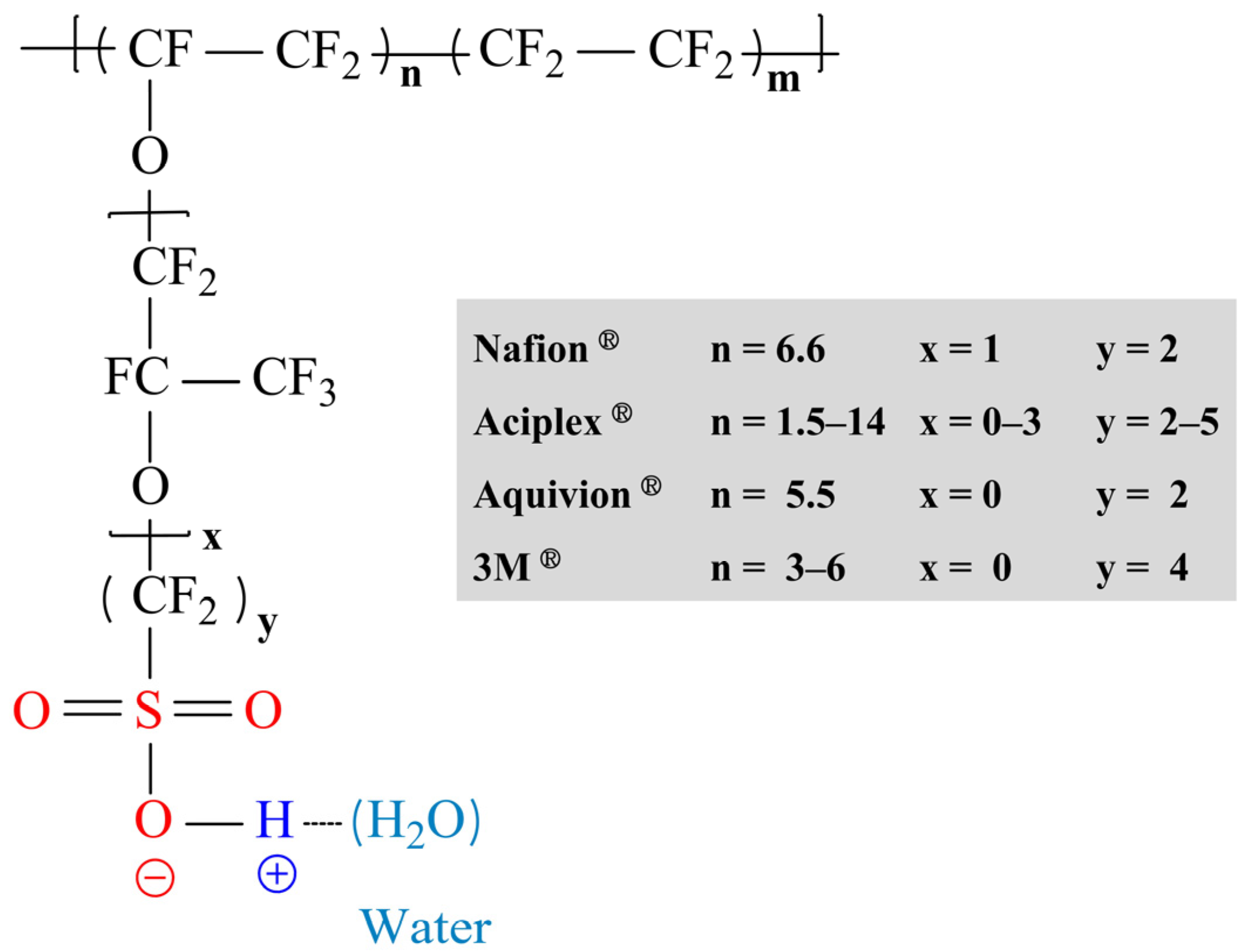
2.1.1. PFSA-Based Composite Membranes with Functionalized Carbon Nanotubes
2.1.2. Hydrocarbon-Based Composite Membranes with Functionalized Carbon Nanotubes
2.2. Composite Membranes with Functionalized Graphene Oxides
2.2.1. PFSA-Based Composite Membranes with Functionalized Graphene Oxides
2.2.2. Hydrocarbon-Based Composite Membranes with Functionalized Graphene Oxides
2.3. Composite Membranes with Functionalized Silica Composites
2.3.1. PFSA-Based Composite Membranes with Functionalized Silica
2.3.2. Hydrocarbon-Based Composite Membrane with Functionalized Silica
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, G.; Yu, Y.; Liu, H.; Gong, C.; Wen, S.; Wang, X.; Tu, Z. Progress on design and development of polymer electrolyte membrane fuel cell systems for vehicle applications: A review. Fuel Process. Technol. 2018, 179, 203–228. [Google Scholar] [CrossRef]
- Ko, H.; Kim, M.; Nam, S.Y.; Kim, K. Research of cross-linked hydrocarbon based polymer electrolyte membranes for polymer electrolyte membrane fuel cell applications. Membr. J. 2020, 30, 395–408. [Google Scholar] [CrossRef]
- Maiyalagan, T.; Pasupathi, S. Components for PEM fuel cells: An overview. In Materials Science Forum; Trans Tech Publications Ltd.: Bäch, Switzerland, 2010. [Google Scholar]
- Souzy, R.; Ameduri, B. Functional fluoropolymers for fuel cell membranes. Prog. Polym. Sci. 2005, 30, 644–687. [Google Scholar] [CrossRef]
- Kusoglu, A.; Weber, A.Z. New Insights into Perfluorinated Sulfonic-Acid Ionomers. Chem. Rev. 2017, 117, 987–1104. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Ko, H.; Nam, S.Y.; Kim, K. Study on control of polymeric architecture of sulfonated hydrocarbon-based polymers for high-performance polymer electrolyte membranes in fuel cell applications. Polymers 2021, 13, 3520. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Moore, R.B. State of understanding of Nafion. Chem. Rev. 2004, 104, 4535–4586. [Google Scholar] [CrossRef]
- Karimi, M.B.; Mohammadi, F.; Hooshyari, K. Recent approaches to improve Nafion performance for fuel cell applications: A review. Int. J. Hydrogen Energy 2019, 44, 28919–28938. [Google Scholar] [CrossRef]
- Kraytsberg, A.; Ein-Eli, Y. Review of advanced materials for proton exchange membrane fuel cells. Energy Fuels 2014, 28, 7303–7330. [Google Scholar] [CrossRef]
- Harun, N.A.M.; Shaari, N.; Zaiman, N.F.H.N. A review of alternative polymer electrolyte membrane for fuel cell application based on sulfonated poly(ether ether ketone). Int. J. Energy Res. 2021, 45, 19671–19708. [Google Scholar] [CrossRef]
- RS, R.R.; Rashmi, W.; Khalid, M.; Wong, W.; Priyanka, J. Recent progress in the development of aromatic polymer-based proton exchange membranes for fuel cell applications. Polymers 2020, 12, 1061. [Google Scholar]
- Han, S.-Y.; Yu, D.M.; Mo, Y.H.; Ahn, S.M.; Lee, J.Y.; Kim, T.-H.; Yoon, S.J.; Hong, S.; Hong, Y.T.; So, S. Ion exchange capacity controlled biphenol-based sulfonated poly(arylene ether sulfone) for polymer electrolyte membrane water electrolyzers: Comparison of random and multi-block copolymers. J. Membr. Sci. 2021, 634, 119370. [Google Scholar] [CrossRef]
- Ghassemi, H.; McGrath, J.E.; Zawodzinski, T.A., Jr. Multiblock sulfonated–fluorinated poly(arylene ether) s for a proton exchange membrane fuel cell. Polymer 2006, 47, 4132–4139. [Google Scholar] [CrossRef]
- Kang, K.; Kim, D. Pendant dual-sulfonated poly(arylene ether ketone) multi-block copolymer membranes for enhanced proton conductivity at reduced water swelling. J. Membr. Sci. 2019, 578, 103–110. [Google Scholar] [CrossRef]
- Holmes, T. Reaction of Hydroxyl Radicals with Sulfonated Phenylated Polyphenylenes. Ph.D. Thesis, Simon Fraser University, Burnaby, BC, Canada, 2019. [Google Scholar]
- Kang, N.R.; Pham, T.H.; Jannasch, P. Polyaromatic perfluorophenylsulfonic acids with high radical resistance and proton conductivity. ACS Macro Lett. 2019, 8, 1247–1251. [Google Scholar] [CrossRef]
- Kim, K.; Kim, S.-K.; Park, J.O.; Choi, S.-W.; Kim, K.-H.; Ko, T.; Pak, C.; Lee, J.-C. Highly reinforced pore-filling membranes based on sulfonated poly(arylene ether sulfone) s for high-temperature/low-humidity polymer electrolyte membrane fuel cells. J. Membr. Sci. 2017, 537, 11–21. [Google Scholar] [CrossRef]
- Le Mong, A.; Yang, S.; Kim, D. Pore-filling polymer electrolyte membrane based on poly(arylene ether ketone) for enhanced dimensional stability and reduced methanol permeability. J. Membr. Sci. 2017, 543, 133–142. [Google Scholar] [CrossRef]
- Kim, Y.M.; Choi, S.H.; Lee, H.C.; Hong, M.Z.; Kim, K.; Lee, H.-I. Organic–inorganic composite membranes as addition of SiO2 for high temperature-operation in polymer electrolyte membrane fuel cells (PEMFCs). Electrochim. Acta 2004, 49, 4787–4796. [Google Scholar] [CrossRef]
- Pu, H.; Liu, L.; Chang, Z.; Yuan, J. Organic/inorganic composite membranes based on polybenzimidazole and nano-SiO2. Electrochim. Acta 2009, 54, 7536–7541. [Google Scholar] [CrossRef]
- Han, M.; Zhang, G.; Li, M.; Wang, S.; Zhang, Y.; Li, H.; Lew, C.M.; Na, H. Considerations of the morphology in the design of proton exchange membranes: Cross-linked sulfonated poly(ether ether ketone) s using a new carboxyl-terminated benzimidazole as the cross-linker for PEMFCs. Int. J. Hydrogen Energy 2011, 36, 2197–2206. [Google Scholar] [CrossRef]
- Kim, K.; Heo, P.; Hwang, W.; Baik, J.-H.; Sung, Y.-E.; Lee, J.-C. Cross-linked sulfonated poly(arylene ether sulfone) containing a flexible and hydrophobic bishydroxy perfluoropolyether cross-linker for high-performance proton exchange membrane. ACS Appl. Mater. Interfaces 2018, 10, 21788–21793. [Google Scholar] [CrossRef]
- Cele, N.P.; Sinha Ray, S.; Pillai, S.K.; Ndwandwe, M.; Nonjola, S.; Sikhwivhilu, L.; Mathe, M.K. Carbon nanotubes based nafion composite membranes for fuel cell applications. Fuel Cells 2010, 10, 64–71. [Google Scholar] [CrossRef]
- Yin, C.; Xiong, B.; Liu, Q.; Li, J.; Qian, L.; Zhou, Y.; He, C. Lateral-aligned sulfonated carbon-nanotubes/Nafion composite membranes with high proton conductivity and improved mechanical properties. J. Membr. Sci. 2019, 591, 117356. [Google Scholar] [CrossRef]
- Sahu, A.K.; Ketpang, K.; Shanmugam, S.; Kwon, O.; Lee, S.; Kim, H. Sulfonated Graphene–Nafion Composite Membranes for Polymer Electrolyte Fuel Cells Operating under Reduced Relative Humidity. J. Phys. Chem. C 2016, 120, 15855–15866. [Google Scholar] [CrossRef]
- Nauman Javed, R.M.; Al-Othman, A.; Tawalbeh, M.; Olabi, A.G. Recent developments in graphene and graphene oxide materials for polymer electrolyte membrane fuel cells applications. Renew. Sustain. Energy Rev. 2022, 168, 112836. [Google Scholar] [CrossRef]
- Lee, D.C.; Yang, H.N.; Park, S.H.; Kim, W.J. Nafion/graphene oxide composite membranes for low humidifying polymer electrolyte membrane fuel cell. J. Membr. Sci. 2014, 452, 20–28. [Google Scholar] [CrossRef]
- Kumar, R.; Xu, C.; Scott, K. Graphite oxide/Nafion composite membranes for polymer electrolyte fuel cells. RSC Adv. 2012, 2, 8777–8782. [Google Scholar] [CrossRef]
- Lee, K.H.; Chu, J.Y.; Kim, A.R.; Yoo, D.J. Effect of functionalized SiO2 toward proton conductivity of composite membranes for PEMFC application. Int. J. Energy Res. 2019, 43, 5333–5345. [Google Scholar] [CrossRef]
- Mishra, A.K.; Bose, S.; Kuila, T.; Kim, N.H.; Lee, J.H. Silicate-based polymer-nanocomposite membranes for polymer electrolyte membrane fuel cells. Prog. Polym. Sci. 2012, 37, 842–869. [Google Scholar] [CrossRef]
- Lee, J.-R.; Won, J.-H.; Yoon, K.-S.; Hong, Y.T.; Lee, S.-Y. Multilayer-structured, SiO2/sulfonated poly(phenylsulfone) composite membranes for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2012, 37, 6182–6188. [Google Scholar] [CrossRef]
- Pinar, F.J.; Canizares, P.; Rodrigo, M.A.; Ubeda, D.; Lobato, J. Titanium composite PBI-based membranes for high temperature polymer electrolyte membrane fuel cells. Effect on titanium dioxide amount. RSC Adv. 2012, 2, 1547–1556. [Google Scholar] [CrossRef]
- Rajendran, S.; Prabhu, M.R. A Study of influence on sulfonated TiO2-Poly (Vinylidene fluoride-co-hexafluoropropylene) nano composite membranes for PEM Fuel cell application. Appl. Surf. Sci. 2017, 418, 64–71. [Google Scholar]
- Baker, A.M.; Wang, L.; Johnson, W.B.; Prasad, A.K.; Advani, S.G. Nafion membranes reinforced with ceria-coated multiwall carbon nanotubes for improved mechanical and chemical durability in polymer electrolyte membrane fuel cells. J. Phys. Chem. C 2014, 118, 26796–26802. [Google Scholar] [CrossRef]
- Yun, Y.; Kumar, A.; Hong, J.; Song, S.-J. Impact of CeO2 Nanoparticle Morphology: Radical Scavenging within the Polymer Electrolyte Membrane Fuel Cell. J. Electrochem. Soc. 2021, 168, 114521. [Google Scholar] [CrossRef]
- Park, K.T.; Jung, U.H.; Choi, D.W.; Chun, K.; Lee, H.M.; Kim, S.H. ZrO2–SiO2/Nafion® composite membrane for polymer electrolyte membrane fuel cells operation at high temperature and low humidity. J. Power Sources 2008, 177, 247–253. [Google Scholar] [CrossRef]
- Mokhtaruddin, S.R.; Mohamad, A.B.; Shyuan, L.K.; Kadhum, A.A.H.; Akhmad, M. Preparation and Characterization of Nafion-Zirconia Composite Membrane for PEMFC. In Advanced Materials Research; Trans Tech Publications Ltd.: Bäch, Switzerland, 2011. [Google Scholar]
- Kim, Y.; Choi, Y.; Kim, H.K.; Lee, J.S. New sulfonic acid moiety grafted on montmorillonite as filler of organic–inorganic composite membrane for non-humidified proton-exchange membrane fuel cells. J. Power Sources 2010, 195, 4653–4659. [Google Scholar] [CrossRef]
- Hasani-Sadrabadi, M.M.; Dashtimoghadam, E.; Majedi, F.S.; Moaddel, H.; Bertsch, A.; Renaud, P. Superacid-doped polybenzimidazole-decorated carbon nanotubes: A novel high-performance proton exchange nanocomposite membrane. Nanoscale 2013, 5, 11710–11717. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Feng, M.; Huang, Y.; Liu, X. SGO/SPEN-based highly selective polymer electrolyte membranes for direct methanol fuel cells. Ionics 2017, 23, 2143–2152. [Google Scholar] [CrossRef]
- Gao, S.; Xu, H.; Fang, Z.; Ouadah, A.; Chen, H.; Chen, X.; Shi, L.; Ma, B.; Jing, C.; Zhu, C. Highly sulfonated poly(ether ether ketone) grafted on graphene oxide as nanohybrid proton exchange membrane applied in fuel cells. Electrochim. Acta 2018, 283, 428–437. [Google Scholar] [CrossRef]
- Chang, Y.-N.; Lai, J.-Y.; Liu, Y.-L. Polybenzimidazole (PBI)-functionalized silica nanoparticles modified PBI nanocomposite membranes for proton exchange membranes fuel cells. J. Membr. Sci. 2012, 403, 1–7. [Google Scholar]
- Cozzi, D.; de Bonis, C.; D’Epifanio, A.; Mecheri, B.; Tavares, A.C.; Licoccia, S. Organically functionalized titanium oxide/Nafion composite proton exchange membranes for fuel cells applications. J. Power Sources 2014, 248, 1127–1132. [Google Scholar] [CrossRef]
- Liu, F.; Wang, S.; Li, J.; Tian, X.; Wang, X.; Chen, H.; Wang, Z. Polybenzimidazole/ionic-liquid-functional silica composite membranes with improved proton conductivity for high temperature proton exchange membrane fuel cells. J. Membr. Sci. 2017, 541, 492–499. [Google Scholar] [CrossRef]
- Jheng, L.-C.; Rosidah, A.A.; Hsu, S.L.-C.; Ho, K.-S.; Pan, C.-J.; Cheng, C.-W. Nanocomposite membranes of polybenzimidazole and amine-functionalized carbon nanofibers for high temperature proton exchange membrane fuel cells. RSC Adv. 2021, 11, 9964–9976. [Google Scholar] [CrossRef] [PubMed]
- Tellez-Cruz, M.M.; Escorihuela, J.; Solorza-Feria, O.; Compañ, V. Proton Exchange Membrane Fuel Cells (PEMFCs): Advances and Challenges. Polymers 2021, 13, 3064. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.-Y.; Oh, J.; Kim, H.J.; Kim, K.Y.; Lee, S.-S.; Lee, J.-C. Effect of antioxidant grafted graphene oxides on the mechanical and thermal properties of polyketone composites. Eur. Polym. J. 2015, 69, 156–167. [Google Scholar] [CrossRef]
- Taufiq Musa, M.; Shaari, N.; Kamarudin, S.K. Carbon nanotube, graphene oxide and montmorillonite as conductive fillers in polymer electrolyte membrane for fuel cell: An overview. Int. J. Energy Res. 2021, 45, 1309–1346. [Google Scholar] [CrossRef]
- Ando, Y. Carbon nanotube: The inside story. J. Nanosci. Nanotechnol. 2010, 10, 3726–3738. [Google Scholar] [CrossRef]
- Liu, H.; Gong, C.; Wang, J.; Liu, X.; Liu, H.; Cheng, F.; Wang, G.; Zheng, G.; Qin, C.; Wen, S. Chitosan/silica coated carbon nanotubes composite proton exchange membranes for fuel cell applications. Carbohydr. Polym. 2016, 136, 1379–1385. [Google Scholar] [CrossRef]
- Vinothkannan, M.; Kim, A.R.; Ryu, S.K.; Yoo, D.J. Structurally modulated and functionalized carbon nanotubes as potential filler for Nafion matrix toward improved power output and durability in proton exchange membrane fuel cells operating at reduced relative humidity. J. Membr. Sci. 2022, 649, 120393. [Google Scholar] [CrossRef]
- Gao, G.; Cagin, T.; Goddard, W.A., III. Energetics, structure, mechanical and vibrational properties of single-walled carbon nanotubes. Nanotechnology 1998, 9, 184. [Google Scholar] [CrossRef]
- Yin, C.; Li, J.; Zhou, Y.; Zhang, H.; Fang, P.; He, C. Enhancement in proton conductivity and thermal stability in nafion membranes induced by incorporation of sulfonated carbon nanotubes. ACS Appl. Mater. Interfaces 2018, 10, 14026–14035. [Google Scholar] [CrossRef]
- Abouzari-Lotf, E.; Etesami, M.; Nasef, M.M. Carbon-based nanocomposite proton exchange membranes for fuel cells. In Carbon-Based Polymer Nanocomposites for Environmental and Energy Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 437–461. [Google Scholar]
- Chew, S.Y.; Ng, S.H.; Wang, J.; Novák, P.; Krumeich, F.; Chou, S.L.; Chen, J.; Liu, H.K. Flexible free-standing carbon nanotube films for model lithium-ion batteries. Carbon 2009, 47, 2976–2983. [Google Scholar] [CrossRef]
- Yamada, T.; Hayamizu, Y.; Yamamoto, Y.; Yomogida, Y.; Izadi-Najafabadi, A.; Futaba, D.N.; Hata, K. A stretchable carbon nanotube strain sensor for human-motion detection. Nat. Nanotechnol. 2011, 6, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Lipomi, D.J.; Vosgueritchian, M.; Tee, B.C.; Hellstrom, S.L.; Lee, J.A.; Fox, C.H.; Bao, Z. Skin-like pressure and strain sensors based on transparent elastic films of carbon nanotubes. Nat. Nanotechnol. 2011, 6, 788–792. [Google Scholar] [CrossRef]
- Costa, R.D.; Feihl, S.; Kahnt, A.; Gambhir, S.; Officer, D.L.; Wallace, G.G.; Lucio, M.I.; Herrero, M.A.; Vázquez, E.; Syrgiannis, Z. Carbon Nanohorns as Integrative Materials for Efficient Dye-Sensitized Solar Cells. Adv. Mater. 2013, 25, 6513–6518. [Google Scholar] [CrossRef]
- Jheng, L.-C.; Huang, C.-Y.; Hsu, S.L.-C. Sulfonated MWNT and imidazole functionalized MWNT/polybenzimidazole composite membranes for high-temperature proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2013, 38, 1524–1534. [Google Scholar] [CrossRef]
- Sun, X.; Simonsen, S.C.; Norby, T.; Chatzitakis, A. Composite membranes for high temperature PEM fuel cells and electrolysers: A critical review. Membranes 2019, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Dyke, C.A.; Tour, J.M. Covalent Functionalization of Single-Walled Carbon Nanotubes for Materials Applications. J. Phys. Chem. A 2004, 108, 11151–11159. [Google Scholar] [CrossRef]
- Yu, D.M.; Sung, I.; Yoon, Y.; Kim, T.H.; Lee, J.; Hong, Y. Properties of Sulfonated Poly(Arylene Ether Sulfone)/Functionalized Carbon Nanotube Composite Membrane for High Temperature PEMFCs. Fuel Cells 2013, 13, 843–850. [Google Scholar] [CrossRef]
- Zhou, W.; Xiao, J.; Chen, Y.; Zeng, R.; Xiao, S.; Nie, H.; Li, F.; Song, C. Sulfonated carbon nanotubes/sulfonated poly(ether sulfone ether ketone ketone) composites for polymer electrolyte membranes. Polym. Adv. Technol. 2011, 22, 1747–1752. [Google Scholar] [CrossRef]
- Ajayan, P.M.; Schadler, L.S.; Giannaris, C.; Rubio, A. Single-Walled Carbon Nanotube–Polymer Composites: Strength and Weakness. Adv. Mater. 2000, 12, 750–753. [Google Scholar] [CrossRef]
- Cele, N.; Ray, S.S. Recent Progress on Nafion-Based Nanocomposite Membranes for Fuel Cell Applications. Macromol. Mater. Eng. 2009, 294, 719–738. [Google Scholar] [CrossRef]
- Shaari, N.; Kamarudin, S.K. Recent advances in additive-enhanced polymer electrolyte membrane properties in fuel cell applications: An overview. Int. J. Energy Res. 2019, 43, 2756–2794. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Su, Y.-H.; Chang, C.-M.; Wang, D.-M.; Lai, J.-Y. Preparation and applications of Nafion-functionalized multiwalled carbon nanotubes for proton exchange membrane fuel cells. J. Mater. Chem. 2010, 20, 4409–4416. [Google Scholar] [CrossRef]
- Kannan, R.; Kakade, B.A.; Pillai, V.K. Polymer Electrolyte Fuel Cells Using Nafion-Based Composite Membranes with Functionalized Carbon Nanotubes. Angew. Chem. Int. Ed. 2008, 47, 2653–2656. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-F.; Wu, J.-S.; Kuo, P.-L. Poly(oxyalkylene)diamine-Functionalized Carbon Nanotube/Perfluorosulfonated Polymer Composites: Synthesis, Water State, and Conductivity. Chem. Mater. 2008, 20, 5756–5767. [Google Scholar] [CrossRef]
- Asgari, M.S.; Nikazar, M.; Molla-Abbasi, P.; Hasani-Sadrabadi, M.M. Nafion®/histidine functionalized carbon nanotube: High-performance fuel cell membranes. Int. J. Hydrogen Energy 2013, 38, 5894–5902. [Google Scholar] [CrossRef]
- Molla-Abbasi, P.; Asgari, M.S.; Sadrabadi, M.M.H. Improving the Performance of Nafion®-Based Fuel Cell Membranes by Introducing Histidine Functionalized Carbon Nanotubes. J. Macromol. Sci. Part B 2017, 56, 234–244. [Google Scholar] [CrossRef]
- Molla-Abbasi, P.; Janghorban, K.; Asgari, M.S. A novel heteropolyacid-doped carbon nanotubes/Nafion nanocomposite membrane for high performance proton-exchange methanol fuel cell applications. Iran. Polym. J. 2018, 27, 77–86. [Google Scholar] [CrossRef]
- Jun, Y.; Zarrin, H.; Fowler, M.; Chen, Z. Functionalized titania nanotube composite membranes for high temperature proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2011, 36, 6073–6081. [Google Scholar] [CrossRef]
- Feng, M.; You, Y.; Zheng, P.; Liu, J.; Jia, K.; Huang, Y.; Liu, X. Low-swelling proton-conducting multi-layer composite membranes containing polyarylene ether nitrile and sulfonated carbon nanotubes for fuel cells. Int. J. Hydrogen Energy 2016, 41, 5113–5122. [Google Scholar] [CrossRef]
- Kim, A.R.; Gabunada, J.C.; Yoo, D.J. Amelioration in physicochemical properties and single cell performance of sulfonated poly(ether ether ketone) block copolymer composite membrane using sulfonated carbon nanotubes for intermediate humidity fuel cells. Int. J. Energy Res. 2019, 43, 2974–2989. [Google Scholar] [CrossRef]
- Steffy, N.J.; Parthiban, V.; Sahu, A.K. Uncovering Nafion-multiwalled carbon nanotube hybrid membrane for prospective polymer electrolyte membrane fuel cell under low humidity. J. Membr. Sci. 2018, 563, 65–74. [Google Scholar] [CrossRef]
- Tohidian, M.; Ghaffarian, S.R. Surface modified multi-walled carbon nanotubes and Nafion nanocomposite membranes for use in fuel cell applications. Polym. Adv. Technol. 2018, 29, 1219–1226. [Google Scholar] [CrossRef]
- Sung, I.H.; Yu, D.M.; Yoon, Y.J.; Kim, T.-H.; Lee, J.Y.; Hong, S.K.; Hong, Y.T. Preparation and properties of sulfonated poly(arylene ether sulfone)/hydrophilic oligomer-g-CNT composite membranes for PEMFC. Macromol. Res. 2013, 21, 1138–1144. [Google Scholar] [CrossRef]
- Kim, A.R.; Vinothkannan, M.; Song, M.H.; Lee, J.-Y.; Lee, H.-K.; Yoo, D.J. Amine functionalized carbon nanotube (ACNT) filled in sulfonated poly(ether ether ketone) membrane: Effects of ACNT in improving polymer electrolyte fuel cell performance under reduced relative humidity. Compos. Part B Eng. 2020, 188, 107890. [Google Scholar] [CrossRef]
- Wang, J.; Gong, C.; Wen, S.; Liu, H.; Qin, C.; Xiong, C.; Dong, L. A facile approach of fabricating proton exchange membranes by incorporating polydopamine-functionalized carbon nanotubes into chitosan. Int. J. Hydrogen Energy 2019, 44, 6909–6918. [Google Scholar] [CrossRef]
- Marroquin, J.B.; Rhee, K.Y.; Park, S.J. Chitosan nanocomposite films: Enhanced electrical conductivity, thermal stability, and mechanical properties. Carbohydr. Polym. 2013, 92, 1783–1791. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Qian, P.; Zhou, Y.; Shi, J.; Shi, H. Sulfonated poly(ether ether ketone)/amine-functionalized graphene oxide hybrid membrane with various chain lengths for vanadium redox flow battery: A comparative study. J. Membr. Sci. 2020, 610, 118232. [Google Scholar] [CrossRef]
- Pandey, R.P.; Shukla, G.; Manohar, M.; Shahi, V.K. Graphene oxide based nanohybrid proton exchange membranes for fuel cell applications: An overview. Adv. Colloid Interface Sci. 2017, 240, 15–30. [Google Scholar] [CrossRef]
- Zarrin, H.; Higgins, D.; Jun, Y.; Chen, Z.; Fowler, M. Functionalized Graphene Oxide Nanocomposite Membrane for Low Humidity and High Temperature Proton Exchange Membrane Fuel Cells. J. Phys. Chem. C 2011, 115, 20774–20781. [Google Scholar] [CrossRef]
- Karim, M.R.; Hatakeyama, K.; Matsui, T.; Takehira, H.; Taniguchi, T.; Koinuma, M.; Matsumoto, Y.; Akutagawa, T.; Nakamura, T.; Noro, S.-I. Graphene Oxide Nanosheet with High Proton Conductivity. J. Am. Chem. Soc. 2013, 135, 8097–8100. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Huang, Y.; Zhang, L.; Wang, Y.; Ma, Y.; Guo, T.; Chen, Y. Molecular-Level Dispersion of Graphene into Poly(vinyl alcohol) and Effective Reinforcement of their Nanocomposites. Adv. Funct. Mater. 2009, 19, 2297–2302. [Google Scholar] [CrossRef]
- Yang, J.; Liu, C.; Gao, L.; Wang, J.; Xu, Y.; He, R. Novel composite membranes of triazole modified graphene oxide and polybenzimidazole for high temperature polymer electrolyte membrane fuel cell applications. RSC Adv. 2015, 5, 101049–101054. [Google Scholar] [CrossRef]
- Choi, B.G.; Huh, Y.S.; Park, Y.C.; Jung, D.H.; Hong, W.H.; Park, H. Enhanced transport properties in polymer electrolyte composite membranes with graphene oxide sheets. Carbon 2012, 50, 5395–5402. [Google Scholar] [CrossRef]
- Gao, W.; Wu, G.; Janicke, M.T.; Cullen, D.A.; Mukundan, R.; Baldwin, J.K.; Brosha, E.L.; Galande, C.; Ajayan, P.M.; More, K.L. Ozonated Graphene Oxide Film as a Proton-Exchange Membrane. Angew. Chem. Int. Ed. 2014, 53, 3588–3593. [Google Scholar] [CrossRef]
- Nicotera, I.; Simari, C.; Coppola, L.; Zygouri, P.; Gournis, D.; Brutti, S.; Minuto, F.D.; Aricò, A.; Sebastian, D.; Baglio, V. Sulfonated Graphene Oxide Platelets in Nafion Nanocomposite Membrane: Advantages for Application in Direct Methanol Fuel Cells. J. Phys. Chem. C 2014, 118, 24357–24368. [Google Scholar] [CrossRef]
- Seo, D.C.; Jeon, I.; Jeong, E.S.; Jho, J.Y. Mechanical Properties and Chemical Durability of Nafion/Sulfonated Graphene Oxide/Cerium Oxide Composite Membranes for Fuel-Cell Applications. Polymers 2020, 12, 1375. [Google Scholar] [CrossRef]
- Peng, K.-J.; Lai, J.-Y.; Liu, Y.-L. Nanohybrids of graphene oxide chemically-bonded with Nafion: Preparation and application for proton exchange membrane fuel cells. J. Membr. Sci. 2016, 514, 86–94. [Google Scholar] [CrossRef]
- Sharma, P.P.; Tinh, V.D.C.; Kim, D. Improved Oxidative Stability by Embedded Cerium into Graphene Oxide Nanosheets for Proton Exchange Membrane Fuel Cell Application. Membranes 2021, 11, 238. [Google Scholar] [CrossRef]
- Vinodh, R.; Atchudan, R.; Kim, H.-J.; Yi, M. Recent advancements in polysulfone based membranes for fuel cell (PEMFCs, DMFCs and AMFCs) applications: A critical review. Polymers 2022, 14, 300. [Google Scholar] [CrossRef]
- Sulaiman, R.R.R.; Walvekar, R.; Wong, W.Y.; Khalid, M.; Pang, M.M. Proton Conductivity Enhancement at High Temperature on Polybenzimidazole Membrane Electrolyte with Acid-Functionalized Graphene Oxide Fillers. Membranes 2022, 12, 344. [Google Scholar] [CrossRef] [PubMed]
- Vinothkannan, M.; Kim, A.R.; Yoo, D.J. Sulfonated graphene oxide/Nafion composite membranes for high temperature and low humidity proton exchange membrane fuel cells. RSC Adv. 2018, 8, 7494–7508. [Google Scholar] [CrossRef] [PubMed]
- Beydaghi, H.; Javanbakht, M. Aligned Nanocomposite Membranes Containing Sulfonated Graphene Oxide with Superior Ionic Conductivity for Direct Methanol Fuel Cell Application. Ind. Eng. Chem. Res. 2015, 54, 7028–7037. [Google Scholar] [CrossRef]
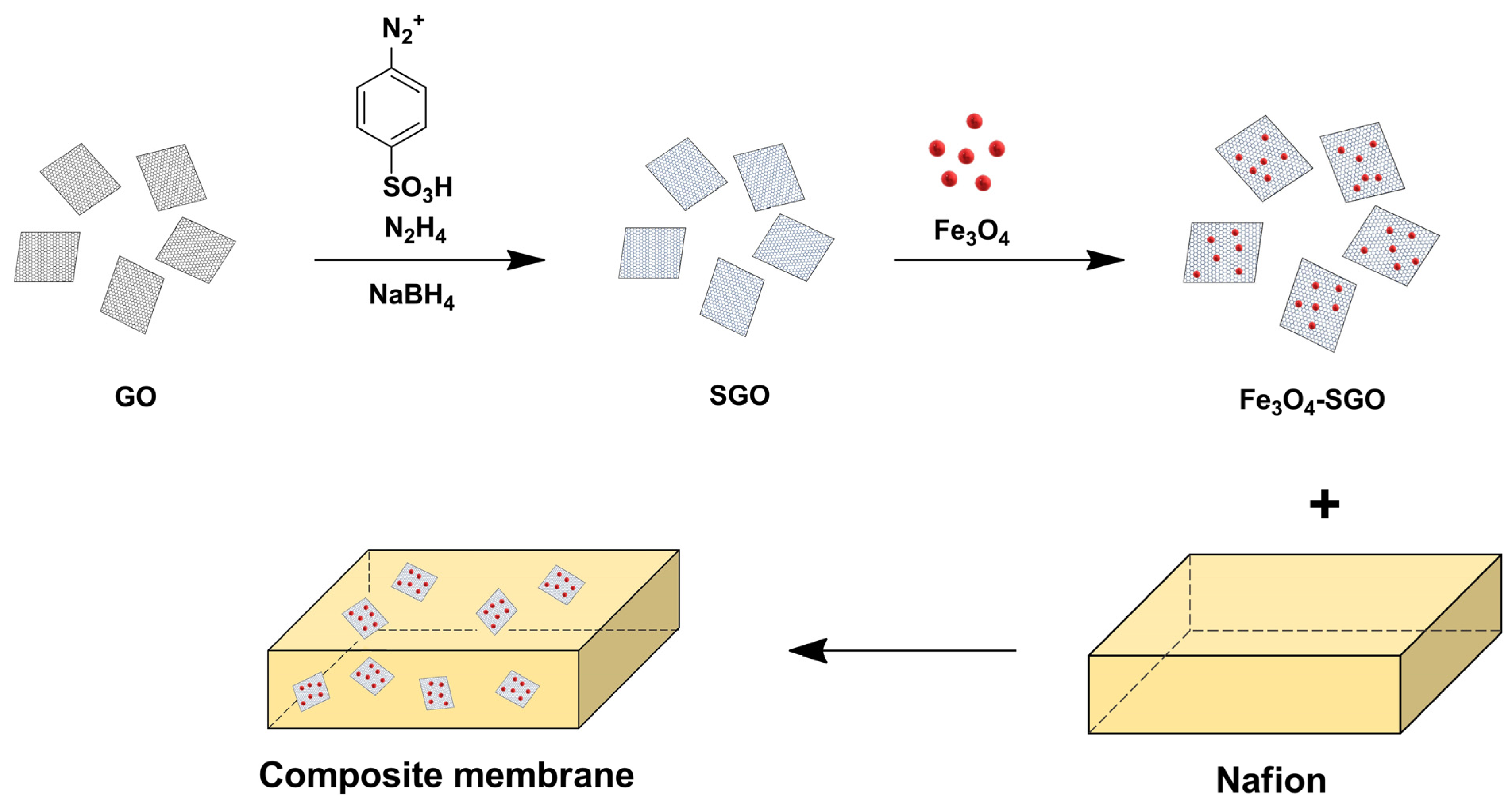
- Beydaghi, H.; Javanbakht, M.; Bagheri, A.; Salarizadeh, P.; Ghafarian-Zahmatkesh, H.; Kashefi, S.; Kowsari, E. Novel nanocomposite membranes based on blended sulfonated poly(ether ether ketone)/poly(vinyl alcohol) containing sulfonated graphene oxide/Fe3O4 nanosheets for DMFC applications. RSC Adv. 2015, 5, 74054–74064. [Google Scholar] [CrossRef]
- Bagheri, A.; Javanbakht, M.; Beydaghi, H.; Salarizadeh, P.; Shabanikia, A.; Amoli, H.S. Sulfonated poly(etheretherketone) and sulfonated polyvinylidene fluoride-co-hexafluoropropylene based blend proton exchange membranes for direct methanol fuel cell applications. RSC Adv. 2016, 6, 39500–39510. [Google Scholar] [CrossRef]
- Ranjani, M.; Yoo, D.J.; Kumar, G.G. Sulfonated Fe3O4@SiO2 nanorods incorporated sPVdF nanocomposite membranes for DMFC applications. J. Membr. Sci. 2018, 555, 497–506. [Google Scholar] [CrossRef]
- Kim, A.R.; Yoo, D.J. A Comparative Study on Physiochemical, Thermomechanical, and Electrochemical Properties of Sulfonated Poly(Ether Ether Ketone) Block Copolymer Membranes with and without Fe3O4 Nanoparticles. Polymers 2019, 11, 536. [Google Scholar] [CrossRef]
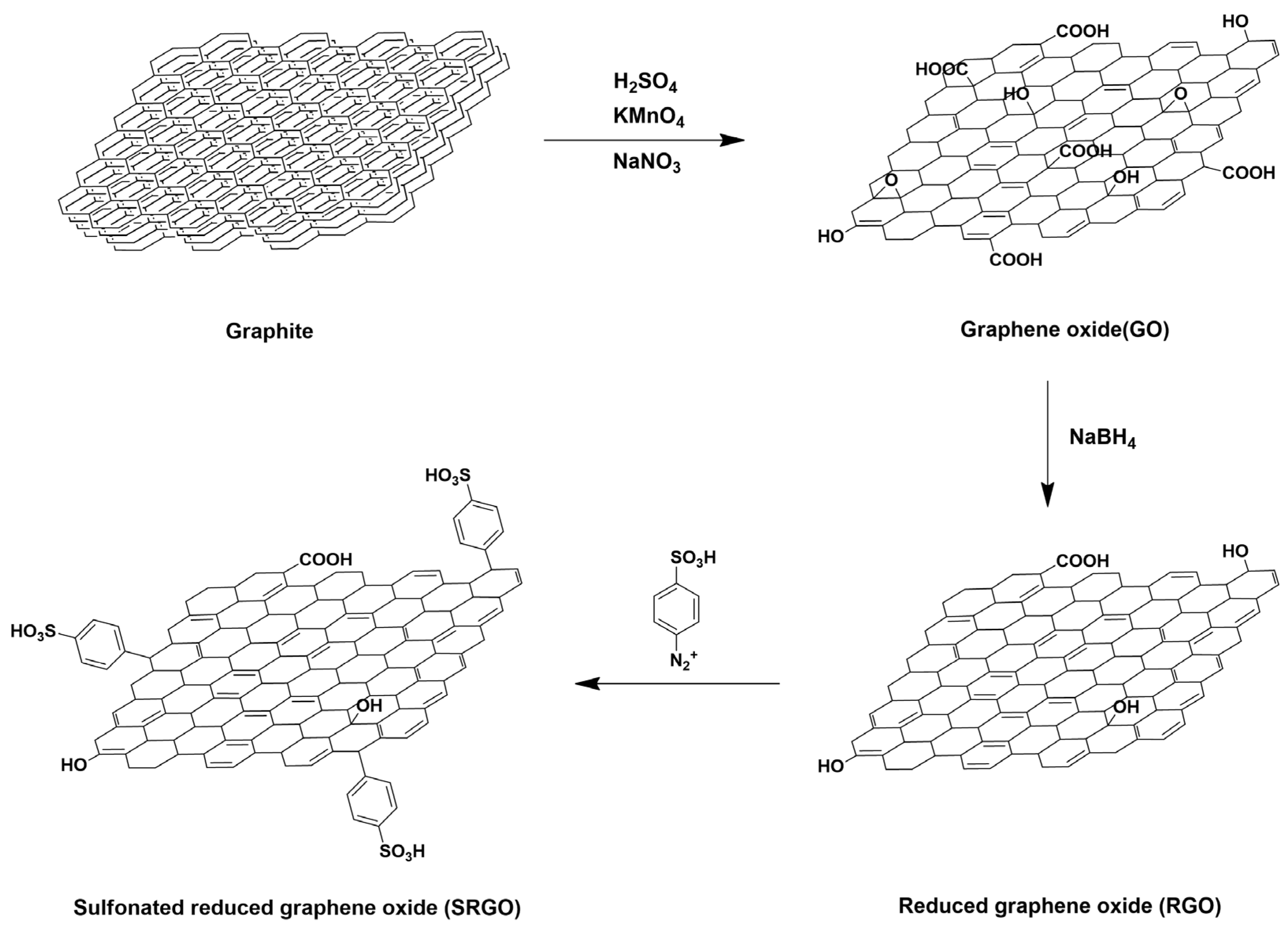
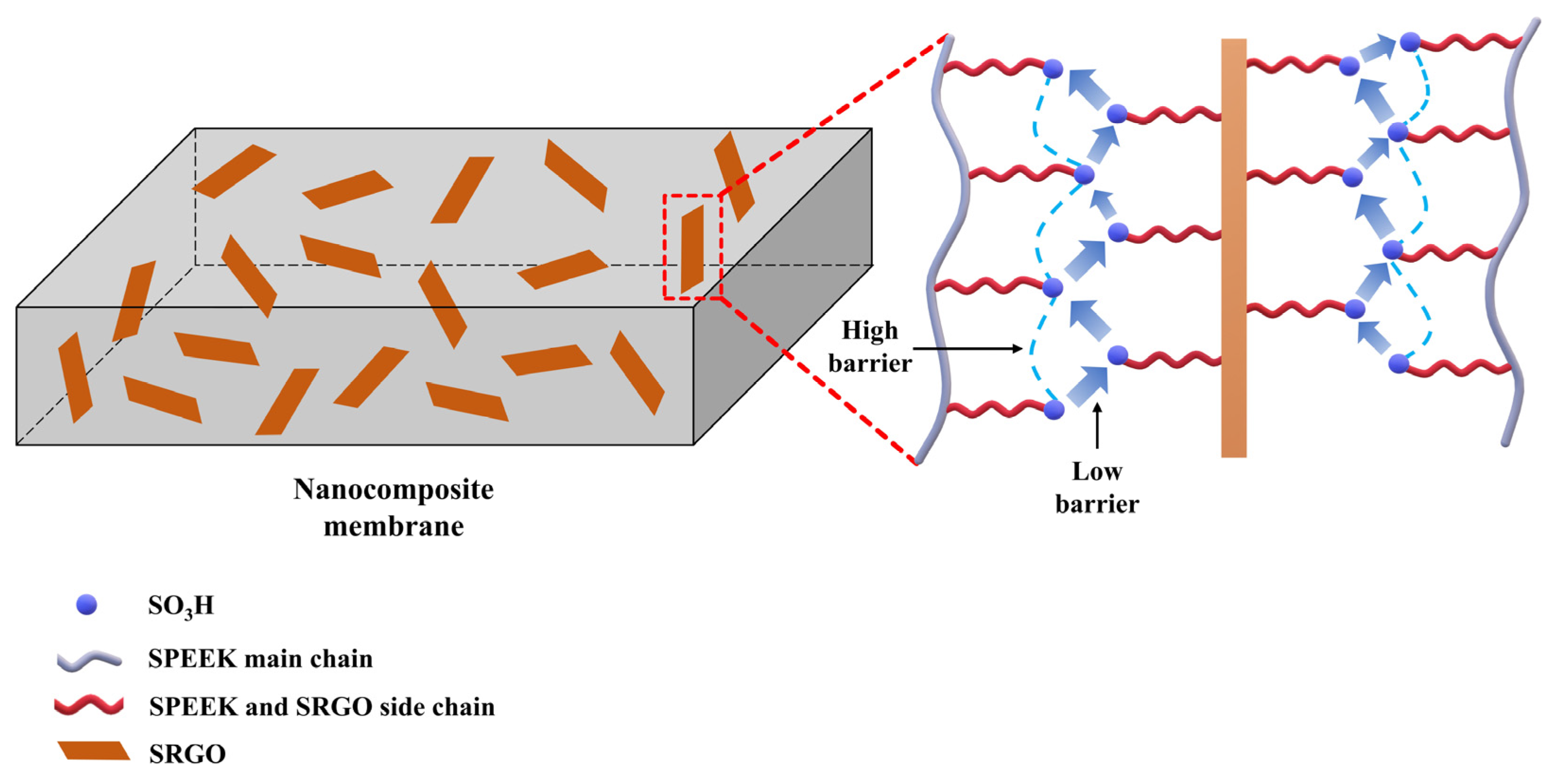
- Qiu, X.; Dong, T.; Ueda, M.; Zhang, X.; Wang, L. Sulfonated reduced graphene oxide as a conductive layer in sulfonated poly(ether ether ketone) nanocomposite membranes. J. Membr. Sci. 2017, 524, 663–672. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhao, X.; Fu, Y.; Manthiram, A. Composite membranes based on sulfonated poly(ether ether ketone) and SDBS-adsorbed graphene oxide for direct methanol fuel cells. J. Mater. Chem. 2012, 22, 24862–24869. [Google Scholar] [CrossRef]
- Han, J.; Lee, H.; Kim, J.; Kim, S.; Kim, H.; Kim, E.; Sung, Y.-E.; Kim, K.; Lee, J.-C. Sulfonated poly(arylene ether sulfone) composite membrane having sulfonated polytriazole grafted graphene oxide for high-performance proton exchange membrane fuel cells. J. Membr. Sci. 2020, 612, 118428. [Google Scholar] [CrossRef]
- Kim, J.; Kim, K.; Ko, T.; Han, J.; Lee, J.-C. Polybenzimidazole composite membranes containing imidazole functionalized graphene oxide showing high proton conductivity and improved physicochemical properties. Int. J. Hydrogen Energy 2021, 46, 12254–12262. [Google Scholar] [CrossRef]
- Rui, Z.; Liu, J. Understanding of free radical scavengers used in highly durable proton exchange membranes. Prog. Nat. Sci. Mater. Int. 2020, 30, 732–742. [Google Scholar] [CrossRef]
- Lim, M.-Y.; Kim, H.J.; Baek, S.J.; Kim, K.Y.; Lee, S.-S.; Lee, J.-C. Improved strength and toughness of polyketone composites using extremely small amount of polyamide 6 grafted graphene oxides. Carbon 2014, 77, 366–378. [Google Scholar] [CrossRef]
- Zuo, Z.; Fu, Y.; Manthiram, A. Novel Blend Membranes Based on Acid-Base Interactions for Fuel Cells. Polymers 2012, 4, 1627–1644. [Google Scholar] [CrossRef]
- Sui, Y.; Du, Y.; Hu, H.; Qian, J.; Zhang, X. Do acid–base interactions really improve the ion conduction in a proton exchange membrane? A study on the effect of basic groups. J. Mater. Chem. A 2019, 7, 19820–19830. [Google Scholar] [CrossRef]
- Li, P.; Wu, W.; Liu, J.; Shi, B.; Du, Y.; Li, Y.; Wang, J. Investigating the nanostructures and proton transfer properties of Nafion-GO hybrid membranes. J. Membr. Sci. 2018, 555, 327–336. [Google Scholar] [CrossRef]
- Ko, T.; Kim, K.; Lim, M.-Y.; Nam, S.Y.; Kim, T.-H.; Kim, S.-K.; Lee, J.-C. Sulfonated poly(arylene ether sulfone) composite membranes having poly(2, 5-benzimidazole)-grafted graphene oxide for fuel cell applications. J. Mater. Chem. A 2015, 3, 20595–20606. [Google Scholar] [CrossRef]
- Lade, H.; Kumar, V.; Arthanareeswaran, G.; Ismail, A. Sulfonated poly(arylene ether sulfone) nanocomposite electrolyte membrane for fuel cell applications: A review. Int. J. Hydrogen Energy 2017, 42, 1063–1074. [Google Scholar] [CrossRef]
- Devrim, Y.; Devrim, H.; Eroglu, I. Polybenzimidazole/SiO2 hybrid membranes for high temperature proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2016, 41, 10044–10052. [Google Scholar] [CrossRef]
- Sarirchi, S.; Rowshanzamir, S.; Mehri, F. Effect of sulfated metal oxides on the performance and stability of sulfonated poly(ether ether ketone) nanocomposite proton exchange membrane for fuel cell applications. React. Funct. Polym. 2020, 156, 104732. [Google Scholar] [CrossRef]
- Rhee, C.H.; Kim, H.K.; Chang, H.; Lee, J.S. Nafion/Sulfonated Montmorillonite Composite: A New Concept Electrolyte Membrane for Direct Methanol Fuel Cells. Chem. Mater. 2005, 17, 1691–1697. [Google Scholar] [CrossRef]
- Lee, G.; Kim, J.; Park, J.; Jeon, Y.; Park, J.; Shul, Y.-G. Nano-Composite Filler of Heteropolyacid-Imidazole Modified Mesoporous Silica for High Temperature PEMFC at Low Humidity. Nanomaterials 2022, 12, 1230. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, N.E.; Easton, E.B. Nafion/Sulfonated Silica Composite Membranes for PEM Fuel Cells. ECS Trans. 2010, 28, 29–38. [Google Scholar] [CrossRef]
- Shao, Z.-G.; Joghee, P.; Hsing, I.M. Preparation and characterization of hybrid Nafion–silica membrane doped with phosphotungstic acid for high temperature operation of proton exchange membrane fuel cells. J. Membr. Sci. 2004, 229, 43–51. [Google Scholar] [CrossRef]
- Amiinu, I.S.; Li, W.; Wang, G.; Tu, Z.; Tang, H.; Pan, M.; Zhang, H. Toward Anhydrous Proton Conductivity Based on Imidazole Functionalized Mesoporous Silica/Nafion Composite Membranes. Electrochim. Acta 2015, 160, 185–194. [Google Scholar] [CrossRef]
- Horvat, C.I.; Zhu, X.; Türp, D.; Vinokur, R.A.; Demco, D.E.; Fechete, R.; Conradi, O.; Graichen, A.; Anokhin, D.; Ivanov, D.A. Perfluorosulfonic acid ionomer–silica composite membranes prepared using hyperbranched polyethoxysiloxane for polymer electrolyte membrane fuel cells. Int. J. Hydrogen Energy 2012, 37, 14454–14462. [Google Scholar] [CrossRef]
- Alashkar, A.; Al-Othman, A.; Tawalbeh, M.; Qasim, M. A Critical Review on the Use of Ionic Liquids in Proton Exchange Membrane Fuel Cells. Membranes 2022, 12, 178. [Google Scholar] [CrossRef]
- Oh, K.; Kwon, O.; Son, B.; Lee, D.H.; Shanmugam, S. Nafion-sulfonated silica composite membrane for proton exchange membrane fuel cells under operating low humidity condition. J. Membr. Sci. 2019, 583, 103–109. [Google Scholar] [CrossRef]
- Alberti, G.; Casciola, M. Composite Membranes for Medium-Temperature PEM Fuel Cells. Annu. Rev. Mater. Res. 2003, 33, 129–154. [Google Scholar] [CrossRef]
- Ying, Y.P.; Kamarudin, S.K.; Masdar, M.S. Silica-related membranes in fuel cell applications: An overview. Int. J. Hydrogen Energy 2018, 43, 16068–16084. [Google Scholar] [CrossRef]
- So, S.Y.; Yoon, Y.J.; Kim, T.-H.; Yoon, K.; Hong, Y.T. Sulfonated poly(arylene ether sulfone)/functionalized silicate hybrid proton conductors for high-temperature proton exchange membrane fuel cells. J. Membr. Sci. 2011, 381, 204–210. [Google Scholar] [CrossRef]
- Ko, T.; Kim, K.; Kim, S.-K.; Lee, J.-C. Organic/inorganic composite membranes comprising of sulfonated Poly(arylene ether sulfone) and core–shell silica particles having acidic and basic polymer shells. Polymer 2015, 71, 70–81. [Google Scholar] [CrossRef]
- Choi, D. Electrochemical Analysis of Polymer Membrane with Inorganic Nanoparticles for High-Temperature PEM Fuel Cells. Membranes 2022, 12, 680. [Google Scholar] [CrossRef] [PubMed]
- Gnana Kumar, G.; Kim, A.; Nahm, K.S.; Elizabeth, R. Nafion membranes modified with silica sulfuric acid for the elevated temperature and lower humidity operation of PEMFC. Int. J. Hydrogen Energy 2009, 34, 9788–9794. [Google Scholar] [CrossRef]
- Hong, Y.T.; Lee, C.H.; Park, H.S.; Min, K.A.; Kim, H.J.; Nam, S.Y.; Lee, Y.M. Improvement of electrochemical performances of sulfonated poly(arylene ether sulfone) via incorporation of sulfonated poly(arylene ether benzimidazole). J. Power Sources 2008, 175, 724–731. [Google Scholar] [CrossRef]
- Sharma, P.P.; Tinh, V.D.C.; Kim, D. Enhanced Ion Cluster Size of Sulfonated Poly(Arylene Ether Sulfone) for Proton Exchange Membrane Fuel Cell Application. Polymers 2021, 13, 1111. [Google Scholar] [CrossRef]
- Kumar, A.; Ibraheem, S.; Ali, S.; Maiyalagan, T.; Javed, M.S.; Gupta, R.K.; Saad, A.; Yasin, G. Polypyrrole and Polyaniline-based Membranes for Fuel Cell Devices: A Review. Surf. Interfaces 2022, 29, 101738. [Google Scholar] [CrossRef]
- Won, J.-H.; Lee, H.-J.; Yoon, K.-S.; Hong, Y.T.; Lee, S.-Y. Sulfonated SBA-15 mesoporous silica-incorporated sulfonated poly(phenylsulfone) composite membranes for low-humidity proton exchange membrane fuel cells: Anomalous behavior of humidity-dependent proton conductivity. Int. J. Hydrogen Energy 2012, 37, 9202–9211. [Google Scholar] [CrossRef]
- Yoon, K.-S.; Choi, J.-H.; Hong, Y.T.; Hong, S.-K.; Lee, S.-Y. Control of nanoparticle dispersion in SPAES/SiO2 composite proton conductors and its influence on DMFC membrane performance. Electrochem. Commun. 2009, 11, 1492–1495. [Google Scholar] [CrossRef]
- Son, B.; Oh, K.; Park, S.; Lee, T.G.; Lee, D.H.; Kwon, O. Study of morphological characteristics on hydrophilicity-enhanced SiO2/Nafion composite membranes by using multimode atomic force microscopy. Int. J. Energy Res. 2019, 43, 4157–4169. [Google Scholar] [CrossRef]




| Polymer | Structure |
|---|---|
| SPSf a |  |
| SPEEK b | 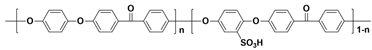 |
| SPAES c | 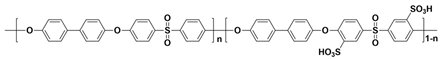 |
| SPI d |  |
| Sample | Water Uptake a (%) | Proton Conductivity b (mS cm−1) | |
|---|---|---|---|
| 20% RH | 80% RH | ||
| Nafion/sMWCNT (0.125 wt.%) | 17.2 | 8.0 | 180 |
| Nafion/sMWCNT (0.25 wt.%) | 29.3 | 23.0 | 198 |
| Nafion/sMWCNT (0.5 wt.%) | 16.4 | 6.0 | 165 |
| Recast Nafion | 15.8 | 2.0 | 160 |
| Composite Membrane (Matrix/Filler) | Filler Content (wt. %) | Functionalization of CNTs | Water Uptake a (%) | IEC b (meq g−1) | Proton Conductivity c (mS cm−1) | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Reagent | Method | 20% RH | 80% RH | |||||
| Nafion/CNT | 5.0 | - | - | 20.7 | 0.8 | 1.0 | 40.0 | [53] |
| Nafion/sMWCNT d | 0.25 | 4-benzendiazonium sulfonic acid | Grafting | 29.3 | - | 2.3 (60 °C) | 198 (60 °C) | [76] |
| Nafion/MWCNT-SO3H e | 0.5 | 1,3-propanesultone | 34.5 | 0.9 | - | 150 | [77] | |
| Nafion/MWCNT-Im f | 0.5 | 4-imidazolecarboxylic acid | Dehydration reaction | 28.0 | 0.9 | - | 210 | [77] |
| SPEEK/CNT | 1.5 | - | - | - | 2.8 | 47.0 | [79] | |
| SPAES/HNT g | 1.0 | SPAES 100 hydrophilic oligomer | Nucleophilic substitution | 58.0 | - | 0.7 | 237 (100% RH) | [78] |
| SPEEK/ACNT h | 1.5 | 3-aminopropyl triethoxysilane | Condensation reaction | 17.5 | 1.2 | 7.3 | 115 | [79] |
| Composite Membrane (Matrix/Filler) | Filler Content (wt. %) | Functionalization of GOs | Water Uptake a (%) | IEC b (meq g−1) | Proton Conductivity c (mS cm−1) | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Agent | Method | 20% RH | 80% RH | |||||
| Nafion/GO | 5.0 | - | - | 57.5 | 0.8 | 2.0 | 79.0 | [110] |
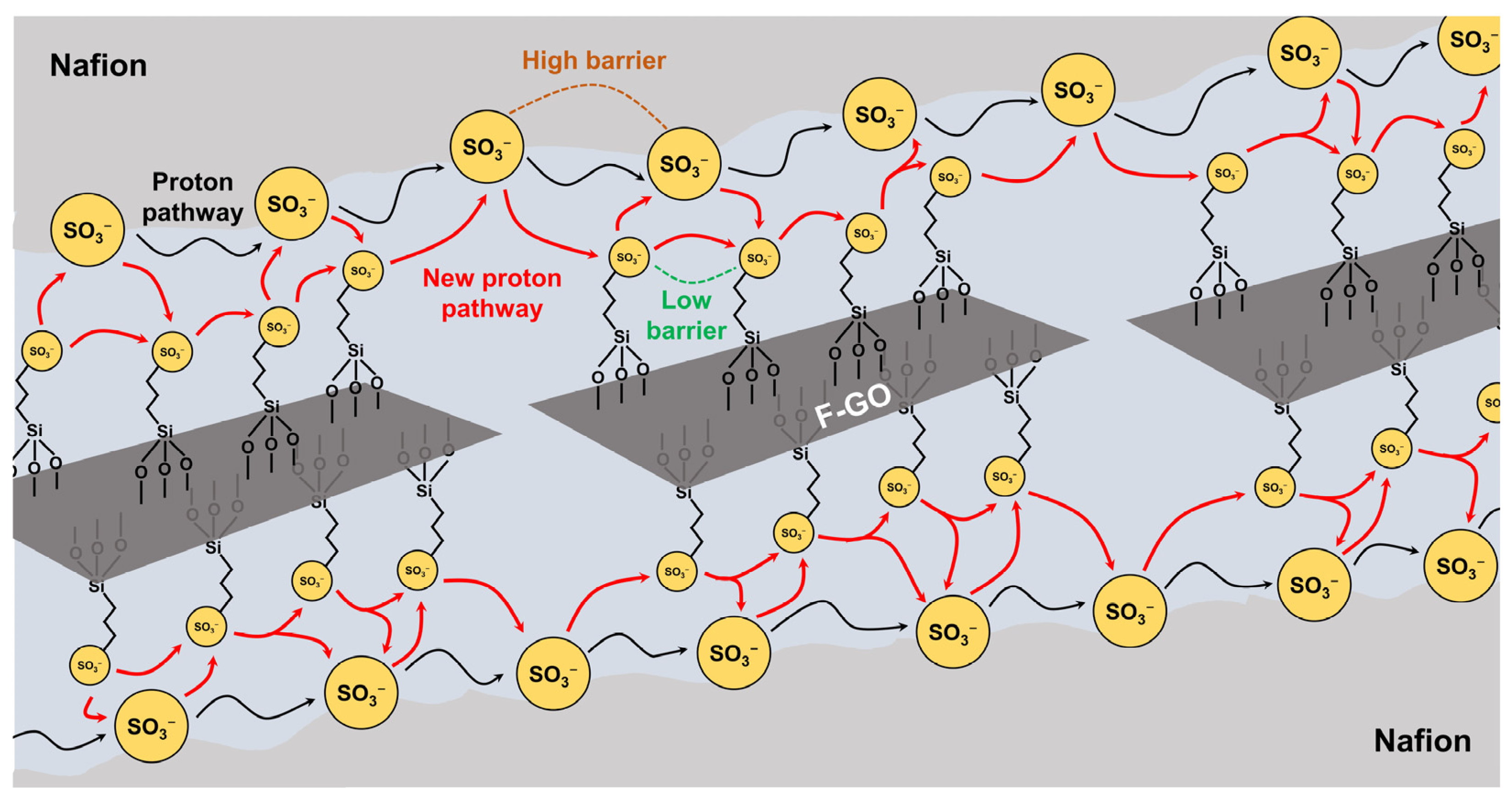
| Nafion/F-GO | 10 | 3-mercaptopropyl trimethoxysilane | Oxidation | 28.8 | 1.0 | 8.0 | 110 | [84] |
| Nafion/Fe3O4-SGO | 3.0 | Sulfanilic acid, Fe3O4 | Arylation, Dispersion | 35.6 (60 °C) | 1.4 | 7.0 | 125 (100% RH) | [96] |
| SPAES/GO | 1.0 | - | - | 27.8 | 1.6 | 1.6 (50% RH) | 114 (90% RH) | [111] |
| SPEEK/SRGO | 1.0 | 4-aminobenzensulfonic acid | Arylation | 31.1 (80 °C) | 1.7 | 8.6 (50% RH) | 147 (95% RH) | [102] |
| SPAES/SPTA-GO | 1.0 | Ethynyl-terminated sulfonated polytriazole | Azide-alkyne click reaction | 57.0 | 2.5 | 20 (40% RH) | 250 | [104] |
| Sample (SiO2 Particle Size) | Nomenclature | Water Uptake (%) | IEC (meq g−1) | Proton Conductivity a (mS cm−1) | |
|---|---|---|---|---|---|
| 30% RH | 80% RH | ||||
| Nafion 117 | N | 23.0 | 0.9 | 4.2 | 108 |
| Nafion/SiO2 (3 nm) | NS-3 | 28.0 | 0.9 | - | - |
| Nafion/SiO2 (90 nm) | NS-90 | 25.0 | 0.9 | - | - |
| Nafion/SiO2 (1000 nm) | NS-1000 | 21.0 | 0.7 | - | - |
| Nafion/SiO2-O-SO3H (3 nm) | NSSH-3 | 32.2 | 1.5 | 49.1 | 193 |
| Nafion/SiO2 SiO2-O-SO3H (90 nm) | NSSH-90 | 30.0 | 1.3 | 30.9 | 180 |
| Nafion/SiO2 SiO2-O-SO3H (1000 nm) | NSSH-1000 | 23.3 | 0.9 | 0.4 | 54.4 |
| Sample | IEC (meq g−1) | Water Uptake (%) | Proton Conductivity a (mS cm−1) | |
|---|---|---|---|---|
| 20% RH | 80% RH | |||
| Nafion/SSA (0.5 wt.%) | 1.2 | 22.0 | 5.9 | 151 |
| Nafion/SSA (1.0 wt.%) | 1.3 | 24.1 | 8.8 | 230 |
| Nafion/SSA (1.5 wt.%) | 1.1 | 20.0 | 5.5 | 143 |
| Recast Nafion | 1.0 | 17.3 | 5.0 | 111 |
| Sample (Matrix/Filler) | Filler Content (wt. %) | Functionalization of SiO2 | Water Uptake a (%) | IEC b (meq g−1) | Proton Conductivity c (mS cm−1) | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Agent | Method | 20% RH | 80% RH | |||||
| Nafion/SiO2 | 2.0 | - | - | 35.6 | - | 7.5 | 80.0 | [134] |
| Nafion/Silica sulfonic acid | - | Chlorosulfonic acid | Sulfonation | 32.2 | 1.5 | 40.0 | 175 | [128] |
| Nafion/SSA d | 1.0 | TEOS, Chlorosulfonic acid | In situ sol-gel methods | 24.1 | 1.3 | 8.8 | 230 (100% RH) | [122] |
| SPAES/SiO2 | 5.0 | - | - | 36.7 | 1.7 | 1.0 (40% RH) | 102 (90% RH) | [126] |
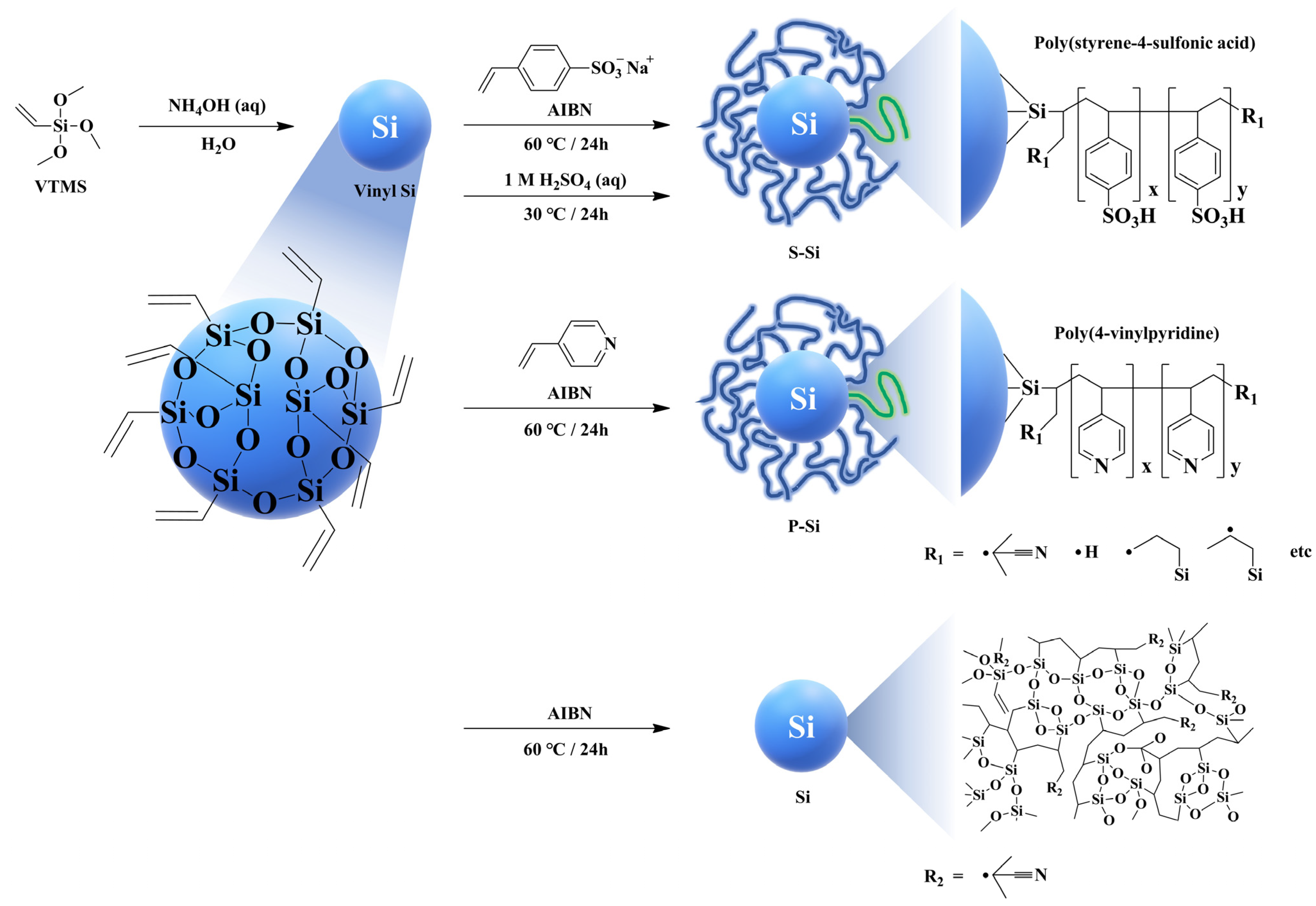
| SPAES/S-Si e | 5.0 | 4-styrensulfonic acid sodium salt hydrate | Radical polymerization | 40.9 | 1.7 | 1.3 (40% RH) | 140 (90% RH) | [126] |
| SPAES/P-Si f | 5.0 | 4-vinylpyridine | Radical polymerization | 34.3 | 1.6 | 2.3 (40% RH) | 146 (90% RH) | [126] |
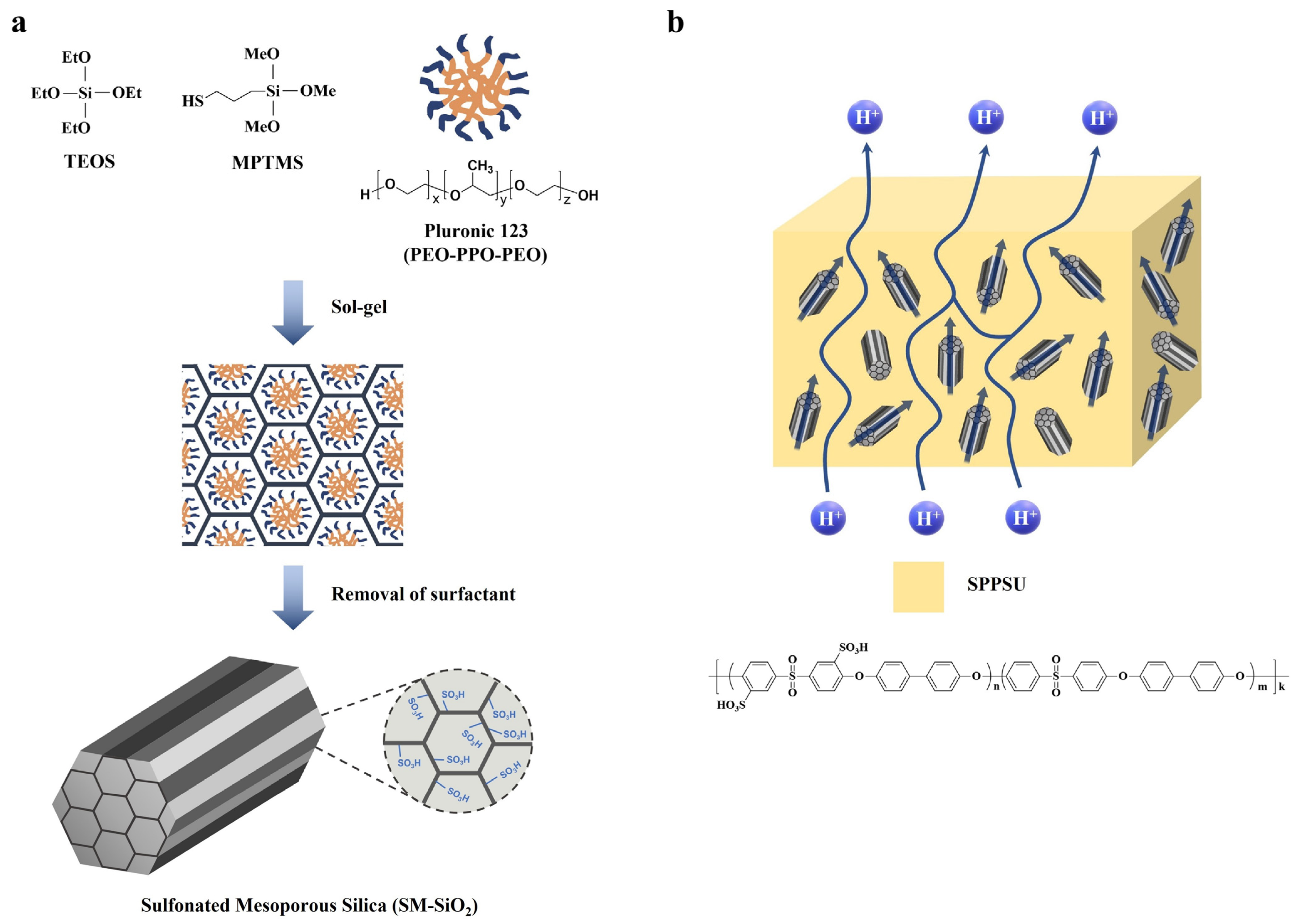
| SPPSU/SM-SiO2 g | - | 3-mercaptoproyl trimethoxysilane | Condensation, oxidation | 17.0 | 2.0 | 6.0 (50% RH) | 183 (100% RH) | [132] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, S.; Lee, H.; Jeong, Y.-G.; Choi, C.; Hwang, I.; Song, S.; Nam, S.Y.; Lee, J.H.; Kim, K. Polymer Electrolyte Membranes Containing Functionalized Organic/Inorganic Composite for Polymer Electrolyte Membrane Fuel Cell Applications. Int. J. Mol. Sci. 2022, 23, 14252. https://doi.org/10.3390/ijms232214252
Hwang S, Lee H, Jeong Y-G, Choi C, Hwang I, Song S, Nam SY, Lee JH, Kim K. Polymer Electrolyte Membranes Containing Functionalized Organic/Inorganic Composite for Polymer Electrolyte Membrane Fuel Cell Applications. International Journal of Molecular Sciences. 2022; 23(22):14252. https://doi.org/10.3390/ijms232214252
Chicago/Turabian StyleHwang, Seansoo, HyeonGyeong Lee, Yu-Gyeong Jeong, Chanhee Choi, Inhyeok Hwang, SeungHyeon Song, Sang Yong Nam, Jin Hong Lee, and Kihyun Kim. 2022. "Polymer Electrolyte Membranes Containing Functionalized Organic/Inorganic Composite for Polymer Electrolyte Membrane Fuel Cell Applications" International Journal of Molecular Sciences 23, no. 22: 14252. https://doi.org/10.3390/ijms232214252
APA StyleHwang, S., Lee, H., Jeong, Y.-G., Choi, C., Hwang, I., Song, S., Nam, S. Y., Lee, J. H., & Kim, K. (2022). Polymer Electrolyte Membranes Containing Functionalized Organic/Inorganic Composite for Polymer Electrolyte Membrane Fuel Cell Applications. International Journal of Molecular Sciences, 23(22), 14252. https://doi.org/10.3390/ijms232214252









