A Combination of Rosa Multiflora and Zizyphus Jujuba Enhance Sleep Quality in Anesthesia-Induced Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. High-Performance Thin-Layer Liquid Chromatography and HPLC/liquid Chromatography-Mass Spectrometry (LC-MS)
2.3. Animal Maintenance
2.4. Tiletamine-Zolazepam/Xylazine-Induced Sleep Experiment in Mouse
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Cellular Experiment
2.7. Open-Field Test
2.8. Statistical Analysis
3. Results
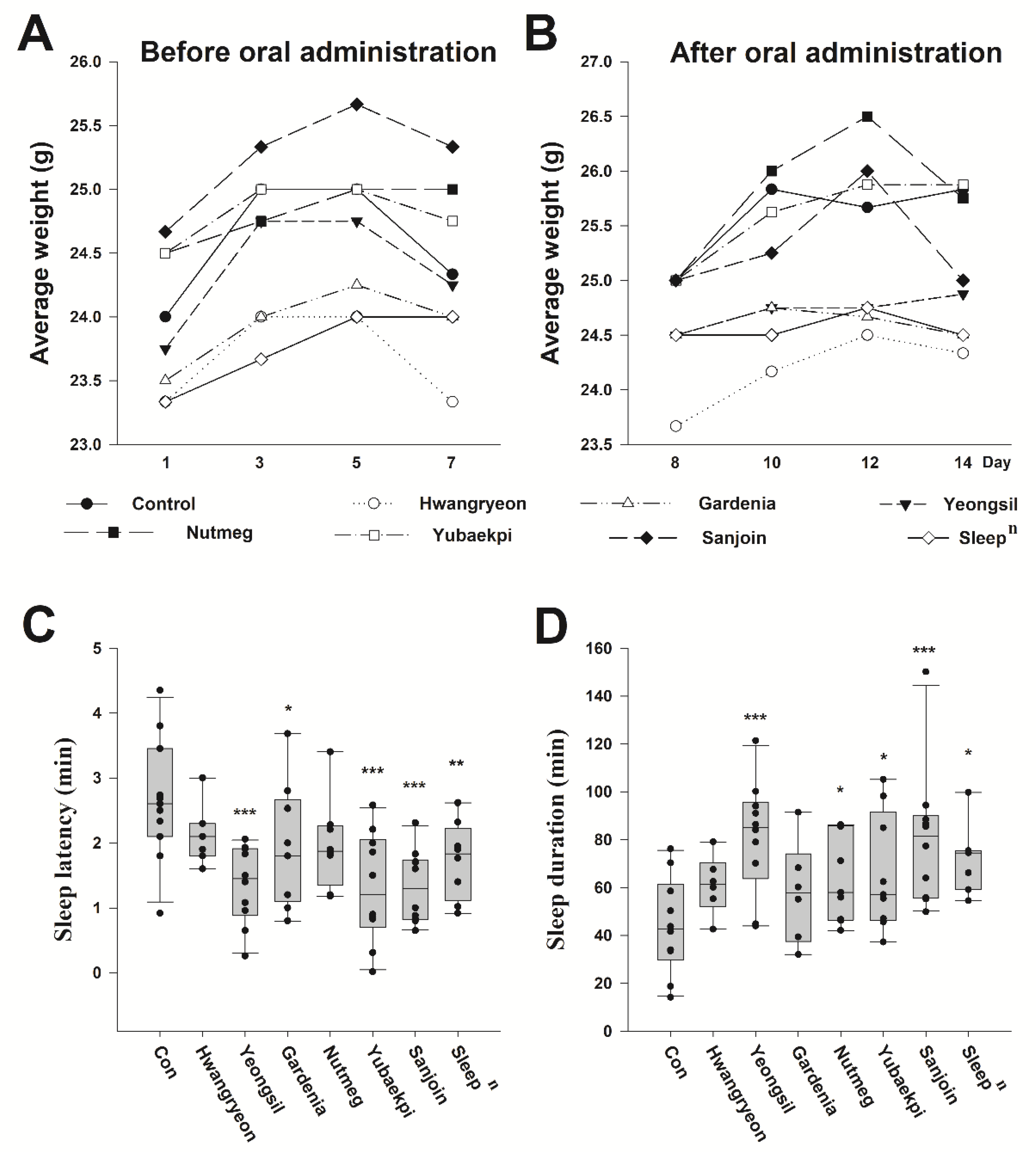
3.1. HPLC/Liquid Chromatography–Mass Spectrometry Analysis and Tiletamine-Zolazepam/Xylazine Sleep Induction Model Mouse for Zizyphus Jujuba Miller (Sanjoin)/Rosa Multiflora Thunb (Yeongsil) Natural Combination Product
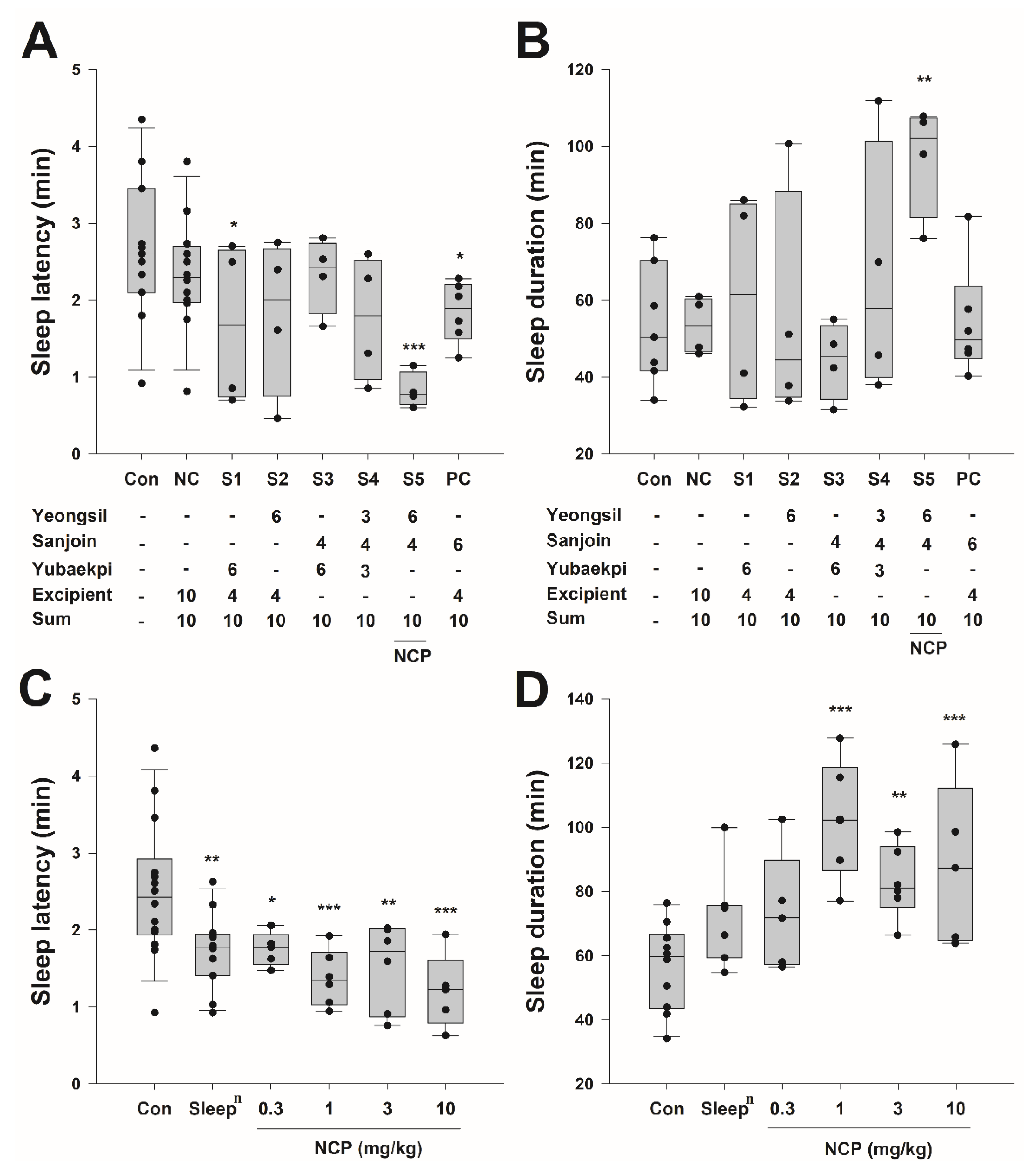
3.2. Selection of Natural Product Candidates for Improving Sleep and Biological Changes in Mice Following Oral Administration of Traditional Korean Medicine
3.3. Mouse Sleep Experiment by Concentration for Each Combination of Traditional Korean Medicine
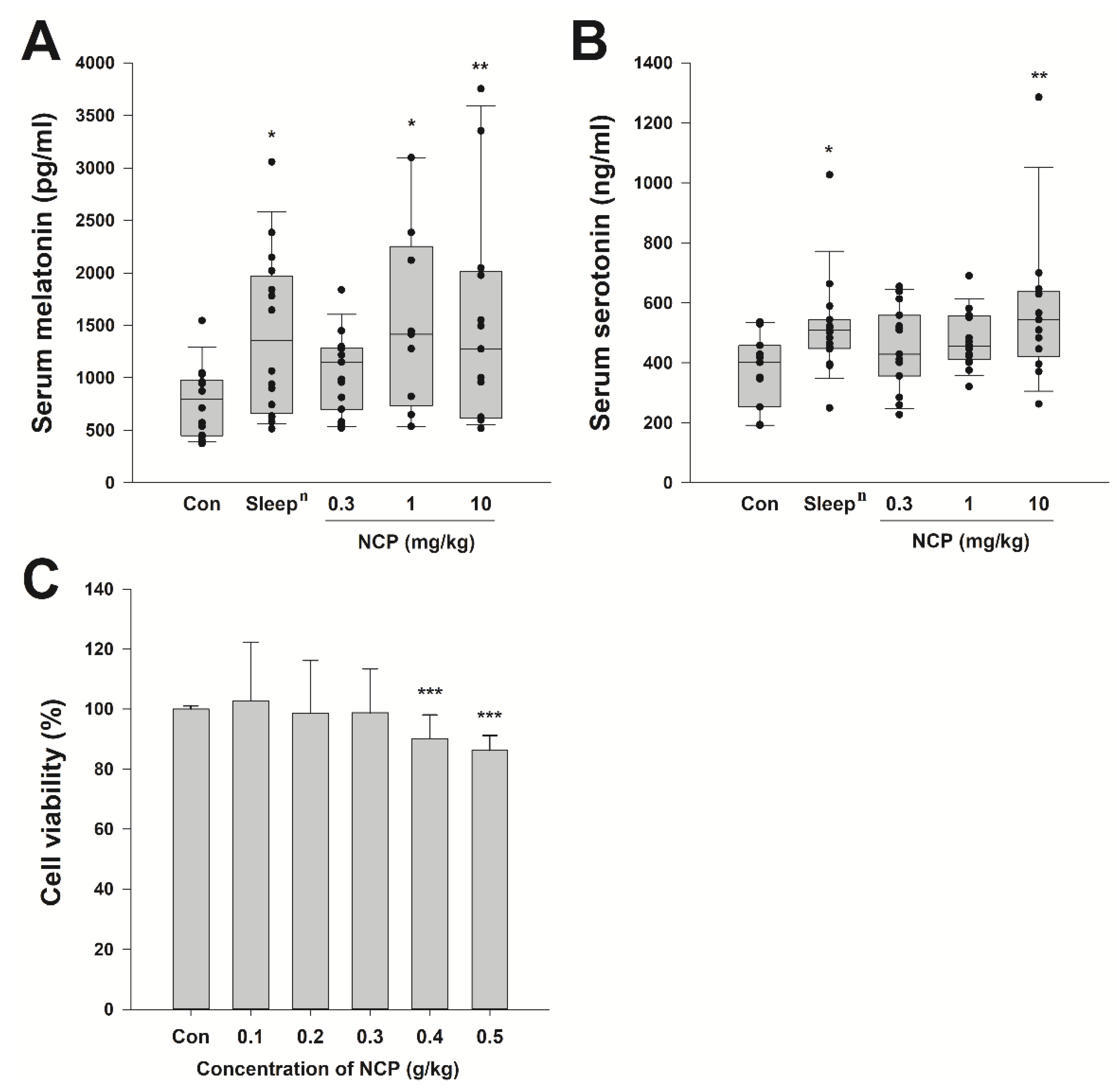
3.4. Blood Melatonin and Serotonin Concentrations and Cytotoxicity of Mice That Ingested with Optimal NCP Ingredients
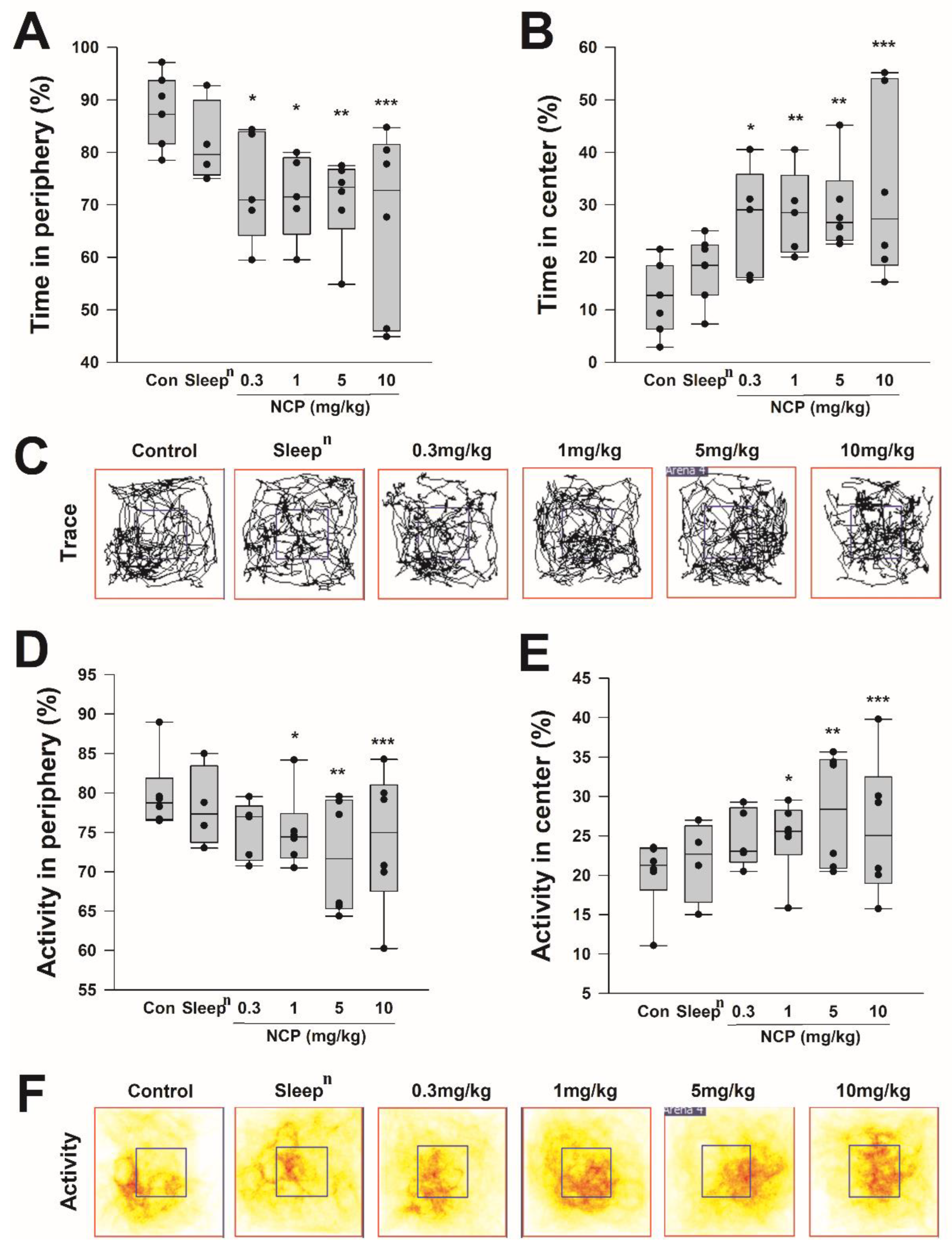
3.5. Open-Field Test According to NCP Intake
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Hillman, D.; Mitchell, S.; Streatfeild, J.; Burns, C.; Bruck, D.; Pezzullo, L. The economic cost of inadequate sleep. Sleep 2018, 41, zsy083. [Google Scholar] [CrossRef] [PubMed]
- Streatfeild, J.; Smith, J.; Mansfield, D.; Pezzullo, L.; Hillman, D. The social and economic cost of sleep disorders. Sleep 2021, 44, zsab132. [Google Scholar] [CrossRef] [PubMed]
- Van Dongen, H.; Maislin, G.; Mullington, J.M.; Dinges, D.F. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep 2003, 26, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Ancoli-Israel, S.; Cole, R.; Alessi, C.; Chambers, M.; Moorcroft, W.; Pollak, C.P. The role of actigraphy in the study of sleep and circadian rhythms. Sleep 2003, 26, 342–392. [Google Scholar] [CrossRef] [PubMed]
- Shepertycky, M.R.; Banno, K.; Kryger, M.H. Differences between men and women in the clinical presentation of patients diagnosed with obstructive sleep apnea syndrome. Sleep 2005, 28, 309–314. [Google Scholar]
- Ohayon, M.M.; Carskadon, M.A.; Guilleminault, C.; Vitiello, M.V. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: Developing normative sleep values across the human lifespan. Sleep 2004, 27, 1255–1273. [Google Scholar] [CrossRef]
- Hwang, L.; Ko, I.-G.; Jin, J.-J.; Kim, S.-H.; Kim, C.-J.; Chang, B.; Rho, J.H.; Moon, E.-J.; Yi, J.-W. Dexmedetomidine ameliorates memory impairment in sleep-deprived mice. Anim. Cells Syst. 2019, 23, 371–379. [Google Scholar] [CrossRef]
- Cheong, E.; Shin, H.-S. T-type Ca2+ channels in normal and abnormal brain functions. Physiol. Rev. 2013, 93, 961–992. [Google Scholar] [CrossRef]
- Crunelli, V.; David, F.; Leresche, N.; Lambert, R.C. Role for T-type Ca2+ channels in sleep waves. Pflügers Arch.-Eur. J. Physiol. 2014, 466, 735–745. [Google Scholar] [CrossRef]
- Paulose, J.K.; Wang, C.; O’Hara, B.F.; Cassone, V.M. The effects of aging on sleep parameters in a healthy, melatonin-competent mouse model. Nat. Sci. Sleep 2019, 11, 113. [Google Scholar] [CrossRef]
- Moon, E.; Partonen, T.; Beaulieu, S.; Linnaranta, O. Melatonergic agents influence the sleep-wake and circadian rhythms in healthy and psychiatric participants: A systematic review and meta-analysis of randomized controlled trials. Neuropsychopharmacology 2022, 47, 1523–1536. [Google Scholar] [CrossRef] [PubMed]
- Chellappa, S.L. Individual differences in light sensitivity affect sleep and circadian rhythms. Sleep 2021, 44, zsaa214. [Google Scholar] [CrossRef] [PubMed]
- Omond, S.E.; Hale, M.W.; Lesku, J.A. Neurotransmitters of sleep and wakefulness in flatworms. Sleep 2022, 45, zsac053. [Google Scholar] [CrossRef] [PubMed]
- Auld, F.; Maschauer, E.L.; Morrison, I.; Skene, D.J.; Riha, R.L. Evidence for the efficacy of melatonin in the treatment of primary adult sleep disorders. Sleep Med. Rev. 2017, 34, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, M.R.; McKenna, J.T.; McCarley, R.W. Functions and mechanisms of sleep. AIMS Neurosci. 2016, 3, 67. [Google Scholar] [CrossRef] [PubMed]
- Eban-Rothschild, A.; Appelbaum, L.; de Lecea, L. Neuronal mechanisms for sleep/wake regulation and modulatory drive. Neuropsychopharmacology 2018, 43, 937–952. [Google Scholar] [CrossRef]
- Mackiewicz, M.; Naidoo, N.; Zimmerman, J.E.; Pack, A.I. Molecular mechanisms of sleep and wakefulness. Ann. N. Y. Acad. Sci. 2008, 1129, 335–349. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; Trakht, I.; Srinivasan, V.; Spence, D.W.; Maestroni, G.J.; Zisapel, N.; Cardinali, D.P. Physiological effects of melatonin: Role of melatonin receptors and signal transduction pathways. Prog. Neurobiol. 2008, 85, 335–353. [Google Scholar] [CrossRef]
- Harrison, N.L. Mechanisms of Sleep Induction by GABA. J. Clin. Psychiatry 2007, 68, 6–12. [Google Scholar]
- Caumo, W.; Hidalgo, M.P.; Souza, A.; Torres, I.L.; Antunes, L.C. Melatonin is a biomarker of circadian dysregulation and is correlated with major depression and fibromyalgia symptom severity. J. Pain Res. 2019, 12, 545. [Google Scholar] [CrossRef]
- Watson, N.F.; Rosen, I.M.; Chervin, R.D. The past is prologue: The future of sleep medicine. J. Clin. Sleep Med. 2017, 13, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Zisapel, N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br. J. Pharmacol. 2018, 175, 3190–3199. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, N.; Uddin, N.; Khan, M.K.U.; Umer, M.; Ali, N.; Ullah, S. Traditional and cultural uses of medicinal plant species in the flora of Kuz Abakhel, for the treatment of various ailments. Adv. Tradit. Med. 2021, 21, 591–607. [Google Scholar] [CrossRef]
- Luo, H.; Sun, S.-j.; Wang, Y.; Wang, Y.-l. Revealing the sedative-hypnotic effect of the extracts of herb pair Semen Ziziphi spinosae and Radix Polygalae and related mechanisms through experiments and metabolomics approach. BMC Complement. Med. Ther. 2020, 20, 1–15. [Google Scholar] [CrossRef]
- Shergis, J.L.; Ni, X.; Sarris, J.; Zhang, A.L.; Guo, X.; Xue, C.C.; Lu, C.; Hugel, H. Ziziphus spinosa seeds for insomnia: A review of chemistry and psychopharmacology. Phytomedicine 2017, 34, 38–43. [Google Scholar] [CrossRef]
- Lin, A.; Shih, C.-T.; Huang, C.-L.; Wu, C.-C.; Lin, C.-T.; Tsai, Y.-C. Hypnotic effects of lactobacillus fermentum PS150TM on pentobarbital-induced sleep in mice. Nutrients 2019, 11, 2409. [Google Scholar] [CrossRef]
- Svorc, P.; Bacova, I.; Gresova, S.; Svorc Jr, P. Chronobiological perspectives on myocardial electrophysiological parameters under three types of general anaesthesia in a rat model. Biol. Rhythm Res. 2017, 48, 343–351. [Google Scholar] [CrossRef]
- Riemann, D.; Baglioni, C.; Bassetti, C.; Bjorvatn, B.; Dolenc Groselj, L.; Ellis, J.G.; Espie, C.A.; Garcia-Borreguero, D.; Gjerstad, M.; Gonçalves, M. European guideline for the diagnosis and treatment of insomnia. J. Sleep Res. 2017, 26, 675–700. [Google Scholar] [CrossRef]
- Lie, J.D.; Tu, K.N.; Shen, D.D.; Wong, B.M. Pharmacological treatment of insomnia. Pharm. Ther. 2015, 40, 759. [Google Scholar]
- Hua, Y.; Guo, S.; Xie, H.; Zhu, Y.; Yan, H.; Tao, W.W.; Shang, E.-X.; Qian, D.-W.; Duan, J. Ziziphus jujuba Mill. var. spinosa (Bunge) Hu ex HF Chou seed ameliorates insomnia in rats by regulating metabolomics and intestinal flora composition. Front. Pharmacol. 2021, 12, 1513. [Google Scholar] [CrossRef]
- Chu, Q.-P.; Wang, L.-E.; Cui, X.-Y.; Fu, H.-Z.; Lin, Z.-B.; Lin, S.-Q.; Zhang, Y.-H. Extract of Ganoderma lucidum potentiates pentobarbital-induced sleep via a GABAergic mechanism. Pharmacol. Biochem. Behav. 2007, 86, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Shergis, J.L.; Hyde, A.; Meaklim, H.; Varma, P.; Da Costa, C.; Jackson, M.L. Medicinal seeds Ziziphus spinosa for insomnia: A randomized, placebo-controlled, cross-over, feasibility clinical trial. Complement. Ther. Med. 2021, 57, 102657. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, Y.-P.; Liu, H.-Y.; Gu, J.-H.; Zhou, X.-F.; Yue-Qin, Z. Long-term oral administration of hyperoside ameliorates AD-related neuropathology and improves cognitive impairment in APP/PS1 transgenic mice. Neurochem. Int. 2021, 151, 105196. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Bai, K.; Liu, X.-H.; Zhang, L.-M.; Yu, G.-R. Hyperoside protects the blood-brain barrier from neurotoxicity of amyloid beta 1–42. Neural Regen. Res. 2018, 13, 1974. [Google Scholar] [PubMed]
- Juergenliemk, G.; Boje, K.; Huewel, S.; Lohmann, C.; Galla, H.-J.; Nahrstedt, A. In vitro studies indicate that Miquelianin (Quercetin 3-O-ß-D-Glucuronopyranoside) is able to reach the CNS from the small intestine. Planta Med. 2003, 69, 1013–1017. [Google Scholar] [PubMed]
- Fricker, G. Drug interactions with natural products at the blood brain barrier. Curr. Drug Metab. 2008, 9, 1019–1026. [Google Scholar] [CrossRef]
- Dubocovich, M.L. Melatonin receptors: Role on sleep and circadian rhythm regulation. Sleep Med. 2007, 8, 34–42. [Google Scholar] [CrossRef]
- Rosenberg, R.S.; Van Hout, S. The American Academy of Sleep Medicine inter-scorer reliability program: Sleep stage scoring. J. Clin. Sleep Med. 2013, 9, 81–87. [Google Scholar] [CrossRef]
- Lee, J.; Kim, D.; Shin, H.-S. Lack of delta waves and sleep disturbances during non-rapid eye movement sleep in mice lacking α1G-subunit of T-type calcium channels. Proc. Natl. Acad. Sci. USA 2004, 101, 18195–18199. [Google Scholar] [CrossRef]
- Yu, J.; Chen, J.; Hu, T.; Guo, S.; Wang, X.; Guo, Y.; Chen, X.; Zhao, C. Effect of Electro-Acupuncture on Expression of Circadian Clock Gene Per2 and Bmal1 in Sleep Deprivation Rat. Chin. Med. 2016, 7, 16–24. [Google Scholar] [CrossRef][Green Version]
- Yi, F.; Tan, X.-l.; Yan, X.; Liu, H.-b. In silico profiling for secondary metabolites from Lepidium meyenii (maca) by the pharmacophore and ligand-shape-based joint approach. Chin. Med. 2016, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Balsalobre, A.; Damiola, F.; Schibler, U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell 1998, 93, 929–937. [Google Scholar] [CrossRef]
- Mirick, D.K.; Davis, S. Melatonin as a biomarker of circadian dysregulation. Cancer Epidemiol. Prev. Biomark. 2008, 17, 3306–3313. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eom, S.; Lee, S.; Lee, J.; Sohn, S.-O.; Lee, J.H.; Park, J. A Combination of Rosa Multiflora and Zizyphus Jujuba Enhance Sleep Quality in Anesthesia-Induced Mice. Int. J. Mol. Sci. 2022, 23, 14177. https://doi.org/10.3390/ijms232214177
Eom S, Lee S, Lee J, Sohn S-O, Lee JH, Park J. A Combination of Rosa Multiflora and Zizyphus Jujuba Enhance Sleep Quality in Anesthesia-Induced Mice. International Journal of Molecular Sciences. 2022; 23(22):14177. https://doi.org/10.3390/ijms232214177
Chicago/Turabian StyleEom, Sanung, Shinhui Lee, Jiwon Lee, Sung-Oh Sohn, Junho H. Lee, and Jaeman Park. 2022. "A Combination of Rosa Multiflora and Zizyphus Jujuba Enhance Sleep Quality in Anesthesia-Induced Mice" International Journal of Molecular Sciences 23, no. 22: 14177. https://doi.org/10.3390/ijms232214177
APA StyleEom, S., Lee, S., Lee, J., Sohn, S.-O., Lee, J. H., & Park, J. (2022). A Combination of Rosa Multiflora and Zizyphus Jujuba Enhance Sleep Quality in Anesthesia-Induced Mice. International Journal of Molecular Sciences, 23(22), 14177. https://doi.org/10.3390/ijms232214177





