Sucrose Consumption during Late Adolescence Impairs Adult Neurogenesis of the Ventral Dentate Gyrus without Inducing an Anxiety-like Behavior
Abstract
1. Introduction
2. Results
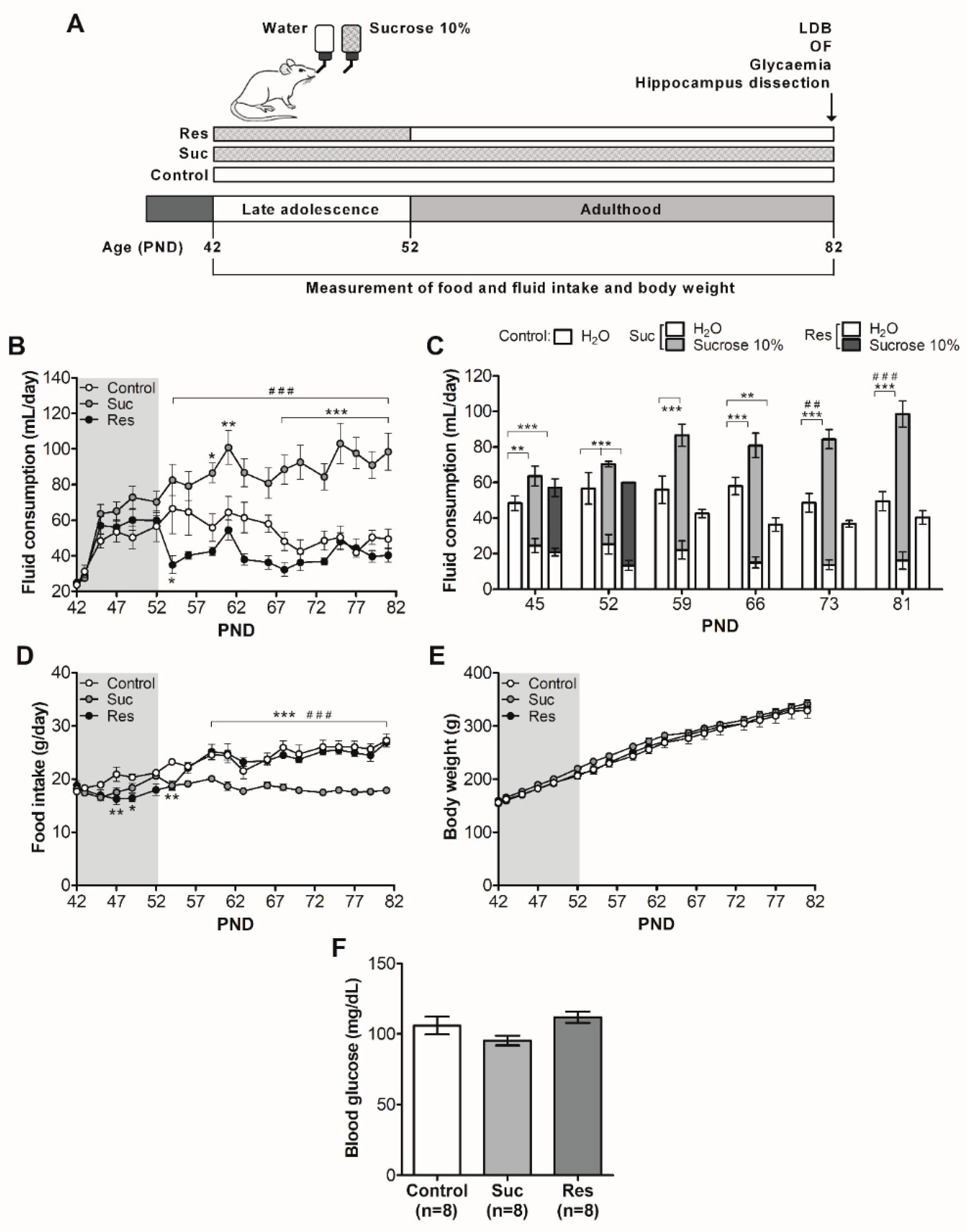
2.1. Sucrose Treatments Do Not Modify Body Weight and Glycaemia
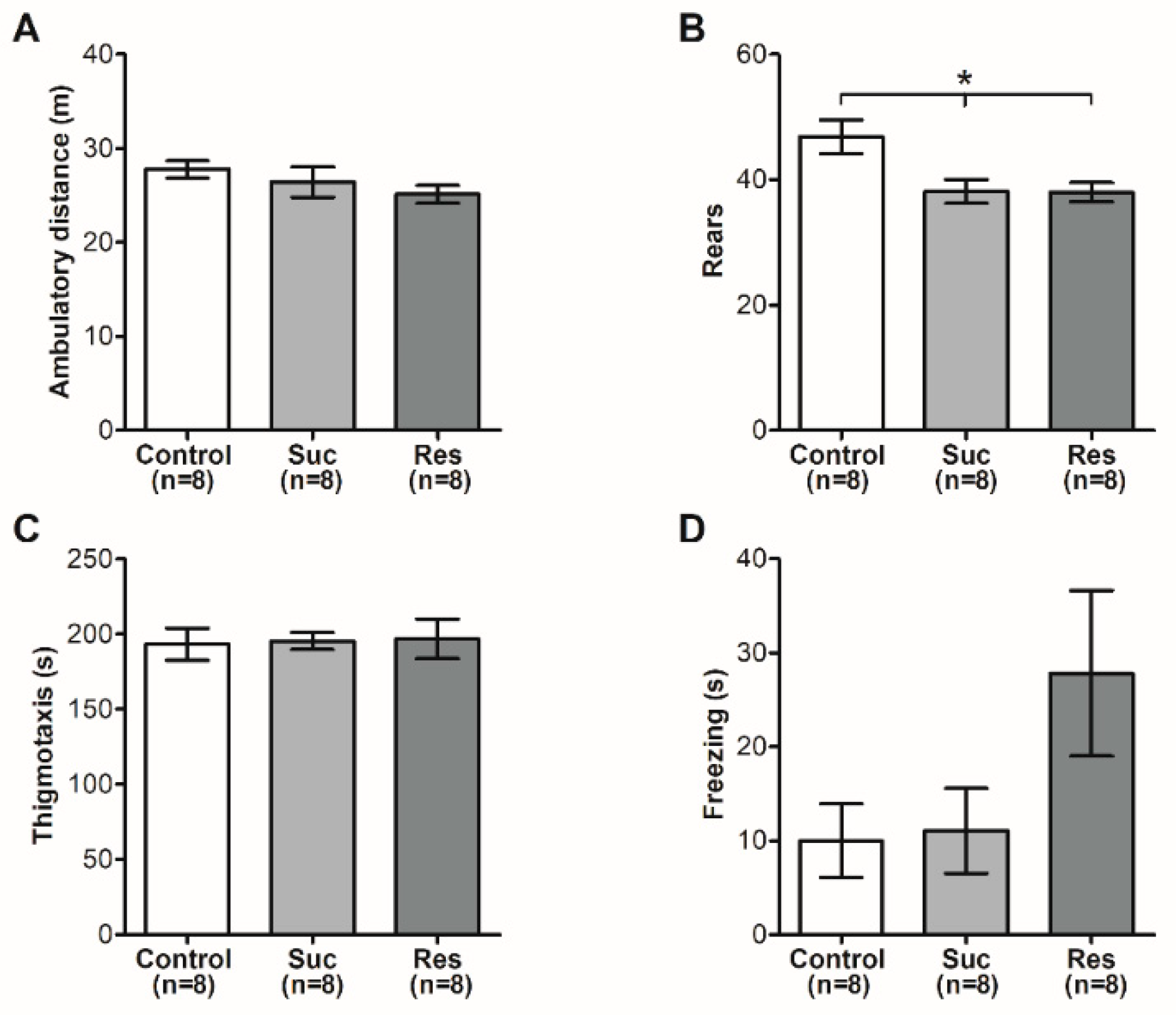
2.2. Sucrose Consumption Starting at Late Adolescence Does Not Induce Anxiety
2.3. Sucrose Intake during Late Adolescence Impaired Adult Neurogenesis in the Ventral DG
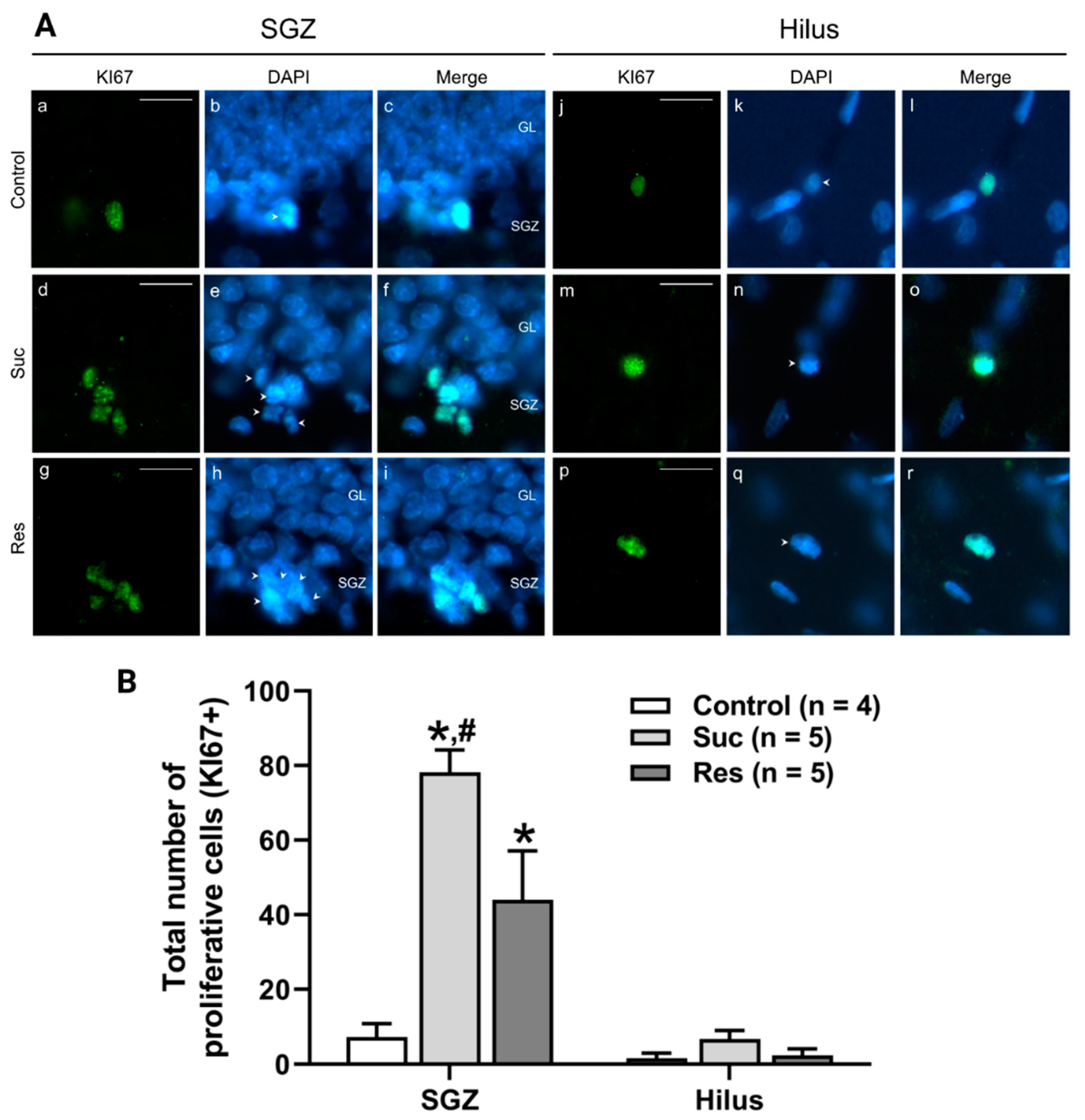
2.3.1. Sucrose Intake during Late Adolescence Increases the Number of Proliferative Cells in the SGZ of the Ventral DG
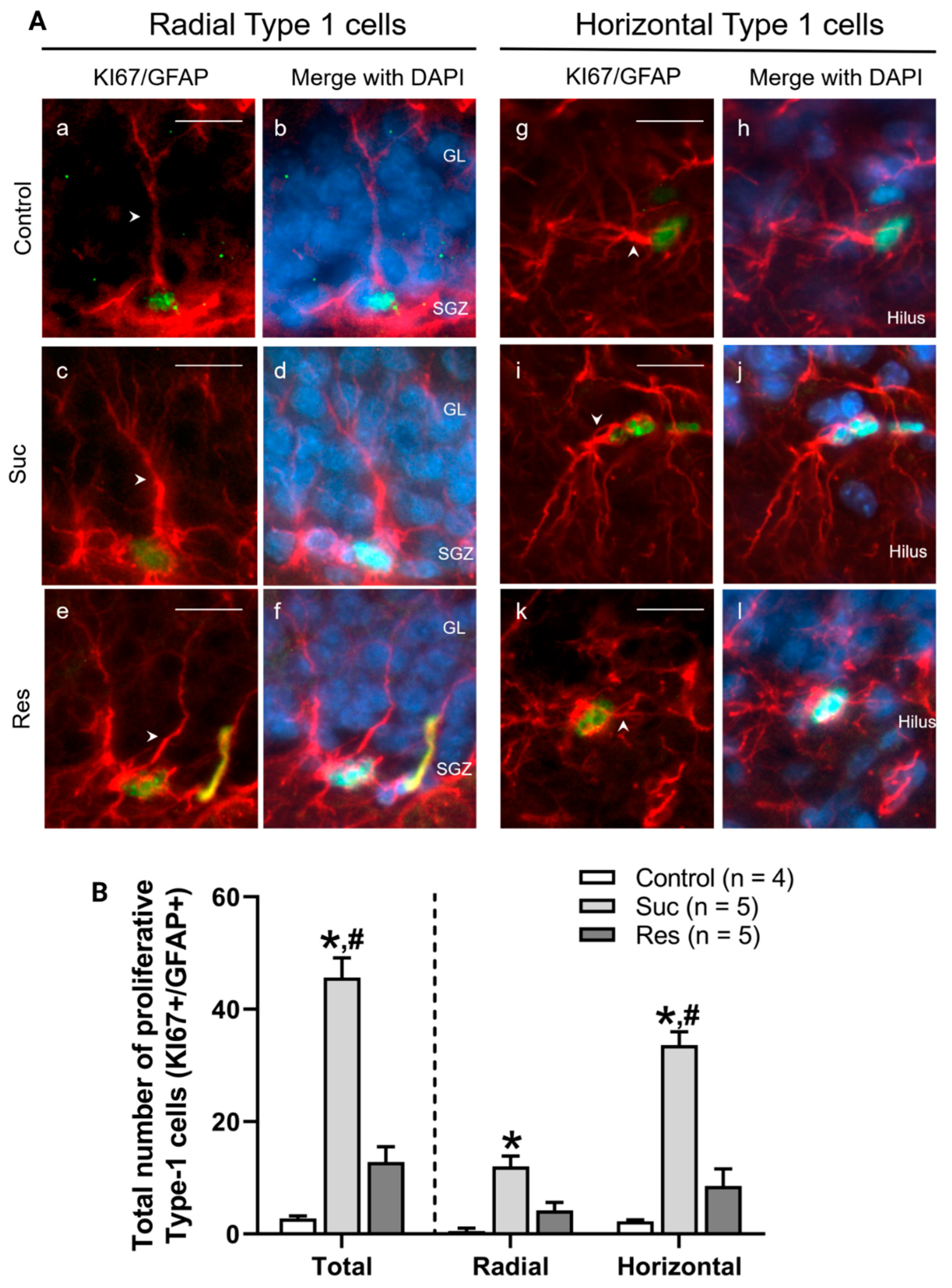
2.3.2. Sucrose Consumption during Late Adolescence Increases the Number of Proliferative Type 1 Cells in the SGZ of the Ventral DG
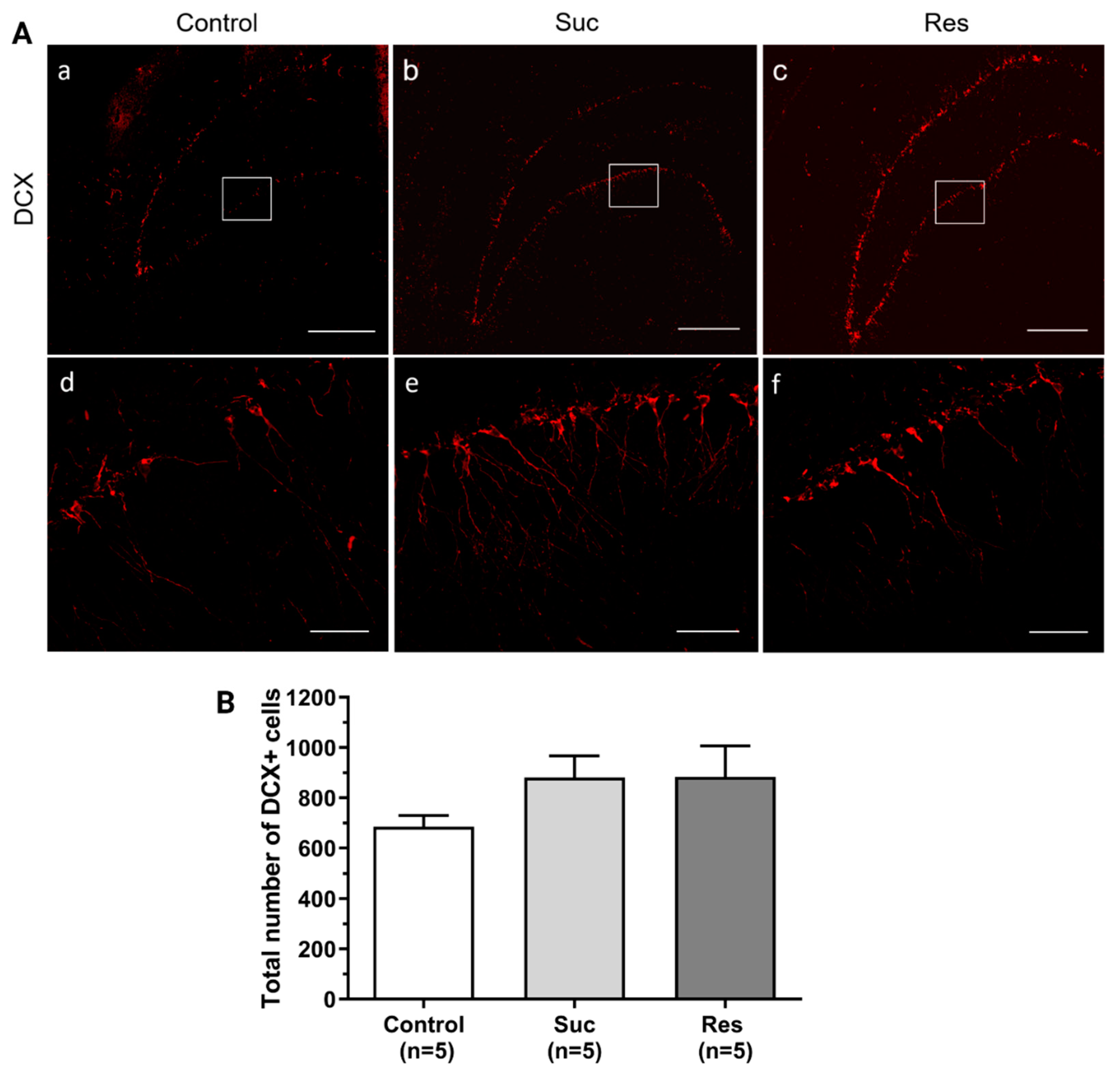
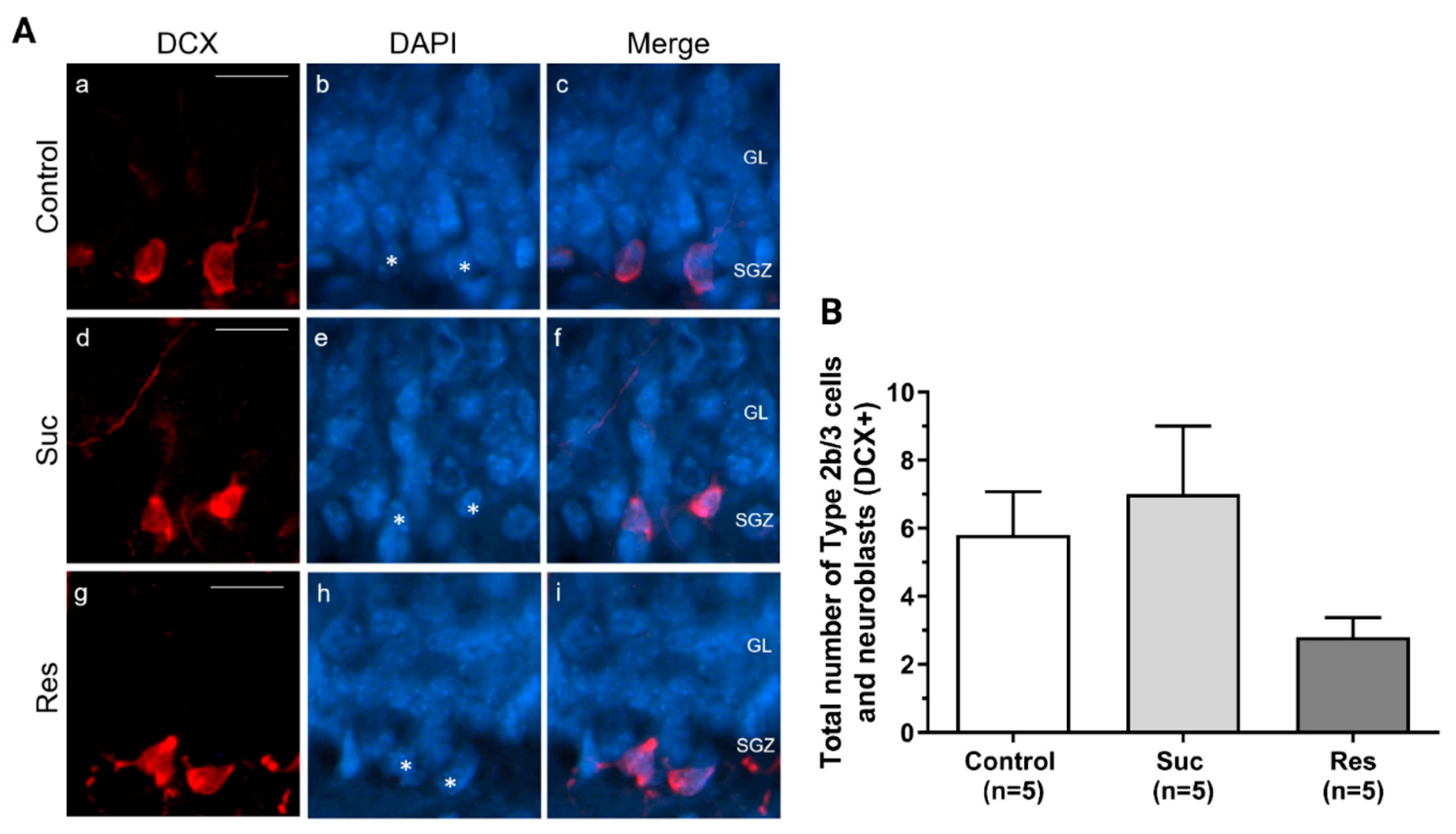
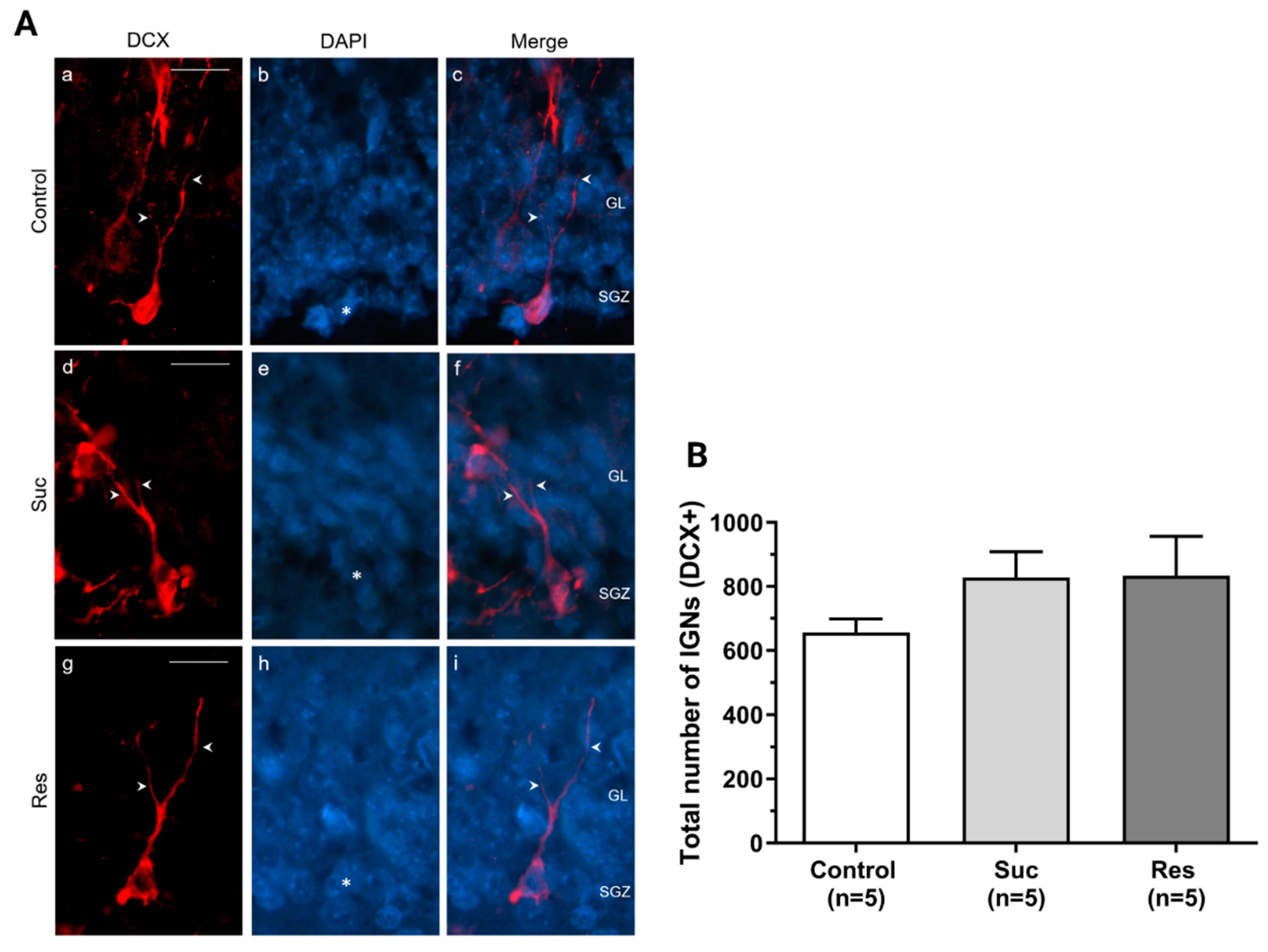
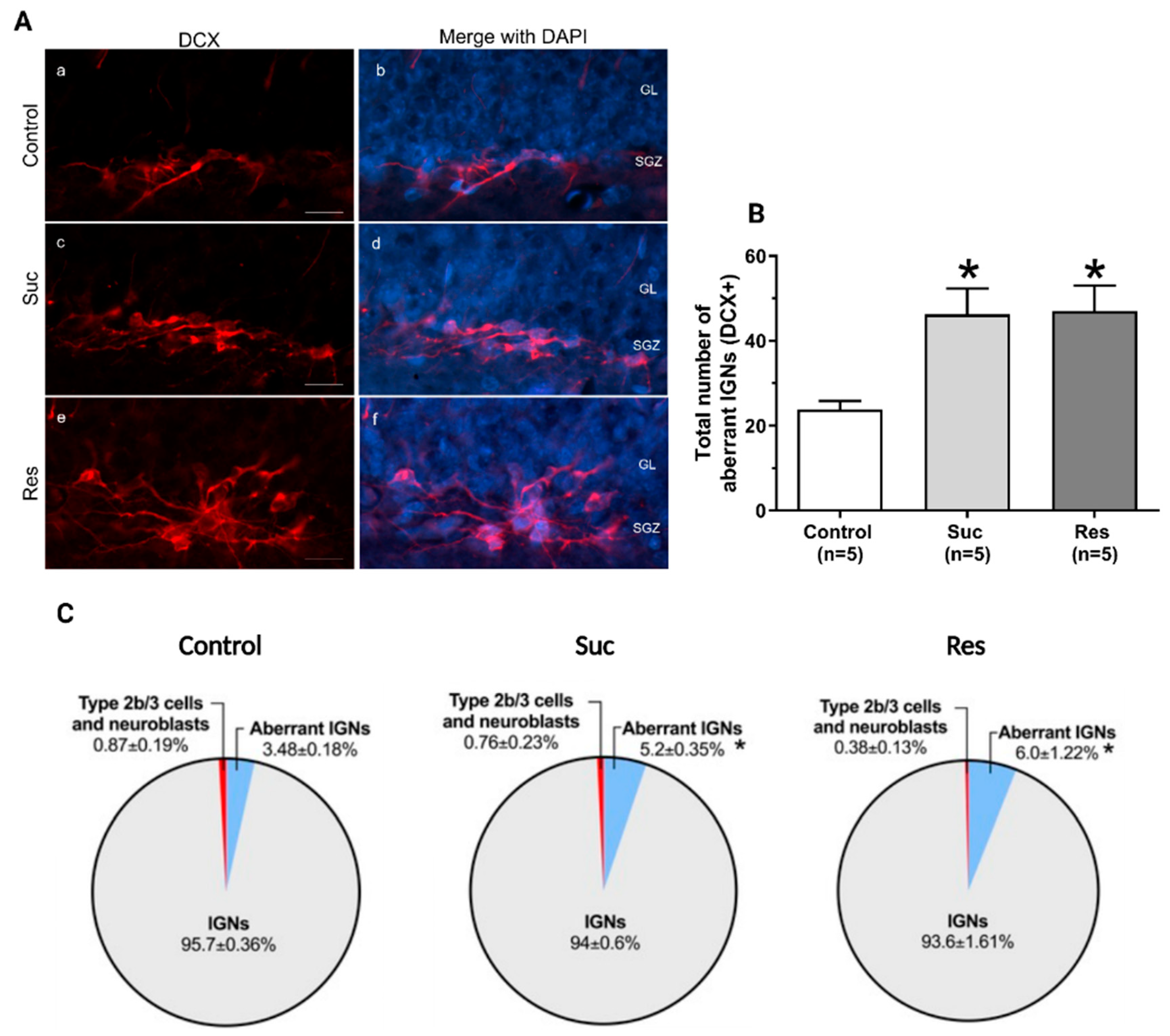
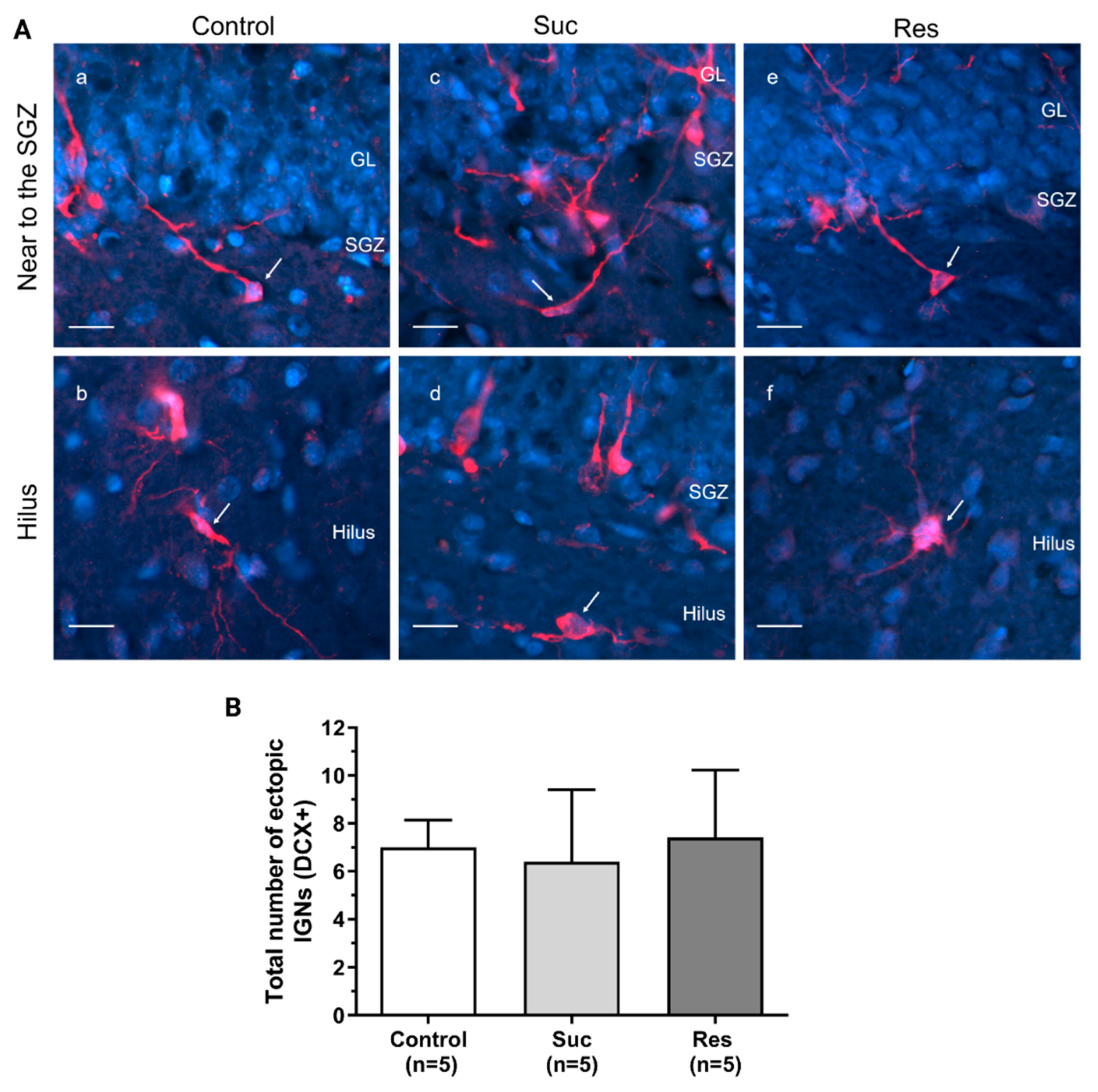
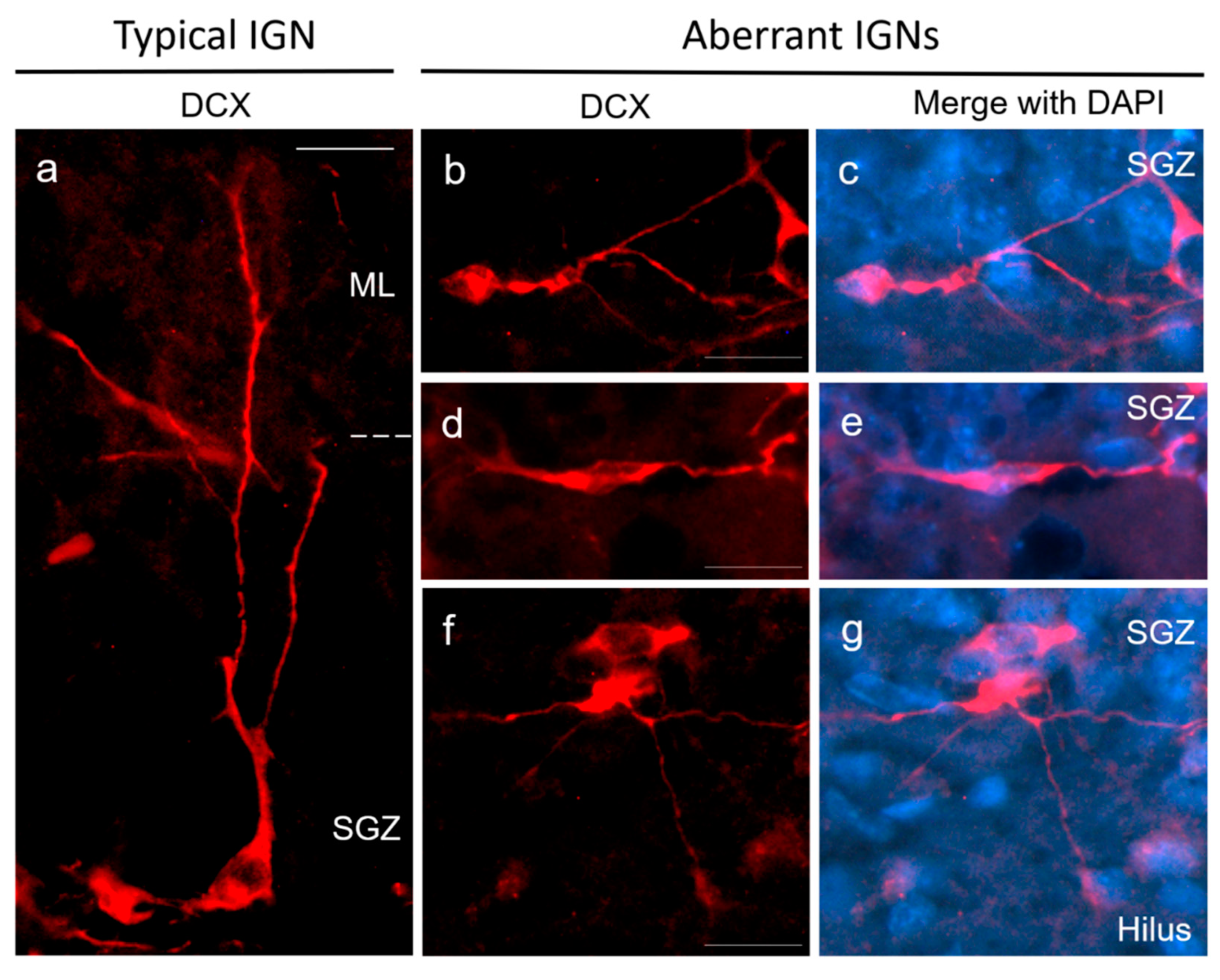
2.3.3. Sucrose Consumption Leads to Alterations in the Population of DCX-Immunoreactive Cells
2.4. Sucrose Consumption Increases Phospho-Erk1/2 Levels in the Hippocampus of Rats Exposed to Sucrose Only during Late Adolescence
3. Discussion
4. Methods and Materials
4.1. Animals and Treatments
4.2. Behavioral Testing
4.2.1. Light/Dark Box Test
4.2.2. Open Field Test
4.3. Tissue Processing and Immunofluorescence
4.4. Morphological Criteria for Cell Identification
4.5. Cell Counting
4.6. Western Blot
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ming, G.L.; Song, H. Adult Neurogenesis in the Mammalian Brain: Significant Answers and Significant Questions. Neuron 2011, 70, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Bonaguidi, M.A.; Song, J.; Ming, G.L.; Song, H. A Unifying Hypothesis on Mammalian Neural Stem Cell Properties in the Adult Hippocampus. Curr. Opin. Neurobiol. 2012, 22, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Sahay, A.; Hen, R. Adult Hippocampal Neurogenesis in Depression. Nat. Neurosci. 2007, 10, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Revest, J.M.; Dupret, D.; Koehl, M.; Funk-Reiter, C.; Grosjean, N.; Piazza, P.V.; Abrous, D.N. Adult Hippocampal Neurogenesis Is Involved in Anxiety-Related Behaviors. Mol. Psychiatry 2009, 14, 959–967. [Google Scholar] [CrossRef]
- Vidal, R.; Garro-Martínez, E.; Díaz, Á.; Castro, E.; Florensa-Zanuy, E.; Taketo, M.M.; Pazos, Á.; Pilar-Cuéllar, F. Targeting β-Catenin in GLAST-Expressing Cells: Impact on Anxiety and Depression-Related Behavior and Hippocampal Proliferation. Mol. Neurobiol. 2019, 56, 553–566. [Google Scholar] [CrossRef]
- Anacker, C.; Hen, R. Adult Hippocampal Neurogenesis and Cognitive Flexibility - Linking Memory and Mood. Nat. Rev. Neurosci. 2017, 18, 335–346. [Google Scholar] [CrossRef]
- Banasr, M.; Soumier, A.; Hery, M.; Mocaër, E.; Daszuta, A. Agomelatine, a New Antidepressant, Induces Regional Changes in Hippocampal Neurogenesis. Biol. Psychiatry 2006, 59, 1087–1096. [Google Scholar] [CrossRef]
- Gualtieri, F.; Brégère, C.; Laws, G.C.; Armstrong, E.A.; Wylie, N.J.; Moxham, T.T.; Guzman, R.; Boswell, T.; Smulders, T.V. Effects of Environmental Enrichment on Doublecortin and BDNF Expression along the Dorso-Ventral Axis of the Dentate Gyrus. Front. Neurosci. 2017, 11. [Google Scholar] [CrossRef]
- Vetreno, R.P.; Crews, F.T. Binge Ethanol Exposure during Adolescence Leads to a Persistent Loss of Neurogenesis in the Dorsal and Ventral Hippocampus That Is Associated with Impaired Adult Cognitive Functioning. Front. Neurosci. 2015, 9, 35. [Google Scholar] [CrossRef]
- Leschik, J.; Lutz, B.; Gentile, A. Stress-Related Dysfunction of Adult Hippocampal Neurogenesis-An Attempt for Understanding Resilience? Int. J. Mol. Sci. 2021, 22, 7339. [Google Scholar] [CrossRef]
- Kempermann, G. Environmental Enrichment, New Neurons and the Neurobiology of Individuality. Nat. Rev. Neurosci. 2019, 20, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, A.; Mohapel, P.; Bouter, B.; Frielingsdorf, H.; Pizzo, D.; Brundin, P.; Erlanson-Albertsson, C. High-Fat Diet Impairs Hippocampal Neurogenesis in Male Rats. Eur. J. Neurol. 2006, 13, 1385–1388. [Google Scholar] [CrossRef]
- Stangl, D.; Thuret, S. Impact of Diet on Adult Hippocampal Neurogenesis. Genes Nutr. 2009, 4, 271–282. [Google Scholar] [CrossRef] [PubMed]
- van der Borght, K.; Köhnke, R.; Göransson, N.; Deierborg, T.; Brundin, P.; Erlanson-Albertsson, C.; Lindqvist, A. Reduced Neurogenesis in the Rat Hippocampus Following High Fructose Consumption. Regul. Pept. 2011, 167, 26–30. [Google Scholar] [CrossRef]
- Reichelt, A.C.; Killcross, S.; Hambly, L.D.; Morris, M.J.; Westbrook, R.F. Impact of Adolescent Sucrose Access on Cognitive Control, Recognition Memory, and Parvalbumin Immunoreactivity. Learn. Mem. 2015, 22, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Gueye, A.B.; Vendruscolo, L.F.; de Ávila, C.; le Moine, C.; Darnaudéry, M.; Cador, M. Unlimited Sucrose Consumption during Adolescence Generates a Depressive-like Phenotype in Adulthood. Neuropsychopharmacology 2018, 43, 2627–2635. [Google Scholar] [CrossRef] [PubMed]
- Arain, M.; Haque, M.; Johal, L.; Mathur, P.; Nel, W.; Rais, A.; Sandhu, R.; Sharma, S. Maturation of the Adolescent Brain. Neuropsychiatr. Dis. Treat. 2013, 9, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Kruse, M.S.; Vadillo, M.J.; Miguelez Fernández, A.M.M.; Rey, M.; Zanutto, B.S.; Coirini, H. Sucrose Exposure in Juvenile Rats Produces Long-Term Changes in Fear Memory and Anxiety-like Behavior. Psychoneuroendocrinology 2019, 104, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A.; Rehm, C.D. Consumption of Added Sugars among US Children and Adults by Food Purchase Location and Food Source. Am. J. Clin. Nutr. 2014, 100, 901–907. [Google Scholar] [CrossRef]
- Barquera, S.; Hernandez-Barrera, L.; Tolentino, M.L.; Espinosa, J.; Shu, W.N.; Rivera, J.A.; Popkin, B.M. Energy Intake from Beverages Is Increasing among Mexican Adolescents and Adults. J. Nutr. 2008, 138, 2454–2461. [Google Scholar] [CrossRef] [PubMed]
- Young, S.E.; Corley, R.P.; Stallings, M.C.; Rhee, S.H.; Crowley, T.J.; Hewitt, J.K. Substance Use, Abuse and Dependence in Adolescence: Prevalence, Symptom Profiles and Correlates. Drug Alcohol Depend. 2002, 68, 309–322. [Google Scholar] [CrossRef]
- Bannerman, D.M.; Sprengel, R.; Sanderson, D.J.; Mchugh, S.B.; Rawlins, J.N.P.; Monyer, H.; Seeburg, P.H. Hippocampal Synaptic Plasticity, Spatial Memory and Anxiety. Nat. Rev. Neurosci. 2014, 15, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Kheirbek, M.A.; Drew, L.J.; Burghardt, N.S.; Costantini, D.O.; Tannenholz, L.; Ahmari, S.E.; Zeng, H.; Fenton, A.A.; Henl, R. Differential Control of Learning and Anxiety along the Dorsoventral Axis of the Dentate Gyrus. Neuron 2013, 77, 955–968. [Google Scholar] [CrossRef] [PubMed]
- Lever, C.; Burton, S.; O’Keefe, J. Rearing on Hind Legs, Environmental Novelty, and the Hippocampal Formation. Rev. Neurosci. 2006, 17, 111–133. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Shou, J.; Abera, S.; Ziff, E.B. Sucrose Withdrawal Induces Depression and Anxiety-like Behavior by Kir2.1 Upregulation in the Nucleus Accumbens. Neuropharmacology 2018, 130, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Chepulis, L.M.; Starkey, N.J.; Waas, J.R.; Molan, P.C. The Effects of Long-Term Honey, Sucrose or Sugar-Free Diets on Memory and Anxiety in Rats. Physiol. Behav. 2009, 97, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Bruel-Jungerman, E.; Veyrac, A.; Dufour, F.; Horwood, J.; Laroche, S.; Davis, S. Inhibition of PI3K-Akt Signaling Blocks Exercise-Mediated Enhancement of Adult Neurogenesis and Synaptic Plasticity in the Dentate Gyrus. PLoS ONE 2009, 4, e7901. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Tong, L.; Balazs, R.; Cotman, C.W. Physical Activity Elicits Sustained Activation of the Cyclic AMP Response Element-Binding Protein and Mitogen-Activated Protein Kinase in the Rat Hippocampus. Neuroscience 2001, 107, 219–229. [Google Scholar] [CrossRef]
- English, J.D.; Sweatt, J.D. A Requirement for the Mitogen-Activated Protein Kinase Cascade in Hippocampal Long Term Potentiation. J. Biol. Chem. 1997, 272, 19103–19106. [Google Scholar] [CrossRef]
- Pinnock, S.B.; Herbert, J. Brain-Derived Neurotropic Factor and Neurogenesis in the Adult Rat Dentate Gyrus: Interactions with Corticosterone. Eur. J. Neurosci. 2008, 27, 2493–2500. [Google Scholar] [CrossRef] [PubMed]
- Shohayeb, B.; Diab, M.; Ahmed, M.; Ng, D.C.H. Factors That Influence Adult Neurogenesis as Potential Therapy. Transl. Neurodegener. 2018, 7, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, H.G.; Biebl, M.; Wilhelm, D.; Li, M.; Friedlander, R.M.; Winkler, J. Increased Generation of Granule Cells in Adult Bcl-2-Overexpressing Mice: A Role for Cell Death during Continued Hippocampal Neurogenesis. Eur. J. Neurosci. 2005, 22, 1907–1915. [Google Scholar] [CrossRef] [PubMed]
- McCormick, C.M.; Nixon, F.; Thomas, C.; Lowie, B.; Dyck, J. Hippocampal Cell Proliferation and Spatial Memory Performance after Social Instability Stress in Adolescence in Female Rats. Behav. Brain Res. 2010, 208, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Jessberger, S.; Zhao, C.; Toni, N.; Clemenson, G.D.; Li, Y.; Gage, F.H. Seizure-Associated, Aberrant Neurogenesis in Adult Rats Characterized with Retrovirus-Mediated Cell Labeling. J. Neurosci. 2007, 27, 9400–9407. [Google Scholar] [CrossRef] [PubMed]
- Jessberger, S.; Nakashima, K.; Clemenson, G.D.; Mejia, E.; Mathews, E.; Ure, K.; Ogawa, S.; Sinton, C.M.; Gage, F.H.; Hsieh, J. Epigenetic Modulation of Seizure-Induced Neurogenesis and Cognitive Decline. J. Neurosci. 2007, 27, 5967–5975. [Google Scholar] [CrossRef]
- Zou, D.; Chen, L.; Deng, D.; Jiang, D.; Dong, F.; McSweeney, C.; Zhou, Y.; Liu, L.; Chen, G.; Wu, Y.; et al. DREADD in Parvalbumin Interneurons of the Dentate Gyrus Modulates Anxiety, Social Interaction and Memory Extinction. Curr. Mol. Med. 2016, 16, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Walf, A.A.; Frye, C.A. The Use of the Elevated plus Maze as an Assay of Anxiety-Related Behavior in Rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Cisternas, P.; Salazar, P.; Serrano, F.G.; Montecinos-Oliva, C.; Arredondo, S.B.; Varela-Nallar, L.; Barja, S.; Vio, C.P.; Gomez-Pinilla, F.; Inestrosa, N.C. Fructose Consumption Reduces Hippocampal Synaptic Plasticity Underlying Cognitive Performance. Biochim. Biophys. Acta 2015, 1852, 2379–2390. [Google Scholar] [CrossRef] [PubMed]
- Cox, B.M.; Shah, M.M.; Cichon, T.; Tancer, M.E.; Galloway, M.P.; Thomas, D.M.; Perrine, S.A. Behavioral and Neurochemical Effects of Repeated MDMA Administration during Late Adolescence in the Rat. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 48, 229–235. [Google Scholar] [CrossRef]
- Sánchez-Huerta, K.; García-Martínez, Y.; Vergara, P.; Segovia, J.; Pacheco-Rosado, J. Thyroid Hormones Are Essential to Preserve Non-Proliferative Cells of Adult Neurogenesis of the Dentate Gyrus. Mol. Cell Neurosci. 2016, 76, 1–10. [Google Scholar] [CrossRef] [PubMed]
- García-Martinez, Y.; Sánchez-Huerta, K.B.; Pacheco-Rosado, J. Quantitative Characterization of Proliferative Cells Subpopulations in the Hilus of the Hippocampus of Adult Wistar Rats: An Integrative Study. J. Mol. Histol. 2020, 51, 437–453. [Google Scholar] [CrossRef]
- Filippov, V.; Kronenberg, G.; Pivneva, T.; Reuter, K.; Steiner, B.; Wang, L.P.; Yamaguchi, M.; Kettenmann, H.; Kempermann, G. Subpopulation of Nestin-Expressing Progenitor Cells in the Adult Murine Hippocampus Shows Electrophysiological and Morphological Characteristics of Astrocytes. Mol. Cell. Neurosci. 2003, 23, 373–382. [Google Scholar] [CrossRef]
- Seri, B.; García-Verdugo, J.M.; McEwen, B.S.; Alvarez-Buylla, A. Astrocytes Give Rise to New Neurons in the Adult Mammalian Hippocampus. J. Neurosci. 2001, 21, 7153–7160. [Google Scholar] [CrossRef]
- Lugert, S.; Basak, O.; Knuckles, P.; Haussler, U.; Fabel, K.; Götz, M.; Haas, C.A.; Kempermann, G.; Taylor, V.; Giachino, C. Quiescent and Active Hippocampal Neural Stem Cells with Distinct Morphologies Respond Selectively to Physiological and Pathological Stimuli and Aging. Cell Stem. Cell 2010, 6, 445–456. [Google Scholar] [CrossRef]
- Steiner, B.; Klempin, F.; Wang, L.; Kott, M.; Kettenmann, H.; Kempermann, G. Type-2 Cells as Link between Glial and Neuronal Lineage in Adult Hippocampal Neurogenesis. Glia 2006, 54, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, G.; Reuter, K.; Steiner, B.; Brandt, M.D.; Jessberger, S.; Yamaguchi, M.; Kempermann, G. Subpopulations of Proliferating Cells of the Adult Hippocampus Respond Differently to Physiologic Neurogenic Stimuli. J. Comp. Neurol. 2003, 467, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Plümpe, T.; Ehninger, D.; Steiner, B.; Klempin, F.; Jessberger, S.; Brandt, M.; Römer, B.; Rodriguez, G.R.; Kronenberg, G.; Kempermann, G. Variability of Doublecortin-Associated Dendrite Maturation in Adult Hippocampal Neurogenesis Is Independent of the Regulation of Precursor Cell Proliferation. BMC Neurosci. 2006, 7. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, H.M. Developmental Regulation of the Growth Plate. Nature 2003, 423, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Huerta, K.; Saldaña-Salinas, R.D.; Bustamante-Nieves, P.E.; Jiménez, A.; Corzo-Cruz, A.; Martínez-Vargas, M.; Guevara-Guzmán, R.; Velasco, I.; Estudillo, E. Sucrose Consumption during Late Adolescence Impairs Adult Neurogenesis of the Ventral Dentate Gyrus without Inducing an Anxiety-like Behavior. Int. J. Mol. Sci. 2022, 23, 14176. https://doi.org/10.3390/ijms232214176
Sánchez-Huerta K, Saldaña-Salinas RD, Bustamante-Nieves PE, Jiménez A, Corzo-Cruz A, Martínez-Vargas M, Guevara-Guzmán R, Velasco I, Estudillo E. Sucrose Consumption during Late Adolescence Impairs Adult Neurogenesis of the Ventral Dentate Gyrus without Inducing an Anxiety-like Behavior. International Journal of Molecular Sciences. 2022; 23(22):14176. https://doi.org/10.3390/ijms232214176
Chicago/Turabian StyleSánchez-Huerta, Karla, Rosaura Debbie Saldaña-Salinas, Pablo Edson Bustamante-Nieves, Adriana Jiménez, Alejandro Corzo-Cruz, Marina Martínez-Vargas, Rosalinda Guevara-Guzmán, Iván Velasco, and Enrique Estudillo. 2022. "Sucrose Consumption during Late Adolescence Impairs Adult Neurogenesis of the Ventral Dentate Gyrus without Inducing an Anxiety-like Behavior" International Journal of Molecular Sciences 23, no. 22: 14176. https://doi.org/10.3390/ijms232214176
APA StyleSánchez-Huerta, K., Saldaña-Salinas, R. D., Bustamante-Nieves, P. E., Jiménez, A., Corzo-Cruz, A., Martínez-Vargas, M., Guevara-Guzmán, R., Velasco, I., & Estudillo, E. (2022). Sucrose Consumption during Late Adolescence Impairs Adult Neurogenesis of the Ventral Dentate Gyrus without Inducing an Anxiety-like Behavior. International Journal of Molecular Sciences, 23(22), 14176. https://doi.org/10.3390/ijms232214176





